Abstract
Background
Rifampin is generally advised in the treatment of acute staphylococcal periprosthetic joint infections (PJI). However, if, when, and how to use rifampin remains a matter of debate. We evaluated the outcome of patients treated with and without rifampin, and analyzed the influence of timing, dose and co-antibiotic.
Methods
Acute staphylococcal PJIs treated with surgical debridement between 1999 and 2017, and a minimal follow-up of 1 year were evaluated. Treatment failure was defined as the need for any further surgical procedure related to infection, PJI-related death or the need for suppressive antimicrobial treatment.
Results
A total of 669 patients were analyzed. Treatment failure was 32.2% (131/407) in patients treated with rifampin and 54.2% (142/262) in whom rifampin was withheld (P < .001). The most prominent effect of rifampin was observed in knees (treatment failure 28.6% versus 63.9%, respectively, P < .001). The use of rifampin was an independent predictor of treatment success in the multi-variate analysis (OR 0.30, 95% CI 0.20 – 0.45). In the rifampin group, the use of a co-antibiotic other than a fluoroquinolone or clindamycin (OR 10.1, 95% CI 5.65 – 18.2) and the start of rifampin within 5 days after surgical debridement (OR 1.96, 95% CI 1.08 – 3.65) were predictors of treatment failure. The dosing of rifampin had no effect on outcome.
Conclusions
Our data supports the use of rifampin in acute staphylococcal PJIs treated with surgical debridement, particularly in knees. Immediate start of rifampin after surgical debridement should probably be discouraged, but requires further investigation.
Keywords: periprosthetic joint infection, acute, staphylococci, rifampin, failure
This multicentre observational study consistently demonstrates the added value of a rifampin-based regimen in the treatment of acute staphylococcal infections. The greatest benefit was observed in knees, and a fluoroquinolone or clindamycin showed the highest success rate as co-antibiotic.
Acute periprosthetic joint infections (PJIs) are classically treated with Debridement, Antibiotics, Irrigation and Retention of the implant (DAIR) [1–3]. Antibiotics active against bacteria embedded in biofilm are the mainstay of the antibiotic treatment regimen. Since the randomized controlled trial performed in the “90 by Zimmerli et al [4], a fluoroquinolone combined with rifampin is considered the most effective antibiotic combination therapy for staphylococcal PJI, and its use has particularly been incorporated in many European hospitals [5]. However, despite the clear benefit of rifampin reported in the study of Zimmerli et al., its necessity has been questioned [6, 7]. The final number of patients included in the study of Zimmerli et al. was limited (i.e. 12 per treatment arm), and monotherapy with other antibiotics than ciprofloxacin has not been studied. Although many observational studies do demonstrate a higher failure rate in patients in whom rifampin was not prescribed [8–10], these results may be due to confounding by indication or survival bias. In addition, lack of efficacy may be due to inadequate dosing, inadequate timing, or the result of an interaction with the co-antibiotic administered. In this retrospective multicentre observational study, we aimed to answer the following questions:
i) Do patients treated with rifampin have a better clinical outcome compared to those in whom rifampin is withheld, taking into account confounding by indication and survival bias?
ii) For those patients treated with rifampin, which timing, dose and co-antibiotic is associated with the best clinical outcome?
MATERIAL AND METHODS
Patient Population
This retrospective study was performed using patient data from 6 different hospitals in 4 countries (Spain [1], Portugal [1], the USA [1] and the Netherlands [3]). All patients diagnosed with an acute PJI of the hip or knee between 1999 and 2017, treated with surgical debridement and implant retention (DAIR) and in which staphylococci were the main or one of the causative pathogens, were included. The 2018 International Consensus Meeting criteria were used to define PJI [11]. An early acute (post-surgical) PJI was defined as an infection occurring within 90 days after the index arthroplasty. A late acute PJI was defined as an acute onset of symptoms occurring >90 days after the index arthroplasty in a prior asymptomatic joint. A polymicrobial infection was defined as an infection where more than 2 different species were identified via MALDI-TOF. Differences in resistance patterns were not taken into account. Cases with a follow-up of less than one year and cases in whom incomplete data was available on the antibiotic regimen were excluded.
