Abstract
Background
The World Health Organization targeted Trypanosoma brucei gambiense human African trypanosomiasis (gHAT) for elimination as a public health problem and for elimination of transmission. To measure gHAT elimination success with prevalences close to zero, highly specific diagnostics are necessary. Such a test exists in the form of an antibody-mediated complement lysis test, the trypanolysis test, but biosafety issues and technological requirements prevent its large-scale use. We developed an inhibition ELISA with high specificity and sensitivity that is applicable in regional laboratories in gHAT endemic countries.
Methods
The T. b. gambiense inhibition ELISA (g-iELISA) is based on the principle that binding of monoclonal antibodies to specific epitopes of T. b. gambiense surface glycoproteins can be inhibited by circulating antibodies of gHAT patients directed against the same epitopes. Using trypanolysis as reference test, the diagnostic accuracy of the g-iELISA was evaluated on plasma samples from 739 gHAT patients and 619 endemic controls and on dried blood spots prepared with plasma of 95 gHAT and 37 endemic controls.
Results
Overall sensitivity and specificity on plasma were, respectively, 98.0% (95% CI 96.7–98.9) and 99.5% (95% CI 98.6–99.9). With dried blood spots, sensitivity was 92.6% (95% CI 85.4–97.0), and specificity was 100% (95% CI 90.5–100.0). The g-iELISA is stable for at least 8 months when stored at 2–8°C.
Conclusion
The g-iELISA might largely replace trypanolysis for monitoring gHAT elimination and for postelimination surveillance. The g-iELISA kit is available for evaluation in reference laboratories in endemic countries.
Keywords: Trypanosoma brucei gambiense, elimination, ELISA, diagnosis
The novel Trypanosoma brucei gambiense inhibition ELISA (g-iELISA) is a high-throughput diagnostic applicable in regional laboratories for monitoring gambiense-HAT elimination. On plasma samples, sensitivity was 98.0% and specificity 99.5%, while on dried blood spots sensitivity was 92.6% and specificity 100%.
Human African trypanosomiasis (HAT) is an infectious disease caused by the protozoan Trypanosoma brucei (T.b.) [1]. Transmission occurs via infected tsetse (Glossina sp.), which confines the disease to sub-Saharan Africa [2]. Two geographically separated sub-species are responsible for the disease in humans: T.b. gambiense type I in West and Central Africa and T.b. rhodesiense in Eastern Africa [2]. Some atypical human infections are due to another trypanosome taxon, called T.b. gambiense type II but are very rare and not considered here [3]. Both gambiense-HAT (gHAT) and rhodesiense-HAT (rHAT) are neglected tropical diseases, however, despite this improved control tools for gHAT recently appeared, such as rapid diagnostic tests (RDTs) for diagnosis, new drugs including nifurtimox-eflornithine combination therapy (NECT) and fexinidazole for treatment, and tiny targets for vector control. Sustained control activities in most affected countries have reduced annual incidence to a point where elimination of gHAT as a public health problem (<2000 reported cases reported annually and 90% reduction of the area at risk reporting ≥1 case/10 000 people/year), seems feasible, and elimination of transmission (EOT, zero human cases of gHAT) is now the new target [4–6]. However, as long as tsetse populations subsist, gHAT may reappear in foci that are considered eliminated. Re-emergence of the disease may be caused by i) an animal reservoir, although the epidemiological role of animals is still under debate, or ii) a human reservoir in the form of patients that are not picked up by active or passive surveys or asymptomatic carriers that harbor the infection for years or decades without developing the disease [7–9].
