Abstract
Objective Access to the infratemporal fossa (ITF) is complicated by its complex neurovascular relationships. In addition, copious bleeding from the pterygoid plexus adds to surgical challenge. This study aims to detail the anatomical relationships among the internal maxillary artery (IMA), pterygoid plexus, V 3, and pterygoid muscles in ITF. Furthermore, it introduces a novel approach that displaces the lateral pterygoid plate (LPP) to access the foramen ovale.
Design and Main Outcome Measures Six cadaveric specimens (12 sides) were dissected using an endonasal approach to the ITF modified by releasing and displacing the LPP and lateral pterygoid muscle (LPTM) as a unit. Subperiosteal elevation of the superior head of LPTM revealed the foramen ovale. The anatomic relationships among the V 3 , pterygoid muscles, pterygoid plexus, and IMA were surveyed.
Results In 9/12 sides (75%), the proximal IMA ran between the temporalis and the LPTM, whereas in 3/12 sides (25%), the IMA pierced the LPTM. The deep temporal nerve was a consistent landmark to separate the superior and inferior heads of LPTM. An endonasal approach displacing the LPP in combination with a subperiosteal elevation of the superior head of LPTM provided access to the posterior trunk of V 3 and foramen ovale while sparing injury of the LPTM and exposing the pterygoid plexus. The anterior trunk of V 3 traveled anterolaterally along the greater wing of sphenoid in all specimens.
Conclusion Displacement of the LPP and LPTM provided direct exposure of foramen ovale and V 3 avoiding dissection of the muscle and pterygoid plexus; thus, this maneuver may prevent intraoperative bleeding and postoperative trismus.
Keywords: infratemporal fossa, lateral pterygoid plate, foramen ovale, V 3, internal maxillary artery
Introduction
Following advances in endoscopic instrumentation and techniques, endoscopic skull base surgery has been increasingly adopted over the past several decades. 1 2 3 Expanded endoscopic approaches (EEA) have been successfully applied to the median anterior, middle, and posterior skull base and respective fossae; craniovertebral junction; and the infratemporal fossa (ITF). 4 5 6 7 The ITF is a complex area containing the neurovascular structures of the upper parapharyngeal space (internal carotid artery, internal jugular vein, and cranial nerves IX–XII) and the masticator space (mandibular nerve or V 3 , internal maxillary artery [IMA], and its distal branches, pterygoid venous plexus, temporalis muscle, and the lateral and medial pterygoid muscles). 8 9 10 While various approaches can be used to access the ITF, endoscopic approaches differ from others in their lack of need of facial or intraoral incisions, transposition of facial nerve, dissection of the parotid gland, and transposition of the temporalis muscle or zygomatic arch. 11 12 This decreases surgical morbidity associated with these steps; thus, better preserving or restoring the postoperative quality of life. 13 Therefore, the indications and application of endoscopic endonasal transpterygoid approaches to access the ITF continue to expand. 9 14
Foramen ovale, V 3, pterygoid plexus, and the IMA are important landmarks as well as obstacles during EEA to the IFT. Extracranially, V 3 branches to provide sensory and motor function to the face and muscles of mastication; V 3 or any of its branches may become the origin for a schwannoma, one of the most common benign tumors of the ITF. 15 16 Another similarly important structure is the IMA, which runs a variable course in relation to the lateral pterygoid muscle. 17 Moreover, the deep pterygoid venous plexus is located at the medial aspect of the lateral pterygoid muscle, and may be the source of copious and frustrating bleeding during approaches to the ITF. 8 11 Its bleeding may be challenging, and in rare instances the surgery needs to be aborted and the wound packed for several days before re-exploration. 18 Therefore, the identification and preservation or control of the aforementioned structures is critical for decreasing comorbidities in endoscopic surgeries of the ITF.
