Abstract
Objective To compare the use of porcine small intestinal submucosal grafts (SISG) and standard autologous material (fascia) in prevention of cerebrospinal fluid (CSF) leak and pseudomeningocele formation after translabyrinthine resection.
Setting Set at the tertiary skull base center.
Methods This is a retrospective chart review. After Institutional Review Board approval, we performed a retrospective cohort study evaluating CSF leak in patients who underwent resection of lateral skull base defects with multilayered reconstruction using either fascia autograft or porcine SISGs. Demographics were summarized with descriptive statistics. Logistic regression was used to compare autograft and xenograft cohorts in terms of CSF complications.
Results Seventy-seven patients underwent lateral skull base resection, followed by reconstruction of the posterior cranial fossa. Of these patients, 21 (27.3%) underwent multilayer repair using SISG xenograft. There were no significant differences in leak-associated complications between autograft and xenograft cohorts. Ventriculoperitoneal shunt was necessary in one (1.8%) autograft and one (4.8) xenograft cases ( p = 0.49). Operative repair to revise surgical defect was necessary in three (5.4%) autograft cases and none in xenograft cases.
Conclusion The use of SISG as a component of complex skull base reconstruction after translabyrinthine tumor resection may help reduce CSF leak rates and need for further intervention.
Keywords: submucosal graft, xenograft, cerebrospinal fluid leak, craniotomy
Introduction
Surgical approaches to lateral skull base resections are guided by tumor and patient characteristics including the presence of serviceable hearing as well as patient and surgeon preference. Regardless of approach, robust and complex reconstruction strategies often involve a multilayer repair to decrease the risk of postoperative cerebrospinal fluid (CSF) leak. Primary closure or a variety of dural substitutes can be used at the dural interface. Small intestinal submucosal grafts (SISGs) provide an alternative to autologous grafts. SISGs are formed from an acellular matrix which promotes wound healing through epidermal differentiation and glycosaminoglycans and have been shown to be effective in many uses, including preventing CSF leaks when used in anterior skull base procedures. 1 2 3
Estimated leak rates following the skull base surgery vary widely, from less than 1 up to 30%. 4 5 6 7 8 9 10 11 12 13 Institutional leak rates at high-volume skull base practices are typically estimated around 8 to12%. 14 15 Risk factors for CSF leak include tumor size, patient age, the degree of pneumatization of the temporal bone, and obesity. 8 12 16 17 High-flow CSF leaks and large pseudomeningoceles usually require readmission with possible additional procedural and surgical interventions, posing potentially avoidable risk at substantial cost. 18 With increasing emphasis on value, surgeons aim to optimize reconstruction strategies and minimize risk for CSF leak.
The purpose of this preliminary study is to compare CSF leak and pseudomeningocele formation after multilayered closure of lateral skull base defects via translabyrinthine approach with cohorts which differed in usage of SISG in lieu of autologous tissues following tumor resection. We hypothesized that leaving an intact fascial layer will yield a more robust musculoperiosteal closure and will yield in decreasing the incidence of pseudomeningocele and incisional leaks. To our knowledge, this is the first study to evaluate the efficacy of SISG in lateral skull base reconstruction.
Materials and Methods
After Institutional Review Board approval, patients who underwent resection of various lateral skull base tumors via a translabyrinthine approach between 2016 and 2018 were retrospectively identified. Following resection, all patients underwent a multilayer repair. Outcomes were then evaluated after dividing patients into cohorts based on whether postresection defects were reconstructed with the use of SISG.
Demographics, tumor characteristics, postoperative complications related to CSF leak and pseudomeningocele, and subsequent interventions (e.g., lumbar drain, incisional overclosure, pharmacologic measures to decrease CSF production, placement of ventriculoperitoneal [VP] shunt, and operative intervention) were documented.