Measuring EOT of gHAT poses new challenges for diagnosis, especially as this infection often persists at extremely low levels. Likewise, there is a threat of re-emergence or re-invasion from another area with ongoing transmission. In epidemic and endemic situations where the goal is to reduce incidence drastically and rapidly, diagnostics should be highly sensitive, which usually compromises their specificity but results in a high negative predictive value (NPV) following the formulaOn the other hand, when prevalence is near zero, it is important to use diagnostics with very high specificity to avoid any false-positive results that may trigger unnecessary alarm and ensuing actions. High specificity results in high positive predictive values (PPV) following the formula
Parasitological diagnosis of gHAT, based on microscopic detection of the parasite in blood, lymph, or cerebrospinal fluid, is highly specific, but moderately sensitive [2]. For this reason, field applicable serological tests have been introduced since the 1970s [10]. They have been instrumental in the control of gHAT but are ineffective for rHAT. The card agglutination test for trypanosomiasis (CATT), of which several million are used each year, is particularly useful for large-scale screening of populations at risk. The sensitivity and specificity of the test are estimated at 91.2% and 97.4%, respectively [11]. RDTs have been available since 2013. A comparative study on archived specimens from West Africa reported sensitivities of 98.5% for the gambiense Sero K-SeT and 99.6% for the SD Bioline HAT; specificities were much lower, 98.6% and 97.1% respectively [12]. With these characteristics, CATT or RDTs cannot be recommended for postelimination monitoring of gHAT—even in current screening programs a single RDT or CATT positive test is not alone considered sufficient for administration of treatment—and confirmation by microscopy is required. Diagnostics for an endgame setting must be increasingly specific, otherwise there will overwhelmingly be more false positives than true positives [13, 14]. An alternative could be the variant-specific trypanolysis test (TL) [15]. This antibody-mediated complement lysis test combines high specificity and high analytical sensitivity and is used to confirm the presence of gambiense-specific antibodies in CATT or RDT positive individuals but in which the parasite cannot be demonstrated by microscopy or molecular tests [16].
The TL test is recognized by the World Health Organization (WHO) as the reference test for contact with T.b. gambiense and, as such, is performed at the WHO Collaborating Centers on HAT (Institute of Tropical Medicine Antwerp, Belgium, and Institut National de Recherche Biomédicale, Democratic Republic of the Congo [DRC]) and in Centre International de Recherche-Développement sur l’Elevage en zone Subhumide, Burkina Faso. The test is applicable in laboratory conditions on serum, plasma, and dried blood spots (DBS) [17]. Its specificity is due to the fact that on intact bloodstream trypomastigotes of T. brucei, only the variable antigen type (VAT)-specific epitopes of the variant surface glycoprotein (VSG) coat are accessible to conventional antibodies (IgM and IgG). VAT-specific antibodies in a test specimen will opsonize the trypanosomes that are subsequently lysed by antibody-mediated complement lysis. As most gHAT patients have antibodies against the VATs Lille Trypanosome antigen types 1.3 and/or 1.5 (LiTat 1.3, LiTat 1.5), TL is carried out with both variants [15]. The primary disadvantage of TL is that it requires in vivo propagation of highly-virulent T.b. gambiense clones, which puts the laboratory personnel under biohazard risk. Secondary disadvantages are the low throughput (400 samples/week) and high cost (5–7 €/test).
We here describe the development of an inhibition ELISA (iELISA) with similar diagnostic accuracy but fewer disadvantages than TL. In the Trypanosoma brucei gambiense-iELISA (g-iELISA), the binding of monoclonal antibodies (mAbs) to VAT-specific epitopes on the VSGs of T.b. gambiense variants LiTat 1.3 and LiTat 1.5, is inhibited by binding of antibodies in the blood of gHAT patients directed to the same epitopes.
MATERIALS AND METHODS
We first established a Target Product Profile (TPP) describing the intended use and test characteristics (Supplementary Material 1). The profile defined for the g-iELISA is that of a tool to monitor the progress towards gHAT elimination and to assess the presence/absence of T.b. gambiense in the human population of a focus where gHAT transmission is thought to have stopped. The test must be applicable in national and regional laboratories in sub-Saharan African countries affected by gHAT. Serum, plasma, or DBS, collected in local health facilities or through population surveys, are sent to the nearest laboratory able to run the g-iELISA. Sensitivity must be at a minimum >90% and optimistically ≥95%, and specificity must be at a minimum ≥99.5% and optimistically 100%. g-iELISA stability should be at a minimum 24 months at 4°C and optimistically 24 months at 30°C. The g-iELISA will be commercialized as in vitro diagnostic device (IVD) submitted to the European Directive 98/79/EC (IVDD 98/79/EC).