This study intends to further define the detailed variations and anatomical relationships among the V 3 , pterygoid muscles, pterygoid plexus, and branches of the IMA in ITF from an endoscopic perspective. Additionally, it introduces a lateral pterygoid plate (LPP) displacement approach to access the potential space lying between the medial and lateral pterygoid muscles; thus, exposing foramen ovale and V 3 . 14 We hypothesized that the LPP displacement approach could spare bleeding from deep pterygoid venous plexus and preserve the lateral pterygoid muscle avoiding postoperative trismus.
Materials and Methods
An endoscopic Denker's approach followed by an endonasal–transantral approach to the ITF, including the displacement of the LPP was performed in six adult cadaveric specimens (12 sides). All dissections were performed at the Anatomy Laboratory Toward Visuospatial Surgical Innovations in Otolaryngology and Neurosurgery (ALT-VISION) at the Wexner Medical Center of The Ohio State University. ALT-VISION, and all researchers involved in the dissections were certified by local regulatory agencies dealing with the use of human tissues and cadaveric studies. Major vessels of the neck, including the common carotid and vertebral arteries and the internal jugular veins, had been identified and injected with red and blue silicone dyes, respectively. All specimens underwent high-resolution CT scans, and their digital data were imported to a Stryker navigational system (Kalamazoo, Michigan, United States).
Endoscopes with 0 and 30 degrees lenses (4 mm diameter and 18 cm length) coupled to high-definition camera and monitor (Karl Storz Endoscopy, Tuttlingen, Germany) were used to provide visualization. Images (TIF format) and videos (MPEG format) were recorded and archived using an AIDA system (Karl Storz Endoscopy, Tuttlingen, Germany). Still photographs and videos were obtained to define and document the anatomic relationships of the endoscopic anatomy and to be correlated with the multiplanar CT views provided by the image guidance system. Bone dissections included high-speed drilling with a straight handpiece equipped with 3 to 4 mm rough diamond (hybrid) burrs (Stryker Co., Kalamazoo, Michigan, United States).
The feasibility of the LPP displacement approach for access to the foramen ovale, and the anatomical relationships of the IMA, pterygoid muscles, the temporalis, pterygoid plexus, V 3, and its branches within the ITF were evaluated on cadaveric specimens (12 sides).
Results
Technical nuances of an endoscopic Denker's approach have been previously reported. 9 19 A posterior septectomy facilitated the use of a four-handed technique. The posterolateral wall of the maxillary sinus and periosteum were removed to expose the soft tissues of the pterygopalatine and infratemporal fossae, identifying their neurovascular structures. After removal of fat to improve a detailed anatomic study, the distal branches of the IMA including the descending palatine artery, sphenopalatine artery, infraorbital artery, and posterosuperior alveolar artery ( Fig. 1A ) were transected to improve the mobilization of the soft tissues and exposure of the pterygoid process. The greater palatine nerve was preserved in all 12 sides ( Fig. 1B ).
Fig. 1.
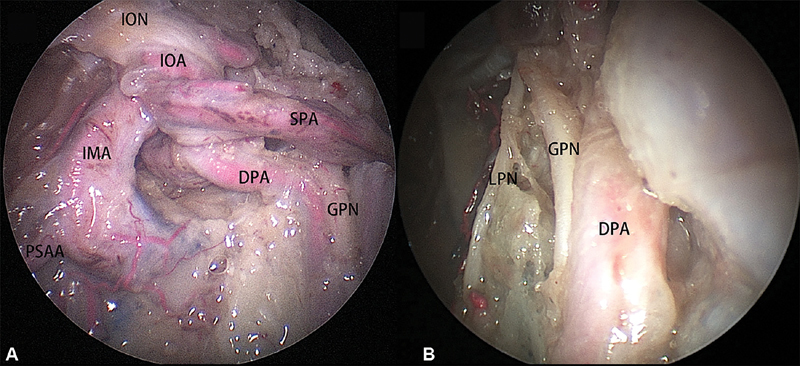
( A ) The branches of the internal maxillary artery on right side. ( B ) The greater palatine nerve and lesser palatine nerve. DPA, descending palatine artery; IOA: infraorbital artery; ION, infraorbital nerve; PSAA, posterosuperior alveolar artery; SPA, sphenopalatine artery.
