Abstract
Background
In October 2012, a maternal pertussis vaccination program was introduced in England for women between 28 and 32 weeks of pregnancy. In April 2016, the recommended optimal window was extended to 20–32 weeks to improve vaccine coverage and protect preterm infants. This study assesses the impact of offering maternal pertussis vaccination earlier in pregnancy on hospitalized infant pertussis cases.
Methods
Hospitalized pertussis cases ≤60 days old in England were extracted from Hospital Episode Statistics pre- and post-policy change. Data were linked to laboratory-confirmed cases, and clinical records were reviewed where cases were not matched. Maternal vaccine status of identified cases was established. Median hospital duration was calculated, and a competing risk survival analysis was undertaken to assess multiple factors.
Results
A total of 201 cases were included in the analysis. Of the 151 cases with reported gestational age, the number of hospitalizations among full-term infants was 60 cases pre-policy and 62 cases post-policy, respectively, while preterm cases declined from 20 to 9 (P = .06). Length of hospital stay did not differ significantly after the policy change. Significantly longer hospital stays were seen in cases aged 0–4 weeks (median of 3 more days than infants aged 5–8 weeks), premature infants (median of 4 more days than term infants), and cases with coinfections (median of 1 more day than those without coinfection).
Conclusions
The number of preterm infants hospitalized with pertussis in England was halved after the policy change and preterm infants were no longer overrepresented among hospitalized cases.
Keywords: maternal pertussis, vaccine, immunization, whooping cough, hospitalization
This study evaluates the impact of offering pertussis vaccination earlier in pregnancy in the second trimester on hospitalized infant pertussis cases in England. We found a decline in hospitalizations in preterm infants from 20 (18.5%) to 9 (9.7%) (P = .06).
(See the Editorial Commentary by Abu-Raya on pages e2509–11.)
Whooping cough (pertussis) is an infectious respiratory disease caused primarily by Bordetella pertussis. Pertussis is severe among infants less than 3 months old who have not been fully protected through primary vaccination [1, 2].
In April 2012, a pertussis outbreak was declared in England and the Department of Health introduced an emergency program to vaccinate women in every pregnancy (between 28 and 32 weeks of pregnancy) with a dose of acellular pertussis–containing vaccine. England was the first country to demonstrate high levels of protection against pertussis disease conferred to infants in the first 2 months of life through maternal vaccination [3]. Several studies from different countries and a 3-year evaluation of the program in England found maternal pertussis vaccination to be safe and effective, protecting young infants against disease, hospitalization, and death [4–6]. Many countries have implemented maternal immunization programs as supplementary strategies to routine childhood immunization as recommended by the World Health Organization, but the timings of vaccination during pregnancy vary [7, 8].
Most countries with a maternal program recommend third-trimester vaccination, when transplacental antibody transfer is maximized [9–12]. In April 2016, the optimal window for offering vaccine in the UK program was extended from 28–32 weeks to 20–32 weeks, although women could receive the vaccine from 16 weeks. This was informed by data suggesting that vaccinating mothers in the early second trimester would significantly increase neonatal antibodies against B. pertussis compared with in the third trimester and could improve seroprotection [13]. Further research indicated that vaccinating mothers from 28 weeks (in the third trimester) may increase protection in infants born prematurely compared with unvaccinated mothers [14]. Previous analyses based on hospital admissions in England, prior to the change in timing recommendations, demonstrated a program impact on the burden of disease requiring hospitalization in term infants. However, preterm infants were not benefiting to the same extent, with proportionately more pertussis hospitalizations than full-term infants [15]. Offering the vaccine earlier provided women with more immunization opportunities, potentially increasing uptake and increasing the likelihood of vaccination before preterm delivery. The maternal immunization program in England also switched from RepevaxTM (Td5aP-IPV) to Boostrix-IPVTM (Td3aP-IPV) from July 2014 due to supply issues and this latter vaccine was supplied for the program throughout the surveillance period.
This study evaluates the impact of the change in timing of maternal pertussis vaccination on the burden of hospitalized pertussis cases in preterm and full-term infants aged 60 days old or less in England, before the effect of the first infant dose offered from 56 days of age.
