Abstract
Background
We previously identified Pseudomonas aeruginosa isolates with characteristics typical of chronic infection in some early infections in children with cystic fibrosis (CF), suggesting that these isolates may have been acquired from other patients. Our objective was to define the extent of P. aeruginosa strain-sharing in early CF infections and its impact on antibiotic eradication treatment failure rates.
Methods
We performed whole genome sequencing on isolates from early pediatric CF pulmonary infections and from the following comparator groups in the same hospital: chronic CF infection, sink drains, sterile site infections, and asymptomatic carriage. Univariate logistic regression was used to assess factors associated with treatment failure.
Results
In this retrospective, observational study, 1029 isolates were sequenced. The CF clones strain B and clone C were present. In 70 CF patients with early infections, 14 shared strains infected 29 (41%) patients over 5 years; 16% (n = 14) of infections had mixed strains. In the 70 children, approximately one-third of shared-strain infections were likely due to patient-to-patient transmission. Mixed-strain infections were associated with strain-sharing (odds ratio, 8.50; 95% confidence interval, 2.2–33.4; P = .002). Strain-sharing was not associated with antibiotic eradication treatment failure; however, nosocomial strain transmission was associated with establishment of chronic infection in a CF sibling pair.
Conclusions
Although early P. aeruginosa CF infection is thought to reflect acquisition of diverse strains from community reservoirs, we identified frequent early CF strain-sharing that was associated with the presence of mixed strains and instances of possible patient-to-patient transmission.
Keywords: Pseudomonas aeruginosa, cystic fibrosis, respiratory tract infections, whole genome sequencing, cross infection
In this study, 41% of children with cystic fibrosis shared Pseudomonas aeruginosa strains with other children. In approximately one-third of patients with shared strains, epidemiologic links were identified, suggesting that patient-to-patient transmission of P. aeruginosa strains may have occurred.
(See the Editorial Commentary by Sala and Jain on pages e2529–30.)
Early Pseudomonas aeruginosa (Pa) infection in individuals with cystic fibrosis (CF) is usually characterized by acquisition of diverse Pa strains from the environment [1]. These Pa strains undergo rapid diversification into a highly heterogeneous population that is adapted to the CF airways (eg, acquisition of mucoid phenotype) [2]. We previously identified early Pa infection with mucoid isolates, suggesting patient-to-patient transmission of Pa, which prompted us to question the degree of genetic diversity and strain-sharing within a population of CF children with initial Pa infection [3].
Although strain-sharing and transmission of Pa between individuals has been described in adult and pediatric patients with chronic Pa infection, it is thought to be a very rare occurrence in early Pa infection [4]. Previous studies that have demonstrated shared Pa strains between CF children, which may be due to either patient-to-patient transmission or transmission from a common environmental source, have been limited by the use of lower-resolution typing techniques, such as pulsed-field gel electrophoresis or multilocus sequence typing (MLST) [5–8]. The advent of newer molecular typing techniques, in particular whole genome sequencing (WGS), has improved our ability to distinguish between nearly identical strains. The one study that used WGS to examine the genetic relatedness of early Pa isolates from children with CF identified strain-sharing in approximately 15% of patients, with epidemiologic data supporting patient-to-patient transmission [9]. That study was limited by a small sample size (35 patients) and limited depth of sampling (1 isolate per clinical specimen). Analysis of multiple bacterial isolates per clinical sample is critical when investigating strain relatedness from infections with intrapatient pathogen subpopulations (ie, that have significant intrahost bacterial diversity), such as Pa infections in CF patients. Furthermore, comparator collections of Pa isolates, such as from the environment and non-CF populations, are also helpful when evaluating the degree of genetic relatedness between strains and are missing in earlier studies.
To address this knowledge gap, we performed a 5-year retrospective observational cohort study of all children with CF and new-onset Pa infection followed at the Hospital for Sick Children (SickKids; Toronto, Canada) to determine the extent of strain-sharing, the potential for patient-to-patient transmission, and the impact on antibiotic eradication treatment. Using WGS of multiple Pa isolates from each sputum sample, we aimed to characterize the level of Pa strain-sharing in our pediatric CF cohort, the largest such studied cohort to date. Additionally, we compared isolates from early Pa infection in children with CF to isolates from chronic Pa infection in CF patients, isolates from the hospital environment, and clinical isolates from invasive infection or asymptomatic intestinal carriage in non-CF patients. From these analyses, we found that a surprising proportion of children with CF (41%) share Pa strains with other children, challenging previous beliefs that early Pa infection occurs due to acquisition of genetically diverse strains from the environment.
