Abstract
Background
Hepatitis B virus (HBV) and fatty liver disease (FLD) are common in human immunodeficiency virus (HIV). Correlates of FLD and its relationship with alanine aminotransferase (ALT) were examined longitudinally in HIV-HBV coinfection.
Methods
From 28/4/2014–7/11/2018, 114 HIV-HBV adults had liver biopsy and were followed for a median of 3 years (ancillary study of Hepatitis B Research Network). Steatohepatitis was based on presence of steatosis, ballooning, and perisinusoidal fibrosis. FLD was defined as ≥5% steatosis and/or steatohepatitis.
Results
Median age was 49 years, 93% were male, 51% black, 93% had HIV RNA <400 copies/mL and 83% HBV DNA <1000 IU/mL. Thirty percent had FLD (20% steatosis, 10% steatohepatitis). Those with FLD had higher median triglyceride (171 vs 100 mg/dL, P < .01) and small, dense LDL (44 vs 29 mg/dL, P < .01) and lower HDL-2-C (9 vs 12 mg/dL, P = .001). After adjusting for age, sex, and alcohol use, white and other versus black race (ORs, 8.49 and 16.54, respectively), ALT (OR, 3.13/doubling), hypertension (OR, 10.93), hyperlipidemia (OR, 4.36), and diabetes family history (OR, 5.38) were associated with having FLD (all P < .05). Steatohepatitis or steatosis alone (vs none) was associated with higher ALT over time (1.93 and 1.34 times higher, respectively; P < .001), with adjustment for age, sex, and HBV DNA.
Conclusions
About 30% with HIV-HBV coinfection had FLD including 10% with steatohepatitis. FLD was associated with non-black race, metabolic risks, an atherogenic lipid profile, and elevated ALT over time. Thus, identification of FLD and management of adverse metabolic profiles are critically important in HIV-HBV coinfection.
Clinical Trial Registration. NCT 01924455.
Keywords: cardiovascular risk, nonalcoholic steatohepatitis, inflammation, adipose tissue insulin resistance
Approximately one-third of Immunodeficiency virus-Hepatitis B virus (HIV-HBV) patients have fatty liver and 10% have steatohepatitis. Fatty liver is associated with increased atherogenic lipids and perisinusoidal liver fibrosis. Persistent ALT elevation despite HBV virologic control requires evaluation for coexisting fatty liver in HIV-HBV coinfection.
(See the Editorial Commentary by Woreta and Chalasani on pages e3286–7.)
Approximately 1.1 million Americans and 33 million individuals globally are living with human immunodeficiency virus (HIV) [1]. Although antiretroviral therapy (ART) improves survival [2], liver disease remains a significant cause of excess morbidity and mortality in HIV [3, 4]. The pathogenesis of liver disease in the ART era may be multifactorial but includes coinfection with hepatitis B (HBV) and hepatitis C (HCV) viruses, fatty liver disease (FLD), and even HIV itself [5–9]. However, similar to the general population, FLD has emerged as a common etiology of liver disease in HIV [10]. There are alcoholic or nonalcoholic forms of FLD (which can coexist) with similar histologic presentations that range from simple steatosis to steatohepatitis to advanced fibrosis and cirrhosis. Because liver biopsies are not systematically performed in patients living with HIV, our understanding of the prevalence of FLD in this population is limited, although epidemiologic studies have reported an estimated nonalcoholic fatty liver disease (NAFLD) prevalence of 50% or more [11, 12].
While metabolic complications increase the risks of dyslipidemia and NAFLD in the aging HIV population [13], a recent study reported a higher prevalence of hepatic steatosis in young adults with HIV compared with an uninfected group (33% vs 10%, respectively) [14]. Importantly, FLD in HIV and its related adverse metabolic effects including dyslipidemia and insulin resistance may signal underlying coronary atherosclerosis and increased risk for cardiovascular events, a major comorbidity in HIV, especially as this population ages [15].
Human immunodeficiency virus can alter the natural history of underlying liver disease. In HIV-HCV coinfection there are high rates of fibrosis [16]. Furthermore, in a recent study of 114 adults with HIV-HBV coinfection, nearly 40% had significant fibrosis on histology despite HBV therapy with ART, although the duration of ART was not known [17]. Studies using transient elastography and cross-sectional imaging have suggested that the severity of hepatic steatosis is associated with advanced fibrosis in those with HIV monoinfection [18, 19]. However, in a study of 30 patients with HIV monoinfection with unexplained abnormal liver tests, while FLD on biopsy was present in a significant proportion of those with fibrosis, the severity of steatosis did not correlate with fibrosis stage [20]. This may be related to the possible reduction in steatosis burden with liver disease progression and that, in those with steatohepatitis, other histologic features of liver injury such as hepatocyte ballooning and lobular inflammation may be more predictive of fibrosis [21]. However, despite the high prevalence of multiple risk factors, there is a paucity of data on the impact of coexisting conditions such as HBV coinfection and FLD on liver disease severity in the HIV population. In HBV monoinfection limited data from predominantly Asian countries have reported an FLD prevalence ranging from 11% using elastography [22] to nearly 38% on liver biopsies prior to HBV therapy [23].
In this study we aimed to determine the presence of coexisting FLD, its disease activity, and its clinical correlates, including cardiovascular disease (CVD) risk, in a well-defined HIV-HBV cohort receiving ART. Additionally, we evaluated the impact of FLD on liver tests (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) as a surrogate for liver disease activity over time.
METHODS
This is a multicenter prospective cohort study of adults with HIV-HBV as previously described (NCT01924455) [17]. Adults who were anti-HIV and hepatitis B surface antigen positive for at least 6 months were recruited from 8 Hepatitis B Research Network (HBRN) sites in the United States and Canada. Those with detectable HCV RNA, decompensated cirrhosis, or hepatocellular carcinoma were excluded. The institutional review board at each center approved the protocol, and participants gave written consent.
In this study, 114 had a liver biopsy within 12 months of enrollment. Participants were evaluated at enrollment and weeks 12, 24, and every 24 weeks thereafter up to 192 weeks.
