Abstract
Background:
Previous reports suggested risk of death and breast cancer varied by comorbidity and age in older women undergoing mammography. However, impacts of functional limitations remain unclear.
Methods:
We used data from 238,849 women in the Breast Cancer Surveillance Consortium-Medicare linked database (1999–2015) who had screening mammogram at ages 66–94 years. We estimated risk of breast cancer, breast cancer death, and non-breast cancer death by function-related indicator (FRI) which incorporated 16 claims-based items and was categorized as an ordinal variable (0, 1, and 2+). Fine and Gray proportional sub-distribution hazards models were applied with breast cancer and death treated as competing events. Risk estimates by FRI scores were adjusted by age and NCI comorbidity index separately and stratified by these factors.
Results:
Overall, 9,252 women were diagnosed with breast cancer, 406 died of breast cancer, and 41,640 died from non-breast cancer causes. The 10-year age-adjusted invasive breast cancer risk slightly decreased with FRI score (FRI=0: 4.0%, 95% CI=3.8%−4.1%; FRI=1: 3.9%, 95% CI=3.7%−4.2%; FRI≥2: 3.5%, 95% CI=3.1%−3.9%). Risk of non-breast cancer death increased with FRI score (FRI=0: 18.8%, 95% CI=18.5%−19.1%; FRI=1: 24.4%, 95% CI=23.9%−25.0%; FRI≥2: 39.8%, 95% CI=38.8%−40.9%). Risk of breast cancer death was low with minimal differences across FRI scores. NCI comorbidity index-adjusted models and stratified analyses yielded similar patterns.
Conclusions:
Risk of non-breast cancer death substantially increases with FRI score, whereas risk of breast cancer death is low regardless of functional status.
Impact:
Older women with functional limitations should be informed that they may not benefit from screening mammography.
Keywords: mammography, functional limitations, breast cancer screening, epidemiology
Introduction
Over the past decades, the elderly population in the United States (US) has grown substantially. The US Census Bureau has estimated that older adults–those at or above 65 years of age–will make up about 24 % of the US population by 2060 (1), including a large number of older people living with functional limitations and co-existing illnesses (2, 3). Because the number of vulnerable older adults continues to increase, it is important to determine the outcomes of specific health services, such as breast cancer screening with mammography, in older women with differential health conditions.
Breast cancer is the most common malignancy among women in the United States (4). It has been estimated that about 276,480 women were diagnosed with invasive breast cancer in the United States during 2020, with 44.5% aged 65 years or older at diagnosis (5). Screening mammography can detect signs of early lesions of breast cancer with high accuracy (6) and reduce breast cancer mortality at the population level (7). However, benefits of screening mammography may be more limited in older women because of aging, co-existing illnesses, and functional impairments (8, 9). Due to pre-existing adverse health conditions and short life expectancy associated with natural aging processes, older women undergoing screening mammography may not be healthy enough to safely undergo mammography (or treatment if cancer is found) or survive long enough to experience the benefits conferred by early breast cancer detection (10, 11). In a prior study of older women with screening mammography in the Breast Cancer Surveillance Consortium (BCSC)-Medicare linked data, we found that older women with a higher Charlson Comorbidity Index (CCI) (≥2 vs. 0) had a substantial increase (1.3 to 3-fold) in risk of non-breast cancer death (12); however, the association between functional limitations and screening outcome was not evaluated in that study.
While comorbidity measures have been used to evaluate disease burden, measures of functional limitations can more accurately reflect older people’s overall wellbeing and be a marker of the severity of comorbid illnesses. For example, comorbidities (e.g., stroke, heart failure, etc.) may affect function or profoundly decrease a person’s ability to perform activities of daily living (13).
In gerontological research, functional limitations and comorbidities are usually considered simultaneously when making decisions about health services utilization for older people due to their strong impacts on general health, life expectancy (14, 15), and risk of mortality (16). Incorporating functional limitations into our analysis along with comorbidity and aging better reflects the severity of pre-existing health conditions which in turn affects the benefits and risks of screening mammography among older women. Therefore, we proposed to investigate if risk of breast cancer or mortality after screening mammography varied by functional limitations in the BCSC-Medicare cohort.
Materials and Methods
BCSC-Medicare and study population
The BCSC is a collaborative research network (https://www.bcsc-research.org/about/sites) of breast imaging registries in the United States used to assess and improve the delivery and quality of breast cancer screening and related outcomes (17). The BCSC registries collect demographic information, risk factors, screening history, pathological characteristics of breast lesions, and mammography indication and results. BCSC data are pooled at a central Statistical Coordinating Center (SCC). All registries and the SCC received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform data analysis. All procedures were Health Insurance Portability and Accountability Act (HIPAA) compliant, and all registries and the SCC had a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities that are subjects of this research (17, 18). In our study, Medicare claims data were linked to data from Carolina Mammography Registry, New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System and claims data from Kaiser Permanente Washington were included.
Women with the following characteristics in the BCSC-Medicare linked data were included for analysis: (1) ≥66 years at screening (because functional limitations were computed based on claims data of the year prior to the screening mammogram, and Medicare eligibility began at 65 years); (2) underwent mammography screening between 1999–2015; (3) were continuously enrolled in Medicare Parts A and B from 1 year before the screening and were not enrolled in a Medicare managed care plan during the same time period; and (4) had no history of breast cancer at the time of screening. Screening mammography was defined as routine bilateral screening views performed in women without breast imaging in the previous nine months and without a history of breast cancer, breast implants, or mastectomy (17). The first screening mammogram that fit these criteria was used for the analysis and referred to as the index screening mammogram.
Exposure and outcome of interest
Functional limitations were the exposure of interest in our analysis, represented by 16 function-related indicators (FRI) present in Medicare Part A and B claims data during the year before index BCSC mammography screening. We used commonly accepted claims-based algorithms to identify these items and assigned 1 point to each; thus, FRI scores represented the summed number of functional limitations and women without functional limitations were assigned 0. Detailed FRI algorithms have been described (15, 19) and we listed these items in Supplementary Table 1. We categorized FRI as 0, 1, and ≥2 for the current analysis due to sample size considerations.
The primary outcomes of interest were incident invasive breast cancer and ductal carcinoma in situ (DCIS) diagnosed after index screening and death from non-breast cancer causes. Breast cancer death was the secondary outcome. Breast cancer diagnosis, mode of cancer detection (screen-detected vs. other), and AJCC stage were obtained by linking BCSC data to pathology databases, SEER programs, and state or regional tumor registries. Date of death was determined using SEER, state cancer registries, state vital records, and Medicare data; cause of death was ascertained using the first three of those sources (17).