Surgical and Antibiotic Treatment
Surgical treatment involved a DAIR procedure in which an arthrotomy was performed, followed by synovectomy and irrigation with a minimum of 6 liters of fluids. Modular components were exchanged if possible. Multiple deep tissue cultures were obtained for culture. Intravenous antibiotics were started following local protocol and adjusted based on the susceptibility of microbiological cultures. Intravenous antibiotic treatment was switched to an oral regimen after a minimum of 14 days. The 5 European centres did routinely add rifampin to the antibiotic regimen, while the US centre did not. Rifampin was withheld in the European centers if the patient did have contra-indications to receive rifampin (e.g. significant drug interaction or allergy / intolerance) or if the staphylococci were rifampin resistant. The dose of rifampin was prescribed according to local protocol (either 450 mg BID or 600 mg QD), and was used during the total antibiotic treatment duration (3 to 6 months).
Outcome Parameters
The primary outcome parameter was treatment failure within 1 year after the DAIR procedure, defined as the need for any further surgical procedure related to infection (i.e. a second surgical debridement, implant removal or amputation), PJI-related death or the need for long-term suppressive antimicrobial treatment due to persistent clinical signs of infection. The secondary outcome parameter was clinical failure, which was defined as the need for implant removal during the follow-up period or PJI related death. The use of rifampin, the timing of rifampin, its dose and the co-antibiotic used in relation to treatment failure and clinical failure were studied in detail.
Statistical Analysis
Continuous variables were presented as mean and standard deviation (SD) or as median and interquartile range (IQR) when not normally distributed. A Chi-square test was used to analyse the difference between groups for categorical variables, and a student t-test (or Mann Witney U test when data was not normally distributed) for continuous variables. A Kaplan Meier survival curve with a cox-regression analysis was used to evaluate treatment failure and clinical failure in time. Logistic regression analysis was performed to identify independent risk factors for treatment failure. Variables with a difference between groups, defined as a P value < .1 in the univariate analysis were included in the multivariate analysis. Statistical significance was defined as a two-tailed P value < .05. Statistical analyses were performed using IBM SPSS Statistics (version 24.0; Chicago, IL, USA).
RESULTS
Patient Population
A total of 669 cases were included, entailing 617 early acute (post-operative) infections and 52 late acute infections. A total of 407 cases were treated with rifampin (61%) and in 262 cases rifampin was withheld (39%). Differences in baseline characteristics, clinical presentation, identified microorganisms and details of surgery known to be associated with failure [10, 12], are shown in Table 1. The percentage of cemented prostheses was higher in the rifampin group (77.3% versus 64.7%, P .01), and the percentage of late acute PJIs was higher in the no rifampin group (15.1% versus 3.2%, P < .001).
Table 1.
Baseline characteristics total cohort (n = 669)
| Total patient group (n= 669) | |||
|---|---|---|---|
| Rifampin (n = 407) | No rifampin (n = 262) | P value | |
| Baseline characteristics | |||
| Male sex | 43.5% (177/407) | 43.9% (115/262) | .92 |
| Age >80 years | 23.4% (95/406) | 18.3% (47/257) | .12 |
| BMI >30 kg/m2 | 48.1 % (177/368) | 55.6% (138/248) | .07 |
| Medical history | |||
| Diabetes | 20.6% (84/407) | 17.9% (47/262) | .39 |
| Renal failure | 6.9% (28/407) | 6.9% (18/262) | .99 |
| COPD | 18.4% (75/407) | 15.6% (41/262) | .35 |
| Liver cirrhosis | 3.7% (15/407) | 5.3% (14/262) | .30 |
| Malignancy | 14.3% (58/407) | 14.5% (38/262) | .93 |
| Rheumatoid arthritis | 7.4% (30/407) | 3.3% (22/262) | .63 |
| Characteristics implant | |||
| Primary | 83% (338/407) | 80.5% (206/256) | .40 |
| Cemented | 77.3% (310/401) | 64.7% (152/235) | .001 |
| Fracture as indication prosthesis | 15.5% (63/407) | 16.5% (42/254) | .72 |
| Clinical presentation | |||
| Serum CRP >115 mg/L | 31.1% (124/399) | 34.3% (87/254) | .40 |
| Serum Leucocytes >12 cells/µL | 28.5% (113/396) | 26.9% (60/223) | .66 |
| Late acute PJI | 3.2% (13/406) | 15.4% (39/253) | <.001 |
| Identified micro-organism | |||
| Staphylococcus aureus | 61.9% (252/407) | 56.9% (149/262) | .19 |
| Polymicrobial | 37.8% (154/407) | 37.8% (99/262) | .98 |
| Surgical treatment | |||
| Exchange modular components | 45.6% (182/399) | 45.2% (104/230) | .92 |
| DAIR >4 wks after surgerya | 18.6% (73/393) | 19.6% (42/214) | .75 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DAIR, debridement, antibiotics and implant retention; PJI, periprosthetic joint infections.
aFor early acute (post-operative) PJI.