Assessment of Liver Histology
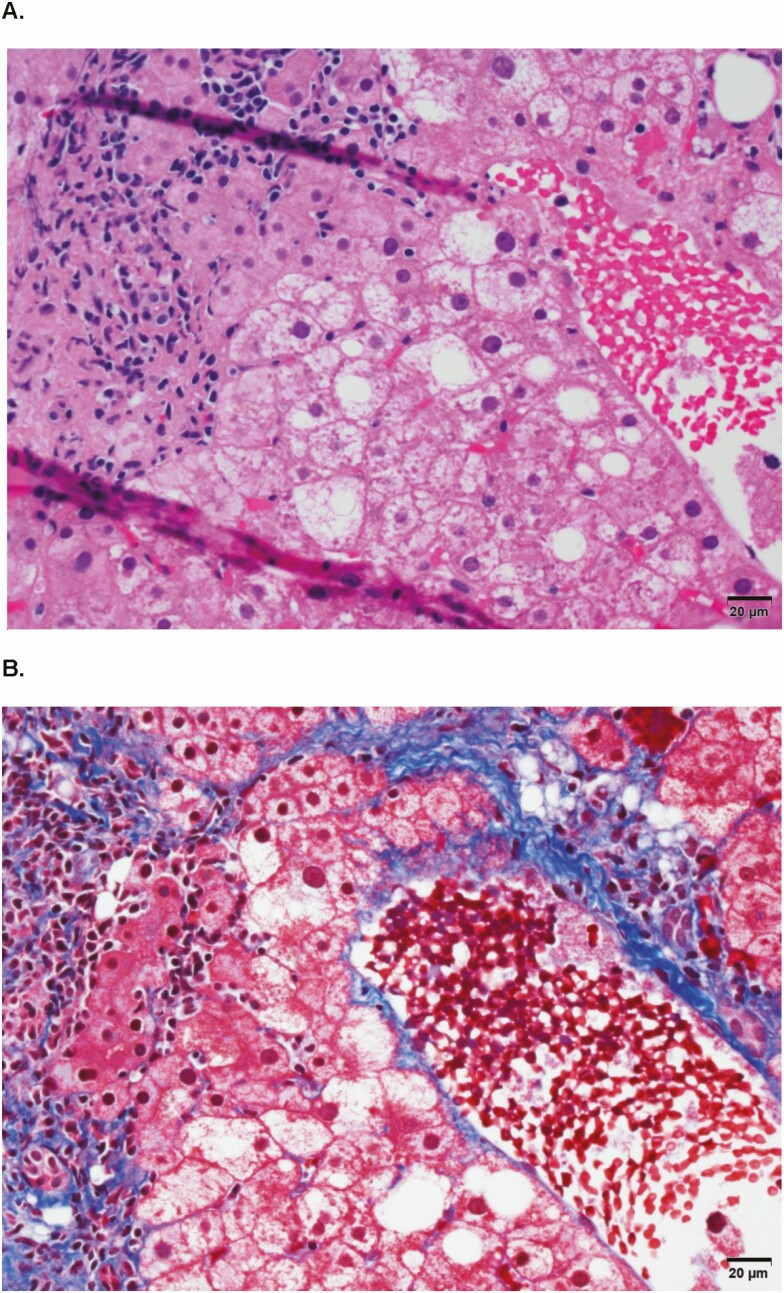
Slides were submitted for staining by a central laboratory. Histological findings (on adequate samples) were scored blindly with respect to clinical data by the HBRN Pathology Committee and for inflammation and fibrosis using the Ishak method [24]. Perisinusoidal fibrosis was assessed as 0 for none, 1 for mild (visible only on trichrome), and 2 for moderate (evident on the hematoxylin and eosin stain). Steatosis was graded as proportion of steatotic hepatocytes: grade 0 indicating none. grade 1 at <5%, grade 2 from 5% to 33%, grade 3 from >33% to 67%, and grade 4 at >67%. As lobular inflammation was always present, steatohepatitis was diagnosed by consensus and based on findings of steatosis and ballooning injury with or without Mallory-Denk bodies and perisinusoidal fibrosis in an appropriate architectural pattern [25] (see Figure 1). Participants were categorized as having (1) steatohepatitis, (2) steatosis (≥5%) without steatohepatitis, or (3) no FLD. Fatty liver disease was defined as having steatosis and/or steatohepatitis.
Figure 1.
A representative liver biopsy (same field): histologic findings of (A) steatosis, inflammation, and hepatocyte ballooning and (B) perisinusoidal fibrosis.
Clinical and Laboratory Data
Detailed assessment of clinical and laboratory data and definitions are provided in the Supplementary Material. Race, ethnicity, and alcohol consumption [26] in the prior 12 months were self-reported and an Alcohol Use Disorder Identification Test (AUDIT) score of 8 or greater was also used to define at-risk drinking [27]. Use of ART and HBV therapy [17] was captured and metabolic syndrome was defined using standard criteria [28]. The Homeostasis Model Assessment Method Index (HOMA-IR) [29] and adipose tissue insulin resistance (Adipo-IR) [30] were calculated. The presence of lipodystrophy was captured [31].
Metabolic, Lipid, and Lipoprotein Characteristics and Subparticle Measurements
The following tests were performed centrally in a commercial laboratory (TrueHealth Diagnostic Laboratories, Inc, Richmond, VA). Metabolic tests included fasting glucose, insulin, and free fatty acids. The lipid and lipoproteins included the following: (1) total cholesterol (TC); (2) LDLs: LDL cholesterol [LDL-C]; LDL particle concentration [LDL-P]; small, dense LDL-C [sdLDL-C]; small LDL-P); (3) HDLs: HDL cholesterol [HDL-C], HDL particle [HDL-P], HDL subclass 2-C [HDL-2-C], apolipoprotein A-1 [apoA1]); and (4) triglycerides (TGs) and apolipoprotein B (apoB). Adiponectin, high-sensitivity C-reactive protein (hs-CRP), and lipoprotein-associated phospholipase A2 (LP-PLA2) activity (markers of inflammation) and homocysteine levels were also measured. Tests that required fasting status were performed after at least 8 hours of fasting.
Statistical Analysis
To compare whether the percentage of participants with FLD differed by participant characteristics, the chi-square test or the Fisher’s exact test, as appropriate, were used for nonordinal categorical variables, and the Cochran-Armitage or the John-Terpstra test for trend, as appropriate, were used for ordinal variables. The percentage of participants with steatohepatitis, steatosis, and no FLD was also compared by participant characteristics and is reported for select variables (ie, ALT, AST, Ishak fibrosis score). The nonparametric Mann–Whitney U test was used to compare continuous variables by presence of FLD. A linear model was used to examine the association between sdLDL-C and small LDL-P, respectively, with TG levels overall and by presence of FLD.
Logistic regression was used to estimate associations between participant characteristics with the presence of FLD. Age, sex, race/ethnicity, and alcohol risk categories were forced in the model. Additionally, log2 ALT, log2 AST, elevated blood pressure, hyperlipidemia, family history of diabetes mellitus, and race-adjusted weight status were considered via stepwise selection and retained if P < .10. Adipo-IR was added to the multivariable model to evaluate its adjusted association. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are reported.
Longitudinal Analysis
Because we hypothesized that FLD would impact ALT, a linear mixed-model fit using maximum likelihood with a person-level random intercept was used to evaluate the adjusted association between the static variable FLD status at baseline as a predictor of the repeated measured ALT over time, with time since liver biopsy date as a continuous fixed effect. Sex, age at biopsy (per decade), and HBV DNA level ≥1000 (vs <1000) IU/mL at each time point were forced in the model as fixed effects. To meet the normality assumption, the log2 scale was used, and therefore results are presented as ratios (factor by which the average value of ALT differs for the comparator vs the reference). Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Reported P values are 2-sided; P < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Overall, the median age of the participants was 49 years. Most participants were male (93.0%) and were born in North America (86.0%). About half were non-Hispanic black (51.3%) and one-third (32.7%) were non-Hispanic white. Approximately 1 in 8 (12.3%) participants reported at-risk alcohol consumption and one-third (32.5%) reported low-risk alcohol consumption. Based on the AUDIT, 6.2% were identified as at risk for alcohol-use disorder. Nearly all participants (99.1%) were on ART and none were taking D-drugs (didanosine and stavudine); 97.4% were on anti-HBV therapy (tenofovir ± emtricitabine or lamivudine [80.7%] or entecavir alone [15.8%]) with HBV DNA <20 IU/mL in 64.9%. Approximately one-tenth (n = 11; 9.7%) had steatohepatitis and an additional 23 participants (20.2%) had steatosis.
Fatty Liver Disease Status by Demographics and Clinical and Histological Findings
The presence of FLD by demographic, clinical, and histological characteristics is reported in Table 1. Fatty liver disease was less prevalent among younger participants, non-Hispanic blacks, and those without metabolic syndrome and more prevalent among those with hyperlipidemia, significant insulin resistance (HOMA-IR ≥4), and family history of diabetes. Additionally, a higher percentage of participants with at least twice normal levels of ALT and abnormal levels of AST had FLD. Indeed, 50.0% with steatohepatitis had at least twice the normal level of ALT (vs 17.4% with steatosis and 7.8% with no FLD) and abnormal AST occurred in 60.0% with steatohepatitis compared with 30.4% in those with steatosis and 15.6% in those with no FLD. There was no significant difference in the presence of FLD by type of ART (Table 1).
Table 1.