METHODS
Hospital Episode Statistics Data for Pertussis Diagnoses
Data on pertussis hospital admissions in England were obtained from the Hospital Episode Statistics (HES) database. The HES database contains details of all National Health Service (NHS) hospital admissions in England [16]. Patients aged 60 days or younger hospitalized with a pertussis code prefix A37 (International Classification of Diseases, 10th revision [ICD-10]) between 1 September 2014 and 31 March 2016, and between 1 September 2016 and 31 March 2018 were extracted from HES [17]. The time periods selected were identical by months of inclusion in view of pertussis seasonality, which peaks in quarter 3 [18]. Pertussis is a cyclical disease that increases every 3–4 years and this study period encompassed a peak year in 2016. Part of 2016 was included in each period. September 2016 was chosen as the earliest date of inclusion to allow the first infants whose mothers were eligible for earlier vaccination to be born. Patient information extracted from HES included the following: date of birth, admission date, discharge date, age at admission, sex, ethnic group, and spell duration. Cases coded as being treated by a consultant with an intensive care unit (ICU) specialty (MAINSPEF 192), being treated with critical care medicine or in a pediatric intensive care unit (TRETSPEF 192 and 242), and those with respiratory Primary Procedure (OPCS) codes with prefixes E85, E89, X58, or X52 were considered as being admitted to ICU [19]. All cases admitted under ordinary admissions, day case admissions, or mothers and infants using only delivery facilities (CLASSPAT 1 and 2) were included in this study.
Enhanced Surveillance of Laboratory-confirmed Pertussis
Under the Health Protection (Notifications) Regulations (2010), all diagnostic laboratories in England are required to notify Public Health England (PHE) when they identify specific infections, including B. pertussis [20]. The PHE maintains the national dataset of laboratory-confirmed pertussis cases, and every infant case in the study was confirmed by culture or polymerase chain reaction (PCR) testing and followed up with the patient’s general practitioner (GP) to collect clinical and epidemiological information, such as gestational age at birth, clinical presentation, coinfections, and maternal vaccination history.
Patients extracted from HES were matched to laboratory-confirmed cases using their NHS number. When a case extracted from HES could not be matched to a laboratory-confirmed case, a form was sent to the GP to request additional information, the hospital discharge summary, and the mother’s vaccination status [6]. Returned forms were independently reviewed by 2 clinicians using a prespecified case definition, to assess whether diagnoses were consistent with pertussis. Cases considered consistent with pertussis were included in the analyses.
In addition to coinfection details on the surveillance forms, ICD-10 diagnostic codes were extracted from HES 30 days before and after each included pertussis case. The ICD-10 codes were reviewed by a clinician, and respiratory infections (excluding pertussis) or clinical syndromes were considered coinfections and included in the analysis. The Second Generation Surveillance System (SGSS) was used to identify additional laboratory-confirmed coinfections using the same approach based on respiratory infections (PCR and culture). The SGSS stores and manages data on laboratory isolates and routine surveillance data on infectious diseases across England [21].
Descriptive and Statistical Analyses Laboratory- and Clinically Confirmed Pertussis Cases
Hospitalized cases included were categorized as follows: 0 to 4 weeks or less and 5 or more to 8 weeks based on their age at first hospital admission; preterm (<37 weeks), full-term (≥37 weeks), and not known; having no coinfections and 1 or more coinfections; admitted to ICU or not admitted to ICU; and by white British, other ethnicity, or ethnicity not known. Information on the cases’ mothers were categorized as mother immunized, not immunized, or not known. Whether the mother was vaccinated against pertussis in a previous pregnancy, regardless of the current pertussis vaccination status, was recorded.
Fisher’s exact test was used to compare proportions pre- and post-policy change for each case characteristic: gender, age at admission, gestational age at birth, coinfection or single infection, ICU admission, ethnicity, maternal vaccine status, and vaccine failure. The proportions of white British ethnicity pre- and post-policy change were compared with the 2016 Office for National Statistics (ONS) birth statistics [22] using Fisher’s exact test.
Hospital duration was calculated by summing the total number of hospital spells with a first admission date between 1 September 2014 and 31 March 2016 and between 1 September 2016 and 31 March 2018 with any diagnostic field coded to pertussis for each case. This included cases discharged from the hospital and readmitted with pertussis within the defined study period and coded for pertussis in a diagnostic field. Each spell was defined as the difference in days between the hospital admission date and the discharge date [19]. Cases with a duration of 0 were recoded to 0.5 days as they spent less than 24 hours in the hospital.