The anterior bony bridge connecting the lateral and medial pterygoid plates ( Fig. 2A ) was drilled superiorly to separate the LPP from the pterygoid process ( Fig. 2B ). The inferior head of the lateral pterygoid muscle and the LPP were displaced laterally as a unit, and the inferior head of the lateral pterygoid muscle was preserved with its fascia intact to keep from rupture of the pterygoid plexus ( Fig. 2C ).
Fig. 2.

( A ) A line illustration of the drilling (green line) between the medial (blue arrow) and LPP (red arrow). ( B ) The bony ridge (enclosed dotted line, right side) was drilled to facilitate the separation of LPP (arrow). ( C ) The lateral pterygoid muscle and LPP were displaced laterally as a unit. DPA, descending palatine artery; LPP, lateral pterygoid plates; MPTM, medial pterygoid muscle.
Then, the posterior trunk of V 3 was identified in the space between medial and lateral pterygoid muscles ( Fig. 3A ). The lingual and inferior alveolar nerves were identified branching from the posterior trunk of V 3 ( Fig. 3B ).
Fig. 3.

( A ) The fascia of the lateral pterygoid muscle (right side) was preserved (arrow), and the posterior trunk of V 3 was exposed. ( B ) The lingual nerve and inferior alveolar nerve lie on the surface of medial pterygoid muscle. TM, temporalis muscle.
The chorda tympani joined the lingual nerve at its posteromedial aspect ( Fig. 4A ). When traced proximally, the chorda tympani were found to travel medial to the sphenoidal spine after exiting the petrotympanic fissure ( Fig. 4B ).
Fig. 4.

( A ) The chorda tympani (right side) joins the lingual nerve. ( B ) The sphenoidal spine. IAN, inferior alveolar nerve; LPTM, lateral pterygoid muscle; SD, styloid diaphragm; SML, sphenomandibular ligament.
To expose foramen ovale and the proximal segment of V 3 , the superior head of the lateral pterygoid muscle was elevated following a subperiosteal plane revealing the undersurface of the greater wing of the sphenoid ( Fig. 5A ). The venous plexus deep to the superior head of lateral pterygoid muscle was separated from the greater wing of the sphenoid by the periosteum overlying the bone ( Fig. 5B ). A venous plexus connecting the pterygoid plexus and the cavernous sinus transmitting the foramen ovale was abundantly present ( Fig. 5C ).
Fig. 5.
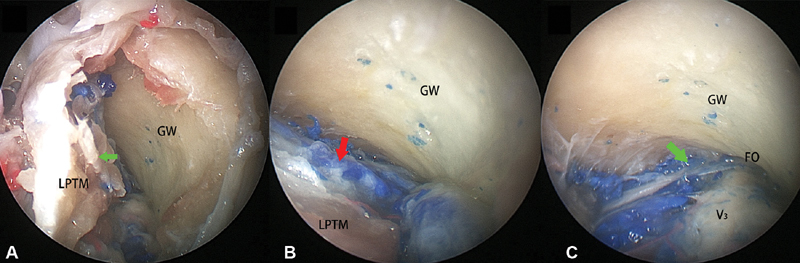
( A ) The superior head of the LPTM is elevated from the greater wing of sphenoid in a subperiosteal fashion (green arrow). ( B ) The pterygoid venous plexus (red arrow) located deep to the LPTM. ( C ) The communicated venous plexus (green arrow) connecting the pterygoid venous plexus and the cavernous sinus crossing the foramen ovale was present. LPTM, lateral pterygoid muscle.