Presence of Fatty Liver Disease by Participant Characteristics
| Variable | Total (N = 114) | No FLD (n = 80) | FLD (n = 34) | P |
|---|---|---|---|---|
| Age | .023a | |||
| <30 years | 3 (2.6) | 3 (100.0) | 0 (0.0) | |
| 30 to <40 years | 10 (8.8) | 10 (100.0) | 0 (0.0) | |
| 40 to <50 years | 46 (40.4) | 36 (78.3) | 10 (21.7) | |
| 50 to <60 years | 42 (36.8) | 20 (47.6) | 22 (52.4) | |
| ≥60 years | 13 (11.4) | 11 (84.6) | 2 (15.4) | |
| Gender | .43b | |||
| Male | 106 (93.0) | 73 (68.9) | 33 (31.1) | |
| Female | 8 (7.0) | 7 (87.5) | 1 (12.5) | |
| Race or ethnicity, n | 113 | 79 | 34 | .002 |
| Non-Hispanic white | 37 (32.7) | 20 (54.1) | 17 (45.9) | |
| Non-Hispanic black | 58 (51.3) | 49 (84.5) | 9 (15.5) | |
| Other | 18 (15.9) | 8 (55.6) | 10 (44.4) | |
| Birth/migration | .57a | |||
| Born in USA/Canada | 98 (86.0) | 69 (70.4) | 29 (29.6) | |
| Migrated <20 years ago | 10 (8.8) | 5 (50.0) | 5 (50.0) | |
| Migrated ≥20 years ago | 6 (5.3) | 6 (100.0) | 0 (0.0) | |
| Race-adjusted weight status, n | 110 | 76 | 34 | .11c |
| Under-/normal weight | 44 (40.0) | 33 (75.0) | 11 (25.0) | |
| Overweight | 36 (32.7) | 26 (72.2) | 10 (27.8) | |
| Obese | 30 (27.3) | 17 (56.7) | 13 (43.3) | |
| High waist circumference, n | 94 | 68 | 26 | .050 |
| No | 62 (66.0) | 49 (79.0) | 13 (21.0) | |
| Yes | 32 (34.0) | 19 (59.4) | 13 (40.6) | |
| Elevated glucose/diabetes status, n | 91 | 63 | 28 | .074a |
| Nondiabetic, glucose <100 mg/dL | 65 (71.4) | 49 (75.4) | 16 (24.6) | |
| IFG | 15 (16.5) | 8 (53.3) | 7 (46.7) | |
| Diabetic | 11 (12.1) | 6 (54.5) | 5 (45.5) | |
| Insulin sensitivity, n | 78 | 53 | 25 | <.001c |
| Nondiabetic, HOMA-IR <4 | 50 (64.1) | 43 (86.0) | 7 (14.0) | |
| Nondiabetic, HOMA-IR ≥4 | 17 (21.8) | 4 (23.5) | 13 (76.5) | |
| Diabetic | 11 (14.1) | 6 (54.5) | 5 (45.5) | |
| Known family history of diabetes, n | 110 | 76 | 34 | .03 |
| No | 39 (35.5) | 32 (82.1) | 7 (17.9) | |
| Yes | 71 (64.5) | 44 (62.0) | 27 (38.0) | |
| Hypertension, n | 113 | 79 | 34 | .10 |
| No | 48 (42.5) | 38 (79.2) | 10 (20.8) | |
| Yes | 65 (57.5) | 41 (63.1) | 24 (36.9) | |
| Hyperlipidemia | .002 | |||
| No | 79 (69.3) | 63 (79.7) | 16 (20.3) | |
| Yes | 35 (30.7) | 17 (48.6) | 18 (51.4) | |
| Metabolic syndrome, n | 95 | 66 | 29 | .012 |
| No | 61 (64.2) | 48 (78.7) | 13 (21.3) | |
| Yes | 34 (35.8) | 18 (52.9) | 16 (47.1) | |
| Alcohol consumption | .49c | |||
| None or minimal | 63 (55.3) | 41 (65.1) | 22 (34.9) | |
| Low-risk | 37 (32.5) | 30 (81.1) | 7 (18.9) | |
| At-risk | 14 (12.3) | 9 (64.3) | 5 (35.7) | |
| AUDIT score ≥8 | .43b | |||
| No | 106 (93.8) | 75 (70.8) | 31 (29.3) | |
| Yes | 7 (6.2) | 4 (57.1) | 3 (42.9) | |
| Duration of HIV >20 years, n | 103 | 72 | 31 | .026 |
| No | 57 (55.3) | 45 (79.0) | 12 (21.1) | |
| Yes | 46 (44.7) | 27 (58.7) | 19 (41.3) | |
| Currently on antiretroviral therapy | .99 | |||
| No | 1 (0.9) | 1 (100.0) | 0 (0.0) | |
| Yes | 113 (99.1) | 79 (69.9) | 34 (30.1) | |
| Currently on NRTI | .63 | |||
| No | 6 (5.3) | 4 (66.7) | 2 (33.3) | |
| Yes | 108 (94.7) | 76 (71.3) | 32 (28.7) | |
| Currently on NNRTI | .99 | |||
| No | 77 (67.5) | 54 (70.1) | 23 (29.9) | |
| Yes | 37 (32.5) | 26 (70.3) | 11 (29.7) | |
| Currently on PI | .67 | |||
| No | 64 (56.1) | 43 (67.2) | 21 (32.8) | |
| Yes | 50 (43.9) | 37 (74.0) | 13 (26.0) | |
| Currently on other antiretroviral therapy, n | 67 | 43 | 24 | .99 |
| No | 48 (71.6) | 31 (64.6) | 17 (35.4) | |
| Yes | 19 (28.4) | 12 (63.2) | 7 (36.8) | |
| Currently on HBV treatment | .55b | |||
| No | 3 (2.6) | 3 (100.0) | 0 (0.0) | |
| Yes | 111 (97.4) | 77 (69.4) | 34 (30.6) | |
| HIV RNA suppressed (<400 copies/mL), n | 104 | 72 | 32 | >.99 |
| No | 8 (7.7) | 6 (75.0) | 2 (25.0) | |
| Yes | 96 (92.3) | 66 (68.8) | 30 (31.3) | |
| Lipodystrophy/lipoatrophy grade, n | 102 | 74 | 28 | >.99b |
| 0 | 88 (86.3) | 64 (72.7) | 24 (27.3) | |
| ≥1 | 14 (13.7) | 10 (71.4) | 4 (28.6) | |
| HBV DNA level (IU/mL) | .99a | |||
| Undetectable (<20 IU/mL) | 74 (64.9) | 53 (71.6) | 21 (28.4) | |
| 20 to <1000 | 20 (17.5) | 12 (60.0) | 8 (40.0) | |
| 1000 to <20 000 | 8 (7.0) | 6 (75.0) | 2 (25.0) | |
| ≥20 000 | 12 (10.5) | 9 (75.0) | 3 (25.0) | |
| HIV and HBV suppression, n | 104 | 72 | 32 | .61 |
| HBV DNA <20 IU/mL/ HIV <400 copies/mL | 68 (65.4) | 49 (72.1) | 19 (27.9) | |
| HBV DNA ≥20 IU/mL/ HIV <400 copies/mL | 28 (26.9) | 17 (60.7) | 11 (39.3) | |
| HBV DNA ≥20 IU/mL/ HIV ≥400 copies/mL | 8 (7.7) | 6 (75.0) | 2 (25.0) | |
| HBeAg (IU/mL) | .54 | |||
| Negative | 50 (43.9) | 37 (74.0) | 13 (26.0) | |
| Positive | 64 (56.1) | 43 (67.2) | 21 (32.8) | |
| ALT (by laboratory-specific ULN), n | 110 | 77 | 33 | <.001c |
| ≤1 | 60 (54.5) | 49 (81.7) | 11 (18.3) | |
| >1 to <2 | 35 (31.8) | 22 (62.9) | 13 (37.1) | |
| ≥2 | 15 (13.6) | 6 (40.0) | 9 (60.0) | |
| AST (by laboratory-specific ULN), n | 110 | 77 | 33 | .012 |
| ≤1 | 85 (77.3) | 65 (76.5) | 20 (23.5) | |
| >1 | 25 (22.7) | 12 (48.0) | 13 (52.0) | |
| HAI score | .02a | |||
| 0 to <5 | 90 (78.9) | 67 (74.4) | 23 (25.6) | |
| 5 to <9 | 20 (17.5) | 12 (60.0) | 8 (40.0) | |
| 9 to <13 | 3 (2.6) | 1 (33.3) | 2 (66.7) | |
| ≥13 | 1 (0.9) | 0 (0.0) | 1 (100.0) | |
| Fibrosis: Ishak score | .25a | |||
| 0 | 30 (26.3) | 26 (86.7) | 4 (13.3) | |
| 1 | 42 (36.8) | 26 (61.9) | 16 (38.1) | |
| 2 | 15 (13.2) | 11 (73.3) | 4 (26.7) | |
| 3 | 17 (14.9) | 10 (58.8) | 7 (41.2) | |
| 4 | 6 (5.3) | 4 (66.7) | 2 (33.3) | |
| 5 | 1 (0.9) | 1 (100.0) | 0 (0.0) | |
| 6 | 3 (2.6) | 2 (66.7) | 1 (33.3) | |
| Fibrosis: perisinusoidal | .026a | |||
| 0 | 84 (73.7) | 63 (75.0) | 21 (25.0) | |
| 1 | 16 (14.0) | 11 (68.8) | 5 (31.3) | |
| 2 | 14 (12.3) | 6 (42.9) | 8 (57.1) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorder Identification Test; FLD, fatty liver disease; HAI, hepatic activity index; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HOMA-IR, Homeostasis Model Assessment Method Index; IFG, impaired fasting glucose; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; ULN, upper limit of normal.