Details of Surgical Repair Following Tumor Resection
For translabyrinthine approaches, the repair involved packing the middle ear and eustachian tube with muscle and oxidized cellulose (Surgicel; Johnson and Johnson Medical, Piscataway, New Jersey, United States). Attic defects were reconstructed with bone pate and a layer of autologous tissue (fascia) or SISG ( Fig. 1A, C ). The internal auditory canal (IAC) and posterior cranial fossa (PCF) dural defects were then bridged with Duraform (Natus Neuro, Middleton, Wisconsin, United States). The PCF defect repair incorporated SISG in the xenograft cohort, whereas harvested fascia was used to repair the defect in the autograft cohort ( Fig. 1B ). In the xenograft cohort, SISG was used in two layers to augment the dural closure as well as the attic defect (to reduce likelihood of CSF rhinorrhea) to leave a very robust fascial envelope for closure, especially as no autologous tissue is harvested. Standard mastoid packing with fat ( Fig. 1D ) and reinforcement with resorbable mesh as previously outlined was performed. 19
Fig. 1.
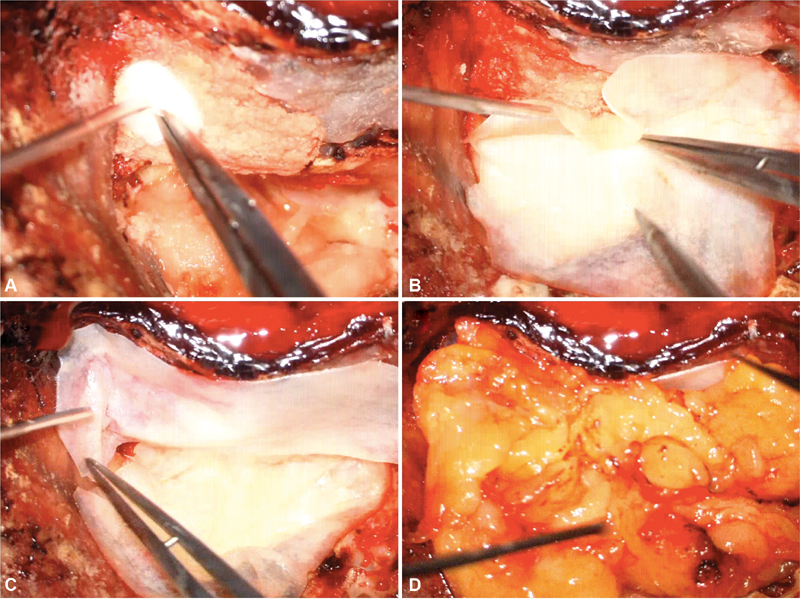
(A) Attic defect reconstruction with muscle and bone pate, (B) SISG used to reinforce the posterior fossa and IAC dural defect, (C) SISG used to reinforce the attic defect, and (D) fat graft used to obliterate the defect before placement of resorbable mesh. IAC, internal auditory canal; SISG, small intestinal submucosal graft.
Data Analysis
Continuous data are presented with mean and standard deviation, and categorical data are presented as proportions. Logistic regression was used to compare autograft and xenograft cohorts in terms of CSF complications. Statistical analysis was performed using Minitab 18 Statistical Software (Minitab, Inc.; State College, Pennsylvania, United States). The p -values < 0.05 were considered statistically significant.
Results
Seventy-seven patients underwent lateral skull base tumor resection, followed by reconstruction of the PCF ( N = 77, Table 1 ). Of these, 21 (27.3%) underwent multilayer repair using SISG xenograft. There were no significant differences in leak-associated complications between autograft and xenograft cohorts ( Table 2 ).
Table 1. Patient characteristics and lesion pathology by location of skull base defect following tumor resection.
| Autograft | Xenograft | p -Value | |
|---|---|---|---|
| Patient characteristics | |||
| Number of subjects | 56 (72.7%) | 21 (27.3%) | |
| Age (y), median (IQR) | 49 (37–60) | 47 (45–62) | 0.583 |
| BMI, median (IQR) | 27.7 (26–32) | 27.7 (26–30) | 0.67 |
| Time to last follow-up, median (IQR) | 15 (11–26) | 14 (12–27) | 0.16 |
| Tumor characteristics | |||
| Tumor size (cm), mean (STD) | 2.2 (0.8) | 2.5 (1.2) | 0.16 |
| Vestibular schwannoma | 54 (96.4%) | 18 (85.7%) | |
| Epidermoid | 0 | 2 (9.5%) | |
| Meningioma | 0 | 1 (4.8%) | |
| FN schwannoma | 2 (3.6%) | 0 | |
| Other | 0 | ||
Abbreviations: BMI, body mass index; FN, facial nerve; IQR, interquartile range; STD, standard deviation.