Outcome Rifampin Versus No Rifampin
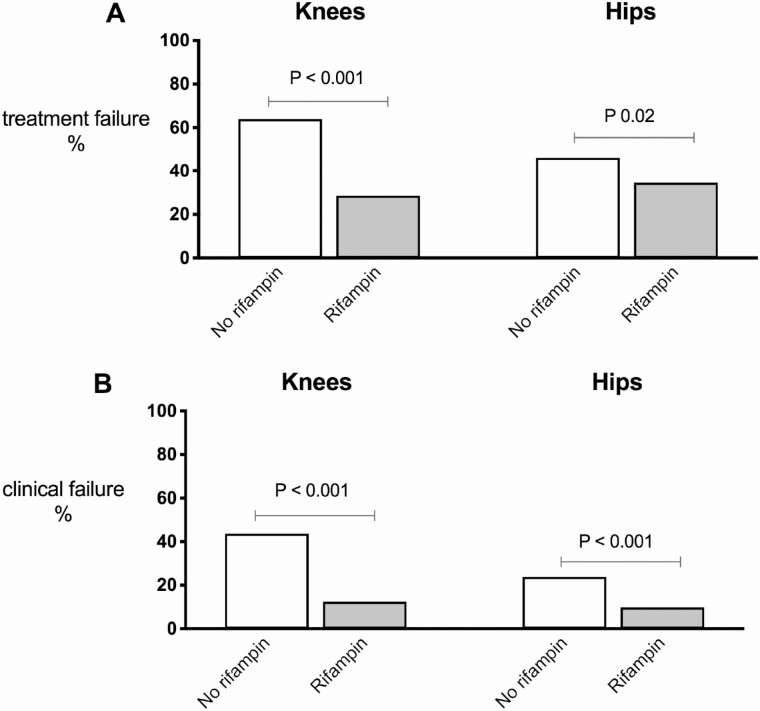
Treatment failure as primary outcome parameter was 40.8% (273/669) in the total cohort. Treatment failure was 32.2% (131/407) in patients treated with rifampin and 54.2% (142/262) in patients in whom rifampin was withheld (P < .001). The most prominent effect of rifampin was observed in knees: treatment failure was 28.6% in the rifampin group versus 63.9% in the no-rifampin group (P < .001). For hips, treatment failure was 34.6% versus 46.2%, respectively (P .02) (Figure 2A). The same effect was observed for clinical failure (Figure 2B). The difference in rifampin effect between both joints could not be explained by a different rate in exchange of mobile components (data not shown).
Figure 2.
Treatment failure (A) and clinical failure (B) rifampin versus no-rifampin according to the type of joint.
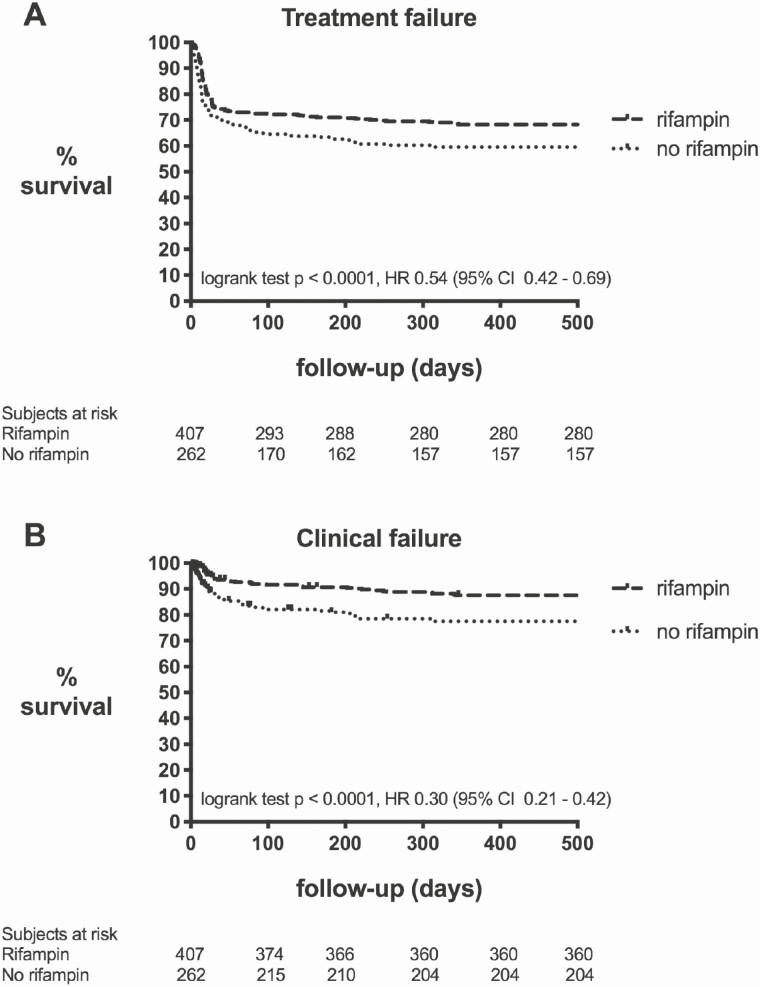
In both the rifampin as the no-rifampin group, most cases failed during antibiotic treatment: the median day of failure after surgical debridement was 17 days (IQR 13 – 28 days) in the rifampin group and 14 days (IQR 8 – 43 days) in the no-rifampin group (P .08) (Figure 1A). We excluded survival bias, by subanalyzing only those patients who “survived” the intravenous period and were switched to an oral regimen (n = 598). Treatment failure w(nas still significantly lower in the rifampin group compared to the no-rifampin group (31.9% versus 45.4%, P .001). In addition, we reduced confounding by indication by subanalyzing those patients who were not routinely treated with rifampin in one of the participating hospitals with those patients who received rifampin in the hospitals that do routinely treat with rifampin (thus, excluding the patients in these hospitals who did not receive rifampin for any reason). Again, treatment failure was still significantly lower in the rifampin group compared to the no-rifampin group (32.2% versus 61.3%, P < .001).
Figure 1.
Survival curve rifampin versus no-rifampin. Survival curve rifampin (n = 407) versus no-rifampin (n = 262) depicted according to treatment failure (A) and clinical failure (B) as defined in the material and method section.