aExact Cochran-Armitage trend test.
bFisher’s exact test.
cCochran-Armitage trend test.
Fatty liver disease was more prevalent in those with higher grades of inflammation, as measured by hepatic activity index (HAI) score, and those with higher degrees of perisinusoidal fibrosis; however, there was no significant difference in the presence of FLD by Ishak fibrosis score (Table 1). The distributions of Ishak fibrosis score (F) among those with FLD versus no FLD (P = .11) were as follows: F0 or F1 at 58.9% versus 65%, F2 or F3 at 32.4% versus 26.3%, and F4 and above at 8.8% versus 8.8%. However, those with steatohepatitis (n = 11) tended to have higher degrees of fibrosis versus those with steatosis (n = 23) and no FLD (n = 80), respectively, as follows: F0 or F1 at 27.3% versus 73.9% and 65%, F2 or F3 at 63.6% versus 17.3% and 26.3%, and F4 and above at 9.1% versus 8.6% and 8.8% (P = .02).
Metabolic and Inflammatory Markers in Those With and Without Fatty Liver Disease
Metabolic and atherogenic risk profile by presence of FLD is summarized in Table 2. Compared with no FLD, patients with FLD had significantly higher insulin (median, 19 vs 8.5 mµ/mL; P < .001) and glucose (median, 94 vs 89 mg/dL; P = .12); FFA levels (median, 0.49 vs 0.39 mg/dL; P = .21) also appeared higher but the differences were not statistically significant. Further, the presence of FLD (vs no FLD) was associated with significantly higher HOMA-IR (median, 5.0 vs 2.0; P < .0001) and Adipo-IR (median, 71.3 vs 26.7; P < .0001), and appeared to be associated with lower adiponectin levels (median, 8.5 vs 11; P = .15), although this was not statistically significant. Other metabolic and inflammatory markers, including homocysteine, hs-CRP, and LP-PLA2 levels, did not significantly differ between the FLD and no-FLD groups (Table 2).
Table 2.
Serum Metabolic and Atherogenic Risk Profile by Presence of Fatty Liver Disease
| Variable | Total (N = 114) | No FLD (n = 80) | FLD (n = 34) | P |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | .23 | |||
| Median (IQR) | 162.5 (146–197) | 159.5 (145–195.5) | 173.5 (148–206) | |
| Low density lipoprotein | ||||
| LDL-C, n | 113 | 79 | 34 | .11 |
| Median (IQR), mg/dL | 96 (78–17) | 91 (76–112) | 98 (81–126) | |
| LDL-P, n | 105 | 75 | 30 | <.001 |
| Median (IQR), nmol/L | 1123 (625–1428) | 1043 (896–1290) | 1429 (1163–1744) | |
| sdLDL-C, n | 112 | 79 | 33 | <.001 |
| Median (IQR), mg/dL | 32 (23–43.5) | 29 (21–38) | 44 (32–56) | |
| Small LDL-P, n | 90 | 61 | 29 | .001 |
| Median (IQR), nmol/L | 633 (436–872) | 553 (387–722) | 912 (498–989) | |
| Very low density lipoprotein | ||||
| Triglycerides, n | 85 | 58 | 27 | <.001 |
| Median (IQR), mg/dL | 114 (77–171) | 100.5 (68–141) | 171 (101–261) | |
| apoB, n | 72 | 50 | 22 | .028 |
| Median (IQR), mg/dL | 83 (71–103.5) | 80.5 (70–94) | 95.5 (81–107) | |
| High density lipoprotein | ||||
| HDL-C, n | 114 | 80 | 34 | .12 |
| Median (IQR), mg/dL | 45 (37–55) | 46 (38.5–56.5) | 40.5 (36–50) | |
| HDL-P, n | 104 | 75 | 29 | .94 |
| Median (IQR), μmol/L | 31.2 (26.7–36.2) | 31.1 (27.2–36.2) | 31.6 (25.4–36.0) | |
| HDL-2-C, n | 111 | 79 | 32 | .001 |
| Median (IQR), mg/dL | 11 (7–17) | 12 (8–18) | 8.5 (6–11.5) | |
| apoA1, n | 72 | 50 | 22 | .60 |
| Median (IQR), mg/dL | 124.5 (108–143.5) | 122 (110–151) | 125.5 (100–139) | |
| Insulin sensitivity | ||||
| Glucose, n | 88 | 61 | 27 | .12 |
| Median (IQR), mg/dL | 90 (82.9–99.5) | 89 (82–97) | 94 (85–111) | |
| Insulin, n | 78 | 54 | 24 | <.001 |
| Median (IQR), μU/mL | 11 (7–19) | 8.5 (5–14) | 19 (11.5–31.5) | |
| Free fatty acids, n | 72 | 50 | 22 | .21 |
| Median (IQR), mmol/L | 0.47 (0.30–0.61) | 0.39 (0.29–0.56) | 0.49 (0.33–0.71) | |
| HOMA-IR, n | 75 | 51 | 24 | <.001 |
| Median (IQR) | 2.4 (1.5–4.9) | 2.0 (1.2–3.3) | 5.0 (2.6–9.2) | |
| Adipo-IR, n | 72 | 50 | 22 | <.001 |
| Median (IQR) | 34.7 (15.8–58.8) | 26.7 (14.1–36.7) | 71.3 (49.3–100.8) | |
| Metabolic | ||||
| Homocysteine, n | 112 | 79 | 33 | .48 |
| Median (IQR), μmol/L | 12 (9–14) | 11 (9–14) | 12 (10–14) | |
| Adiponectin, n | 62 | 46 | 16 | .15 |
| Median (IQR), μg/mL | 10 (7–15) | 11 (7–15) | 8.5 (6.5–11) | |
| Inflammatory | ||||
| hs-CRP, n | 109 | 78 | 31 | .90 |
| Median (IQR), mg/L | 2.0 (1.0–6.8) | 2.0 (1.0–7.0) | 2.1 (0.9–5.8) | |
| LP-PLA2, n | 111 | 78 | 33 | .74 |
| Median (IQR), nmol/minute/mL | 161 (125–191) | 165 (125–189) | 160 (133–197) |
Abbreviations: Adipo-IR, adipose tissue insulin resistance; apo, apolipoprotein; FLD, fatty liver disease; HBV, hepatitis B virus; HDL-2-C, HDL subclass 2-C; HDL-C, HDL cholesterol; HDL-P, HDL particle; HOMA-IR, Homeostasis Model Assessment Method Index; hs-CRP- high-sensitivity C-reactive protein; IQR, interquartile range; LDL-C, LDL cholesterol; LDL-P, LDL particle; LP-PLA2, lipoprotein-associated phospholipase A2; sdLDL-C, small, dense LDL cholesterol.