METHODS
Early Pa Cohort
This was a retrospective observational cohort study. The primary study population consisted of the early cohort that included all children with CF from SickKids with at least 1 new-onset Pa infection between 2011 and 2015 and who underwent antibiotic eradication therapy (AET) [10]. Pa isolates from the time of initial infection were recovered from the CF Sputum Biobank, which has been prospectively storing frozen sputum samples from SickKids CF patients since 2011 [3].
Comparator Cohorts
Comparator cohorts included the following [1]: chronic, environmental, carriage, and sterile site. The chronic cohort consisted of a CF patient population chronically infected with Pa and included 24 children who were enrolled in a randomized control trial of CF Pa biofilm antimicrobial susceptibility testing at SickKids between January 2009 and September 2013 [11]. One Pa isolate from up to 3 morphotypes was prospectively isolated and stored from each sputum culture. We required children in the chronic cohort to have 2 or more positive cultures between 2011 and 2013. Two patients in the early cohort who subsequently developed chronic infection were also included in the chronic cohort [2]. An environmental cohort consisted of isolates cultured from sink drain sampling performed in CF clinical areas in 2018 (see the Supplementary Methods for details) [3]. The carriage cohort consisted of isolates cultured from stool or rectal swabs during an inpatient point prevalence screen for carbapenemase-producing Enterobacteriaceae performed in 2017 (no CF patients) [4]. The sterile site cohort consisted of Pa cultured from children without CF who had invasive Pa infection (includes 1 isolate of each morphotype of Pa from each blood or sterile body fluid sample) at the hospital between 2000 and 2017.
We have included all the isolate numbers for all cohorts with sequence types, collection dates, and shared-strain number in Supplementary Table 1.
Whole Genome Sequencing and Analysis
All Pa isolates were sequenced and analyzed as described in the Supplemental Methods.
Definitions of Strain-sharing
We defined a shared strain as a set of identical isolates found to infect multiple individuals with CF [4]. We chose a cutoff of 4 or fewer single-nucleotide polymorphism (SNP) differences between isolates to define a strain based on our observations that intrapatient sequence diversity from new-onset infections was up to 4 SNPs (excluding outliers >50 SNPs) and that 3 to 4 SNPs per year were accumulated in patients who experienced recurrent new-onset infection years apart (Supplementary Methods) [12]. An exception to the SNP cutoff was made for 2 strains with complex phylogenetic relationships and hypermutator genotype Pa, which substantially increased the SNP distance between isolates.
Mixed-strain infection was defined as the presence of 2 or more Pa strains from different clonal complexes (ie, differing in at least 3 of 7 MLST alleles) [13] in a new-onset sputum sample. Superinfection occurred when a chronically Pa-infected individual was coinfected with a different strain of Pa at a later point in time that may or may not have supplanted the original strain.
Statistical Analyses
Univariate logistic regressions were used to assess associations between mixed-strain infection and AET failure, strain-sharing and AET failure, and mixed-strain infection and strain-sharing. All statistical analyses were done using SAS 9.04.01 (SAS Institute). The Hospital for Sick Children Research Ethics Board approved the study.
RESULTS
Pa Sequencing Results
A total of 435 early cohort isolates were cultured and sequenced from 87 new-onset episodes, in 70 patients (in those with repeated new-onset episodes, 35% (5 of 14 patients) had reinfection with a Pa strain of the same ST). A median of 4 isolates (interquartile range [IQR], 3–8) and 2 morphotypes (IQR, 1–2) were sequenced per episode. Pa could not be recovered from frozen sputum in 41 eligible episodes (32%), thus they were excluded from the analysis. The clinical characteristics of patients included in the early cohort are shown in Table 1 and were similar to those of patients excluded from the early cohort (Supplementary Table 2).
Table 1.
Clinical Characteristics of Patients in the Early Cohort (N = 70)
| Characteristic | Value |
| Age at commencement of study, mean (SD), y | 9.7 (3.5) |
| Female, n (%) | 36 (51) |
| Mutation class, n (%) | |
| Class I–III | 68 (97) |
| Class IV and V | 2 (3) |
| Complications | |
| Cystic fibrosis–related diabetes, n (%) | 3 (4) |
| Pancreatic insufficiency, n (%) | 68 (97) |
| Baseline forced expiratory volume in 1 second | |
| L, mean (SD) | 1.72 (0.77) |
| % predicted, mean (SD) | 89.7 (19.8) |
| Body mass index, mean (SD) (centile) | 39.0 (29.8) |
Abbreviation: SD, standard deviation.