Duration of stay was compared with case characteristics by calculating mean, median, and interquartile range (IQR) duration in days, as described in previous papers [15, 23]. A competing risk regression analysis (survival on time to discharge) [24] was conducted where the competing event was death of a case. Hazard ratios (HRs) represent the relative time to final discharge; an HR less than 1 relates to a longer duration of stay compared with the baseline group. Univariate competing risk models were conducted, and a multivariable model was adjusted for the policy change (categorical variable) and all significant characteristics from the univariable models. Analyses were conducted in STATA 15.0 (StataCorp). A post hoc analysis was conducted excluding gestational age at birth to determine whether there was a reduction in duration of stay post-policy change, mediated through the reduction in premature cases.
RESULTS
Descriptive Results of All Hospital Episode Statistics Cases
A total of 346 infants aged 60 days or less were recorded in the HES dataset with a diagnostic code of pertussis between 1 September 2014 and 31 March 2016 (pre-policy change), and from 1 September 2016 to 31 March 2018 (post-policy change). A total of 189 (54.6%) cases were admitted prior to the policy change and 157 (45.4%) cases were admitted after the policy change. The distribution of infants recorded in HES in the 2016 peak year was similar between 1 January and 31 March 2016 (n = 20, average = 6.7 hospitalizations per month) and 1 September–31 December 2016 (n = 21, average = 7.0 hospitalizations per month).
Descriptive Results of Confirmed Pertussis Cases
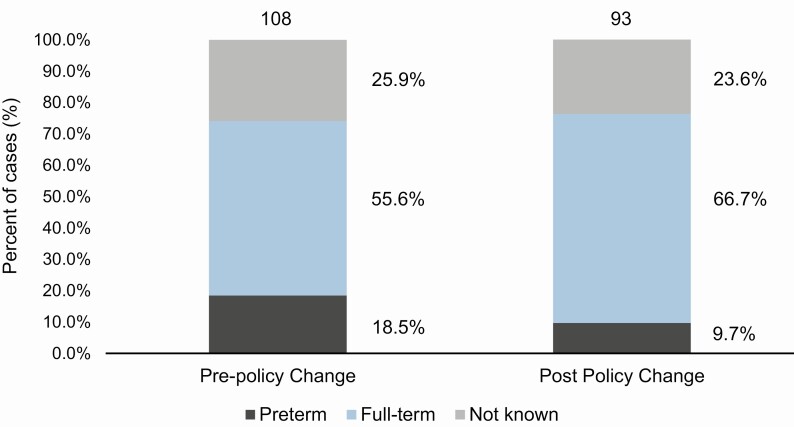
A total of 164 of 346 (47.4%) HES cases were linked to laboratory-confirmed cases. Four cases were excluded due to missing GP details for follow-up. The remaining unlinked cases were reviewed by the 2 clinicians. Of the 178 cases reviewed, 37 additional cases were classified as consistent with pertussis based on clinical review, totaling 201 cases classified as pertussis; 108 infants were reported pre-policy change and 93 post-policy change (Figure 1).
Figure 1. .
Distribution of infants ≤60 days old by gestational age between 1 September 2014 and 31 March 2016 (pre-policy change) and 1 September 2016 and 31 March 2018 (post-policy change) in England.
Results from Fisher’s exact test comparing proportions pre-policy change and post-policy change showed no significant difference in gender and age at admission (Table 1). The proportion of white British cases was overrepresented pre-policy change compared with the ONS birth statistics (80.6% compared with 71.8%, P = .04). After the policy change, the proportion of white British cases was no longer significantly different from the ONS birth statistics (73.7% compared with 71.8%, P = .71) [22].
Table 1. .