Lipid and Lipoprotein Profiles in Those With and Without Fatty Liver Disease
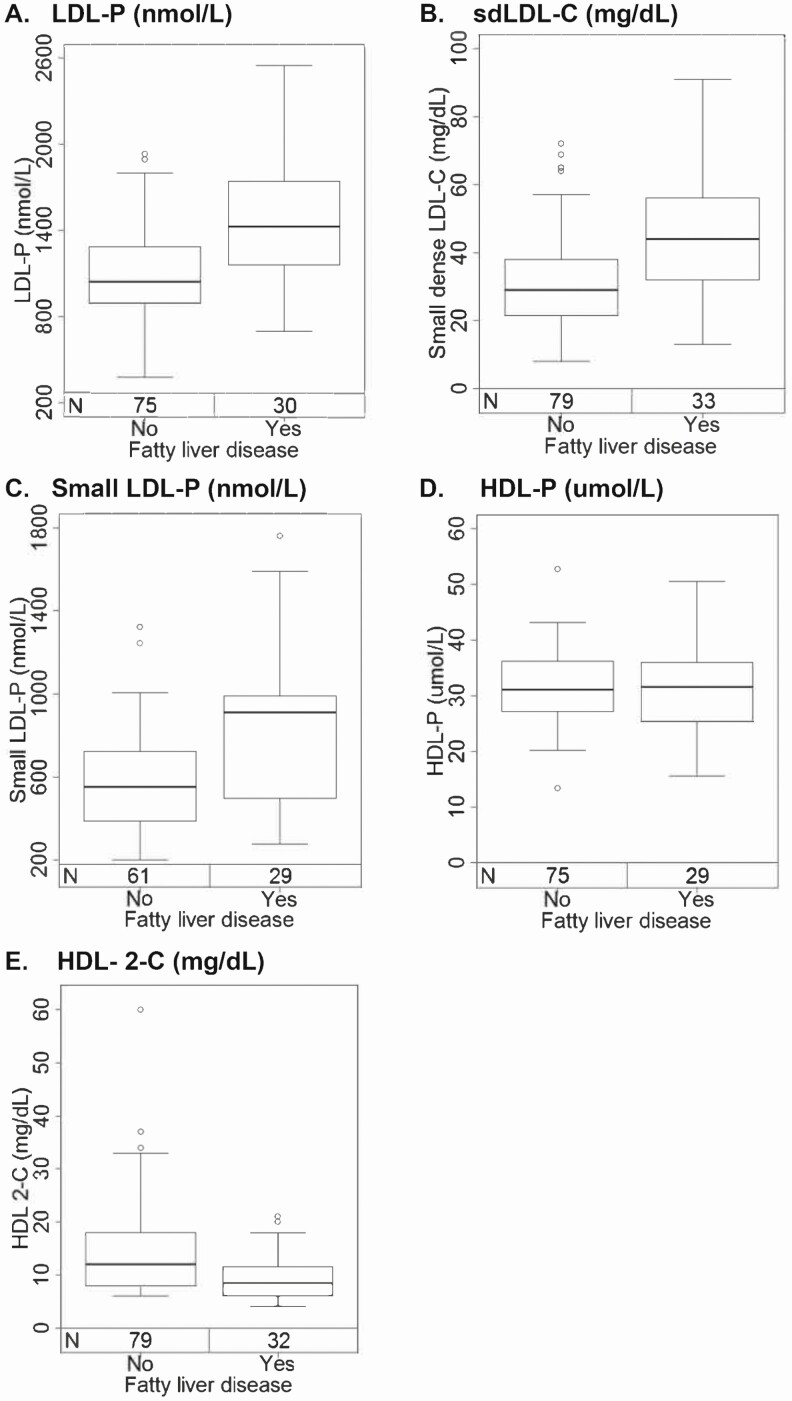
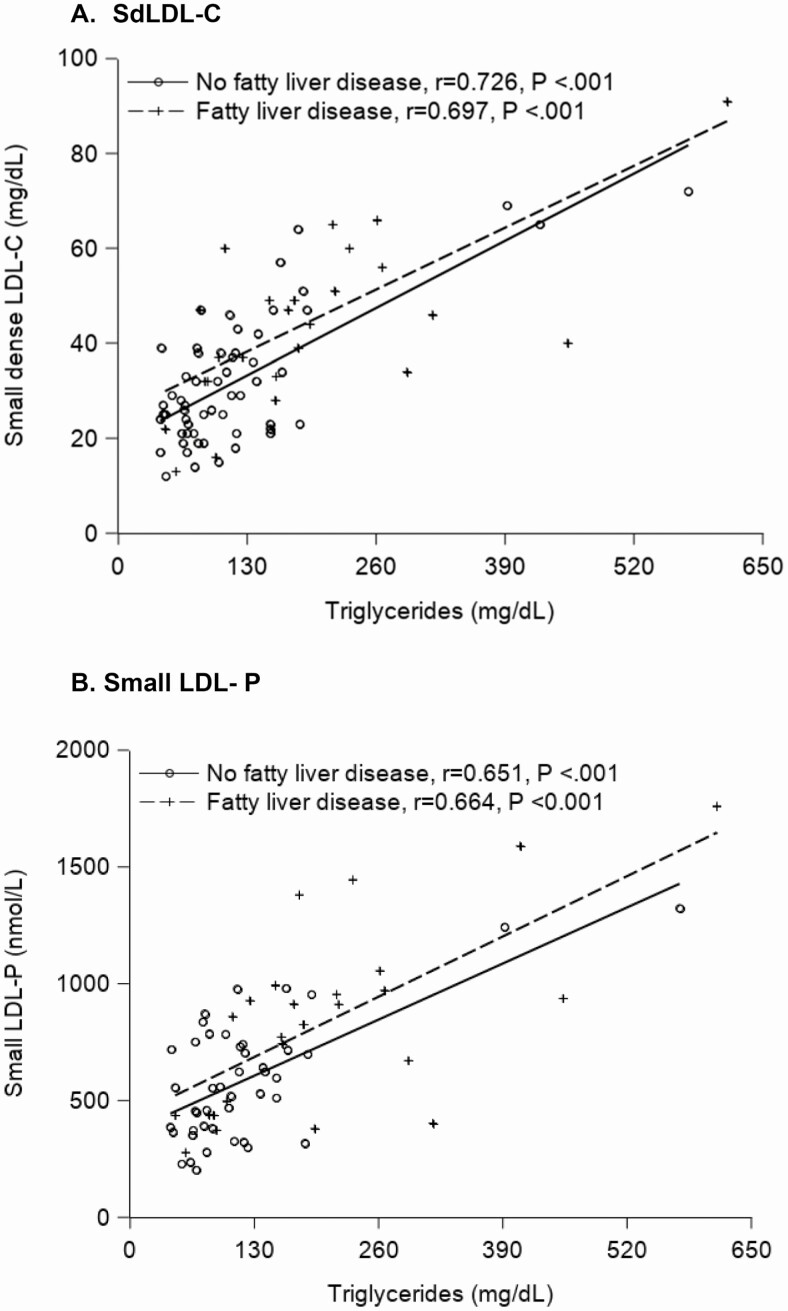
Traditional lipid profiles of TC, LDL-C, and HDL-C were not significantly different among those with and without FLD (median values: 173.5 vs 159.5 mg/dL [P = .23], 98 vs 91 mg/dL [P = .11], and 40.5 vs 46 [P = .12], respectively). However, participants with FLD had significantly higher median TG (171 vs 100.5 mg/dL, P < .001) and apoB (95.5 vs 80.5 mg/dL, P = .028) levels compared with no FLD. In addition, those with versus without FLD had significantly higher LDL-P, sdLDL-C, and small LDL-P, and lower HDL-2-C (P ≤ .001), but HDL-P concentrations did not differ by FLD (P = .94) (Table 2, Figure 2). Triglycerides positively correlated with small LDL-P and sdLDL-C levels (r = 0.70, P < .001, and r = 0.75, P < .001, respectively). This was true both among those with and without FLD (Figure 3). Although HDL-2-C was lower in those with FLD, the apoA1 levels did not significantly differ by presence of FLD (Table 2).