Other covariates
Age, race/ethnicity, education level, family history of breast cancer in a first degree relative, and time since last mammogram were reported by participants in self-administered questionnaires or obtained from the electronic medical record. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2) based on self-reported height and weight or obtained from the electronic medical record. Breast density at index screening was interpreted in clinical practice by radiologists based on Breast Imaging Reporting and Data System (BI-RADS) and categorized as: almost entirely fat, scattered fibroglandular density, heterogeneously dense, or extremely dense (20). Pre-existing comorbidities were represented by the National Cancer Institute (NCI) comorbidity index, a weighted index based on 16 diseases identified from hospital and physician claims data (21); the NCI comorbidity index was computed using algorithms suggested by NCI (22) and categorized as 0, 1, and ≥2 in our study.
Statistical analysis
We descriptively summarized distributions of NCI comorbidity index, sociodemographic characteristics (age, race/ethnicity, and education), BMI, first-degree family history of breast cancer, breast density, time since prior mammogram, mode of diagnosis, and stage of breast cancer at diagnosis in the overall sample and for each category of FRI. Distributions of FRI and other covariates were examined by status at the end of follow-up (alive, breast cancer death, death from non-breast cancer causes, invasive breast cancer, and DCIS) within the 10-year period after index mammography. In the primary analysis, women were followed from the date of the index screening to death from non-breast cancer causes, invasive breast cancer, DCIS, disenrollment, end of complete information for cancer data, end of complete information for vital status data, or end of the 10-year follow-up period, whichever occurred first. To estimate the 10-year cumulative incidence of each outcome, we used Fine and Gray proportional subdistribution hazards models that accounted for competing events (23). A model including FRI level was fit for each age group (66–74, 75–94), comorbidity level (0, 1, ≥ 2), and age group by comorbidity level. This provided cumulative incidence estimates of FRI level by age group and/or comorbidity level. These estimates were then adjusted by the distribution of age group or comorbidity level to obtain the overall estimated cumulative incidence for each FRI level. Estimated risk of DCIS was not computed for FRI level by age group by comorbidity level due to sparse data. In the primary analysis, when evaluating risk of one outcome (death from non-breast cancer causes, invasive breast cancer, or DCIS), the other two outcomes were treated as competing events. Due to the similarity between point estimates of the risk of invasive breast cancer and DCIS across FRI scores, we conducted a trend test by including FRI as an ordinal variable to examine if cumulative incidence of these outcomes changed significantly by FRI categories. To further explore risk patterns of women aged 75–94, we calculated risk of non-breast cancer death and incident breast cancer by FRI scores for women aged 75–84 and 85–94 years separately; the same analysis was not performed for breast cancer death due to small sample sizes in women aged 85–94. Moreover, as compared to White women, Black women had a higher prevalence of co-existing illnesses and different risk of breast cancer and mortality (12, 24), thus we calculated risk of non-breast cancer death and incident breast cancer by FRI in White and Black women separately and compared the estimates; the analysis was not conducted for other race/ethnicity groups and breast cancer death because of smaller sample sizes. In the secondary analysis, which treated breast cancer death as the outcome, follow-up was not truncated by breast cancer diagnosis and death from non-breast cancer causes was a competing event. We did not adjust for other confounders in analysis because minimally adjusted results would provide a direct view of cumulative incidence by FRI scores among older women. Confidence intervals (CI) were calculated via bootstrapping by using the 2.5th and 97.5th percentiles from 1,000 random bootstrap samples. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Table 1 presents distributions of study characteristics according to FRI. A total of 238,849 women ages 66 to 94 years undergoing mammography screening between 1999–2015 were included with a median follow-up time of 101 months. Overall, 66.7% of the women had an FRI score of 0, 24.5% had a score of 1, and 8.8% had a score ≥2. Distribution of NCI comorbidity index was as follows: 0: 72.4%, 1: 20.4%, ≥2: 7.2%. The median age of participants was 69 years (Quartile 1, Quartile 3 [Q1, Q3]: 67, 75) and approximately half (50.8%) were between ages 65–69 years. Most of the women self-reported as White (83.7%), 6.1% were Black, 2.5% Hispanic, 6.2% Asian/Pacific Islander, and 1.6% self-classified as other race/ethnicity groups. Over half (54.7%) of participants had some college education, 58.8% were overweight or obese (BMI≥25 kg/m2), and 17.8% had family history of breast cancer. In terms of breast density, over half of the women (52.8%) had scattered fibroglandular density in their breast tissue and only a very small fraction (3.4%) were classified as extremely dense. Most women (82.2%) had a prior mammogram within 1–2 years before index screening. Among women diagnosed with breast cancer, 75.4% were detected via screening and 20.7% were diagnosed with DCIS. Women with FRI score ≥2 appeared to have higher NCI comorbidity index score, be older, and be less likely to have a prior mammogram within 1–2 years compared to those with lower FRI scores.