Characteristics of Hospital Admissions in Infants ≤60 Days Old That Were Linked To Confirmed Pertussis Cases With Maternal Vaccination Information Between 1 September 2014 and 31 March 2016 and From 1 September 2016 to 31 March 2018 in England
| All Cases | Pre-Policy Change | Post-Policy Change | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | n | % (95% CI)a | n | % (95% CI)a | n | % (95% CI)a | Fisher’s Exact TestbP Value |
| Gender | |||||||
| Male | 88 | 43.8 (36.8–50.9) | 46 | 42.6 (33.1–52.5) | 42 | 45.2 (34.8–55.8) | .78 |
| Female | 113 | 56.2 (49.1–63.2) | 62 | 57.4 (47.5–66.9) | 51 | 54.8 (44.2–65.2) | |
| Admission week | |||||||
| ≤4 weeks | 44 | 21.9 (16.4–28.3) | 20 | 18.5 (11.7–27.1) | 24 | 25.8 (17.3–35.9) | .23 |
| ≥5 weeks | 157 | 78.1 (71.7–83.6) | 88 | 81.5 (72.9–88.3) | 69 | 74.2 (64.1–82.7) | |
| Gestational age at birth | |||||||
| Premature | 29 | 19.2 (13.3–26.4) | 20 | 25.0 (16.0–35.9) | 9 | 12.7 (6.0–22.7) | .06 |
| Full-term | 122 | 80.8 (73.6–86.7) | 60 | 75.0 (64.1–84.0) | 62 | 87.3 (77.3–94.0) | |
| Coinfections/clinical syndromes | |||||||
| Single infection | 115 | 57.2 (50.1–64.2) | 61 | 56.5 (46.6–66.0) | 54 | 58.1 (47.4–68.2) | .89 |
| Coinfection | 86 | 42.8 (35.8–49.9) | 47 | 43.5 (34.0–53.4) | 39 | 41.9 (31.8–52.6) | |
| Admitted to ICU | |||||||
| Admitted | 39 | 19.4 (14.2–25.6) | 24 | 22.2 (14.8–31.2) | 15 | 16.1 (9.3–25.2) | .29 |
| Not admitted | 162 | 28.6 (22.2–35.6) | 84 | 77.8 (68.8–85.2) | 78 | 83.9 (74.8–90.7) | |
| Ethnic group | .12 | ||||||
| White British | 135 | 71.4 (64.4–77.8) | 79 | 76.7 (41.7–84.5) | 56 | 65.1 (54.1–75.1) | |
| Other white ethnicity | 15 | 7.9 (4.5–12.8) | 5 | 4.9 (1.6– 11.0) | 10 | 11.6 (5.7–20.3) | |
| Other ethnicity | 39 | 20.6 (15.1–27.1) | 19 | 18.4 (11.5–27.3) | 20 | 23.3 (14.8–33.6) | |
| Mother immunized | |||||||
| Not immunized | 143 | 73.3 (66.5–79.4) | 74 | 71.2 (61.4–79.6) | 69 | 75.8 (65.7–84.2) | .52 |
| Immunized | 52 | 26.7 (20.6–33.5) | 30 | 28.8 (20.4–38.6) | 22 | 24.2 (15.8–34.3) | |
| Gestational age of mother when vaccinated | |||||||
| <28 weeks | 13 | 28.3 (16.0–43.5) | 1 | 3.7 (.9–19.0) | 12 | 63.2 (38.4–83.7) | <.001 |
| ≥28 weeks | 33 | 71.7 (56.5–84.0) | 26 | 96.3 (81.0–99.9) | 7 | 36.8 (16.3–61.6) | |
Abbreviations: CI, confidence interval; ICU, intensive care unit.
aDoes not include “not known.”
bFisher’s exact test comparing the program pre-policy change and post-policy change was used.
One hundred fifty-one cases (75.1%) had gestational age at birth recorded. Term cases were similar pre- to post-policy change, at 60 and 62, respectively, but decreased from 20 to 9 in preterm cases, meaning that the proportion of preterm cases decreased nonsignificantly (P = .06) from 20 of 80 (25%) to 9 of 71 (12.7%) (Table 1). However, before the policy change, 25% of pertussis cases were in preterm infants—significantly more than in the ONS birth statistics (54 142 preterm and 639 321 full-term) where only 7.8% of infants were preterm (P < .001) in 2016. After the policy change, the proportion of preterm infants fell to 12.7% and was no longer significantly different from the ONS birth statistics (P = .12). There was no overall significant difference in the proportion of vaccine failures between infants born prematurely and at term (P = .82), and this was the case both pre- and post-policy change.