Factors associated with treatment failure according to the uni- and multivariate analysis are shown in Table 2. The use of rifampin (OR 0.30) and infected primary arthroplasties (OR 0.59) were independent variables associated with treatment success. Risk factors for treatment failure were a serum CRP > 115 mg/L (OR 2.31), infections caused by Staphylococcus aureus (OR 1.88), cemented protheses (OR 1.69), male sex (OR 1.59), and a serum leucocyte count of >12 cells/µL at presentation (OR 1.55).
Table 2.
Risk factors for treatment failure total cohort (n = 669)
| Non-failures (n = 396) | Failures (n = 273) | P value | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Male sex | 39.4% (156/396) | 49.8% (136/273) | .01* | 1.59 (1.09 – 2.31) | .02 |
| Age >80 years | 18.4% (72/392) | 25.8% (70/271) | .02* | 1.47 (0.92 – 2.36) | .11 |
| BMI >30 kg/m2 | 51.2% (187/365) | 51.0% (128/251) | .95 | ||
| Medical history | |||||
| Diabetes | 18.7% (74/396) | 20.9% (57/273) | .48 | ||
| Renal failure | 6.3% (25/396) | 7.7% (21/273) | .48 | ||
| COPD | 17.4% (69/396) | 17.2% (47/273) | .94 | ||
| Liver cirrhosis | 3.8% (15/396) | 5.1% (14/273) | .40 | ||
| Malignancy | 15.2% (60/396) | 13.2% (36/273) | .48 | ||
| Rheumatoid arthritis | 6.8% (27/396) | 9.2% (25/273) | .27 | ||
| Characteristics implant | |||||
| Knee | 39.9% (158/396) | 44.7% (122/274) | .22 | ||
| Primary | 84.9% (333/392) | 77.9 (211/271) | .02* | 0.59 (0.36 – 0.95) | .03 |
| Cemented | 70.1% (262/374) | 76.3% (200/262) | .08* | 1.69 (1.09 – 2.63) | .02 |
| Fracture | 13.5% (53/392) | 19.3% (52/269) | .05* | 1.40 (0.84 – 2.33) | .20 |
| Clinical presentation | |||||
| CRP >115 mg/L | 22.0% (85/386) | 47.2% (126/267) | <.001* | 2.31 (1.53 – 3.49) | <.001 |
| Leucocytes >12 cells/uL | 21.4% (79/369) | 37.6% (94/250) | <.001* | 1.55 (1.02 – 2.35) | .04 |
| Late acute PJI | 5.3% (21/393) | 11.7% (31/266) | <.001* | 1.79 (0.72 – 4.40) | .21 |
| Identified micro-organism | |||||
| Staphylococcus aureus | 53.5% (212/396) | 69.2% (189/273) | <.001* | 1.88 (1.26 – 2.79) | .002 |
| Polymicrobial | 40.4% (160/396) | 34.1% (93/273) | .09* | 0.87 (0.60 – 1.28) | .49 |
| Surgical treatment | |||||
| Exchange modular components | 46.0% (171/372) | 44.7% (115/257) | .76 | ||
| DAIR >4 wks after surgerya | 20.7% (77/372) | 16.2% (38/235) | .17 | ||
| Antibiotic treatment | |||||
| Rifampin used | 69.7% (276/396) | 48.0% (131/273) | <.001* | 0.30 (0.20 – 0.45) | <.001 |
*Variables with a P value <.1 were included in the multivariate binary logistic regression analysis.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DAIR, debridement, antibiotics, and implant retention; PJI, periprosthetic joint infections.