Figure 2.
The distribution of (A) LDL-P, (B) sdLDL-C, (C) small LDL-P, (D) HDL-P, and (E) HDL-2-C by FLD. Each box represents the first (lower end) to third (upper end) quartiles of lipid values (IQR), and the horizontal line in each box represents the median lipid value. The vertical line at either end of the box extends to the most extreme values or is cut off at 1.5 times the IQR; observations beyond this cutoff are displayed as circles. Abbreviations: FLD, fatty liver disease; IQR, interquartile range; HDL-2-C, HDL subclass 2-C; HDL-P, HDL particle; LDL-P, LDL particle; sdLDL-C, small, dense LDL cholesterol.
Figure 3.
The associations between (A) sdLDL-C and (B) small LDL-P with triglycerides by FLD. Abbreviations: FLD, fatty liver disease; LDL-P, LDL particle; sdLDL-C, small, dense LDL cholesterol.
Clinical and Laboratory Factors Associated With Fatty Liver Disease
In a multivariable model of FLD (n = 104), including age and sex, non-Hispanic white (vs non-Hispanic black; adjusted OR [aOR], 8.49; P = .004), other race (vs non-Hispanic black; aOR, 16.54; P = .004), higher ALT (aOR, 3.13 per doubling of ALT; P = .003), elevated blood pressure (aOR, 10.93; P = .002), hyperlipidemia (aOR, 4.36; P = .04), and known family history of diabetes (aOR, 5.38; P = .02) were independently associated with higher odds of FLD (Table 3). Moderate alcohol use (vs none/minimal use) was associated with lower odds of FLD (aOR, 0.19; P = .03), and although not statistically significant, heavy use appeared to be associated with higher risk (aOR, 5.80; P = .08). The C-statistic was 0.892 indicating good model fit. Controlling for HBV DNA detectable status (<20 IU/mL vs ≥20 IU/mL) did not significantly change the model estimates (data not shown). When replacing ALT with AST level in the model, AST was also independently associated with higher odds of FLD (aOR, 2.72 per doubling of AST; P = .02). In addition, when Adipo-IR was added to the model, it was independently associated with higher odds of FLD (aOR, 1.13; P = .01).
Table 3.
Multivariable Analysis of Factors Associated With Fatty Liver Disease
| n | Adjusted OR | 95% CI | P | |
|---|---|---|---|---|
| Age, per 10 years | 104 | 1.65 | .70, 3.87 | .25 |
| Sex (ref = female) | 7 | |||
| Male | 97 | 3.20 | .23, 45.17 | .39 |
| Race (ref = non-Hispanic black) | 53 | .004 | ||
| Non-Hispanic white | 33 | 8.49 | 1.96, 36.72 | .004 |
| Other | 18 | 16.54 | 2.42, 113.21 | .004 |
| Alcohol use (ref = none or minimal) | 56 | .01 | ||
| Moderate | 35 | .19 | .04, .83 | .03 |
| Heavy | 13 | 5.80 | .80, 41.91 | .08 |
| ALT, log2a | 104 | 3.13 | 1.49, 6.57 | .003 |
| Elevated blood pressure (ref = no) | 47 | |||
| Yes | 57 | 10.93 | 2.44, 48.99 | .002 |
| Hyperlipidemia (ref = no) | 71 | |||
| Yes | 33 | 4.36 | 1.09, 17.44 | .04 |
| Known diabetes family history (ref = no) | 35 | |||
| Yes | 69 | 5.38 | 1.29, 22.49 | .02 |
N = 104.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; OR, odds ratio; ref, reference.
aGiven the high correlation between ALT and AST, separate models evaluated each with the additional independent variables that met the multivariable model criteria. If AST log2 replaces ALT log2, N = 104, C-statistic = 0.879, adjusted OR = 2.72 (1.19–6.21); P = .02.
As a sensitivity analysis, modeling was repeated replacing the 3-level alcohol risk variable based on consumption alone with the 2-level variable (no/yes at-risk) from the AUDIT, which signifies alcohol-use disorder. The aOR for having FLD was 18.94 (P = .04). However, the 95% CI was very large (1.20 to 298.61) indicating uncertainty of the estimate. The model fit was similar (C = .885).
Impact of Fatty Liver Disease on ALT and AST Levels Over Time
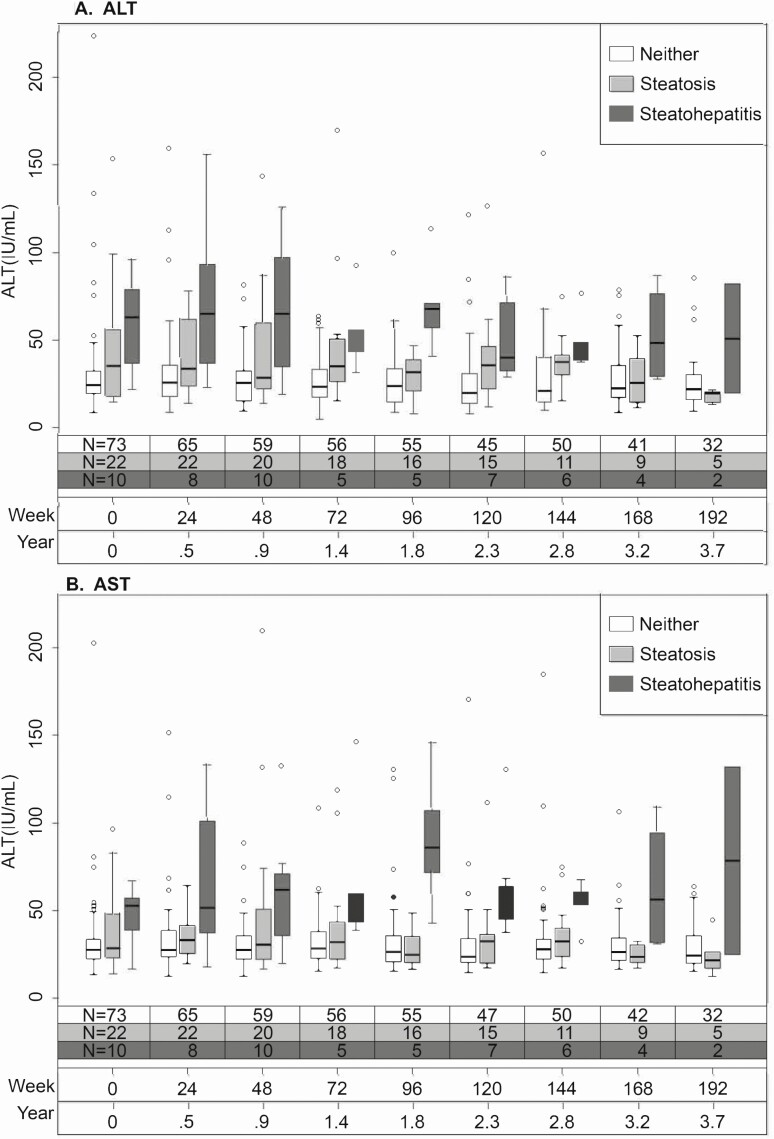
Participants (N = 112) had a median of 7 (interquartile range [IQR], 4–8) ALT and AST measurements over a median of 3.0 years (IQR, 1.8–3.6 years). Figure 4 shows AST and ALT levels longitudinally by baseline FLD status. Participants had a stepwise increase in ALT from no FLD to steatosis to steatohepatitis that persisted over time. Although AST levels appeared similar among those with no FLD and those with steatosis, AST remained higher in those with steatohepatitis throughout follow-up.