Twenty-five ICD-10 codes of respiratory coinfections or clinical syndromes coded in HES and 6 respiratory pathogens confirmed in SGSS data were considered respiratory coinfections after clinical review. Eighty-six (42.8%) infants had at least 1 coinfection (17 preterm, 47 full-term, 22 gestational age not known). Of these 86 infants, 10 were identified as having a coinfection in both HES and SGSS databases and 6 infants were only identified in SGSS. Seventeen (19.8%) had 2 coinfections and the remaining 69 (80.2%) had 1 coinfection. The most common coinfection was “acute bronchiolitis” (45 infants), followed by “acute upper respiratory infection/nasopharyngitis” (14 infants) (Table 2).
Table 2. .
Total Number of Respiratory Coinfections and Clinical Syndromes Identified in HES and SGSS 30 Days Before and After Each Included Pertussis Case (Infants ≤60 Days Old) Was Sent to the Hospital for Pertussis Between 1 September 2014 and 31 March 2016 and from 1 September 2016 to 31 March 2018 in England
| Coinfection | ICD-10 Code(s) | Total Codes in HES/SGSS/GP | Cumulative Frequency, % |
|---|---|---|---|
| Acute bronchiolitis | J219, J218 | 45 | 38.5 |
| Acute upper respiratory infection / nasopharyngitis | J069, J00X | 14 | 12.0 |
| Pneumonia | J181, P239, J22X, J189, J209, J180 | 11 | 9.4 |
| Respiratory syncytial virus | J210, B974 | 12 | 10.3 |
| Rhinovirusa | n/aa | 8 | 6.8 |
| Viral infections | B349, B348 | 7 | 6.0 |
| Enterovirus, unspecified | B971, B341 | 5 | 4.3 |
| Streptococcus | B995, J13X, B953 | 4 | 3.4 |
| Coronavirus | B972 | 3 | 2.6 |
| Influenza | J101 | 3 | 2.6 |
| Haemophilus influenzae infection | A492 | 2 | 1.7 |
| Adenovirus infection, unspecified | B340 | 2 | 1.7 |
| Human metapneumovirus | J211 | 1 | 0.9 |
| Total | 117 | 100.0 |
Abbreviations: GP, general practitioner; HES, Hospital Episode Statistics; ICD-10, International Classification of Diseases, 10th revision; n/a, not applicable; SGSS, Second Generation Surveillance System.
aRhinovirus was only determined in the SGSS database. Some cases of enterovirus, unspecified, may be rhinovirus.
Of the 151 cases with known gestational age, 20 of 122 (16.4%) full-term infants and 19 of 29 (65.5%) preterm infants were admitted to ICU. Of the 39 cases admitted to ICU all had a recorded gestational age. There was no significant difference in the proportion of cases admitted to ICU pre-policy change and post-policy change (P = .29) (Table 1). The median duration in the hospital was 5 days for infants not admitted to ICU and 10 days for those admitted to ICU.
There was no difference in the overall proportion of immunized mothers pre- and post-policy change (P = .52) (Table 1). The median infant gestational age at maternal vaccination was 31 weeks (IQR, 28–34 weeks) pre-policy change and 27 weeks (IQR, 23–28 weeks) post-policy change. Two women were recorded as having received RepevaxTM vaccine (83%, 43/52 had records available) in the current pregnancy despite Boostrix-IPVTM being recommended; both infants were born at term with one born preceding the policy change in timing and one born after. Twelve mothers had records of being vaccinated in a previous pregnancy, of whom 8 did not receive the pertussis vaccine in the current pregnancy.
Analysis of Length of Stay
Five deaths were observed pre-policy change and no deaths were recorded post-policy change. Two infants who died were preterm, 2 were full-term, and 1 infant did not have a known gestational age. Four mothers of the infants who died (including both with preterm deliveries) were not immunized and 3 of the infants had coinfections. The mother who was immunized received the vaccine at 34 weeks’ gestation and delivered 1 week later. Death was considered the competing risk, as this may impact the duration in hospital. Gestational age at vaccination and infant admission to ICU were considered on the causal pathway and not included in the analyses.
Univariable analyses were not significant for sex, ethnicity, mothers’ vaccination status, and whether the mother was vaccinated in a previous pregnancy. Data for maternal vaccination status and vaccination in a previous pregnancy were incomplete, which may have contributed to the lack of significance.
The multivariable analyses indicated the change in policy timing had no significant impact on the duration of hospital stay (Table 3). Post hoc analysis removing gestational age from the multivariable model indicated that hospital duration decreased post-policy change (with the reduction in premature cases), but remained insignificant (HR, 1.24; 95% confidence interval, .96–1.61).