The IMA traveled in a plane between the lateral pterygoid and the temporalis muscles, in 9/12 sides (75%, Fig. 6A ). The inferior head of the lateral pterygoid muscle was resected to further explore the anatomical relationships within this area. When traced proximally, the IMA was travelling along the medial aspect of the temporalis muscle after crossing behind the ramus of the mandible, at which point the auriculotemporal nerve was identified superior to the IMA ( Fig. 6B ). The middle meningeal artery was identified between the auriculotemporal nerve and the bundle comprising the lingual and inferior alveolar nerves prior to entering the foramen spinosum ( Fig. 6C ).
Fig. 6.

( A ) The IMA (right side) travels between the temporalis muscle and the lateral pterygoid muscle. ( B ) The auriculotemporal nerve runs superior to IMA (right side). ( C ) The middle meningeal artery (right side) travels upward between the branches of V 3 . ATN, auriculotemporal nerve; IAN: inferior alveolar nerve; IMA, internal maxillary artery; IOA, infraorbital artery; ION, infraorbital nerve; LN, lingual nerve; MR: mandible ramus; SML, stylomandibular ligament.
The IMA pierced the superior and inferior heads of lateral pterygoid muscle in 3/12 sides (25%, Fig. 7A ). After separating and elevating the inferior head of the lateral pterygoid muscle inferiorly and laterally, the main trunk of the IMA was identified, enclosed by the fascia of the inferior head of lateral pterygoid muscle. Moreover, the middle meningeal artery had a similar relationship with V 3 branches as abovementioned before entering the foramen spinosum ( Fig. 7B ).
Fig. 7.

( A ) The IMA (right side) pierces between the superior (LPTM-S) and inferior (LPTM-I) heads of lateral pterygoid muscle. ( B ) The middle meningeal artery (right side) also travels upward between branches of V 3 . ATN, auriculotemporal nerve; IMA, internal maxillary artery; IAN, inferior alveolar nerve; LN, lingual nerve; MR, mandible ramus; TM, temporalis muscle.
The deep temporal nerve was consistently identified at the medial aspect of the temporalis muscle in all 12 sides ( Fig. 8A ). When traced proximally, the deep temporal nerve was identified crossing between the two heads of the lateral pterygoid muscle ( Fig. 8B ) and the anterior trunk of V 3 could be identified after resecting the superior head of lateral pterygoid muscle. Branches from the anterior trunk to the masseter muscle and the buccal mucosa, run anterolaterally under the greater wing of sphenoid ( Fig. 8C ).
Fig. 8.

( A ) The deep temporal nerve (right side) located at the medial border of the temporalis muscle; ( B ) the DTN constitutes a separation between superior (LPTM-S) and inferior (LPTM-I) heads of lateral pterygoid muscle. ( C ) The anterior trunk of V 3 (right side). BN, buccal nerve; MN, masseteric nerve; LPTN, lateral pterygoid nerve (cut in this specimen).
Discussion
Endoscopic transpterygoid approaches have been applied successfully to manage lesions of the ITF; however, the high complexity of these approaches is compounded by the variable relationships among the V 3 , pterygoid muscles, and branches of the IMA, as well as bleeding from the pterygoid plexus. 20 21 Displacement of the LPP offers a direct access to V3 and foramen ovale with the advantage of avoiding the manipulation of the fascia of lateral pterygoid muscle; thus, avoiding potential bleeding from the pterygoid plexus which lies lateral to the fascia. A dry surgical field will facilitate the identification the V3 branches and avoiding their accidental injury. Furthermore, sparing the lateral pterygoid muscle may theoretically decrease postoperative scar formation and trismus. The attachment of the lateral pterygoid muscle at its origin, on the LPP, is firmly adherent and requires sharp transection. 11 Previously reported endoscopic endonasal approaches to access foramen ovale require elevation of the lateral pterygoid muscle from its corresponding origin at the LPP. 7 8 One should anticipate copious bleeding from the deep pterygoid plexus, which is most prominent at medial aspect of the lateral pterygoid muscle, when the dissection misses the subperiosteal plane. 18 Lateral displacement of the LPP, however, preserves the integrity of fascia of the lateral pterygoid muscle and separates the pterygoid plexus from the surgical corridor (e.g., resection of a V 3 schwannoma); and as such, the potential venous bleeding from the deep pterygoid plexus could be avoided. 16 However, when the fascia of the lateral pterygoid muscle is invaded by the tumor (e.g., malignancy) or the separation of the superior and inferior heads of the lateral pterygoid muscle is necessary, copious bleeding from the pterygoid venous plexus may be encountered; therefore, displacement of the LPP cannot adequately satisfy the demands, and additional preparations for hemostasis of pterygoid venous plexus is required.