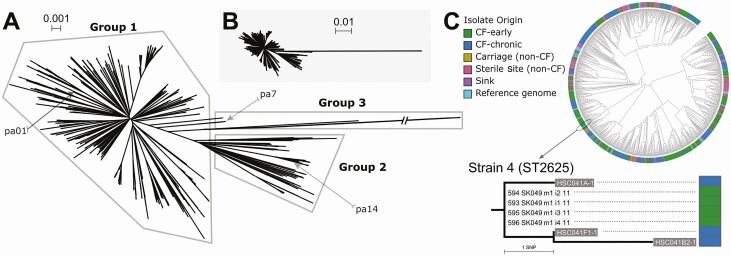
In the chronic cohort, 331 isolates were sequenced from 24 patients enrolled in the biofilm antimicrobial susceptibility trial (including 2 siblings from the early cohort), with a mean of 14 isolates sequenced per patient. Twenty environmental cohort isolates were collected and sequenced from 5 sinks installed in separate patient rooms on the CF inpatient ward (2–6 isolates per sink); no Pa was recovered from sink drain cultures taken from the CF clinic or pulmonary function test (PFT) laboratory. Twenty-two carriage cohort isolates were sequenced (1 per patient), as well as 221 sterile site cohort isolates from 201 patients. In total, 1029 SickKids Pa sequences and 81 reference genomes were included in the first-pass analysis (Figure 1).
Figure 1.
A, Unrooted neighbour-joining mashtree of 1029 de novo assemblies from the Hospital for Sick Children Pseudomonas aeruginosa (Pa) isolates and 81 complete Pa reference genomes. Most sequences cluster in either group 1, which contains reference strain pa01, or group 2, which contains reference strain pa14. This population structure is similar to those from previous reports of phylogenetic analyses of diverse Pa isolates. The remaining outlier sequences cluster in group 3 together with the pa7 reference genome. Note that pa7 is a phylogenetic outlier that diverged early in evolutionary history from other Pa lineages. Note that a branch leading to a sterile site isolate (STS031) in group 3 is truncated. B, Neighbour-joining tree from panel A with the group 3 branch shown to scale. C, A circular mashtree cladogram (branch lengths ignored). Isolates from the different cohorts (CF and non-CF patients and hospital sinks) are dispersed across the tree. A group of closely related sequences from the early and chronic cohorts is circled and shown in an enlarged phylogram (branch lengths proportional to evolutionary distance) on the right. Sequences from early cohort case 49 (SK049) and chronic cohort case 14 (HSC014) appear highly related. In fact, some sequences from SK049 appear as closely related to HSC014 sequences as they are to other SK049 sequences. This group was therefore subjected to further analysis by mapping sequencing reads to the most closely related reference genome, in this case PAER_119, to generate a pairwise single-nucleotide polymorphism distance matrix and maximum likelihood (ML) tree and thereby determine if these sequences represent a shared strain. Isolate coding system: early cohort, 594 (isolate number) SK (early cohort) 049 (patient number) m1 (morphotype number) i2 (isolate number) 11 (collection year 2011); chronic cohort, HSC (chronic cohort) 041 (patient number) B2 (visit type and number; A: enrollment, B: baseline, E: exacerbation, F: follow up) -1(isolate number); environmental cohort, ENV (environmental cohort) 64 (room number where isolate collected)-3 (isolate number). Abbreviation: CF, cystic fibrosis.
Strain B (ST439), a well-recognized clone that accounts for 7% of isolates from adult CF patients in Ontario [13], was identified in 1 early cohort patient. Clone C (ST17), which is widely disseminated in the general environment, was detected in 8 CF patients (5 early cohort and 3 chronic cohort patients). However, using WGS, only 4 patients were classified as harboring a shared strain (see Supplementary Table 1 for more detail).
Mixed strains (up to 4 strains) were present in 14 new-onset infections (16%) from 13 early cohort patients and could not be reliably predicated from the morphotypic appearance of isolates alone. However, we found an association between the presence of multiple morphotypes in a sample and mixed-strain infection (odds ratio [OR], 2.18; 95% confidence interval [CI], 1.14–4.20; P = .02).