Table 1.
Characteristics of 238,849 older women undergoing screening mammography in 1999–2015 in BCSC by function-related indicator score
| Overall (N=238,849) | FRI=0 (N=159,332) | FRI=1 (N=58,450) | FRI≥2 (N=21,067) | |
|---|---|---|---|---|
| Characteristics | No. (%)a | No. (%)a | No. (%)a | No. (%)a |
|
| ||||
| Follow-up time in months (median, Q1-Q3) | 101 (42, 120) | 105 (47, 120) | 97 (40, 120) | 75 (30, 120) |
| NCI comorbidity index | ||||
| 0 | 172,956 (72.4) | 122,969 (77.2) | 39,878 (68.2) | 10,109 (48.0) |
| 1 | 48,637 (20.4) | 29,661 (18.6) | 13,267 (22.7) | 5,709 (27.1) |
| ≥ 2 | 17,256 (7.2) | 6,702 (4.2) | 5,305 (9.1) | 5,249 (24.9) |
| Age (median, Q1-Q3) | 69 (67, 75) | 69 (66, 74) | 70 (67, 76) | 72 (67, 78) |
| Age group | ||||
| 65–69 | 121,364 (50.8) | 84,383 (53.0) | 28,525 (48.8) | 8,456 (40.1) |
| 70–74 | 54,519 (22.8) | 36,857 (23.1) | 13,089 (22.4) | 4,573 (21.7) |
| 75–79 | 35,737 (15.0) | 22,690 (14.2) | 9,149 (15.7) | 3,898 (18.5) |
| 80–84 | 18,723 (7.8) | 10,969 (6.9) | 5,192 (8.9) | 2,562 (12.2) |
| 85–94 | 8,506 (3.6) | 4,433 (2.8) | 2,495 (4.3) | 1,578 (7.5) |
| Race/ethnicity | ||||
| White | 186,009 (83.7) | 122,856 (82.6) | 46,578 (86.0) | 16,575 (86.4) |
| Black | 13,515 (6.1) | 9,780 (6.6) | 2,741 (5.1) | 994 (5.2) |
| Hispanic | 5,466 (2.5) | 3,619 (2.4) | 1,363 (2.5) | 484 (2.5) |
| Asian/Pacific Islander | 13,676 (6.2) | 10,428 (7.0) | 2,547 (4.7) | 701 (3.7) |
| Other | 3,456 (1.6) | 2,119 (1.4) | 916 (1.7) | 421 (2.2) |
| Missing | 16,727 (7.0) | 10,530 (6.6) | 4,305 (7.4) | 1,892 (9.0) |
| Education | ||||
| <High school graduate | 28,931 (14.2) | 18,959 (14.0) | 7,129 (14.3) | 2,843 (16.2) |
| High school graduate or GED | 62,986 (31.0) | 42,612 (31.5) | 15,093 (30.2) | 5,281 (30.1) |
| Some college or technical school | 52,640 (25.9) | 34,921 (25.8) | 13,199 (26.4) | 4,520 (25.8) |
| College graduate | 58,471 (28.8) | 38,997 (28.8) | 14,587 (29.2) | 4,887 (27.9) |
| Missing | 35,821 (15.0) | 23,843 (15.0) | 8,442 (14.4) | 3,536 (16.8) |
| Body mass index, kg/m2 | ||||
| Underweight (<18.5) | 2,311 (1.9) | 1,263 (1.6) | 671 (2.2) | 377 (3.4) |
| Normal weight (18.5–24.9) | 47,028 (39.3) | 31,249 (40.1) | 11,578 (37.6) | 4,201 (38.2) |
| Overweight (25–29.9) | 39,036 (32.6) | 25,718 (33.0) | 10,058 (32.6) | 3,260 (29.6) |
| Class I obesity (30–34.9) | 19,829 (16.6) | 12,658 (16.3) | 5,302 (17.2) | 1,869 (17.0) |
| Class II/III obesity (≥35) | 11,485 (9.6) | 6,992 (9.0) | 3,197 (10.4) | 1,296 (11.8) |
| Missing | 119,160 (49.9) | 81,452 (51.1) | 27,644 (47.3) | 10,064 (47.8) |
| Family history of breast cancer | ||||
| No | 170,929 (82.2) | 113,310 (82.4) | 42,278 (82.0) | 15,341 (81.4) |
| Yes | 36,999 (17.8) | 24,185 (17.6) | 9,305 (18.0) | 3,509 (18.6) |
| Missing | 30,921 (12.9) | 21,837 (13.7) | 6,867 (11.7) | 2,217 (10.5) |
| Breast density | ||||
| Almost entirely fat=a | 27,065 (12.5) | 17,597 (12.2) | 6,842 (13.0) | 2,626 (13.9) |
| Scattered fibroglandular density=b | 113,869 (52.8) | 76,196 (52.8) | 27,632 (52.5) | 10,041 (53.3) |
| Heterogeneously dense=c | 67,427 (31.2) | 45,530 (31.5) | 16,396 (31.2) | 5,501 (29.2) |
| Extremely dense=d | 7,427 (3.4) | 4,999 (3.5) | 1,762 (3.3) | 666 (3.5) |
| Missing | 23,061 (9.7) | 15,010 (9.4) | 5,818 (10.0) | 2,233 (10.6) |
| Time since prior mammogram | ||||
| No prior | 4,813 (2.2) | 3,237 (2.2) | 1,101 (2.0) | 475 (2.5) |
| 1 year | 3,388 (1.5) | 2,188 (1.5) | 866 (1.6) | 334 (1.8) |
| 1–2 years | 181,464 (82.2) | 122,586 (82.9) | 44,121 (81.8) | 14,757 (77.8) |
| 3 or more years | 31,158 (14.1) | 19,936 (13.5) | 7,823 (14.5) | 3,399 (17.9) |
| Missing | 18,026 (7.5) | 11,385 (7.1) | 4,539 (7.8) | 2,102 (10.0) |
| Mode of diagnosis b | ||||
| Screen-detected | 5,657 (75.4) | 3,843 (75.5) | 1,379 (75.3) | 435 (74.7) |
| Other | 1,847 (24.6) | 1,247 (24.5) | 453 (24.7) | 147 (25.3) |
| Missing | 1,748 (18.9) | 1,264 (19.9) | 374 (17.0) | 110 (15.9) |
| AJCC stage at diagnosis b | ||||
| DCIS | 1,773 (20.7) | 1,258 (21.3) | 378 (18.6) | 137 (21.3) |
| Stage I-IIA | 5,770 (67.3) | 3,926 (66.6) | 1,423 (70.0) | 421 (65.4) |
| Stage IIB, III, IV | 1,031 (12.0) | 713 (12.1) | 232 (11.4) | 86 (13.4) |
| Missing | 678 (7.3) | 457 (7.2) | 173 (7.8) | 48 (6.9) |
Abbreviations: AJCC: American Joint Committee on Cancer, BCSC: Breast Cancer Surveillance Consortium, DCIS: ductal carcinoma in situ, FRI: function-related indicator, GED: General Educational Development, IQR: interquartile range, NCI: National Cancer Institute
Number of observations in the table represented individual women
Column percent
Denominators used for calculating column percentages of non-missing categories were the sum of non-missing observations.
Based on screens with cancer diagnosed during follow-up
A total of 9,252 women were diagnosed with breast cancer (7,479 invasive, 1,773 DCIS) during follow-up. Among women with breast cancer diagnosis, 406 died of breast cancer and 1,359 died of non-breast cancer causes. Among women without a breast cancer diagnosis during follow-up, 189,316 were alive and 40,281 died by the end of follow-up (Figure 1). Table 2 summarizes study characteristics by outcome of interest. Among women without breast cancer diagnosis, those who died during follow-up had higher scores of FRI and NCI comorbidity index and were older compared with those who were alive. Compared to women diagnosed with invasive breast cancer, women diagnosed with DCIS had slightly lower scores of FRI and NCI comorbidity index and younger ages. Women dying of breast cancer had lower scores of FRI and NCI comorbidity index and were younger than those dying from non-breast cancer causes.
Figure 1.