For development of the g-iELISA and to define a cut-off value, we used plasma samples collected during a previous study conducted in the DRC [18].
Purified VSGs were produced following Büscher and co-workers with some modifications [19] (Supplementary Material 2). Reactivity of the VSGs was assessed in indirect ELISA by testing 2-fold dilutions, ranging from 4 to 0.125 µg/mL, with both variant-specific mAbs described below.
Mouse mAbs against VSG LiTat 1.3 (clone 7B1D7) and LiTat 1.5 (clone 1A11G10) were generated using standard protocols at Icosagen Cell Factory (Tartumaa, Estonia).
VAT-specific chicken IgY were produced at the University of KwaZulu-Natal. IgY antibodies were affinity purified on VSG LiTat 1.3 and LiTat 1.5 columns and (cross)-reactivity was verified in ELISA (Supplementary Material 3).
The Research Use Only (RUO) prototype g-iELISA was developed at Advanced Practical Diagnostics (Supplementary Material 4).
Diagnostic accuracy of this RUO g-iELISA (index test) on plasma was assessed on 1358 samples from gHAT patients and controls from the WHO Human African Trypanosomiasis Specimen Bank and originating from DRC, Guinea, Chad, and Uganda [20]. Controls were individuals living in endemic areas with negative serology (CATT) and parasitology for gHAT, and without previous gHAT infection. To assess the diagnostic accuracy of the g-iELISA on DBS, samples were prepared with healthy donor blood of which plasma was replaced by plasma of 95 TL-positive gHAT patients and 37 endemic controls [18, 21] (Supplementary Material 5).
Trypanolysis was used as reference test. All plasma samples were tested in TL with two VATs of T.b. gambiense type I (ie, LiTat 1.3 and LiTat 1.5), according to [15] with a cut-off of 30%.
RESULTS
Cut-off Value
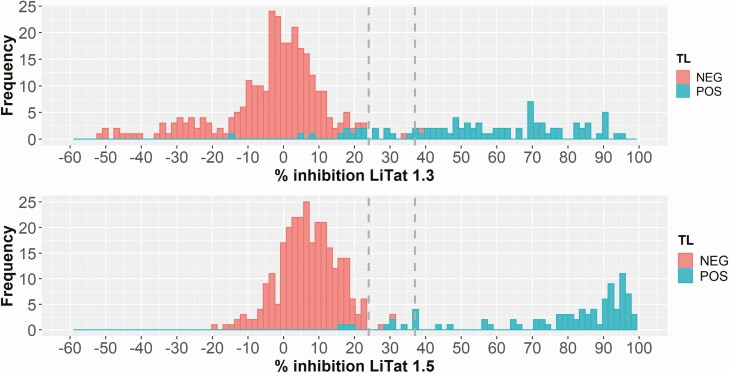
To define a percent inhibition cut-off value, 87 TL-positive and 275 TL-negative samples from DRC were tested. Combining the results obtained with each VSG antigen separately (Figure 1), the sensitivity and specificity were calculated at varying % inhibition cut-off values. The Youden index (sensitivity + specificity—1) was highest between 24% and 37% inhibition cut-off value (0.977 and 0.966, respectively). Percent inhibition values ranged from −52.5% to 95.3% in the test with LiTat 1.3 VSG and from −19.6% to 98.3% in the test with LiTat 1.5 VSG. Despite all attempts to avoid the negative percent inhibition often observed with TL-negative samples and with the LiTat 1.3 VSG, we were not able to overcome this unexpected phenomenon that, on the other hand, did not hinder to score the final result as positive or negative.
Figure 1.
Frequency plots of % inhibition results obtained with 87 TL-positive and 275 TL-negative samples in the g-iELISA with LiTat 1.3 and LiTat 1.5 antigen. Dashed lines indicate the cut-off value range with highest Youden index. Abbreviations: TL, trypanolysis test; NEG, negative; POS, positive.