The foramen ovale is located deep to the superior head of the lateral pterygoid muscle, and abundant communication between the pterygoid venous plexus and the cavernous sinus is present ( Fig. 5 ). 22 A subperiosteal elevation with preservation of an intact periosteum can avoid injury of the pterygoid venous plexus within the superior head of the lateral pterygoid muscle. For lesions arising from V 3 located deep to the lateral pterygoid muscle, the combination of displacement of the LPP technique and a subperiosteal elevation of the superior head of lateral pterygoid muscle facilitates exposure of the region from the foramen ovale to the distal segment of the V 3 while avoiding injury of the pterygoid plexus. However, lesions arising from the superior portion of the ITF, especially those with intracranial extension through the foramen ovale, often require opening and enlargement of the foramen ovale; therefore, copious venous bleeding should be anticipated, and strategies for hemostasis should be adequately prepared prior to the surgery. 22
The presence of the IMA running on the surface or deep to the lateral pterygoid muscle impacts any surgery in the ITF; 11 however, the incidence of this anatomical variation has not been described well. This study found that the IMA travelled on the surface of the lateral pterygoid muscle in 75% of the specimens. When facing this variation, inadvertent injury to the IMA would not be encountered by operating in the medial aspect of lateral pterygoid muscle. In the remaining 25% of the specimens, however, the IMA pierced the lateral pterygoid muscle. In specimens with this type of variation, the IMA was enclosed by the fascia of the inferior head of the lateral pterygoid muscle, which separated the artery from the posterior trunk of V 3 . Surgeries via a LPP displacement approach (e.g., resection of a V 3 schwannoma) within the space enclosed by lateral and medial pterygoid muscles present in this study, the damage to IMA could be avoided if the fascia of the lateral pterygoid muscle is intact.
Identification and control of the middle meningeal artery is a critical step for traditional ITF approaches. 23 However, the methods and landmarks to identify the middle meningeal artery during an EEA corridor are rarely reported. In the present study, the middle meningeal artery consistently traveled cephalad between the auriculotemporal nerve and the bundle of lingual and inferior alveolar nerves before entering the foramen spinosum in all 12 sides, regardless of the variations of the IMA. The constant trajectory of middle meningeal artery may provide an additional landmark for endonasal procedures around the foramen spinosum for procedures within the ITF.
The chorda tympani originates from the facial nerve and exits the petrotympanic fissure to enter the ITF. 24 The chorda tympani is a thin fiber, joining the lingual nerve at the posteromedial aspect. 25 A detailed dissection at the posteromedial aspect of the lingual nerve is required to identify and preserve the chorda tympani; thus, preserving taste function. However, the lesions arising from the extracranial V 3 may displace or disturb the normal distribution of the chorda tympani. Therefore, exploration of the chorda tympani was feasible on the cadaveric dissection; however, during live surgery identifying and preserving the chorda tympani is challenging, especially when active bleeding existed.