Table 3. .
Duration of Stay and Competing Risks Regression Hazard Ratios for Time to Final Hospital Discharge Among Infants ≤60 Days Old Hospitalized With Pertussis by Predictors of Severity Between 1 September 2014 and 31 March 2016 (Pre-Policy Change) and 1 September 2016 and 31 March 2018 (Post-Policy Change) in England
| Characteristics | Number of Cases | Mean Duration, Days | Median Duration, Days (IQR) | HR (95% CI) | P |
|---|---|---|---|---|---|
| Pre-policy change | 108 | 9.28 | 5 (2–10.5) | Reference | |
| Post-policy change | 93 | 8.13 | 6 (3–10) | 1.14 (.88–1.49) | .321 |
| Admitted at 0–4 weeks | 44 | 10.44 | 8 (4–13.5) | Reference | |
| Admitted at 5–8 weeks | 157 | 8.28 | 5 (2–10) | 1.85 (1.32–2.57) | .001 |
| Preterm | 29 | 15.47 | 9 (5–20) | Reference | |
| Full-term | 122 | 7.02 | 5 (2–9) | 2.05 (1.35–3.10) | |
| Not known | 50 | 9.09 | 5 (3–10) | 1.87 (1.17–3.02) | .010 |
| Single infection | 115 | 6.79 | 5 (2–9) | Reference | |
| Coinfection | 86 | 11.38 | 6 (4–14) | .59 (.45–.78) | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
Cases aged 0–4 weeks had a significantly longer hospital stay (median of 3 more days) than infants aged 5–8 weeks. Cases with a coinfection (median of 1 more day) and premature infants (median of 4 more days) had a significantly longer hospital duration (Table 3).
DISCUSSION
Maternal pertussis vaccination is highly effective in preventing infant hospitalizations and deaths. While a number of studies to evaluate antibody concentrations in infants of mothers vaccinated at different times in the third trimester have been conducted, few have compared antibody concentrations in infants of mothers vaccinated before and during the third trimester [25, 26]. Eberhardt et al [27] reported higher infant anti-pertussis toxin and -filamentous hemagglutinin concentrations in both term and preterm infants whose mothers were immunized in the second trimester. There are an increasing number of studies demonstrating the safety of offering pertussis vaccines as early as the first trimester [28]. However, there is still debate on the optimal timing for maternal pertussis vaccination, and country-specific recommendations on the timing of vaccination vary in Europe, the United States, South America, and Australia [7, 11, 12, 29, 30]. This study presents the first evaluation of the impact of this policy change on hospitalized infant pertussis cases in England. Our results suggest that earlier vaccination in pregnancy has reduced hospitalized pertussis cases in young preterm infants, who are at risk of severe outcomes and have a significantly longer stay in the hospital than term infants with pertussis. Where the mother’s gestational age at vaccination was known, gestational age was earlier after the policy change, which would be expected to lead to fewer preterm cases.
Hospitalized cases of pertussis in full-term infants were previously shown to have fallen following the introduction of maternal vaccination [15]. Since the policy change there does not appear to have been an additional benefit to full-term infants. However, numbers in preterm infants were halved, with preterm infants no longer overrepresented among pertussis hospital admissions when compared with ONS population data. These findings are consistent with protection of infants at an earlier gestational age as a result of women being vaccinated earlier in pregnancy. These findings align with a study in Australia that found that 66.5% of preterm infants did not benefit when the vaccine was offered at 28 weeks of gestation and full-term infants were more than twice as likely to have mothers who received the vaccine [31].
Previous analyses have demonstrated that the maternal program has had an impact on duration of stay in the hospital, with a shorter duration in infants born to vaccinated mothers [15]. Our analyses suggest that earlier delivery of maternal vaccination has not had a significant additional impact on the duration of stay in the hospital, although this is likely to reflect the small number of preterm hospitalized cases. Since the policy change vaccine coverage increased, reaching just over 70.0% in March 2018 compared with 60.7% in March 2016 and 55.6% in September 2014 [32, 33]. Increased coverage may also be associated with changes in data-extraction methods from April 2016 [34]. An unpublished internal report in England suggests that most women receive the pertussis vaccine on the same day or shortly after their 20-week scan.