aFor early acute (post-operative) PJI.
Clinical failure as secondary outcome parameter was 10.8% (44/407) in the rifampin group, and 32.8% (86/262) in the no rifampin group (P < .001) (Figure 1B).
Outcome Rifampin Based Regimen According to Timing, Dose, and Co-Antibiotic
A subanalysis was performed to determine whether the timing of rifampin, the dose and the co-antibiotic administered with rifampin were associated with treatment failure.
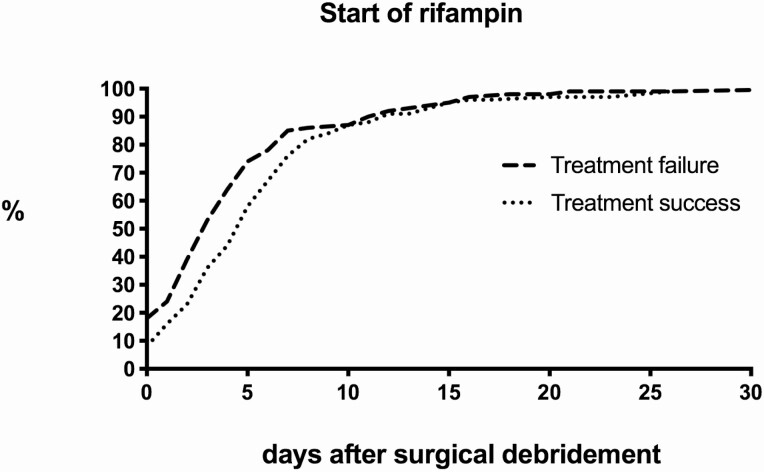
i) Timing. There was a wide range in the timing when rifampin was started (Figure 3). When rifampin was started within 5 days after surgical debridement, treatment failure was observed in 40.8% (80/196) of cases. Interestingly, treatment failure was significantly lower when rifampin was started after this time point: 20.9% (28/134) when started between day 5 and 9, and 21.4 % (17/58) when started after 10 days (P .001). Subanalyses demonstrated that patients who received rifampin within 5 days after surgical debridement had a significantly higher rate of S. aureus infections (74% versus 51%, P < .001) and mobile components were less often exchanged (32% versus 59%, P < .001) compared to patients in whom rifampin was started after this time point. In addition, for early acute (post-surgical) infections, surgical debridement performed more than 4 weeks after arthroplasty was more commonly observed in patients in whom rifampin was started within 5 days (24% versus 13%, P .005). Despite these differences, the timing of rifampin remained a significant predictor for treatment failure in the multivariate analysis (Table 3).
ii) Dose. In 39.8% (162/407) of cases, a rifampin dose of 600 mg QD was prescribed, in 55.8% (227/407) a dose of 450 mg BID and in 0.5% (2/407) a dose of 600 mg BID. In the univariate analysis, a daily dose of more than 600 mg was associated with treatment failure, but this association disappeared in the multivariate analysis (Table 3). We additionally analyzed whether the BMI/mg rifampin ratio affected outcome. The mean ratio was 27 (7.5 SD, range 12 – 55), but the ratio was not associated with treatment failure (Table 3). Since rifampin may reduce serum levels of multiple antibiotics [13], a subanalysis was performed for each oral co-antibiotic used, but in none of the antibiotic regimens, a higher dose of rifampin was associated with a higher rate of treatment failure (data not shown).