Shared Strains in the Early Cohort
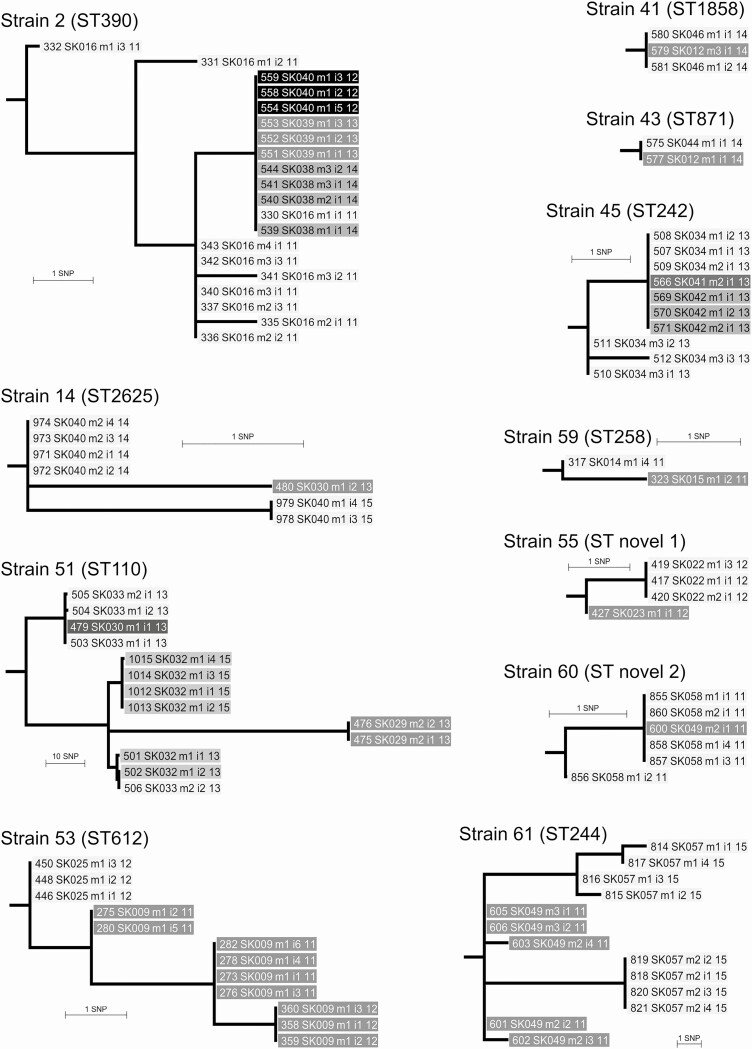
The majority of shared strains were found in the early cohort. A total of 78 different Pa strains from 60 STs were identified in the early cohort (8 of these STs have previously been reported in other early CF cohorts [5]). Of these, 64 strains (82%) were recovered only once, while 14 were shared among patients. These 14 shared strains were found among 29 (41%) patients, with shared strains found in 33 (38%) of the new-onset episodes. Eleven of the 14 shared strains were shared only among early cohort patients. Of these 11 strains, 8 (strains 14, 41, 43, 53, 55, 59, 60, 61) were found in patient pairs, 1 (strain 45) was shared among 3 patients, and 2 (strains 2, 51) were shared among 4 patients. One strain (strain 51) included a patient with isolates that had a hypermutator genotype (Figure 2).
Figure 2.
Maximum likelihood trees for 11 shared strains in early cohort patients rooted on reference genome (not shown) used for SNP calling as detailed in Supplementary Table 1. Strain 51 contains a long branch to 2 hypermutator isolates from case SK029 (475 and 476). These isolates are 89 to 90 SNPs different from isolates from infections that occurred in 1 case (SK032) in 2013 and 2015. We identified a mutS DNA mismatch repair gene frameshift mutation at codon 333 (of total 2568), resulting in nonfunctional MutS in isolates 475 and 476 only, and so consider them part of the shared strain. The SK032 isolates are paraphyletic with respect to 475 and 476, which suggests the direction of Pseudomonas aeruginosa transmission was from SK032 to SK029. Abbreviation: SNP, single-nucleotide polymorphism.
Overall, we found few epidemiological links for the 11 early cohort shared strains. No siblings were affected, no social links outside the hospital were identified, and cases were not coinfected with similar pathogens other than Pa. One shared strain may have been transmitted between 2 patients at a clinic visit in 2011. Another was potentially transmitted between 2 patients during a same-day PFT laboratory visit in 2012; however, 2 other patients who acquired the same strain a year later had no epidemiological links. Nine shared strains had no epidemiological link between any patients; the gap between detection of infection ranged from 2 days to 14 months (less than 6 months for 8 strains). All infections were detected between late March and mid-November, and only 1 shared strain was newly identified in a patient after 2014.
Mixed-strain infection was associated with strain-sharing (OR, 8.50; 95% CI, 2.2–33.4; P = .002). There was no association between shared-strain infections and AET failure or mixed-strain infection and AET failure, using Pa sputum culture positivity at 5 weeks or 3 months after the time of initial Pa infection to define AET failure or using development of chronic infection [14] after 18 months.