Study limitations include missing and misclassification of information in the HES dataset. We observed an overestimation of pertussis cases in the HES dataset compared with total confirmed cases. Inflated HES cases have been observed in other studies and the accuracy of the HES data relies on correct diagnoses of cases [35]. Additionally, data on weeks of gestation at vaccination were not complete and completeness declined during the study period. Women in England are increasingly receiving maternal pertussis vaccination in maternity clinics rather than in general practice. It is important that the vaccination status is transferred from maternity services and recorded in the GP patient record [36].
While immunogenicity studies have shown that maternal vaccination blunts infants’ response to their own pertussis vaccination [37], studies on the effect of maternal vaccination against pertussis disease have not found an increased disease risk in immunized infants with vaccinated mothers compared with those with unvaccinated mothers [6, 38]. One immunogenicity study showed that any blunting effects diminish in infants born prematurely by 12 months of age [14]. This blunting effect needs to be considered in light of the higher risk of hospitalization and deaths in young infants against potential risks among older vaccinated infants.
Country-specific recommendations vary on the optimal timing of maternal vaccination and have generally been informed by immunogenicity studies. Our study is highly relevant for policymakers considering introducing a maternal pertussis program and those with established programs to optimize protection in preterm infants. The results from our study have been important in the recent review of the UK maternal pertussis program, which was initially introduced as an outbreak-control measure. In 2019, it was recommended by a national independent advisory committee of experts that maternal pertussis vaccination should become a routine national program [39]. Countries that offer the maternal pertussis vaccine may wish to consider the implications of these findings to increase maternal pertussis vaccine coverage and optimize protection in the preterm infant population.
Notes
Acknowledgments. The authors thank Adolphe Bukasa, Joanne Lacy, and Kim Taylor for their support on the pertussis surveillance program. They also thank all the GPs, practice nurses, and receptionists who completed the enhanced surveillance forms.
Potential conflicts of interest. The Immunisation Department has provided vaccine manufactures with post-marketing surveillance reports, which the Marketing Authorization Holders are required to submit to the UK Licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 2016; 12:1972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller E, Gay N. Effect of age on outcome and epidemiology of infectious diseases. Biologicals 1997; 25:137–42. [DOI] [PubMed] [Google Scholar]
- 3. Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014; 384:1521–8. [DOI] [PubMed] [Google Scholar]
- 4. Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis 2017; 64:9–14. [DOI] [PubMed] [Google Scholar]
- 5. Winter K, Nickell S, Powell M, Harriman K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis 2017; 64:3–8. [DOI] [PubMed] [Google Scholar]
- 6. Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis 2016; 63:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards KM. Maternal immunisation in pregnancy to protect newborn infants. Arch Dis Child 2019; 104:316–9. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Revised guidance on the choice of pertussis vaccines: July 2014. Releve Epidemiologique Hebdomadaire 2014; 89:337–40. [PubMed] [Google Scholar]
- 9. Campbell H, Gupta S, Dolan GP, et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol 2018; 67:1426–56. [DOI] [PubMed] [Google Scholar]
- 10. Joint Committee on Vaccination and Immunisation. Minutes of the meeting on 3 February 2016. London, 2016. Available at: https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation#minutes. Accessed 10 October 2019.
- 11. European Center for Disease Prevention and Control. Vaccination schedules for individual European countries and specific age groups. Available at: https://www.ecdc.europa.eu/en/immunisation-vaccines/EU-vaccination-schedules. Accessed 11 October 2019.
- 12. Centers for Disease Control and Prevention. Tdap (pertussis) vaccine and pregnancy. Available at: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/tdapvaccine-pregnancy.html. Accessed 11 October 2019.
- 13. Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis 2016; 62:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kent A, Ladhani SN, Andrews NJ, et al. Pertussis antibody concentrations in infants born prematurely to mothers vaccinated in pregnancy. Pediatrics 2016; 138:1–5. [DOI] [PubMed] [Google Scholar]
- 15. Byrne L, Campbell H, Andrews N, Ribeiro S, Amirthalingam G. Hospitalisation of preterm infants with pertussis in the context of a maternal vaccination programme in England. Arch Dis Child 2018; 103:224–9. [DOI] [PubMed] [Google Scholar]
- 16. NHS Digital. Hospital Episode Statistics (HES). 2019. Available at: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospitalepisode-statistic. Accessed 16 October 2020.