Results on Plasma Samples
All 1358 plasma samples were tested in TL against T.b. gambiense LiTat 1.3 and LiTat 1.5. With TL as the reference test and using 35% inhibition as cut-off in the index test, the overall diagnostic accuracy of the g-iELISA is 99.5% with a 95% confidence interval (CI) of 99.0% to 99.8 (computed using the exact Clopper-Pearson method), assuming a 0.05% prevalence as reported for active screening [4]. The sensitivity is 98.0%, and the specificity is 99.5% (Table 1). Thus, values for sensitivity and specificity are above the values set in the TPP, ≥95% (optimistic) for sensitivity and (minimum) >99.5% for specificity. This is also the case when stratifying the results per country, except for DRC where observed specificity was slightly lower (99.47%) than the minimum value proposed in the TPP (99.5%).
Table 1.
Diagnostic Parameters Obtained in g-iELISA on Plasma with TL as Reference Test
| TP | FN | TN | FP | Sensitivity % | 95% CI | Specificity % | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| All countries | 724 | 15 | 616 | 3 | 98.0 | 96.7–98.9 | 99.5 | 98.6–99.9 |
| DRC | 529 | 13 | 567 | 3 | 97.6 | 95.9–98.7 | 99.5 | 98.5–99.9 |
| Guinea | 89 | 0 | 30 | 0 | 100.0 | 95.9–100.0 | 100.0 | 88.4–100.0 |
| Chad | 75 | 2 | 19 | 0 | 97.4 | 90.9–99.7 | 100.0 | 82.3–100.0 |
| Uganda | 31 | 0 | - | - | 100.0 | 88.8–100.0 | - | - |
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Results on DBS
With TL as reference test and 35% inhibition cut-off in the index test, the diagnostic accuracy of the g-iELISA on plasma samples is 100.0% (95% CI 97.2–100.0). The sensitivity is 94.7%, which is somewhat lower than the optimistic value but well above the minimum value (>90.0%) set in the TPP; the specificity is 100% (Table 2). With 20% inhibition cut-off, the diagnostic accuracy on DBS is 100.0% (97.2% to 100.0%) with a sensitivity of 92.6% and a specificity of 100.0% (Table 2). Compared to the sensitivity obtained with the corresponding plasma samples, testing DBS induced a small loss in sensitivity (2.1%) but still above the minimum value set in the TPP. Agreement between results obtained in g-iELISA with plasma and DBS is almost perfect (Cohen’s kappa k = 0.897, 95% CI: .73–1.067) [22].
Table 2.
Diagnostic Parameters Obtained in g-iELISA with DBS and Corresponding Plasma Samples
| TP | FN | TN | FP | Sensitivity % | 95% CI | Specificity % | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Plasma | 90 | 5 | 37 | 0 | 94.7 | 88.1–98.3 | 100 | 90.5–100.0 |
| DBS | 88 | 7 | 37 | 0 | 92.6 | 85.4–97.0 | 100 | 90.5–100.0 |
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
TL is used as reference test.
Precision
Repeatability, expressed as coefficient of variation (%CV) of 20 tests on the same sample in the same run, varied between 1.6% with antigen LiTat 1.5 and 5.8% with antigen LiTat 1.3 (Table 3). Reproducibility, expressed as the %CV on 6 samples tested in 4 different runs, varied between 1.7% with LiTat 1.5 and 18.9% with LiTat 1.3 (Table 4).
Table 3.
Repeatability of Results Obtained in g-iELISA by Testing 2 Trypanolysis-Positive Samples 20× in the Same Run
| Antigen LiTat 1.3 | Antigen LiTat 1.5 | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | |
| Average % inhibition | 58.8 | 78.9 | 56.0 | 78.5 |
| Standard deviation | 3.4 | 2.1 | 2.8 | 1.2 |
| % coefficient variation | 5.8 | 2.6 | 5.0 | 1.6 |
Table 4.