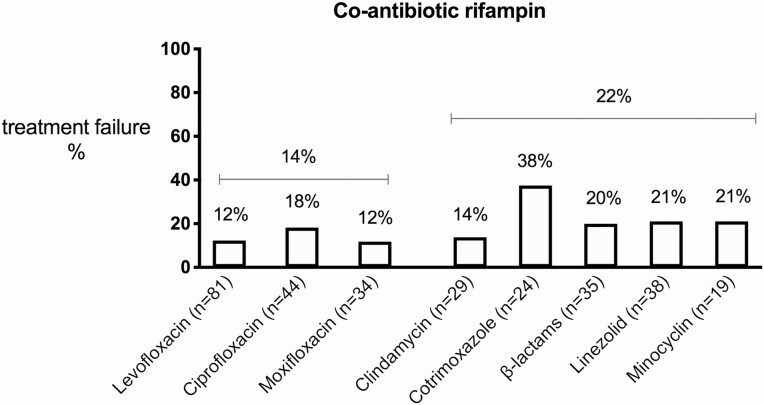
iii) Co-antibiotic. Fluoroquinolones were prescribed as co-antibiotic of rifampin in 46.9% (191/407) of cases, these included: levofloxacin (47.1%), ciprofloxacin (29.3%) and moxifloxacin (20.4%). In 3.1% of cases multiple fluoroquinolones were used, most likely due to an antibiotic switch during therapy. Treatment failure did not differ between the types of fluoroquinolones used, but a trend was observed in favour of levofloxacin and moxifloxacin (Figure 4). Other oral antibiotics combined with rifampin were β-lactams (13.1%), linezolid (11.3%), clindamycin (9.6%), cotrimoxazole (6.6%) and minocyclin (5.7%). When excluding patients treated with triple therapy, the co-antibiotic of rifampin associated with the highest treatment failure was cotrimoxazole (38%), and the co-antibiotic with the lowest treatment failure clindamycin (14%) (Figure 3).
Figure 3.
Timing of rifampin after surgical debridement.
Table 3.
Risk factors for treatment failure rifampin cohort (n = 407)
| Non-failures (n = 276) | Failures (n = 131) | P value | Adjusted OR (95% CI)a | P value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Male sex | 40.2%(111/276) | 50.4% (66/131) | .05* | 2.07 (1.19 – 3.58) | .009 |
| Age >80 years | 21.1% (58/275) | 28.2% (37/131) | .11 | ||
| BMI >30 kg/m2 | 48.8% (122/250) | 46.6% (55/118) | .70 | ||
| Medical history | |||||
| Diabetes | 17.8% (49/276) | 26.7% (35/131) | .04* | 2.16 (1.12 – 4.15) | .022 |
| Renal failure | 6.2% (17.276) | 8.4% (11/131) | .41 | ||
| COPD | 17.8% (49/276) | 19.8% (26/131) | .61 | ||
| Liver cirrhosis | 3.6% (10/276) | 3.8% (5/131) | .92 | ||
| Malignancy | 13.8% (38/276) | 15.3% (20/131) | .67 | ||
| Rheumatoid arthritis | 6.9% (19/276) | 8.4% (11/131) | .59 | ||
| Characteristics implant | |||||
| Knee | 41.7% (115/276) | 35.1% (46/131) | .21 | ||
| Primary | 85.1% (235/276) | 78.6% (103/131) | .10 | ||
| Cemented | 75.4% (205/272) | 81.4% (105/129) | .18 | ||
| Fracture | 13.4% (37/276) | 19.8% (26/131) | .09* | 1.40 (0.68 – 2.91) | .36 |
| Clinical presentation | |||||
| CRP >115 mg/L | 23.3% (63/270) | 47.3% (61/129) | <.001* | 1.54 (0.85 – 2.79) | .16 |
| Leucocytes >12 cells/µL | 21.1% (57/270) | 44.4% (56/126) | <.001* | 2.79 (1.48 – 5.27) | .002 |
| Late acute PJI | 2.2% (6/276) | 5.4% (7/130) | .09 | ||
| Identified micro-organism | |||||
| Staphylococcus aureus | 57.2% (158/276) | 71.8% (94/131) | .01* | 1.63 (0.89– 2.97) | .11 |
| Polymicrobial | 37.3 (103/276) | 38.9% (51/131) | .75 | ||
| Surgical treatment | |||||
| Exchange modular components | 48.3% (131/271) | 39.8% (51/128) | .11 | ||
| Antibiotic treatment | |||||
| Co-antibiotic other than a fluoroquinolone or clindamycin | 30.4% (84/276) | 78.6% (103/131) | <.001* | 10.1 (5.65 – 18.2) | <.001 |
| Rifampin dose >600 mg/24h | 52.1% (139/267) | 72.6% (90/124) | <.001* | 1.23 (0.65 – 2.32) | .52 |
| BMI/mg rifampin ratio >30 | 87.6% (242/276) | 81.6% (107/131) | .12 | ||
| Start rifampin <5 days after surgical debridement | 44.1% (116/263) | 64.0% (80/125) | <.001* | 1.96 (1.08 – 3.56) | .03 |
* Variables with a P value <.1 were included in the multivariate binary logistic regression analysis.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DAIR, debridement, antibiotics, and implant retention; PJI, periprosthetic joint infections. a For early acute (post-operative) PJI.