- 17. World Health Organization. International classification of diseases. Tenth revision. Geneva, Switzerland: WHO Library Cataloging-in Publication Data, World Health Organization, 2010. Available at: https://apps.who.int/iris/handle/10665/42980. Accessed 12 July 2019.
- 18. Campbell H, Amirthalingam G, Andrews N, Fry NK, George RC, Harrison T. Accelerating control of pertussis in England and Wales. Emerg Infect Dis 2012; 18:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NHS Digital. Hospital episode statistics data dictionary. 2019. Available at: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary. Accessed 22 November 2019.
- 20. Public Health England. The health protection (notification) regulations 2010. Available at: http://www.legislation.gov.uk/uksi/2010/659/introduction/made. Accessed 10 July.
- 21. Public Health England. Laboratory reporting to Public Health England: a guide for diagnostic laboratories. Public Health England in London, 2016. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/739854/PHE_Laboratory_Reporting_Guidelines.pdf. Accessed 10 October 2019.
- 22. Office for National Statistics. Birth characteristics. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthcharacteristicsinenglandandwales. Accessed 29 August 2019.
- 23. Keene CM, Dondorp A, Crawley J, Ohuma EO, Mukaka M. A competing-risk approach for modeling length of stay in severe malaria patients in South-East Asia and the implications for planning of hospital services. Clin Infect Dis 2018; 67:1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 25. Healy CM, Rench MA, Swaim LS, et al. Association between third-trimester tdap immunization and neonatal pertussis antibody concentration. JAMA 2018; 320:1464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abu Raya B, Srugo I, Kessel A, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels—a prospective study. Vaccine 2014; 32:5787–93. [DOI] [PubMed] [Google Scholar]
- 27. Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Pertussis antibody transfer to preterm neonates after second- versus third-trimester maternal immunization. Clin Infect Dis 2017; 64:1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu-Raya B, Edwards KM. Optimizing the timing of vaccine administration during pregnancy. JAMA 2019; 321:935–6. [DOI] [PubMed] [Google Scholar]
- 29. Australian Government Department of Health. Pertussis (whooping cough). Available at: https://immunisationhandbook.health.gov.au/vaccinepreventable-diseases/pertussis-whooping-cough. Accessed 10 October 2019.
- 30. Romanin V, Acosta AM, Juarez MdV, et al. Maternal vaccination in Argentina: tetanus, diphtheria, and acellular pertussis vaccine effectiveness during pregnancy in preventing pertussis in infants <2 months of age. Clin Infect Dis 2019; 70:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janagaraj PD, Gurusamy PSR, Webby R. Current antenatal pertussis vaccination guidelines miss preterm infants: an epidemiological study from the Northern Territory. Aust N Z J Obstet Gynaecol 2019; 59:436–43. [DOI] [PubMed] [Google Scholar]
- 32. Public Health England. Pertussis vaccination programme for pregnant women update: vaccine coverage in England, October to December 2018. Health Protection Report Vol. 13. 2019. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/797814/HPR1419_prntl-prtsss_vaccine_coverage_October_to_December_2018.pdf. Accessed 16 September 2019.
- 33. Public Health England. Pertussis vaccination programme for pregnant women: vaccine coverage estimates in England, September to December 2014. Health Protection Report. London: 2015. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/408500/hpr0715_prtsss-vc.pdf. Accessed 16 September 2019.
- 34. Public Health England. Pertussis vaccination programme for pregnant women: vaccine coverage estimates in England, April to September 2016. London: 2016. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/572731/hpr4116_prntl-prtsss-vc.pdf. Accessed 16 September 2019.
- 35. Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis 2016; 213:243–9. [DOI] [PubMed] [Google Scholar]
- 36. Public Health England. Pertussis vaccination programme for pregnant women update: vaccine coverage in England, October to December 2017. London: Health Protection Report Vol 12. 2018. [Google Scholar]
- 37. Zimmermann P, Perrett KP, Messina NL, et al. The effect of maternal immunisation during pregnancy on infant vaccine responses. EClinicalMedicine 2019; 13:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baxter R, Bartlett J, Fireman B, Lewis N, Klein N. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017; 139:e20164091. [DOI] [PubMed] [Google Scholar]
- 39. Joint Committee on Vaccination and Immunisation. Minutes of the meeting held on 05 June 2019. London: 2019. Available at: https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation#minutes. Accessed 10 October 2019.