Reproducibility of Results Obtained in g-iELISA by Testing 6 Trypanolysis-Positive Samples in 4 Different Runs
| Antigen LiTat 1.3 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|
| Mean % inhibition | 60.6 | 47.9 | 66.7 | 43.0 | 0.2 | 55.2 |
| Standard deviation | 3.5 | 6.1 | 5.5 | 8.1 | 4.0 | 7.2 |
| % coefficient variation | 5.7 | 12.8 | 8.2 | 18.9 | 5.7 | 13.1 |
| Antigen LiTat 1.5 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
| Mean % inhibition | 58.2 | 79.1 | 80.8 | 37.5 | 37.7 | 70.4 |
| Standard deviation | 3.8 | 2.1 | 1.4 | 6.4 | 5.3 | 3.2 |
| % coefficient variation | 6.5 | 2.7 | 1.7 | 17.2 | 14.0 | 4.5 |
Stability Testing
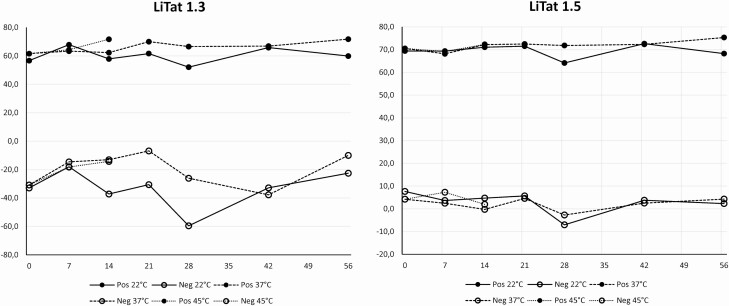
Accelerated stability testing, performed according to ISO 23640, revealed considerable variation in optical density (O.D.) values obtained with samples and controls in the test with LiTat 1.3 antigen, in contrast to LiTat 1.5 antigen. The % inhibition remained stable over time for all storage conditions except for the TL-negative samples tested with LiTat 1.3 antigen (Figure 2).
Figure 2.
Accelerated stability results. Four trypanolysis-positive and 2 trypanolysis-negative samples were tested with g-ELISA kits after 7, 14, 21, 28, 42, and 56 days storage at 22°C and 37°C, and after 7 and 14 days storage at 45°C. Abbreviations: Neg, negative; Pos, positive.
So far, real-time stability testing of g-iELISA kits stored at the storage temperature prescribed in the IFU (2–8°C), showed that the kits remain stable after 8 months storage at that temperature. Stability will further be followed up to 24 months.
DISCUSSION AND CONCLUSION
This study was undertaken to develop a high-throughput surveillance test for monitoring the EOT of T.b. gambiense and to assess its sustained absence or re-emergence in gHAT foci in the postelimination phase. Since the test will be deployed in situations where infection rates are very low or zero, it should have a high positive predictive value and therefore a very high specificity to avoid raising the alarm unnecessarily [23]. We opted for an ELISA since this test format is cheap and can be implemented in national and regional laboratories in sub-Saharan Africa, in contrast to TL, which is the WHO recommended reference test for anti-T.b. gambiense antibodies in humans, but that is performed in only3 laboratories world-wide. At least 3 times more samples can be tested simultaneously in g-iELISA, while for TL the limit is 400 samples per week. Aiming at high specificity of the new test, we designed it as an inhibition ELISA based on the recognition of 2 specific epitopes by two mAbs, each conjugated with horseradish peroxidase; thus i) avoiding reaction of test sample antibodies with other, less specific epitopes present on native VSGs, and ii) obviating the need for host-specific antibody conjugates when testing other host species that may harbor T.b. gambiense infections, such as domestic animals. As positive controls, we selected chicken antibodies (IgY) over mammalian antibodies since they can be produced in a less invasive, more cost-effective way, and in much larger amounts. Purified IgYs are stable up to 60°C [24] and remain reactive following storage at 4°C for several years (Coetzer, unpublished observation).