Figure 4.
Treatment failure according to the co-antibiotic administered with rifampin.
Table 3 shows the results of the uni- and multivariate analysis for all patients treated with rifampin. The strongest factor independently associated with treatment failure was the use of a co-antibiotic other than a fluoroquinolone or clindamycin (OR 10.1 [95% CI 5.56 – 18.2], P < .001). Other factors associated with treatment failure in the multivariate analysis were: male sex, diabetes, serum leucocytes >12 cells/µL and the start of rifampin within 5 days after surgical debridement. A daily rifampin dose of more than 600 mg was not associated with treatment failure in the multi-variate analysis.
DISCUSSION
The efficacy of rifampin in acute staphylococcal PJI has been recently questioned [6], and in addition, the best timing to start rifampin, which dose to use, and the optimal co-antibiotic to prescribe, especially when a fluoroquinolone is not an option, is unclear. In this large retrospective multicentre study, including 669 patients with acute staphylococcal PJIs that were managed with surgical debridement, we demonstrated a clear benefit of a rifampin based regimen, even after correcting for potential sources of bias and confounding. The most prominent effect of rifampin was observed in knees. Rifampin started within 5 days after surgical debridement was associated with a worse outcome, and a consistent superiority of the use of a fluoroquinolone was observed as co-antibiotic next to rifampin, but a clindamycin based regimen showed similar efficacy. We did not find an association between the rifampin dose and treatment failure.
i) Should rifampin be used? In a recent randomized controlled trial conducted in Norway, Karlsen et al. questioned the added value of rifampin in acute staphylococcal PJIs [6]. With a follow-up period of 2 years, the authors demonstrated a remission rate of 72% (18/25) in patients treated with monotherapy without rifampin and 74% (17/23) in patients treated with duo therapy with rifampin. Unfortunately, the study was underpowered to draw definitive conclusions, and in addition, rifampin was not combined with a fluoroquinolone or other agents active against biofilms, nor with antibiotics with adequate oral availability and bone penetration. The clear benefit of rifampin demonstrated in observational studies has been criticized because of confounding by indication and survival bias [8, 14, 15]. However, in our study, subanalyses in which confounding and bias were reduced to a minimum did not change the results, and consistently supported the use of rifampin in acute staphylococcal PJIs managed with DAIR. These results were most prominent in patients with a PJI of the knee.
ii) What should be the timing to start rifampin? Our analysis demonstrated an association between the early start of rifampin (i.e. within 5 days after surgical debridement) and treatment failure. Although this observation may be partially explained by the fact that patients who received rifampin within this time period entailed patients with more severe infections (e.g. infection caused by S. aureus), this association remained significant in the multivariate analysis. To avoid the induction of rifampin resistance, it has been proposed by experts in the field [16–18], to wait for the start of rifampin after the wound is dry, drains have been removed, and at least 3–5 days of intravenous antibiotics have been given to secure adequate load reduction of bacteria. Therefore, early start may, theoretically, result in a higher failure rate. Unfortunately, we did not collect data on the development of rifampin resistant strains during follow-up. A previous study did not report the development of rifampin resistant strains in a cohort where rifampin was started immediately after surgical debridement [19]. A possible explanation for the higher failure rate could be that rifampin works antagonistically with the co-antibiotic administered when bacteria are still in the planktonic phase [20], but future studies are necessary to draw definite conclusions on this matter.