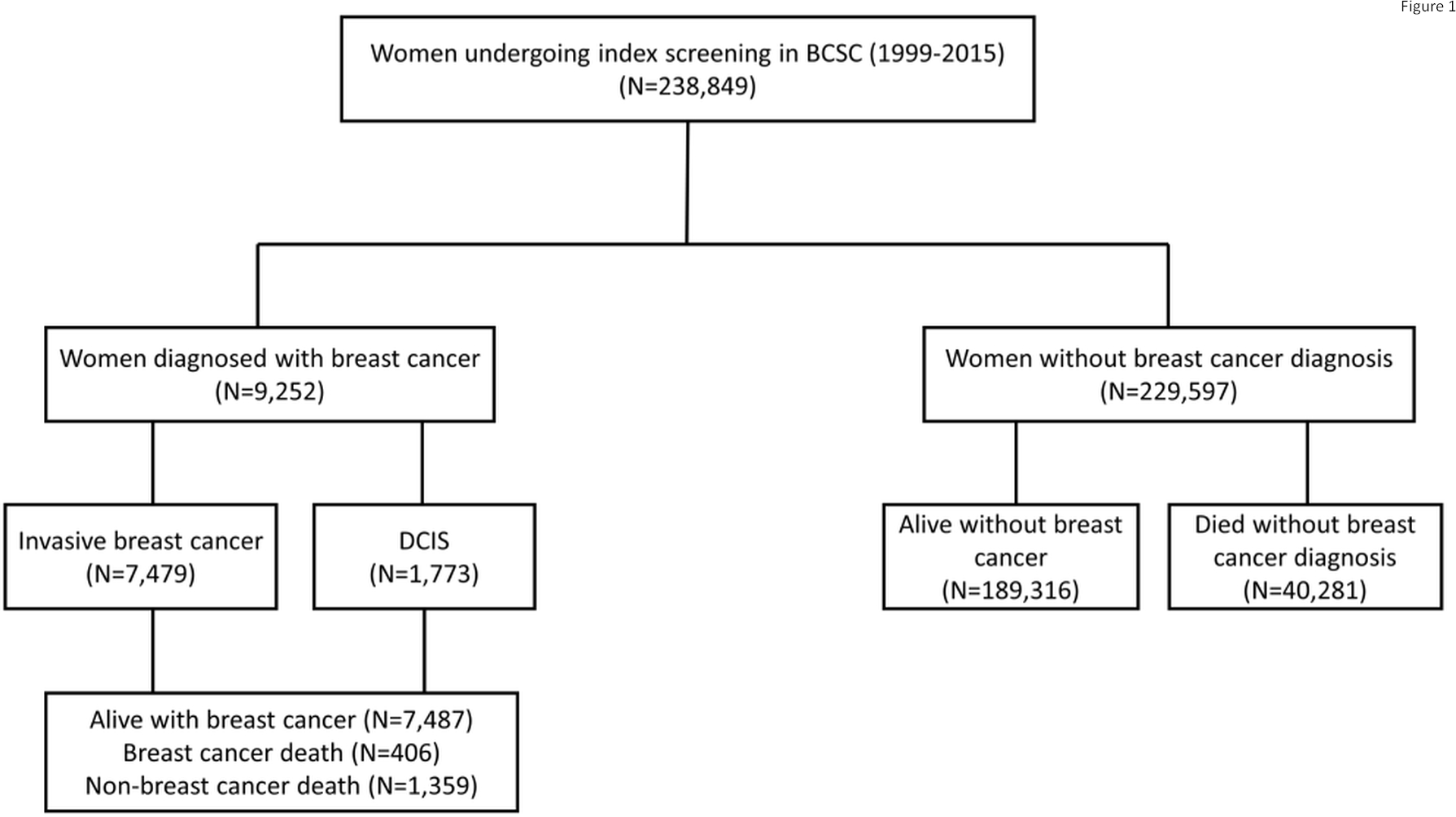
Flowchart showing the numbers of people with different outcomes of interest. Abbreviations: BCSC: Breast Cancer Surveillance Consortium, DCIS: ductal carcinoma in situ.
Table 2.
Characteristics of 238,849 older women at index screening mammogram in BCSC (1999–2015) by vital status and breast cancer diagnosis.
| No breast cancer during follow-up |
Breast cancer during follow-up |
||||
|---|---|---|---|---|---|
| Alive without breast cancera (N=189,316) | Died from non-breast cancer causesb (N=40,281) | Diagnosed with invasive breast cancerc (N=7,479) | Diagnosed with DCISc (N=1,773) | Died of breast cancerd (N=406) | |
| Characteristics | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
|
| |||||
| Follow-up time in months (median, Q1-Q3) | 120 (46, 120) | 73 (45, 96) | 38 (12, 73) | 35 (10, 64) | 75 (48, 96) |
| FRI | |||||
| 0 | 130,616 (69.0) | 22,362 (55.5) | 5,096 (68.1) | 1,258 (71.0) | 274 (67.5) |
| 1 | 45,040 (23.8) | 11,204 (27.8) | 1,828 (24.4) | 378 (21.3) | 85 (20.9) |
| ≥ 2 | 13,660 (7.2) | 6,715 (16.7) | 555 (7.4) | 137 (7.7) | 47 (11.6) |
| NCI comorbidity index | |||||
| 0 | 143,919 (76.0) | 22,173 (55.0) | 5,503 (73.6) | 1,361 (76.8) | 269 (66.3) |
| 1 | 35,549 (18.8) | 11,254 (27.9) | 1,515 (20.3) | 319 (18.0) | 102 (25.1) |
| ≥ 2 | 9,848 (5.2) | 6,854 (17.0) | 461 (6.2) | 93 (5.2) | 35 (8.6) |
| Age group | |||||
| 66–74 | 151,393 (80.0) | 17,711 (44.0) | 5,403 (72.2) | 1,376 (77.6) | 245 (60.3) |
| 75–84 | 34,737 (18.3) | 17,502 (43.4) | 1,855 (24.8) | 366 (20.6) | 142 (35.0) |
| 85–94 | 3,186 (1.7) | 5,068 (12.6) | 221 (3.0) | 31 (1.7) | 19 (4.7) |
| Race/ethnicity | |||||
| White | 148,014 (83.1) | 30,446 (86.0) | 6,161 (87.6) | 1,388 (82.7) | 311 (83.4) |
| Black | 10,440 (5.9) | 2,546 (7.2) | 404 (5.7) | 125 (7.4) | 37 (9.9) |
| Hispanic | 4,579 (2.6) | 729 (2.1) | 128 (1.8) | 30 (1.8) | 9 (2.4) |
| Asian/Pacific Islander | 12,155 (6.8) | 1,177 (3.3) | 233 (3.3) | 111 (6.6) | 10 (2.7) |
| Other | 2,831 (1.6) | 494 (1.4) | 106 (1.5) | 25 (1.5) | 6 (1.6) |
| Missing | 11,297 (6.0) | 4,889 (12.1) | 447 (6.0) | 94 (5.3) | 33 (8.1) |
| Education | |||||
| <High school graduate | 20,732 (12.8) | 7,167 (21.6) | 850 (12.9) | 182 (11.4) | 77 (22.1) |
| High school graduate or GED | 48,259 (29.9) | 12,155 (36.6) | 2,062 (31.3) | 510 (32.0) | 95 (27.2) |
| Some college or technical school | 42,769 (26.5) | 7,635 (23.0) | 1,825 (27.7) | 411 (25.8) | 90 (25.8) |
| College graduate | 49,866 (30.9) | 6,268 (18.9) | 1,847 (28.1) | 490 (30.8) | 87 (24.9) |
| Missing | 27,690 (14.6) | 7,056 (17.5) | 895 (12.0) | 180 (10.2) | 57 (14.0) |
| Body mass index, kg/m2 | |||||
| Underweight (<18.5) | 1,617 (1.6) | 647 (3.7) | 30 (0.8) | 17 (2.0) | 0 |
| Normal weight (18.5–24.9) | 38,250 (39.0) | 7,264 (42.1) | 1,218 (34.3) | 296 (35.3) | 62 (33.0) |
| Overweight (25–29.9) | 32,328 (33.0) | 5,239 (30.3) | 1,192 (33.6) | 277 (33.1) | 66 (35.1) |
| Class I obesity (30–34.9) | 16,338 (16.7) | 2,627 (15.2) | 710 (20.0) | 154 (18.4) | 44 (23.4) |
| Class II/III obesity (≥35) | 9,497 (9.7) | 1,495 (8.7) | 399 (11.2) | 94 (11.2) | 16 (8.5) |
| Missing | 91,286 (48.2) | 23,009 (57.1) | 3,930 (52.5) | 935 (52.7) | 218 (53.7) |
| Family history of breast cancer | |||||
| No | 137,029 (82.4) | 27,877 (82.6) | 4,847 (76.7) | 1,176 (76.9) | 265 (77.7) |
| Yes | 29,315 (17.6) | 5,861 (17.4) | 1,470 (23.3) | 353 (23.1) | 76 (22.3) |
| Missing | 22,972 (12.1) | 6,543 (16.2) | 1,162 (15.5) | 244 (13.8) | 65 (16.0) |
| Breast density | |||||
| Almost entirely fat | 21,950 (12.8) | 4,546 (12.7) | 447 (6.7) | 122 (7.8) | 28 (7.9) |
| Scattered fibroglandular density | 90,299 (52.5) | 19,369 (54.3) | 3,407 (51.2) | 794 (50.7) | 172 (48.5) |
| Heterogeneously dense | 53,743 (31.3) | 10,543 (29.6) | 2,558 (38.4) | 583 (37.3) | 143 (40.3) |