The g-iELISA makes use of a combination of native T.b. gambiense antigens that are also used in other serodiagnostic tests for gHAT, like the RDTs HAT Sero-K-SeT and SD Bioline HAT [25, 26], latex agglutination [27] ,and other ELISAs [28, 29]. None of these tests had a specificity higher than the minimum of 99.5% proposed in the TPP. For example, an ELISA with a mixture of VSGs LiTat 1.3, LiTat 1.5, and LiTat 1.6 was 99.2% (95% CI: 95.7–100) specific with sera from 128 negative controls and 98.7% (95% CI: 93.1–100) sensitive with sera from 78 gHAT patients [28], which, at 0.05% prevalence, would yield a PPV of 6.0% (95% CI: .9–30.8%). At the same prevalence, the here described g-iELISA on plasma would yield a PPV of 9.2% (95% CI: 3.2–23.8%), which is still far from optimal. Interestingly, maximum PPV was obtained when testing DBS instead of plasma (due to 100% specificity). For large-scale surveillance, the easiest specimen to collect is DBS, which is used in other disease surveillance programs. Active, village-based screening for gHAT by means of collecting DBS for remote testing in ELISA or other antibody detection tests has already been proposed a long time ago as an alternative to active screening by mobile teams [23, 30]. Its cost-effectiveness is evaluated in an ongoing study in Côte d’Ivoire, Burkina Faso, Guinea, and DRC (https://www.ditect-hat.eu/). More detailed analysis is required to assess the level of certainty the g-iELISA would provide in verifying whether the elimination of transmission goal has been reached using this collection framework. The benefits of other plausible sampling schemes for measurement of the elimination goal could be quantified using state-of-the-art Bayesian statistical frameworks [31], historical data, and mechanistic modelling approaches.
DBS specimen are currently collected in gHAT sentinel sites established with the help of the WHO in 15 endemic countries [32]. They are subsequently sent to the few reference laboratories where TL is performed, thus allowing detection of gHAT cases in nonendemic foci [33]. Replacing TL by g-iELISA opens perspectives to set up a larger network of sentinel sites in the most remote foci of an endemic country linked to national or regional laboratories equipped for ELISA testing. This will cut operational costs and the delay between sampling and test result, which, for obvious reasons, is beneficial to the national or international programs involved in gHAT elimination. Operational costs will further be reduced by the lower price of g-iELISA (3 €/test) compared to TL (5–7 €/test).
Results obtained with the current version of the g-iELISA are promising, in particular since diagnostic accuracy was similar on samples originating from West and Central Africa. However, further investigations may be considered to overcome inherent disadvantages. For instance, accelerated stability testing showed the limited stability of the LiTat 1.3 antigen at 22°C and higher, thus necessitating storage and long-distance transport of the kit between 2°–8°C. Furthermore, the need to cut out 8 × 6 mm diameter discs from each DBS prior to actually testing the eluted fraction, puts a limit on the number of samples that can be tested simultaneously by one lab technician. As an alternative, small blood volumes could be dried in the wells of a microfiltration plate that contains a suitable absorbing filter pad from which the test sample can be eluted via vacuum or centrifugation. Also, replacing the native antigens, which are produced in laboratory rodents, by recombinant antigens or peptides, would be a major achievement in the context of the 3Rs (Replacement, Reduction, and Refinement of animals in research). In previous studies, we developed alternative diagnostic antigens, derived from the VSGs LiTat 1.3 and LiTat 1.5, in the form of synthetic peptides and recombinant antigens produced in Pichia pastoris and Leishmania tarentolae [34–37]. Unfortunately, none of these antigens reacted with the highly VAT-specific monoclonal antibodies currently used in the g-iELISA, thus necessitating the development of new monoclonal antibodies before an inhibition ELISA with these alternative antigens can be constructed.
The present study has a limitation that makes it difficult to compare the sensitivity and specificity of the g-iELISA with those reported in other studies on gHAT serodiagnostics. Most gHAT patients, from which the plasma samples were collected, were screened with CATT/T.b. gambiense before undergoing parasitological confirmation. In addition, endemic controls were generally defined as negative in CATT/T.b. gambiense. Thus, we observed TL negatives among the gHAT patients and TL positives among the controls. However, since the aim was to develop an ELISA with similar characteristics as TL, we used the latter as a reference test and not the positive or negative status in parasitological examination. To assess its clinical accuracy and robustness, the g-iELISA should be evaluated under conditions prevailing in reference laboratories in the endemic countries.
In conclusion, the diagnostic sensitivity and specificity of this prototype g-iELISA comply with the minimum requirements set in the TPP, in particular when testing with DBS. As such, the test might largely replace the TL for monitoring the gHAT elimination progress and for postelimination surveillance. The RUO ELISA kit is available for evaluation in reference laboratories in gHAT endemic countries.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Plasma samples originate from the WHO HAT Specimen Bank and were provided by Pasteur Institute Paris.