Shared Strains in the Chronic Cohort
Overall, 24 Pa strains were identified in the chronic cohort; 17 (71%) strains recovered from 15 patients were unique, while 4 strains recovered from 4 patients were shared with other cohorts and are discussed separately. The remaining 3 strains were shared by 5 patients (Supplementary Figure E1). Strain 102 was found in a sibling pair consistently throughout their longitudinal sampling. It was also briefly present in 2 other chronically infected nonsibling patients in whom it caused superinfection. One nonsibling patient was at baseline status at the time (isolate HSC022B2–3), and the other nonsibling was experiencing an exacerbation (isolate HSC034E2–1). Strain 102 did not supplant the original chronic strain for the nonsibling patient in whom follow-up samples were obtained. Two other shared strains (strains 100 101) involved pairs of nonsibling patients who attended the clinic on the same day and superinfected each other; the superinfecting strains did not supplant the original patient strain.
Shared Strains Between Cohorts
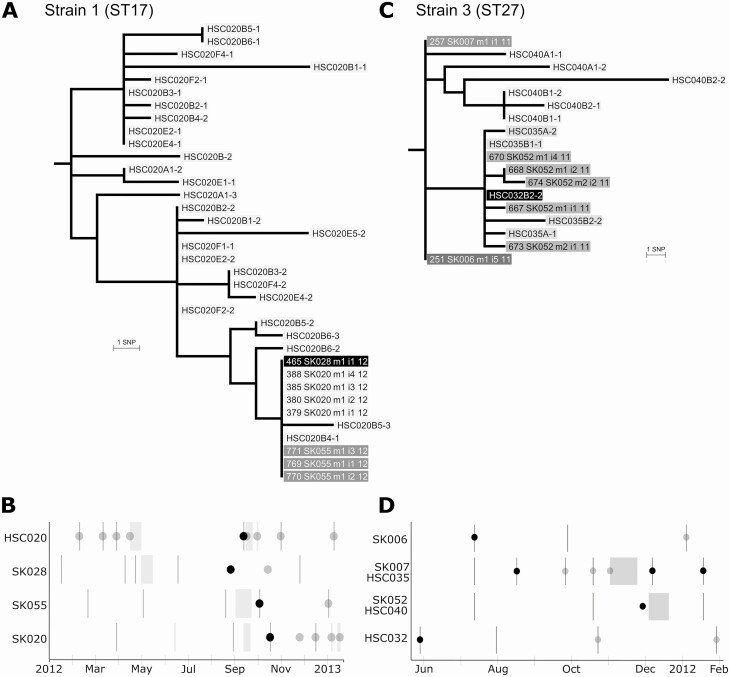
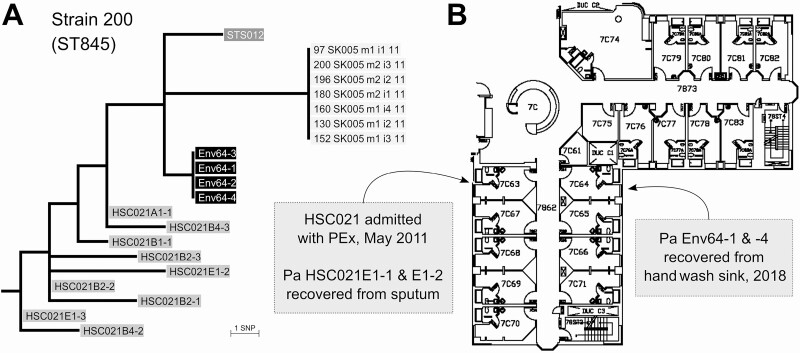
Three strains were shared between patients in the early and chronic cohorts. For 2 of these strains, there was evidence of possible patient-to-patient transmission. Genomic and epidemiological analyses demonstrate that a strain (strain 01) was shared between a chronic patient and 3 patients in the early cohort on the inpatient CF ward (Figure 3A, B). Another shared strain (strain 03) may have been transmitted in the CF clinic from an early cohort patient to a pair of early cohort siblings, with the siblings then becoming chronically infected. Additionally, a patient in the chronic cohort became superinfected with this strain around the same time but had no clear epidemiological link (Figure 3C, D). The third strain (strain 04) was shared between an early cohort case and a chronic cohort case with no epidemiological link. The final strain shared between cohorts (strain 200) consisted of 4 isolates collected from the hand-wash sink in a room on the CF ward in 2018 and isolates from 1 patient each in the chronic and sterile site cohorts (Figure 4). The CF patient was hospitalized in the room across the hall in 2011. However, the sterile site isolate came from a blood culture taken from a non-CF patient hospitalized 16 years earlier on a different ward.
Figure 3.