| Extremely dense | 5,906 (3.4) | 1,207 (3.4) | 248 (3.7) | 66 (4.2) | 12 (3.4) |
| Missing | 17,418 (9.2) | 4,616 (11.5) | 819 (11.0) | 208 (11.7) | 51 (12.6) |
| Time since prior mammogram | |||||
| No prior | 3,120 (1.8) | 1,469 (4.0) | 183 (2.6) | 41 (2.5) | 13 (3.5) |
| 1 year | 2,497 (1.4) | 713 (2.0) | 145 (2.1) | 33 (2.0) | 10 (2.7) |
| 1–2 years | 147,089 (83.7) | 27,271 (74.6) | 5,707 (81.8) | 1,397 (83.9) | 272 (73.9) |
| 3 or more years | 22,932 (13.1) | 7,088 (19.4) | 944 (13.5) | 194 (11.7) | 73 (19.8) |
| Missing | 13,678 (7.2) | 3,740 (9.3) | 500 (6.7) | 108 (6.1) | 38 (9.4) |
Abbreviations: AJCC: American Joint Committee on Cancer, BCSC: Breast Cancer Surveillance Consortium, DCIS: ductal carcinoma in situ, FRI: function-related indicator, GED: General Educational Development, IQR: interquartile range, NCI: National Cancer Institute
Number of observations in the table represented individual women
Column percentages are shown
Denominators used for calculating percentages of non-missing categories were the sum of non-missing observations.
Alive without breast cancer—women who are alive at the end of follow-up who do not have a diagnosis of breast cancer during follow-up
Death from non-breast cancer causes—women without a diagnosis of breast cancer whose follow-up was terminated due to death
Invasive breast cancer/DCIS—women with a diagnosis of breast cancer during follow-up
Died of breast cancer—women who had a diagnosis of breast cancer and follow-up was terminated due to death caused by breast cancer.
Overall, age-adjusted 10-year cumulative incidence (Table 3) of invasive breast cancer (FRI=0: 4.0%, 95% CI=3.8%−4.1%; FRI=1: 3.9%, 95% CI=3.7%−4.2%; FRI≥2: 3.5%, 95% CI=3.1%−3.9%) and DCIS (FRI=0: 1.0%, 95% CI=0.9%−1.0%; FRI=1: 0.8%, 95% CI=0.7%−0.9%; FRI≥2: 0.8%, 95% CI=0.7%−1.0%) slightly decreased as FRI score increased. In the overall sample, risk decrease across FRI scores was significant for invasive breast cancer (p-trend<0.01) and DCIS (p-trend=0.01). Age-adjusted cumulative risk of death from non-breast cancer causes substantially increased as FRI score increased (FRI=0: 18.8%, 95% CI=18.5%−19.1%; FRI=1: 24.4%, 95% CI=23.9%−25.0%; FRI≥2: 39.8%, 95% CI=38.8%−40.9%). The cumulative incidence of breast cancer death was low, and women with FRI score≥2 (0.30%, 95% CI=0.18%−0.42%) had a slightly higher age-adjusted risk of breast cancer death compared to those with FRI score at 0 (0.24%, 95% CI=0.20%−0.28%), although risk difference between these two groups was minimal and largely non-significant. In subgroups stratified by age (66–74 vs. 75–94), risk of invasive breast cancer decreased as FRI score increased in both subgroups but statistical significance was only observed in those aged 75–94 years (p-trend<0.01). In both age groups, risk of DCIS decreased with minimal difference as FRI score increased (Table 3), although the trend test suggested statistical significance (p-trend=0.04) in the subgroup aged 66–74 years. Risk of non-breast cancer death increased by FRI in all age groups, and the relative increment (from FRI=0 to FRI≥2) was more substantial in younger women (percent change in risk of non-breast cancer death for 66–74: 157.3%, 75–84: 71.4%, 85–94: 30.0%) (Table3, Supplementary Table 2). Risk of death due to breast cancer was low in both subgroups and women with FRI≥2 had a slightly higher risk than those with FRI=0. Subgroup analysis by race suggested that risk of non-breast cancer death increased by FRI score (Supplementary Table 3) in both White (FRI=0: 18.1%, 95% CI=17.8%−18.5%; FRI=1: 23.5%, 95% CI=22.9%−24.1%; FRI=2: 38.2%, 95% CI=37.0%−39.4%) and Black women (FRI=0: 20.7%, 95% CI=19.4%−21.9%; FRI=1: 29.2%, 95% CI=26.6%−31.9%; FRI=2: 46.0%, 95% CI=41.0%−50.8%), but the estimated risk was higher in Black women.
Table 3.