Note: Denominators of all percentages reflect number of subjects by approach and graft type listed in the given columns.
Table 2. Postoperative complications and interventions by postresection defect site and the incorporation of an SISG in the multilayer repair.
| Autograft | Xenograft | p -Value | |
|---|---|---|---|
| Postoperative symptoms | |||
| Rhinorrhea | 6 (10.7%) | 1 (4.8%) | 0.46 |
| Otorrhea | 1 (1.8%) | 0 | N/A |
| Incisional leak | 2 (3.6%) | 0 | N/A |
| Pseudomeningocele | 5 (8.9%) | 1 (4.8%) a | 0.53 |
| Postoperative interventions | |||
| Lumbar drain | 6 (10.7%) | 2 (9.5) a | 0.878 |
| VP shunt | 1 (1.8%) | 1 (4.8%) a | 0.49 |
| Steroids for pseudomeningocele | 2 (3.6%) | 1 (4.8%) a | 0.814 |
| Incisional overclosure | 2 (3.6%) | 0 | N/A |
| EAC packing | 1 (1.8%) | 0 | N/A |
| Operative repair | 3 (5.4%) | 0 | N/A |
Abbreviations: EAC, external auditory canal; N/A, not available; SISG, small intestine submucosal graft; VP, ventriculoperitoneal.
Note : Denominators of all percentages reflect number of subjects by approach and graft type listed in the given columns.
One patient in each marked category was identified as having hydrocephalus preoperatively.
Two xenograft patients (9.5%) experienced postoperative complications and subsequent postoperative intervention ( Table 2 ). No patient from the xenograft patient required operative repair to address a postoperative leak. Eleven autograft patients (39.3%) experienced CSF complications postoperatively, with 10 requiring postoperative intervention, including 6 lumbar drain placements. More specifically, postoperative CSF rhinorrhea occurred in six (10.7%) of autograft patients and one (4.8%) of xenograft patients ( p = 0.43). One (1.8%) and two (3.6%) autograft patients experienced otorrhea and incisional leak, respectively, while no xenograft patients experienced these complications. Pseudomeningocele occurred in five (8.9%) autograft and one (4.8%) xenograft patients, while steroids given specifically to treat pseudomeningocele were necessary in two (3.6%) autograft cases and one (4.8%) xenograft case ( p = 0.53 and 0.81, respectively). Lumbar drain was used in six (10.7%) autograft and two (9.5%) xenograft patients ( p = 0.88). VP shunt was necessary in one (1.8%) autograft and one (4.8) xenograft cases ( p = 0.49). The xenograft patient who developed a pseudomeningocele and subsequently required a lumbar drain and VP shunt was noted to have hydrocephalus preoperatively. Incisional overclosure and external auditory canal packing each occurred once (1.8%) in the autograft cohort and did not occur in the xenograft case. Operative repair to revise surgical defect was necessary in three (5.4%) autograft cases and was not necessary in any xenograft cases.
Discussion
CSF leaks are an infrequent but costly complication following lateral skull base resections. Several strategies have been proposed to mitigate this risk in skull base reconstruction, including a multilayer repair with a wide range of graft materials at the surgeon's disposal. 20 21 Defect-specific reconstruction is employed with plan to achieve closure and limit egress of CSF via the eustachian tube as well as direct incisional leak. For translabyrinthine approaches, the dural defect is usually bridged with a substitute (Duraform, dura repair, etc.) as watertight closure is not possible. Perimeatal air cells can be obliterated with bone wax as well. Eustachian tube and middle ear space are packed and attic defect is obliterated. Fat is then used to reinforce the IAC defect as well as fill up the mastoid defect. At our institution, a resorbable mesh cranioplasty is employed to secure the fat graft and has resulted in reduced rates of pseudomeningocele and incisional leaks. 21 Watertight closure of musculoperiosteal flap and subcutaneous layer is done before skin closure and a pressure dressing in applied. Cueva has highlighted various technical aspects of wound closure for translabyrinthine, retrosigmoid, as well as middle fossa defects for optimal prevention of CSF leak. 23 These include design for thick musculoperiosteal layer, no overlapping skin and periosteal cuts, as well as robust closure of eustachian tube orifice.