Disclaimer. The funders had no role in the design, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Bill & Melinda Gates Foundation [grant numbers OPP1174221 to PB, OPP1177824 to KSR].
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet 2017; 390:2397–409. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Control and surveillance of human African trypanosomiasis. World Health Organ Tech Rep Ser 2013; 984:1–237. [PubMed] [Google Scholar]
- 3. Jamonneau V, Truc P, Grébaut P, et al. Trypanosoma brucei gambiense Group 2: the unusual suspect. Trends Parasitol 2019; 35:983–95. [DOI] [PubMed] [Google Scholar]
- 4. Franco JR, Cecchi G, Priotto G, et al. Monitoring the elimination of human African trypanosomiasis: Update to 2016. PLoS Neglect Trop Dis 2018; 12:e0006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simarro PP, Cecchi G, Franco JR, et al. Monitoring the progress towards the elimination of gambiense human African trypanosomiasis. PLoS Negl Trop Dis 2015; 9:e0003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franco JR, Simarro PP, Diarra A, Ruiz-Postigo JA, Jannin JG. The journey towards elimination of gambiense human African trypanosomiasis: not far, nor easy. Parasitology 2014; 141:748–60. [DOI] [PubMed] [Google Scholar]
- 7. Alvar J, Alves F, Bucheton B, et al. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Semin Immunopathol 2020; 42:231–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Büscher P, Bart JM, Boelaert M, et al. Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol 2018; 34:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehlitz D, Molyneux DH. The elimination of Trypanosoma brucei gambiense? Challenges of reservoir hosts and transmission cycles: Expect the unexpected. Parasite Epidemiol Control 2019; 6:e00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magnus E, Vervoort T, Van Meirvenne N. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T.b.gambiense trypanosomiasis. Ann Soc Belg Méd Trop 1978; 58:169–76. [PubMed] [Google Scholar]
- 11. Checchi F, Chappuis F, Karunakara U, Priotto G, Chandramohan D. Accuracy of five algorithms to diagnose gambiense human African trypanosomiasis. PLoS Negl Trop Dis 2011; 5:e1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jamonneau V, Camara O, Ilboudo H, et al. Accuracy of individual rapid tests for serodiagnosis of gambiense sleeping sickness in West Africa. PLoS Negl Trop Dis 2015; 9:e0003480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medley GF, Hollingsworth TD, Olliaro PL, Adams ER. Health-seeking behaviour, diagnostics and transmission dynamics in the control of visceral leishmaniasis in the Indian subcontinent. Nature 2015; 528:S102–8. [DOI] [PubMed] [Google Scholar]
- 14. Unnasch TR, Golden A, Cama V, Cantey PT. Diagnostics for onchocerciasis in the era of elimination. Int Health 2018; 10:i20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Meirvenne N, Magnus E, Büscher P. Evaluation of variant specific trypanolysis tests for serodiagnosis of human infections with Trypanosoma brucei gambiense. Acta Trop 1995; 60:189–99. [DOI] [PubMed] [Google Scholar]
- 16. Mumba Ngoyi D, Ali Ekangu R, Mumvemba Kodi MF, et al. Performance of parasitological and molecular techniques for the diagnosis and surveillance of gambiense sleeping sickness. PLoS Negl Trop Dis 2014; 8:e2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Camara O, Camara M, Lejon V, et al. Immune trypanolysis test with blood spotted on filter paper for epidemiological surveillance of sleeping sickness. Trop Med Int Health 2014; 19:828–31. [DOI] [PubMed] [Google Scholar]
- 18. Büscher P, Mertens P, Leclipteux T, et al. Sensitivity and specificity of HAT Sero-K-SeT, a rapid diagnostic test for serodiagnosis of sleeping sickness caused by Trypanosoma brucei gambiense: a case-control study. Lancet Glob Health 2014; 2:e359–63. [DOI] [PubMed] [Google Scholar]