Figure 4.
The distribution of (A) ALT and (B) AST over time by FLD status at enrollment. Each box represents the first (lower end) to third (upper end) quartiles of ALT or AST values (IQR), and the horizontal line in each box represents the median value. The vertical line at either end of the box extends to the most extreme values or is cut off at 1.5 times the IQR; observations beyond this cutoff are displayed as circles. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FLD, fatty liver disease; IQR, interquartile range.
When adjusting for HBV DNA level, age, and sex, in comparison to no FLD having steatohepatitis was associated with, on average, 1.93 (95% CI, 1.37–2.72) times higher ALT across time, while having steatosis without steatohepatitis was associated with, on average, 1.34 (95% CI, 1.05–1.70) times higher ALT across time (P < .001). Furthermore, HBV DNA levels of 1000 IU/mL or greater were associated with higher ALT (ratio, 1.27; 95% CI, 1.12–1.44; P < .001). While age did not appear to be associated with ALT (ratio, .97/decade; 95% CI, .87–1.08; P = .54), the small sample size resulted in large 95% CIs and lack of statistical power to detect other potential associations (eg, male vs female ratio = 1.32; 95% CI, .91–1.93; P = .15).
DISCUSSION
In this HIV-HBV cohort residing in North America we found a moderately high prevalence (ie, 30%) of FLD. Risk factors for FLD largely mirrored those observed in persons without HIV and were dominated by metabolic factors. While not necessarily evident by traditional lipid profiles, there was an increase in other atherogenic lipid profiles signaling cardiovascular risk in those with FLD. Notably, there were no specific HBV-related measures that were associated with likelihood of histologic FLD. However, over 90% of this cohort was treated for HBV and the substantial majority (over 80%) had HBV DNA levels below 1000 IU/mL. Thus, whether HBV contributes to coexisting FLD cannot be addressed in this cohort on ART.
Participants with FLD had higher glucose, insulin, and FFA levels. Adipose tissue dysfunction is considered central to the pathogenesis of FLD [32] and HIV has known adverse effects on insulin sensitivity in the periphery, including muscle and adipose tissue, resulting in lipotoxicity [33]. Indeed, similar to that observed in other populations, adipose tissue insulin resistance was associated with the presence of FLD in our HIV-HBV cohort.
Cardiovascular disease has emerged as a leading cause of mortality in FLD [34, 35]. Chronic HIV is also a risk for adverse cardiovascular outcomes [36]. In a recent meta-analysis and systematic review, risk factors such as hypertension, dyslipidemia, and smoking were significant contributors to CVD risk, suggesting the importance of identification and aggressive management of these risk factors in the management of patients with HIV [36]. When assessing traditional lipid profiles by the presence of FLD, although values were in the expected direction, we did not find a significant difference in LDL-C or HDL-C between those participants with and without FLD. However, a more detailed evaluation of lipid subfractions revealed significantly higher levels of the atherogenic lipid subfractions small LDL-P and sdLDL-C and lower HDL-2-C in the FLD group compared with those without FLD. Other studies in patients with FLD without HIV or HBV infection reveal similar patterns of atherogenic fractions [37]. Moreover, an increase in TG levels appeared to correlate with the trends in each of these subfractions. This suggests that TG levels can act as a proxy for assessment of atherogenic risk. We also observed that, although the HDLs were lower in those with FLD, the apoA1 levels did not significantly differ by presence of FLD. This suggests that there may be other defects such as pre–B-HDL particle formation or altered clearance of HDL that play a role in this population with coinfection. Confirmation is required to determine the generalizability of these findings.
Evaluation of liver enzymes revealed that those with steatohepatitis had the highest sustained ALT levels over time compared with those with steatosis or no FLD. In those with HBV DNA of 1000 IU/mL or greater, however, elevated ALT levels were also observed. These findings suggest that, in those persons with HBV-HIV with low HBV viral levels, FLD should be considered when ALT levels are persistently elevated. This is an important consideration, particularly given the contribution of FLD, HBV, and HIV each to progressive liver disease or even hepatocellular carcinoma. In light of our prior finding that histologic HBV-related liver injury appears more severe in HBV-HIV despite suppression with ART [17], it becomes even more important to identify and control other forms of liver injury such as FLD.
The limitations of this study include the self-selection of patients willing to undergo liver biopsy, limiting generalizability to the total HIV-HBV population. In addition, similar to other studies [20, 38], data were not available on clinical parameters at the time of HIV diagnosis, baseline severity of HIV disease, duration of anti–HBV therapy prior to ART, and older ART regimens prior to enrollment, which may influence FLD prevalence and severity. Likewise, data were not available on clinical parameters prior to onset of FLD. Cross-sectionally, ALT was higher with FLD, and while we demonstrated that this relationship persists over time when accounting for HBV viral levels, we could not determine if ALT had increased with the initial development of FLD. However, we were able to assess these parameters at the time of enrollment and evaluated laboratory parameters prospectively to account for changes over time. While liver biopsy remains the gold standard for diagnosis of steatohepatitis, sampling errors can occur. Nevertheless, this study represents the largest prospective HIV-HBV cohort with detailed histologic, clinical, and laboratory assessment and longitudinal follow-up. With the planned assessment of follow-up histologic assessment in this population, the impact of FLD on liver disease progression can be evaluated in the future.
In summary, nearly one-third of this HIV-HBV cohort had FLD and 10% had coexisting steatohepatitis. Fatty liver disease was associated with higher degrees of perisinusoidal fibrosis and persistent ALT elevation over time. Thus, elevated ALT despite HBV control should prompt evaluation for metabolic risk and coexisting FLD. While traditional metabolic abnormalities predicted FLD, TG levels or lipid subfractions, rather than standard lipid measurements, may be required to unmask CVD risk and target preventive management. Our results further highlight the importance of identification of FLD and management of adverse metabolic profiles in patients with HIV-HBV coinfection. The influence of these metabolic derangements on liver disease progression in this prospective cohort will be explored in future studies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant number R01-DK94818) as an ancillary study (NCT01924455) of the Hepatitis B Research Network. M. K. was also partially supported by the National Institutes of Health (grant number K24AA022523), M. S. was partially supported by the National Institutes of Health (grant number K24DA034621), and R. T. C. was supported by the National Institutes of Health (grant number K24DK078772) and the Massachusetts General Hospital (MGH) Research Scholars Program.