A, Maximum likelihood tree of strain 1 isolates rooted on reference genome (not shown) used for SNP calling as detailed in Supplementary Table 1. Isolates of ST17 Pseudomonas aeruginosa (Pa) from 3 early cohort cases (SK020, SK028, and SK055) are identical to each other and to 1 isolate from a chronic cohort case (HSC020). The chronic case exhibits significant intrapatient Pa sequence diversity from the time of enrollment (sample A) through baseline pulmonary status assessments (B), pulmonary exacerbations (E), and follow-up visits postexacerbation (F). However, only 1 HSC020 isolate, B4–1, is identical to the early patient isolates, which allowed us to date the approximate period that strain transmission occurred to when this isolate was collected in September 2012. B, Timeline of Pa infections and hospital visits. Each row represents 1 patient. Dark vertical lines: clinic or pulmonary function test (PFT) laboratory visits. Light gray boxes: inpatient admissions. Dark circles: sputum was positive for Pa and isolates were sequenced, with presence of shared strain confirmed. Light gray circles: sputum positive for Pa, but isolates were not sequenced. Sibling cases SK020 and SK055 were admitted to the cystic fibrosis ward in September 2012 and overlapped for 6 days with the stay of the chronic cohort patient. Shortly thereafter, SK020 and SK055 had new-onset infection with the chronic patient’s strain. Another early cohort patient, SK028, was infected in August 2012, several months after an admission to the ward that commenced on the day after the chronic patient had been discharged. C, Maximum likelihood tree of strain 3 isolates rooted on the reference genome. Isolates from early cohort cases SK006 and SK007 are identical and separated by 4 SNPs from an isolate from SK052 (the sibling of SK007) and an isolate from chronic cohort case HSC032. The siblings developed chronic infection with this Pa strain and were subsequently enrolled in the biofilm trial. All subsequent chronic isolates from the siblings were with the same strain and were sequenced under the study code numbers HSC040 (for SK0007) and HSC035 (for SK052). D, Timeline of Pa infections and hospital visits as in (B). Each row represents 1 patient (SK007 and SK052 also have chronic cohort study codes HSC035 and HSC040). SK006 attended the clinic in July 2011 and had a new-onset infection detected. On the same day, the siblings also attended the clinic; SK007 developed infection with the shared strain 1 month later, while SK052 developed infection with the shared strain in December. SK052 could have acquired the strain from SK006 at the clinic visit or from their sibling subsequently. In May 2012, HSC032 was superinfected with the shared strain but had no overlapping visits with the other patients in the prior 6 months. Afterward, HSC032 reverted to their preexisting Pa strain. Abbreviation: SNP, single-nucleotide polymorphism.
Figure 4.
A, Maximum likelihood tree of isolates rooted on the reference genome (not shown) used for SNP calling as detailed in Supplementary Table 1. One isolate from chronic case HSC021 (HSC021-A1–1, collected at trial enrollment in 2010) is 3 SNPs from isolates from a sink in cystic fibrosis (CF) ward room 64 (Env64 1–4). The other 9 isolates from HSC020 (collected in 2010–2012) are 5 to 9 SNPs distant. An isolate from a sterile site infection (STS012) is also 3 SNPs from the sink isolates and 4 SNPs from HSC021-A1–1. Isolates from early cohort case SK005 were included as they appeared closely related to the other isolates on the mashtree. However, we found at least 6 SNP differences from all other isolates, so we deemed the SK005 isolates to be unique, that is, they are not part of the shared strain. B, Floor map of the inpatient CF ward with locations of sink samples and inpatient admissions. Case HSC021 was admitted in May 2011 to a room opposite room 64 where the sink isolates were retrieved 7 years later. This suggests isolates from HSC021 were introduced to the ward environment during the 2011 admission and persisted in the ward sinks. The STS012 isolate came from a postoperative blood culture from a 2001 patient (non-CF) with no epidemiological links to this ward. Abbreviation: Pa, Pseudomonas aeruginosa; PEx, pulmonary exacerbation; SNP, single-nucleotide polymorphism.
A summary of the degree of strain-sharing between cohorts is provided in Supplementary Figure E2.
Discussion
Our study demonstrates that strain-sharing occurred in 41% of patients with early Pa infection. Mixed-strain infection was relatively frequent in the early cohort (16% of episodes) and was strongly associated with strain-sharing. Epidemiological links between patients were found for 4 of 29 (14%) shared-strain–infected patients; these links were comprised of overlapping ward, clinic or PFT laboratory visits, and occasionally sibling relationships. Strain-sharing was not associated with antibiotic eradication treatment failure; however, potential nosocomial strain transmission was associated with establishment of chronic infection in a CF sibling pair.