10-year cumulative incidence of non-breast cancer death, invasive breast cancer, DCIS, and death due to breast cancer by FRI score and age at index screening
| Death from non-breast cancer causesc |
Invasive breast cancerc |
DCISc |
Death due to breast cancerd |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) |
|
| ||||||||
| FRI a | ||||||||
| 0 | 22,362 | 18.8 (18.5, 19.1) | 5,096 | 4.0 (3.8, 4.1) | 1,258 | 1.0 (0.9, 1.0) | 274 | 0.24 (0.20, 0.28) |
| 1 | 11,204 | 24.4 (23.9, 25.0) | 1,828 | 3.9 (3.7, 4.2) | 378 | 0.8 (0.7, 0.9) | 85 | 0.20 (0.14, 0.26) |
| ≥2 | 6,715 | 39.8 (38.8, 40.9) | 555 | 3.5 (3.1, 3.9) | 137 | 0.8 (0.7, 1.0) | 47 | 0.30 (0.18, 0.42) |
| Age at screening b | ||||||||
| 66–74 | 17,711 | 15.3 (14.9, 15.6) | 5,403 | 4.0 (3.8, 4.2) | 1,376 | 1.0 (0.9, 1.1) | 245 | 0.21 (0.17, 0.26) |
| 75–94 | 22,570 | 40.8 (40.2, 41.5) | 2,076 | 3.6 (3.4, 3.9) | 397 | 0.7 (0.6, 0.8) | 161 | 0.30 (0.23, 0.37) |
| Age 66–74 | ||||||||
| FRI=0 | 10,367 | 12.4 (12.2, 12.7) | 3,781 | 4.0 (3.9, 4.2) | 995 | 1.0 (1.0, 1.1) | 171 | 0.21 (0.18, 0.24) |
| FRI=1 | 4,729 | 17.0 (16.6, 17.5) | 1,273 | 4.0 (3.8, 4.3) | 292 | 0.9 (0.8, 1.0) | 52 | 0.19 (0.14, 0.25) |
| FRI≥2 | 2,615 | 31.9 (31.0, 32.9) | 349 | 3.7 (3.3, 4.1) | 89 | 0.9 (0.7, 1.1) | 22 | 0.27 (0.16, 0.39) |
| Age 75–94 | ||||||||
| FRI=0 | 11,995 | 36.5 (36.0, 37.0) | 1,315 | 3.8 (3.6, 4.0) | 263 | 0.7 (0.7, 0.8) | 103 | 0.31 (0.25, 0.37) |
| FRI=1 | 6,475 | 45.0 (44.2, 45.8) | 555 | 3.6 (3.3, 3.9) | 86 | 0.6 (0.4, 0.7) | 33 | 0.23 (0.15, 0.31) |
| FRI≥2 | 4,100 | 61.9 (60.9, 63.1) | 206 | 2.8 (2.5, 3.2) | 48 | 0.7 (0.5, 0.9) | 25 | 0.37 (0.24, 0.52) |
Abbreviations: DCIS: ductal carcinoma in situ, FRI: function-related indicator
Estimates for FRI were adjusted by distribution of age at screening
Estimates for age were adjusted by distribution of FRI
Results based on 6 models (one per age group and outcome); model includes FRI and treats the other 2 outcomes as competing events
Results based on 2 models (one per age group); model includes FRI and treats “death from non-breast cancer causes” as a competing event. In this model follow-up time is not terminated by a diagnosis of breast cancer and women who have breast cancer and die of other causes would be included in “death from non-breast cancer causes”.
Patterns of cumulative incidence of invasive breast cancer, DCIS, death from non-breast cancer causes, and breast cancer death in NCI comorbidity index-adjusted models (Table 4) were largely consistent with outcomes derived from age-adjusted models.
Table 4.
10-year cumulative incidence of non-breast cancer death, invasive breast cancer, DCIS, and death due to breast cancer by FRI score and comorbidity index at index screening
| Death from non-breast cancer causesc |
Invasive breast cancerc |
DCISc |
Death due to breast cancerd |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) | No. of events | Cumulative Incidence (95% CI) |
|
| ||||||||
| FRI a | ||||||||
| 0 | 22,362 | 20.6 (20.2, 21.0) | 5,096 | 3.9 (3.7, 4.1) | 1,258 | 0.9 (0.9, 1.0) | 274 | 0.24 (0.19, 0.29) |
| 1 | 11,204 | 25.8 (25.1, 26.5) | 1,828 | 3.9 (3.6, 4.2) | 378 | 0.8 (0.7, 0.9) | 85 | 0.20 (0.14, 0.27) |
| ≥2 | 6,715 | 37.7 (36.5, 38.9) | 555 | 3.4 (3.0, 3.9) | 137 | 0.9 (0.7, 1.1) | 47 | 0.27 (0.15, 0.41) |
| NCI index b | ||||||||
| 0 | 22,173 | 18.5 (18.1, 18.9) | 5,503 | 3.9 (3.8, 4.1) | 1,361 | 1.0 (0.9, 1.0) | 269 | 0.21 (0.17, 0.26) |
| 1 | 11,254 | 31.4 (30.6, 32.1) | 1,515 | 3.8 (3.5,4.1) | 319 | 0.8 (0.7, 0.9) | 102 | 0.30 (0.21, 0.39) |
| ≥2 | 6,854 | 49.4 (48.0, 50.9) | 461 | 3.3 (2.8, 3.8) | 93 | 0.7 (0.5, 1.0) | 35 | 0.24 (0.10, 0.40) |
| NCI index=0 | ||||||||
| FRI=0 | 14,073 | 15.9 (15.7, 16.2) | 3,955 | 4.0 (3.9, 4.1) | 1,010 | 1.0 (0.9, 1.1) | 198 | 0.22 (0.19, 0.25) |
| FRI=1 | 5,810 | 20.5 (20.0, 21.0) | 1,263 | 3.9 (3.7, 4.2) | 271 | 0.8 (0.7, 0.9) | 55 | 0.19 (0.14, 0.25) |
| FRI≥2 | 2,290 | 32.3 (31.2, 33.4) | 285 | 3.6 (3.2, 4.0) | 80 | 1.0 (0.8, 1.2) | 16 | 0.22 (0.13, 0.33) |
| NCI index=1 | ||||||||
| FRI=0 | 6,138 | 28.3 (27.7, 28.9) | 964 | 3.9 (3.7, 4.2) | 201 | 0.8 (0.7, 0.9) | 66 | 0.31 (0.24, 0.39) |
| FRI=1 | 3,291 | 34.5 (33.6, 35.5) | 404 | 3.8 (3.4, 4.1) | 83 | 0.8 (0.6, 0.9) | 20 | 0.21 (0.12, 0.31) |
| FRI≥2 | 1,825 | 46.0 (44.4, 47.4) | 147 | 3.2 (2.7, 3.8) | 35 | 0.8 (0.5, 1.0) | 16 | 0.41 (0.23, 0.62) |
| NCI index≥2 | ||||||||
| FRI=0 | 2,151 | 45.0 (43.6, 46.4) | 177 | 3.2 (2.7, 3.7) | 47 | 0.8 (0.6, 1.1) | <11 | 0.21 (0.08, 0.36) |
| FRI=1 | 2,103 | 54.4 (52.9, 56.0) | 161 | 3.7 (3.1, 4.3) | 24 | 0.5 (0.3, 0.8) | <11 | 0.26 (0.11, 0.43) |
| FRI≥2 | 2,600 | 68.7 (67.3, 70.2) | 123 | 2.9 (2.4, 3.4) | 22 | 0.5 (0.3, 0.7) | 15 | 0.40 (0.22, 0.61) |
Abbreviations: DCIS: ductal carcinoma in situ, FRI: function-related indicator, NCI: National Cancer Institute
Due to privacy concerns, we do not report Medicare data cell counts <11.