In this study, SISG has been used as an alternative of fascia used for attic closure with resultant thicker musculoperiosteal flap. The safety, biocompatibility, and long-term viability of SISG have been demonstrated in anterior skull base reconstruction, repair of medial orbital wall and septal defects, and in various gynecologic and urologic applications. 3 22 23 24 25 26 27 28 29 It has also been used in otologic repair of tympanic membrane perforations with similar success profile compared with autologous tissue. 30 When used to cover exposed cartilage or bone after elevation of nasoseptal flaps in anterior skull base reconstructions, SISG use results are faster in remucosolization and healing as compared with autologous grafts. 31 While safe and effective SISG use becomes increasingly common in various otolaryngology procedures, SISG use in lateral skull base reconstruction has yet to be examined.
Based on the emerging safety profile and efficaciousness of SISG in other otolaryngologic operations, we studied incorporation of SISGs in lateral skull base reconstruction. To our knowledge, this is the first study to evaluate the efficacy of SISG in lateral skull base reconstruction following tumor resection. We did not find any significant differences in the incidence of leak complications between cohorts related to CSF rhinorrhea, otorrhea, and incisional leaks. There was no significant difference in rates of development of pseudomeningocele. While this preliminary study did not identify statistically significant results between cohorts, this may be due to the relatively low number of procedures performed as well as the already low risk of CSF leak. Nevertheless, SISG complication and intervention rates are lower than those in the autograft cohort in all categories except steroid usage and VP shunt placement where the only xenograft patient to receive the interventions was previously determined to have hydrocephalus which likely impacted the need for these interventions ( Table 2 ). This suggests that a larger sample size may be able to identify statistical significance, though such a study would require involvement of multiple sites to achieve necessary power.
While a variety of surgical materials techniques are used in the closure of translabyrinthine defects, leak rates still approach 8 to 12%. 14 32 33 Similar to autologous tissue, SISG xenografts support a safe and effective multilayered lateral skull base reconstruction. Though preliminary, this study suggests use of SISG xenografts as a component of multilayer repair may provide a more robust repair of translabyrinthine defects.
This study is limited by a small sample size, surgeon preference, and retrospective review of cases at a single institution and more numbers will be needed to achieve required power to determine significant differences. As a comparison of two small leak rates, multi-institutional cooperation will be needed to achieve this goal, though this preliminary study suggests usage of SISG xenografts in translabyrinthine defect repair is safe, effective, and may be able to reduce CSF leak rates.
Conclusion
This series reports comparable postresection CSF leak rates when SISG is used in place of autologous fascia during lateral skull base reconstruction. Although these results are preliminary and based on a small sample size, the use of SISG xenograft as a component of multilayer translabyrinthine defect repair enables the fascial layer to remain undisturbed and may provide a robust closure of the musculoperiosteal flaps, thereby reducing need for additional intervention. Further investigation is needed with a larger sample size, ideally in a multicenter study.
Funding Statement
Funding No funding or other support was provided for this study.
Conflict of Interest A.R.: Consultant for Med-El, Advanced Bionics, Cochlear, Grace Medical, Stryker, Cook Medical.
D.H.: Consultant for Med-El, Advanced Bionics, Stryker, and Cochlear.
Institutional Review Board Approval
Vanderbilt University IRB Approval 181441.