Previous studies have reported that between 0% and 62% of CF patients may share strains of Pa, although few studies have focused specifically on early Pa acquisition in children with CF [4]. Kidd et al demonstrated that the majority of Pa strains that infect CF children aged <5 years were unique and commonly found in different ecological settings; however, there was limited within-patient sampling of Pa colonies [1, 5]. With low-resolution typing techniques of single Pa colonies from respiratory tract specimens and the absence of proven epidemiological links, it is reasonable to conclude that genotypically similar strains were acquired independently from the general environment. In a smaller, retrospective study performed at the Copenhagen CF Centre, 474 isolates of Pa sampled from the airways of 34 children and young individuals with CF were genotyped by WGS. In only a few cases (n = 5) were strains closely related, differing by only a few SNPs, suggesting patient-to-patient transmission, supported by epidemiologic links [9]. Although this degree of patient-to-patient transmission (approximately 15%) is similar to that in our study, we found a significantly higher percentage of strain-sharing overall (41%).
Our shared-strain infections occurred in a pediatric CF center that adheres to national CF infection prevention and control (IPAC) recommendations [15]: no designated waiting room, environmental cleaning of the pulmonary function testing laboratory (not under negative pressure) after every patient, standard precautions and single-room isolation in all CF care areas from 2011 to 2014, and additional contact precautions (gloving and gowning for staff) from 2014 onward. Given that our study occurred from 2011–2015, we cannot judge the impact of such changes in infection control practices on strain-sharing due to the unequal follow-up times pre- and post-2014. Due to the retrospective nature of the study, we could not establish how strains were shared during overlapping hospital visits, although cough aerosol generation and fomites have been implicated in previous studies [16, 17]. Hospital water distribution networks, including sink outlets, may be the source of outbreaks of waterborne bacteria. Although we did not sample water distribution networks, we did not find evidence of widespread dissemination of a single Pa strain, which would be typical of point-source transmission. Of note, we did not find Pa in CF clinic sinks. A shared Pa strain was present in a CF ward sink, but it is possible this represents unidirectional contamination of sink drains by patients. Shared strains may have come from other unsampled reservoirs in CF clinical areas. If a community reservoir was the main source, we would expect considerable sharing between cohorts; however, only 1 Pa strain was shared between CF and non-CF patients. In fact, there was little to no strain-sharing among the environmental, carriage, and sterile site cohorts, suggesting that inclusion in the CF cohort played a role in strain-sharing. Movement of patients between CF centers has previously been shown to be a risk factor for the acquisition of shared Pa strain infections [18].
This study had several limitations as well as strengths. We were able to recover Pa from only two-thirds of the frozen patient samples, limiting our study population. Additionally, comparator cohort isolates were obtained during time periods that were different from those of the early cohort, and fewer isolates per sample were sequenced. Hospital environmental sampling was performed 3 years after the last infections occurred and, therefore, was restricted to potential long-term reservoirs, not surfaces or fomites that might be transiently contaminated. Although 1 strain was found shared among a sink drain and CF and non-CF patients, the samples were collected 16 years apart, and there were insufficient environmental samples to put the SNP differences in context (eg, to determine if sink isolates have lower diversity and or mutation rates than clinical isolates). The main strength of this study was the depth of sampling in the early CF cohort that allowed us to identify the frequent presence of nearly identical isolates shared between patients. Furthermore, we were able to set a clear definition of “shared strain” with a SNP threshold derived from intrahost isolate diversity observed with deep sampling. Although the term “strain” is frequently used to describe nearly identical CF Pa isolates, these shared strains may be more accurately referred to as clones, evolved from a common bacterial ancestor.
In conclusion, our study demonstrated that 41% of CF patients with early Pa infection had shared strains, of which approximately one-third were potentially associated with patient-to-patient transmission. Although strain-sharing was not associated with failure of antibiotic eradication therapy, nosocomial transmission of a shared strain was associated with the establishment of chronic infection in a pair of CF siblings. Additional studies are warranted to determine whether silent Pa strain-sharing is common in other pediatric CF centers and how this may be prevented with enhanced infection prevention and control policies.
Supplementary Material
Notes
Author contributions . P. J. S., V. W., Y. Y., and D. S. G. conceived of, planned, and directed the study. P. J. S. performed environmental sampling and bacterial cultures. S. C. and P. W. W. sequenced the isolates. C. I. and P. J. S. developed the methods for bioinformatics analyses. A. B. performed the statistical analyses and assisted P. J. S. with epidemiological investigations. P. J. S. performed the main analyses and wrote the manuscript. All authors discussed the results and provided critical feedback on the manuscript.
Acknowledgments. The authors thank the following for their expertise and assistance: Julio Diaz Caballero and Yunchen Gong for assistance with bioinformatics analyses, Michelle Klingel for Toronto Cystic Fibrosis (CF) Database support, and Alvin Li for help with the sputum biobank cultures. We are indebted to the SickKids clinical microbiology laboratory, infection control, and CF clinical care teams for help with Pseudomonas aeruginosa cultures and epidemiological investigations. Finally, we express our sincere gratitude to the patients with CF and their families for their participation in the Toronto CF database and this research study.