Estimates for FRI were adjusted by distribution of NCI comorbidity index
Estimates for NCI comorbidity index were adjusted by distribution of FRI
Results based on 9 models (one per NCI index category and outcome); model includes FRI and treats the other 2 outcomes as competing events
Results based on 3 models (one per NCI index category); model includes FRI and treats “death from non-breast cancer causes” as a competing event. In this model follow-up time is not terminated by a diagnosis of breast cancer and women who have breast cancer and die of other causes would be included in “death from non-breast cancer causes”.
Patterns of estimates for FRI were similar in subgroup analysis stratified by composite of age and NCI comorbidity index (Supplementary Table 4). In this set of analysis, risk of death from non-breast cancer causes significantly increased as FRI score became higher. Risk of breast cancer death increased substantially if people had older age, more comorbidities, and higher FRI scores simultaneously. Cumulative incidence of invasive breast cancer slightly decreased or remained unchanged as FRI score increased.
Discussion
We found that functional limitations were common among older women, with approximately one-third of our study population having some functional limitations. In this screening cohort, women with a higher burden of functional limitations had a significantly increased risk of death from non-breast cancer causes and the increment was more substantial for subgroups with younger ages. Compared to women with younger age and lower burdens of functional limitations, risk of incident invasive breast cancer was slightly lower in older women with higher burdens of functional limitations. While cumulative risk of breast cancer death increased slightly with increasing FRI, comorbidity, and age, the absolute risk differences were minimal and remained very low in each FRI, comorbidity, and age group.
Several population-based studies yielded similar outcomes as ours and suggested a positive association between functional limitations and mortality among older people with cancer diagnosis, although they had smaller sample sizes (25, 26). For example, Brown et al. (25) followed 428 cancer survivors aged ≥60 years for 11 years on average and measured 5 functional indexes reflecting mobility, frailty, and strengths of arms and legs. They reported that risk of all-cause mortality increased significantly when number of functional limitations increased; specifically, as compared to patients without functional limitations, there was a 35%−171% relative increase in risk of all-cause mortality among patients with 1–5 types of functional limitations. Another cohort study (26) followed 975 female breast cancer survivors (mean age=63 years) for 11 years on average and identified 753 deaths. This study used 10 items to reflect functional impairment and women were treated as having functional limitations if they had at least 1 item. Using multivariable analysis, they reported that women with functional limitations had a 47% relative increase in risk of non-breast cancer mortality. However, these previous studies included only cancer survivors as the study population and had small sample sizes, which could reduce statistical precision and generalizability. Our research used nationwide registry data and a more comprehensive measure of functional limitations, which greatly improves representativeness and better reflects risk variation by functional status in older women undergoing screening mammography.
In stratified analysis by age and NCI comorbidity index, we found that although risk of death from non-breast cancer causes increased with FRI score in all subgroups, the relative increase was larger among women with younger age (66–74 years) and lower NCI index score. This suggests that impact of functional limitations on non-breast cancer death is much stronger among younger or healthier women compared with their older or sicker counterparts in BCSC-Medicare data.
The underlying biological mechanisms that can explain why older women with functional limitations have a higher risk of death are not fully uncovered, but chronic inflammation may play an important role (26, 27). Several studies indicated a link between physical functional decline and chronic inflammation and suggested that functional limitations, comorbidities, and older age could synergistically increase levels of inflammatory biomarkers in the elderly and contribute to a shorter life expectancy (26–30). In addition, functional limitations usually represent illness severity and reflect lower physiologic reserve in multiple organ systems (31), suggesting that older people with a higher burden of functional limitations may have increased mortality due to aggressive, late-stage, or advanced diseases (32).
Interestingly, on the other hand, older women in our cohort with a higher FRI score tended to have a lower risk of breast cancer, especially invasive breast cancer. This is contrary to our presumption because poor functional status can be associated with a decline in immune surveillance among older people, and such decline may contribute to tumor promotion (33–36). Since Fine and Gray proportional subdistribution hazards models were used in this analysis, this result was not likely due to competing risks. One speculation is that older women who had an FRI score≥2 were less likely to undergo invasive medical procedures (e.g. breast biopsy and surgery) because these medical services could cause complications without bringing survival benefits for older women with functional limitations (37–39). Thus, physicians might not be able to obtain pathological evidence to confirm the existence of breast cancer among these women despite suspicious lesions on mammograms. In addition, inadequate utilization of mammography in functionally impaired older women (40), which has also been suggested by our data, may also partially explain the inverse relationship between FRI score and breast cancer risk.
Our research has strengths and limitations that should be considered. We used a robust and comprehensive measurement of functional limitations which have been validated and can predict mortality in older people (19). The BCSC mammography registries, which were linked to Medicare claims data, ensured a large sample of women seen in US clinical practice followed for incident breast cancer and death. Moreover, subgroup analysis by age and NCI comorbidity index allowed us to explore how cumulative incidence of screening outcomes varied by these 3 factors that are fundamental in gerontological research. However, limitations should be noted. First, our cohort was a screening population, and the percentage of non-White race/ethnicity groups (e.g. Black women) (41) was lower than that of the US as a whole, suggesting that generalizability might be compromised. Specifically, risk of breast cancer might be overestimated due to the high rate of screening in our cohort. Second, the number of women who were diagnosed with DCIS or died of breast cancer was low, which could introduce imprecision when estimating risk.
In this screening cohort, older women living with functional limitations had a significantly higher cumulative risk of death from causes other than breast cancer, whereas risk of breast cancer death appeared to be low regardless of functional status. Based on our results, we recommend that physicians consider functional limitations along with major comorbidities and age when offering breast cancer screening to older women even if they are just in early stage of late adulthood, and women should be notified that benefits of screening mammography may be diminished because of these unfavorable health conditions.
Supplementary Material
Acknowledgments:
The collection of cancer and vital status data was supported in part by several state public health departments and cancer registries in the US (https://www.bcsc-research.org/about/work-acknowledgement). The statements in this report, including findings and conclusions, are those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee, nor those of the National Cancer Institute or National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided. You can learn more about the BCSC at: http://www.bcscresearch.org/. This research was supported by grant 1R01CA207361-01A1 from the National Cancer Institute (Dr. Dejana Braithwaite). Data collection for this work was additionally supported by the Breast Cancer Surveillance Consortium with funding from the National Cancer Institute (P01CA154292, U54CA163303, R01CA149365) and the Patient-Centered Outcomes Research Institute (PCS-1504-30370).