Anatomical descriptions of the anterior trunk of V 3 from an endonasal endoscopic perspective are sparse. This study suggests that the V 3 anterior trunk, innervating the temporalis, masseter muscle, and mucosa of the buccal area, is smaller than its posterior trunk. The deep temporal nerve is found consistently at the medial border of the temporalis muscle; thus, it can be used as a landmark for the identification of temporalis muscle and the space medial to it. 9 Moreover, the deep temporal nerve also crosses the superior and inferior heads of the lateral pterygoid muscle, constituting a landmark and a divider of the two muscle heads. Resection of the superior head of the lateral pterygoid muscle was required to adequately expose the anterior trunk of V 3 . However, due to the scarcity of lesions arising from anterior trunk of V 3 , the resection of superior head of lateral pterygoid muscle and the anterior trunk of V 3 should be dependent on the necessity of exposure within this area.
A technique that includes the lateral displacement of the LPP and muscle together present in this study provides an alternative for the exposure of lesions arising from the extracranial V 3 with seemingly less invasiveness. However, there are significant limitations to this study. The success of the procedure is dependent on the thickness of pterygoid process; the thick bony connection between the medial and LPPs may become a contraindication for successful realization of this corridor. Moreover, careful separation is also needed to keep intact the origin of lateral the pterygoid muscle and its fascia. In addition, this study is a preclinical cadaveric study; and as such, the usefulness of the lateral pterygoid displacement approach still deserves further validation in clinical scenarios. However, the anatomical principles as described in this study are sound.
Conclusion
An endoscopic modified transpterygoid approach with displacement of the LPP and muscle together provides direct exposure of V 3 and foramen ovale while preserving the fascia of the lateral pterygoid muscle, which may be helpful for prevention of intraoperative bleeding from the deep pterygoid plexus as well as injury to the IMA, and postoperative trismus.
Funding Statement
Funding This study was partially funded by the Lynne Shepard Jones endowment.
Footnotes
Conflict of Interest N.R.L. holds stock in Navigen Pharmaceuticals and was a consultant for Cooltech Inc. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(01):E3. [PubMed] [Google Scholar]
- 2.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(01):E4. [PubMed] [Google Scholar]
- 3.Prevedello D M, Doglietto F, Jane J A, Jr, Jagannathan J, Han J, Laws E R., Jr History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107(01):206–213. doi: 10.3171/JNS-07/07/0206. [DOI] [PubMed] [Google Scholar]
- 4.Kassam A B, Snyderman C, Gardner P, Carrau R, Spiro R.The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report Neurosurgery 20055701E213, discussion E213 [DOI] [PubMed] [Google Scholar]
- 5.Gardner P A, Kassam A B, Thomas A.Endoscopic endonasal resection of anterior cranial base meningiomas Neurosurgery 2008630136–52., discussion 52–54 [DOI] [PubMed] [Google Scholar]
- 6.Kassam A B, Gardner P A, Snyderman C H, Carrau R L, Mintz A H, Prevedello D M. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(04):715–728. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 7.Kasemsiri P, Carrau R L, Ditzel Filho L F. Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg. 2014;82(06):S12–S21. doi: 10.1016/j.wneu.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Kasemsiri P, Solares C A, Carrau R L. Endoscopic endonasal transpterygoid approaches: anatomical landmarks for planning the surgical corridor. Laryngoscope. 2013;123(04):811–815. doi: 10.1002/lary.23697. [DOI] [PubMed] [Google Scholar]