Potential conflicts of interest. M. K. is a recipient of research grant (to her institution) from Gilead Sciences and grants from the National Institutes of Health (NIH) and Intercept Pharmaceuticals and she has served as consultant for Gilead Sciences. R. T. C. has received research grants (to his institution) from Gilead Sciences Inc, AbbVie Inc, Bristol Myers Squibb, Merck, Boehringer Ingelheim, RHoffmann-La Roche, and JJanssen Pharmaceutica. M. L.-M. serves on the speaker’s bureau for AbbVie Inc, Gilead Sciences, and SimplySpeaking. M. K. J. has received research funding from Gilead Sciences, Janssen Pharmaceuticals, Merck, and GlaxoSmithKline/ViiV. She has served on the scientific advisory board for Gilead Sciences. R. K. S. has received research grants from Abbott Laboratories, AbbVie Inc, Gilead Sciences, and Hoffman La Roche Life Sciences and serves on the data safety and monitoring board for Pfizer and Baxter. A. S. reports stock options from Genfit, Tiziana Life Sciences, Indalo Therapeutics, Durect, and Exhalenz; grants from Gilead Sciences, Intercept Pharmaceuticals, AstraZeneca, Eli Lilly and Company, Allergan, Novartis, Novo Nordisk, Boehringer Ingelheim, Zydus Pharmaceuticals, Echosense, Perspectum, Bristol Myers Squibb, Malinckrodt, and Valeant Pharmaceuticals; personal fees from Merck, Pfizer, AstraZeneca, Eli Lilly and Company, Novartis, Terns, Novo Nordisk, Boehringer Ingelheim, Sun Pharma, Siemens, Histoindex, Bristol Myers Squibb, Chiasma, and Ferring; research collaboration from Second Genome; ownership in Sanyal Bio; royalties from Uptodate and Elsevier, and a patent mouse model of nonalcoholic steatohepatitis pending to Sanyal Bio. M. S. reports grants from AbbVie Inc, Assembly Biosciences, Gilead Sciences, and the NIH and personal fees from AbbVie Inc, Arbutus, Assembly Biosciences, Atea, Biomarin, Immunocore LCC, Gilead Sciences, Clinical Care Options LLC, Viral Ed LLC, DKBmed, Practice Point Communications, and Wiley. D. K. W. reports grants from the NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. AIDS by the numbers 2015. Available at: http://www.unaids.org/sites/default/files/media_asset/AIDS_by_the_numbers_2015_en.pdf. Accessed 28 February 2020.
- 2. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acharya C, Dharel N, Sterling RK. Chronic liver disease in the human immunodeficiency virus patient. Clin Liver Dis 2015; 19:1–22. [DOI] [PubMed] [Google Scholar]
- 4. Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010; 24:1537–48. [DOI] [PubMed] [Google Scholar]
- 5. Cai J, Osikowicz M, Sebastiani G. Clinical significance of elevated liver transaminases in HIV-infected patients. AIDS 2019; 33:1267–82. [DOI] [PubMed] [Google Scholar]
- 6. Kim HN, Nance R, Van Rompaey S, et al. Poorly controlled HIV infection: an independent risk factor for liver fibrosis. J Acquir Immune Defic Syndr 2016; 72:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kovari H, Ledergerber B, Battegay M, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis B or C virus co-infection. Clin Infect Dis 2010; 50:502–11. [DOI] [PubMed] [Google Scholar]
- 8. Sherman KE, Thomas D, Chung RT. Human immunodeficiency virus and liver disease forum 2012. Hepatology 2014; 59:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szabo G, Zakhari S. Mechanisms of alcohol-mediated hepatotoxicity in human-immunodeficiency-virus-infected patients. World J Gastroenterol 2011; 17:2500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 11. Seth A, Sherman KE. Fatty liver disease in persons with HIV infection. Top Antivir Med 2019; 27:75–82. [PMC free article] [PubMed] [Google Scholar]
- 12. Morse CG, McLaughlin M, Matthews L, et al. Nonalcoholic steatohepatitis and hepatic fibrosis in HIV-1-monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis 2015; 60:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guaraldi G, Lonardo A, Maia L, Palella FJ Jr. Metabolic concerns in aging HIV-infected persons: from serum lipid phenotype to fatty liver. AIDS 2017; 31Suppl 2:147–56. [DOI] [PubMed] [Google Scholar]
- 14. Aepfelbacher JA, Balmaceda J, Purdy J, et al. Increased prevalence of hepatic steatosis in young adults with lifelong HIV. J Infect Dis 2019; 220:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crum-Cianflone N, Krause D, Wessman D, et al. Fatty liver disease is associated with underlying cardiovascular disease in HIV-infected persons. HIV Med 2011; 12:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konerman MA, Mehta SH, Sutcliffe CG, et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology 2014; 59:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterling RK, Wahed AS, King WC, et al. ; HIV-HBV Cohort Study of the Hepatitis B Research Network . Spectrum of liver disease in hepatitis B virus (HBV) patients co-infected with human immunodeficiency virus (HIV): results of the HBV-HIV cohort study. Am J Gastroenterol 2019; 114:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Squillace N, Soria A, Bozzi G, Gori A, Bandera A. Nonalcoholic fatty liver disease and steatohepatitis in people living with HIV. Expert Rev Gastroenterol Hepatol 2019; 13:643–50. [DOI] [PubMed] [Google Scholar]
- 19. Torgersen J, So-Armah K, Freiberg MS, et al. Comparison of the prevalence, severity, and risk factors for hepatic steatosis in HIV-infected and uninfected people. BMC Gastroenterol 2019; 19:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingiliz P, Valantin MA, Duvivier C, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 2009; 49:436–42. [DOI] [PubMed] [Google Scholar]
- 21. Pelusi S, Cespiati A, Rametta R, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without Steatohepatitis. Clin Gastroenterol Hepatol 2019; 17:2310–2319.e6. [DOI] [PubMed] [Google Scholar]
- 22. Wang B, Li W, Fang H, Zhou H. Hepatitis B virus infection is not associated with fatty liver disease: evidence from a cohort study and functional analysis. Mol Med Rep 2019; 19:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen F, Mi YQ, Xu L, et al. Moderate to severe hepatic steatosis leads to overestimation of liver stiffness measurement in chronic hepatitis B patients without significant fibrosis. Aliment Pharmacol Ther 2019; 50:93–102. [DOI] [PubMed] [Google Scholar]
- 24. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696–9. [DOI] [PubMed] [Google Scholar]
- 25. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012; 32:3–13. [DOI] [PubMed] [Google Scholar]
- 26. National Institute on Alcohol Abuse and Alcoholism. What is “low-risk” drinking? Available at: http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsLowRiskDrinking.asp. Accessed 3 March 2020.
- 27. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995; 56:423–32. [DOI] [PubMed] [Google Scholar]
- 28. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–5. [DOI] [PubMed] [Google Scholar]
- 29. Lam KD, Bacchetti P, Abbasi F, et al. Comparison of surrogate and direct measurement of insulin resistance in chronic hepatitis C virus infection: impact of obesity and ethnicity. Hepatology 2010; 52:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bell LN, Wang J, Muralidharan S, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology 2012; 56:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr A, Law M; HIV Lipodystrophy Case Definition Study Group . An objective lipodystrophy severity grading scale derived from the lipodystrophy case definition score. J Acquir Immune Defic Syndr 2003; 33:571–6. [DOI] [PubMed] [Google Scholar]
- 32. Rosso C, Kazankov K, Younes R, et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol 2019; 71:1012–21. [DOI] [PubMed] [Google Scholar]
- 33. Coronel-Castillo CE, Qi X, Contreras-Carmona J, Ramírez-Pérez OL, Méndez-Sánchez N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in HIV infection: a metabolic approach of an infectious disease. Expert Rev Gastroenterol Hepatol 2019; 13:531–40. [DOI] [PubMed] [Google Scholar]
- 34. Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol 2014; 20:13306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality risk detected by atherosclerotic cardiovascular disease score in patients with nonalcoholic fatty liver disease. Hepatol Commun 2019; 3:1050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rao SG, Galaviz KI, Gay HC, et al. Factors associated with excess myocardial infarction risk in HIV-infected adults: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2019; 81:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corey KE, Misdraji J, Gelrud L, Zheng H, Chung RT, Krauss RM. Nonalcoholic steatohepatitis is associated with an atherogenic lipoprotein subfraction profile. Lipids Health Dis 2014; 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martín-Carbonero L, Teixeira T, Poveda E, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS 2011; 25:73–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.