Footnotes
Conflict of interest: None of the authors had conflict of interest
References
- 1.The U.S. Joins Other Countries With Large Aging Populations. https://www.census.gov/library/stories/2018/03/graying-america.html [Last accessed Sept 10th 2020].
- 2.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR: Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005–2012. Prev Chronic Dis 2016, 13:E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Population Ageing. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf [Last accessed Aug 10th 2020].
- 4.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA et al. : American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin 2016, 66(1):43–73. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html [Last accessed Apr 28 2020].
- 6.Kemp Jacobsen K, O’Meara ES, Key D, D SMB, Kerlikowske K, Vejborg I, Sprague BL, Lynge E, von Euler-Chelpin M: Comparing sensitivity and specificity of screening mammography in the United States and Denmark. Int J Cancer 2015, 137(9):2198–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns LE, Coleman DA, Swerdlow AJ, Moss SM: Effect of population breast screening on breast cancer mortality up to 2005 in England and Wales: an individual-level cohort study. Br J Cancer 2017, 116(2):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divo MJ, Martinez CH, Mannino DM: Ageing and the epidemiology of multimorbidity. Eur Respir J 2014, 44(4):1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter LC, Schonberg MA: Screening mammography in older women: a review. JAMA 2014, 311(13):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braithwaite D, Walter LC, Izano M, Kerlikowske K: Benefits and Harms of Screening Mammography by Comorbidity and Age: A Qualitative Synthesis of Observational Studies and Decision Analyses. J Gen Intern Med 2016, 31(5):561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings SR: Continuing screening mammography in women aged 70 to 79 years: impact on life expectancy and cost-effectiveness. JAMA 1999, 282(22):2156–2163. [DOI] [PubMed] [Google Scholar]
- 12.Demb J, Abraham L, Miglioretti DL, Sprague BL, O’Meara ES, Advani S, Henderson LM, Onega T, Buist DSM, Schousboe JT et al. : Screening mammography outcomes: risk of breast cancer and mortality by comorbidity score and age. J Natl Cancer Inst 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain AM, Rutten LJF, Jacobson DJ, Fan C, Wilson PM, Rocca WA, Roger VL, St Sauver JL: Multimorbidity, functional limitations, and outcomes: Interactions in a population-based cohort of older adults. J Comorb 2019, 9:2235042X19873486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisnado DM, Moore AA, Levin JR, Rosen S: Developing and testing a decision aid for use by providers in making recommendations: about mammography screening in older women. J Appl Gerontol 2015, 34(3):343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrischilles EA, Schneider KM, Schroeder MC, Letuchy E, Wallace RB, Robinson JG, Brooks JM: Association Between Preadmission Functional Status and Use and Effectiveness of Secondary Prevention Medications in Elderly Survivors of Acute Myocardial Infarction. J Am Geriatr Soc 2016, 64(3):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeler E, Guralnik JM, Tian H, Wallace RB, Reuben DB: The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci 2010, 65(7):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, Barlow WE, Geller BM, Kerlikowske K, Edwards BK et al. : Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol 1997, 169(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 18.Sprague BL, Arao RF, Miglioretti DL, Henderson LM, Buist DS, Onega T, Rauscher GH, Lee JM, Tosteson AN, Kerlikowske K et al. : National Performance Benchmarks for Modern Diagnostic Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017, 283(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrischilles E, Schneider K, Wilwert J, Lessman G, O’Donnell B, Gryzlak B, Wright K, Wallace R: Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care 2014, 52 Suppl 3:S75–84. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson BT, LoRusso AP, Smolkin M, Bovbjerg VE, Petroni GR, Harvey JA: Accuracy of assigned BI-RADS breast density category definitions. Acad Radiol 2006, 13(9):1143–1149. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL: Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000, 53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 22.NCI Comorbidity Index Overview. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html [Last accessed May 14th 2020].
- 23.JP F RJ G: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999, 94(446):496. [Google Scholar]
- 24.Hirschman J, Whitman S, Ansell D: The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control 2007, 18(3):323–333. [DOI] [PubMed] [Google Scholar]
- 25.Brown JC, Harhay MO, Harhay MN: Patient-reported versus objectively-measured physical function and mortality risk among cancer survivors. J Geriatr Oncol 2016, 7(2):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izano M, Satariano WA, Hiatt RA, Braithwaite D: The impact of functional limitations on long-term outcomes among African-American and white women with breast cancer: a cohort study. BMJ Open 2013, 3(10):e003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ: Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 2009, 64(4):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE: Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 2002, 50(4):638–644. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM: Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002, 50(12):1947–1954. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM: Predictors of interleukin-6 elevation in older adults. J Am Geriatr Soc 2009, 57(9):1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al. : Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001, 56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 32.Kono Y, Yamada S, Iwatsu K, Nitobe S, Tanaka Y, Shimizu Y, Shinoda N, Okumura T, Hirashiki A, Murohara T: Predictive value of functional limitation for disease severity in patients with mild chronic heart failure. J Cardiol 2012, 60(5):411–415. [DOI] [PubMed] [Google Scholar]
- 33.Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, Baehl S, Camous X, Witkowski JM, Larbi A: Frailty, Inflammation and Immunosenescence. Interdiscip Top Gerontol Geriatr 2015, 41:26–40. [DOI] [PubMed] [Google Scholar]
- 34.Malaguarnera L, Ferlito L, Di Mauro S, Imbesi RM, Scalia G, Malaguarnera M: Immunosenescence and cancer: a review. Arch Gerontol Geriatr 2001, 32(2):77–93. [DOI] [PubMed] [Google Scholar]
- 35.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS: The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 1997, 52(4):M201–208. [DOI] [PubMed] [Google Scholar]
- 36.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL: Physical activity and immune function in elderly women. Med Sci Sports Exerc 1993, 25(7):823–831. [DOI] [PubMed] [Google Scholar]
- 37.Rocco N, Rispoli C, Pagano G, Rengo G, Compagna R, Danzi M, Accurso A, Amato B: Breast cancer surgery in elderly patients: postoperative complications and survival. BMC Surg 2013, 13 Suppl 2:S25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Eamer G, Gibson JA, Gillis C, Hsu AT, Krawczyk M, MacDonald E, Whitlock R, Khadaroo RG: Surgical frailty assessment: a missed opportunity. BMC Anesthesiol 2017, 17(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Avenia S, Conti C, Barillaro I, Rondelli F, Bugiantella W, Avenia N: Breast cancer in older women: what factors affect the treatment? Int J Surg 2014, 12 Suppl 2:S177–S180. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Advani S, Zhu Z, Dang L, Walter LC, Braithwaite D: Mammography use in relation to comorbidities and functional limitations among older breast cancer survivors. J Cancer Surviv 2021, 15(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcadis AR, Davies L, Marti JL, Morris LGT: Racial Disparities in Cancer Presentation and Outcomes: The Contribution of Overdiagnosis. JNCI Cancer Spectr 2020, 4(2):pkaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