- 9.Li L, London N R, Jr, Prevedello D M, Carrau R L. Anatomy based corridors to the infratemporal fossa: Implications for endoscopic approaches. Head Neck. 2019;00:1–8. doi: 10.1002/hed.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, London N R, Jr, Prevedello D M, Carrau R L. Endonasal endoscopic transpterygoid approach to the upper parapharyngeal space. Head Neck. 2020;00:1–7. doi: 10.1002/hed.26127. [DOI] [PubMed] [Google Scholar]
- 11.Falcon R T, Rivera-Serrano C M, Miranda J F. Endoscopic endonasal dissection of the infratemporal fossa: Anatomic relationships and importance of eustachian tube in the endoscopic skull base surgery. Laryngoscope. 2011;121(01):31–41. doi: 10.1002/lary.21341. [DOI] [PubMed] [Google Scholar]
- 12.Youssef A, Carrau R L, Tantawy A. Endoscopic versus open approach to the infratemporal fossa: a cadaver study. J Neurol Surg B Skull Base. 2015;76(05):358–364. doi: 10.1055/s-0035-1549003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oakley G M, Harvey R J. Endoscopic resection of pterygopalatine fossa and infratemporal fossa malignancies. Otolaryngol Clin North Am. 2017;50(02):301–313. doi: 10.1016/j.otc.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Dallan I, Lenzi R, Bignami M.Endoscopic transnasal anatomy of the infratemporal fossa and upper parapharyngeal regions: correlations with traditional perspectives and surgical implications Minim Invasive Neurosurg 201053(5-6):261–269. [DOI] [PubMed] [Google Scholar]
- 15.Raza S M, Amine M A, Anand V, Schwartz T H. Endoscopic endonasal resection of trigeminal schwannomas. Neurosurg Clin N Am. 2015;26(03):473–479. doi: 10.1016/j.nec.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Hu L, Zhao W, Zhang H, Liu Q, Wang D. Endoscopic endonasal approach for trigeminal schwannomas: our experience of 39 patients in 10 years. Eur Arch Otorhinolaryngol. 2018;275(03):735–741. doi: 10.1007/s00405-018-4871-1. [DOI] [PubMed] [Google Scholar]
- 17.Fortes F S, Sennes L U, Carrau R L. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118(01):44–49. doi: 10.1097/MLG.0b013e318155a492. [DOI] [PubMed] [Google Scholar]
- 18.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(01):E6. [PubMed] [Google Scholar]
- 19.Lee J T, Suh J D, Carrau R L, Chu M W, Chiu A G. Endoscopic Denker's approach for resection of lesions involving the anteroinferior maxillary sinus and infratemporal fossa. Laryngoscope. 2017;127(03):556–560. doi: 10.1002/lary.26237. [DOI] [PubMed] [Google Scholar]
- 20.Taylor R J, Patel M R, Wheless S A. Endoscopic endonasal approaches to infratemporal fossa tumors: a classification system and case series. Laryngoscope. 2014;124(11):2443–2450. doi: 10.1002/lary.24638. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia P, Turri-Zanoni M, Dallan I. Endoscopic endonasal transpterygoid transmaxillary approach to the infratemporal and upper parapharyngeal tumors. Otolaryngol Head Neck Surg. 2014;150(04):696–702. doi: 10.1177/0194599813520290. [DOI] [PubMed] [Google Scholar]
- 22.Leonel L CPC, de Sousa S DG, Liberti E A. Topographic and microscopic anatomical description of the emissary sinus of foramen ovale in adult humans. Clin Neurol Neurosurg. 2018;169:77–85. doi: 10.1016/j.clineuro.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu F, Komatsu M, Di Ieva A, Tschabitscher M. Endoscopic extradural subtemporal approach to lateral and central skull base: a cadaveric study. World Neurosurg. 2013;80(05):591–597. doi: 10.1016/j.wneu.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 24.McManus L J, Dawes P J, Stringer M D. Clinical anatomy of the chorda tympani: a systematic review. J Laryngol Otol. 2011;125(11):1101–1108. doi: 10.1017/S0022215111001873. [DOI] [PubMed] [Google Scholar]
- 25.Sittitavornwong S, Babston M, Denson D, Zehren S, Friend J. Clinical Anatomy of the Lingual Nerve: A Review. J Oral Maxillofac Surg. 2017;75(05):9260–9.26E11. doi: 10.1016/j.joms.2017.01.009. [DOI] [PubMed] [Google Scholar]