References
- 1.Lindberg K, Badylak S F. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns. 2001;27(03):254–266. doi: 10.1016/s0305-4179(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 2.Hodde J P, Badylak S F, Brightman A O, Voytik-Harbin S L. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2(03):209–217. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- 3.Illing E, Chaaban M R, Riley K O, Woodworth B A. Porcine small intestine submucosal graft for endoscopic skull base reconstruction. Int Forum Allergy Rhinol. 2013;3(11):928–932. doi: 10.1002/alr.21206. [DOI] [PubMed] [Google Scholar]
- 4.Mangham C A. Complications of translabyrinthine vs. suboccipital approach for acoustic tumor surgery. Otolaryngol Head Neck Surg. 1988;99(04):396–400. doi: 10.1177/019459988809900408. [DOI] [PubMed] [Google Scholar]
- 5.Fishman A J, Hoffman R A, Roland J T, Jr, Lebowitz R A, Cohen N L. Cerebrospinal fluid drainage in the management of CSF leak following acoustic neuroma surgery. Laryngoscope. 1996;106(08):1002–1004. doi: 10.1097/00005537-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fishman A J, Marrinan M S, Golfinos J G, Cohen N L, Roland J T., Jr Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope. 2004;114(03):501–505. doi: 10.1097/00005537-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Goddard J C, Oliver E R, Lambert P R. Prevention of cerebrospinal fluid leak after translabyrinthine resection of vestibular schwannoma. Otol Neurotol. 2010;31(03):473–477. doi: 10.1097/MAO.0b013e3181cdd8fc. [DOI] [PubMed] [Google Scholar]
- 8.Brennan J W, Rowed D W, Nedzelski J M, Chen J M. Cerebrospinal fluid leak after acoustic neuroma surgery: influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001;94(02):217–223. doi: 10.3171/jns.2001.94.2.0217. [DOI] [PubMed] [Google Scholar]
- 9.Charpiot A, Tringali S, Zaouche S, Ferber-Viart C, Dubreuil C. Perioperative complications after translabyrinthine removal of large or giant vestibular schwannoma: outcomes for 123 patients. Acta Otolaryngol. 2010;130(11):1249–1255. doi: 10.3109/00016481003762316. [DOI] [PubMed] [Google Scholar]
- 10.Merkus P, Taibah A, Sequino G, Sanna M. Less than 1% cerebrospinal fluid leakage in 1,803 translabyrinthine vestibular schwannoma surgery cases. Otol Neurotol. 2010;31(02):276–283. doi: 10.1097/MAO.0b013e3181cc06ad. [DOI] [PubMed] [Google Scholar]
- 11.Shea M C, Robertson J T. Acoustic neuroma removal: a comparative study of translabyrinthine and suboccipital approaches. Am J Otol. 1979;1(02):94–99. [PubMed] [Google Scholar]
- 12.Becker S S, Jackler R K, Pitts L H. Cerebrospinal fluid leak after acoustic neuroma surgery: a comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003;24(01):107–112. doi: 10.1097/00129492-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Falcioni M, Mulder J J, Taibah A, De Donato G, Sanna M. No cerebrospinal fluid leaks in translabyrinthine vestibular schwannoma removal: reappraisal of 200 consecutive patients. Am J Otol. 1999;20(05):660–666. [PubMed] [Google Scholar]
- 14.Peng K A, Chen B S, Lorenz M B et al. Revision surgery for vestibular schwannomas. J Neurol Surg B Skull Base. 2018;79(06):528–532. doi: 10.1055/s-0038-1635256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russel A, Hoffmann C P, Nguyen D T, Beurton R, Parietti-Winkler C. Can the risks of cerebrospinal fluid leak after vestibular schwannoma surgery be predicted? Otol Neurotol. 2017;38(02):248–252. doi: 10.1097/MAO.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 16.Stieglitz L H, Giordano M, Gerganov V.Petrous bone pneumatization is a risk factor for cerebrospinal fluid fistula following vestibular schwannoma surgery Neurosurgery 20106702, Suppl Operative ):509–515. [DOI] [PubMed] [Google Scholar]
- 17.Murphy M E, McCutcheon B A, Kerezoudis P et al. Morbid obesity increases risk of morbidity and reoperation in resection of benign cranial nerve neoplasms. Clin Neurol Neurosurg. 2016;148:105–109. doi: 10.1016/j.clineuro.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Chern A, Hunter J B, Bennett M L. Cost analysis of cerebrospinal fluid leaks and cerebrospinal fluid leak prevention in patients undergoing cerebellopontine angle surgery. Otol Neurotol. 2017;38(01):147–151. doi: 10.1097/MAO.0000000000001252. [DOI] [PubMed] [Google Scholar]