- 19. Büscher P, Draelants E, Magnus E, Vervoort T, Van Meirvenne N. An experimental latex agglutination test for antibody detection in human African trypanosomiasis. Ann Soc Belg Med Trop 1991; 71:267–73. [PubMed] [Google Scholar]
- 20. Franco JR, Simarro PP, Diarra A, Ruiz-Postigo JA, Jannin JG. The Human African trypanosomiasis specimen biobank: a necessary tool to support research of new diagnostics. PLoS Negl Trop Dis 2012; 6:e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bisser S, N’Siesi FX, Lejon V, et al. Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness. J Infect Dis 2007; 195:322–9. [DOI] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 23. Hasker E, Lutumba P, Mumba D, et al. Diagnostic accuracy and feasibility of serological tests on filter paper samples for outbreak detection of T.b. gambiense human African trypanosomiasis. Am J Trop Med Hyg 2010; 83:374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz-Asplund J, Terzolo HR. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim 2005; 33:129–54. [DOI] [PubMed] [Google Scholar]
- 25. Büscher P, Mumba Ngoyi D, Pyana PP, et al. New rapid tests for antibody detection serodiagnosis in Trypanosoma brucei gambiense sleeping sickness. HAT Platform Newslett 2013; 13:21–2. [Google Scholar]
- 26. Bisser S, Lumbala C, Nguertoum E, et al. Sensitivity and specificity of a prototype rapid diagnostic test for the detection of Trypanosoma brucei gambiense infection: a multi-centric prospective study. PLoS Negl Trop Dis 2016;10:e0004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Büscher P, Lejon V, Magnus E, Van Meirvenne N. Improved latex agglutination test for detection of antibodies in serum and cerebrospinal fluid of Trypanosoma brucei gambiense infected patients. Acta Trop 1999; 73:11–20. [DOI] [PubMed] [Google Scholar]
- 28. Lejon V, Jamonneau V, Solano P, et al. Detection of trypanosome-specific antibodies in saliva, towards non-invasive serological diagnosis of sleeping sickness. Trop Med Int Health 2006; 11:620–7. [DOI] [PubMed] [Google Scholar]
- 29. Lejon V, Kwete J, Büscher P. Towards saliva-based screening for sleeping sickness? Trop Med Int Health 2003; 8:585–8. [DOI] [PubMed] [Google Scholar]
- 30. Laveissière C, Meda AH, Doua F, Sane B. [Detecting sleeping sickness: comparative efficacy of mobile teams and community health workers]. Bull World Health Organ 1998; 76:559–64. [PMC free article] [PubMed] [Google Scholar]
- 31. Fronterre C, Amoah B, Giorgi E, Stanton MC, Diggle PJ. Design and analysis of elimination surveys for neglected tropical diseases. J Infect Dis 2020; 221:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Report of the third WHO stakeholders meeting on gambiense human African trypanosomiasis elimination, Geneva, Switzerland, 18–20 April 2018. Geneva: World Health Organization, 2020. [Google Scholar]
- 33. Dama E, Drabo A, Kaboré J, et al. Description of the first sleeping sickness case diagnosed in Burkina Faso since two decades. PLoS Negl Trop Dis 2018; 12:e0006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rogé S, Van Nieuwenhove L, Meul M, et al. Recombinant antigens expressed in Pichia pastoris for the diagnosis of sleeping sickness caused by Trypanosoma brucei gambiense. PLoS Negl Trop Dis 2014; 8:e3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Nieuwenhove L, Büscher P, Balharbi F, et al. Identification of mimotopes with diagnostic potential for Trypanosoma brucei gambiense variant surface glycoproteins using human antibody fractions. PLoS Negl Trop Dis 2012; 6:e1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Nieuwenhove LC, Rogé S, Balharbi F, et al. Identification of peptide mimotopes of Trypanosoma brucei gambiense variant surface glycoproteins. PLoS Negl Trop Dis 2011; 5:e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rooney B, Piening T, Büscher P, Rogé S, Smales CM. Expression of Trypanosoma brucei gambiense Antigens in Leishmania tarentolae. Potential for use in Rapid Serodiagnostic Tests (RDTs). PLoS Negl Trop Dis 2015; 9:e0004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.