Financial support. This work was supported by a Collaborative Health Research Project grant awarded to D. S. G., with joint funding provided by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada (CP-151952). P. J. S. and A. C. are recipients of Cystic Fibrosis Canada post-doctoral research fellowships.
Potential conflicts of interest . V. W. reports grants from Gilead and consultancy fees from Astra Zeneca outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ranganathan SC, Skoric B, Ramsay KA, et al. ; Australian Respiratory Early Surveillance Team for Cystic Fibrosis . Geographical differences in first acquisition of Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc 2013; 10:108–14. [DOI] [PubMed] [Google Scholar]
- 2. Hogardt M, Heesemann J. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 2010; 300:557–62. [DOI] [PubMed] [Google Scholar]
- 3. Vidya P, Smith L, Beaudoin T, et al. Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2016; 35:67–74. [DOI] [PubMed] [Google Scholar]
- 4. Parkins MD, Somayaji R, Waters VJ. Epidemiology, biology, and impact of clonal Pseudomonas aeruginosa infections in cystic fibrosis. Clin Microbiol Rev 2018; 31:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kidd TJ, Ramsay KA, Vidmar S, et al. ; ACFBAL Study Investigators . Pseudomonas aeruginosa genotypes acquired by children with cystic fibrosis by age 5-years. J Cyst Fibros 2015; 14:361–9. [DOI] [PubMed] [Google Scholar]
- 6. Johansson E, Welinder-Olsson C, Gilljam M, Pourcel C, Lindblad A. Genotyping of Pseudomonas aeruginosa reveals high diversity, stability over time and good outcome of eradication. J Cyst Fibros 2015; 14:353–60. [DOI] [PubMed] [Google Scholar]
- 7. Hall AJ, Fothergill JL, McNamara PS, Southern KW, Winstanley C. Turnover of strains and intraclonal variation amongst Pseudomonas aeruginosa isolates from paediatric CF patients. Diagn Microbiol Infect Dis 2014; 80:324–6. [DOI] [PubMed] [Google Scholar]
- 8. Speert DP, Campbell ME, Henry DA, et al. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am J Respir Crit Care Med 2002; 166:988–93. [DOI] [PubMed] [Google Scholar]
- 9. Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet 2015; 47:57–64. [DOI] [PubMed] [Google Scholar]
- 10. Blanchard AC, Horton E, Stanojevic S, Taylor L, Waters V, Ratjen F. Effectiveness of a stepwise Pseudomonas aeruginosa eradication protocol in children with cystic fibrosis. J Cyst Fibros 2017; 16:395–400. [DOI] [PubMed] [Google Scholar]
- 11. Yau YC, Ratjen F, Tullis E, et al. Randomized controlled trial of biofilm antimicrobial susceptibility testing in cystic fibrosis patients. J Cyst Fibros 2015; 14:262–6. [DOI] [PubMed] [Google Scholar]
- 12. Marvig RL, Johansen HK, Molin S, Jelsbak L. Genome analysis of a transmissible lineage of Pseudomonas aeruginosa reveals pathoadaptive mutations and distinct evolutionary paths of hypermutators. PLoS Genet 2013; 9:e1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Middleton MA, Layeghifard M, Klingel M, et al. Epidemiology of clonal Pseudomonas aeruginosa infection in a Canadian cystic fibrosis population. Ann Am Thorac Soc 2018; 15:827–36. [DOI] [PubMed] [Google Scholar]
- 14. Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2003; 2:29–34. [DOI] [PubMed] [Google Scholar]
- 15. Saiman L, Siegel JD, LiPuma JJ, et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infection control and hospital epidemiology Infect Control Hosp Epidemiol. 2014; 35Suppl 1: S1–S67. [DOI] [PubMed] [Google Scholar]
- 16. Wainwright CE, France MW, O’Rourke P, et al. Cough-generated aerosols of Pseudomonas aeruginosa and other gram-negative bacteria from patients with cystic fibrosis. Thorax 2009; 64:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panagea S, Winstanley C, Walshaw MJ, Ledson MJ, Hart CA. Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis centre, and study of its survival on dry surfaces. J Hosp Infect 2005; 59:102–7. [DOI] [PubMed] [Google Scholar]
- 18. Kidd TJ, Soares Magalhães RJ, Paynter S, Bell SC; ACPinCF Investigator Group . The social network of cystic fibrosis centre care and shared Pseudomonas aeruginosa strain infection: a cross-sectional analysis. Lancet Respir Med 2015; 3:640–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.