- 19.Hunter J B, Sweeney A D, Carlson M L et al. Prevention of postoperative cerebrospinal fluid leaks after translabyrinthine tumor resection with resorbable mesh cranioplasty. Otol Neurotol. 2015;36(09):1537–1542. doi: 10.1097/MAO.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 20.Oakley G M, Christensen J M, Winder M et al. Collagen matrix as an inlay in endoscopic skull base reconstruction. J Laryngol Otol. 2018;132(03):214–223. doi: 10.1017/S0022215117001499. [DOI] [PubMed] [Google Scholar]
- 21.Cueva R A, Mastrodimos B. Approach design and closure techniques to minimize cerebrospinal fluid leak after cerebellopontine angle tumor surgery. Otol Neurotol. 2005;26(06):1176–1181. doi: 10.1097/01.mao.0000176174.94764.3b. [DOI] [PubMed] [Google Scholar]
- 22.Phillips J, Riley K O, Woodworth B A. Porcine small intestine submucosal grafts for post-tumor resection orbital reconstruction. Laryngoscope. 2014;124(06):E219–E223. doi: 10.1002/lary.24515. [DOI] [PubMed] [Google Scholar]
- 23.Yoo F, Wang M B, Bergsneider M, Suh J D. Single layer repair of large anterior skull base defects without vascularized mucosal flap. J Neurol Surg B Skull Base. 2017;78(02):139–144. doi: 10.1055/s-0036-1593438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt R G, Raff G, Van Gundy J, Rodgers-Ohlau M, Si M S. Short-term experience of porcine small intestinal submucosa patches in paediatric cardiovascular surgery. Eur J Cardiothorac Surg. 2013;44(01):72–76. doi: 10.1093/ejcts/ezs638. [DOI] [PubMed] [Google Scholar]
- 25.Madhu C, Cooke J, Harber P, Holmes D. Functional outcomes of posterior vaginal wall repair and prespinous colpopexy with biological small intestinal submucosal (SIS) graft. Arch Gynecol Obstet. 2014;290(04):711–716. doi: 10.1007/s00404-014-3254-0. [DOI] [PubMed] [Google Scholar]
- 26.Staerman F, Pierrevelcin J, Ripert T, Menard J. Medium-term follow-up of plaque incision and porcine small intestinal submucosal grafting for Peyronie's disease. Int J Impot Res. 2010;22(06):343–348. doi: 10.1038/ijir.2010.28. [DOI] [PubMed] [Google Scholar]
- 27.Farahat Y A, Elbahnasy A M, El-Gamal O M, Ramadan A R, El-Abd S A, Taha M R. Endoscopic urethroplasty using small intestinal submucosal patch in cases of recurrent urethral stricture: a preliminary study. J Endourol. 2009;23(12):2001–2005. doi: 10.1089/end.2009.0074. [DOI] [PubMed] [Google Scholar]
- 28.Knoll L D. Use of porcine small intestinal submucosal graft in the surgical management of tunical deficiencies with penile prosthetic surgery. Urology. 2002;59(05):758–761. doi: 10.1016/s0090-4295(02)01607-2. [DOI] [PubMed] [Google Scholar]
- 29.Durasis Study Group . Bejjani G K, Zabramski J. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J Neurosurg. 2007;106(06):1028–1033. doi: 10.3171/jns.2007.106.6.1028. [DOI] [PubMed] [Google Scholar]
- 30.Yawn R J, Dedmon M M, O'Connell B P, Virgin F W, Rivas A. Tympanic membrane perforation repair using porcine small intestinal submucosal grafting. Otol Neurotol. 2018;39(05):e332–e335. doi: 10.1097/MAO.0000000000001792. [DOI] [PubMed] [Google Scholar]
- 31.Nayak J V, Rathor A, Grayson J W et al. Porcine small intestine submucosal grafts improve remucosalization and progenitor cell recruitment to sites of upper airway tissue remodeling. Int Forum Allergy Rhinol. 2018;8(10):1162–1168. doi: 10.1002/alr.22156. [DOI] [PubMed] [Google Scholar]
- 32.Mangus B D, Rivas A, Yoo M J et al. Management of cerebrospinal fluid leaks after vestibular schwannoma surgery. Otol Neurotol. 2011;32(09):1525–1529. doi: 10.1097/MAO.0b013e318232e4a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selesnick S H, Liu J C, Jen A, Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25(03):387–393. doi: 10.1097/00129492-200405000-00030. [DOI] [PubMed] [Google Scholar]