iii) What dose should be administered? Different practices on rifampin dose exist in hospitals. Prescribing an insufficient low dose would theoretically lead to treatment failure, while prescribing a dose above the necessary threshold may result in more significant interactions with certain co-antibiotics, and therefore may lead to treatment failure. Although our analysis showed an association with treatment failure when a dose of >600 mg was prescribed, this association disappeared in the multivariate analysis. These data are in line with the study performed by Nguyen et al., demonstrating in 154 analysed cases no influence of dosing (≤ 600 mg to ≥ 1200 mg) when levofloxacin was used as co-antibiotic.[21] A recently published study of Tonnelier et al. showed similar results [22]. In our subanalysis of other co-antibiotics, the prescribed dose did not seem to have a substantial effect on treatment outcome either, suggesting that the enzyme induction of CYP3A4 by rifampin is not dose dependent.
iv) What co-antibiotic should be prescribed? Our results confirm the superiority of the use of fluoroquinolones, in which a trend was observed in favour of levofloxacin and moxifloxacin compared to ciprofloxacin., andclindamycin. The latter finding is somewhat surprising, as rifampin lowers the serum concentration of clindamycin [23], and this has been shown in a previous analysis of Tornero et al. to be associated with a worse outcome [13]. However, in the study of Tornerno et al. a clindamycin dose of 300mg TID was used, and demonstrates that rifampin induction becomes clinically relevant when using low doses of the co-antibiotic prescribed. Indeed, a previous report, demonstrated a good clinical outcome when a clindamycin dose of 600mg TID is prescribed [24, 25]. The reduction of serum levels of antibiotics induced by rifampin is not always clinically relevant and depends on the intrinsic activity of the antibiotic, the degree of reduction and the dosage of the co-antibiotic administered. This is supported by the high success rate of moxifloxacin combination therapy [26], an antibiotic that is also affected by rifampin [27, 28]. In concordance with the study of Tornero et al., cotrimoxazole showed the highest treatment failure in our analysis.
Our study has some limitations. First, we only analysed treatment failure and clinical failure, but did not collect data on microbiological failure or on the development of resistance. Second, with a minimum follow-up of 1 year, infection relapses with coagulase negative staphylococci may be missed. Third, since our study is observational, causality cannot be inferred and residual confounding may be present. However, the use of multivariable regression techniques and subanalyses consistently showed identical results.
In conclusion, even after minimizing potential confounding and bias, our data confirms the efficacy of rifampin in acute staphylococcal PJIs treated with surgical debridement, especially in prosthetic knees. Next to a fluoroquinolone, clindamycin (600 mg TID) is a good alternative to use as co-antibiotic. Our data indicates, that the early start of rifampin (i.e. within 5 days) should be avoided, but these findings need to be confirmed in future analyses.
Note
Potential conflicts of interest. A. S. reports grants and personal fees from PFIZER, personal fees from MSD, personal fees from SHIONOGY, personal fees from MENARINI, personal fees from ANGELINI, personal fees from GILEAD, during the conduct of the study. J. P. reports board membership with Eastern Orthopaedic Association, 3M, Muller Foundation, and United Healthcare; J. P. received personal fees for consultancy for Zimmer Biomet, Stryker, TissueGene, CeramTec, Corentec, Ethicon, Tenor, KCI, and Heraus; J. P. reports royalties paid to them by DataTrace, Elsevier, Jaypee, SLACK Incorporated, Wolters Kluwer, and Corentec; J. P. reports holding stock/stock options with Parvizi Surgical Innovations, Hip Innovation Technology, Cross Current Business Intelligence, Alphaeon, Joint Purification Systems, Ceribell, MedAp, Physician Recommended Nutriceuticals, PRN-Veterinary, MDValuate, Intellijoint, MicroGenDx, and Corentec, all outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Mark Beldman, Department of Orthopaedic Surgery, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Claudia Löwik, Department of Orthopaedic Surgery, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Alex Soriano, Department of Infectious Diseases, University of Barcelona, IDIBAPS, Hospital Clinic of Barcelona, Barcelona, Spain.
Laila Albiach, Department of Infectious Diseases, University of Barcelona, IDIBAPS, Hospital Clinic of Barcelona, Barcelona, Spain.
Wierd P Zijlstra, Department of Orthopaedic Surgery, Medical Center Leeuwarden , Leeuwarden, The Netherlands.
Bas A S Knobben, Department of Orthopaedic Surgery, Martini Hospital, Groningen, The Netherlands.
Paul Jutte, Department of Orthopaedic Surgery, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Ricardo Sousa, Department of Orthopaedic Surgery, Centro Hospitalar do Porto, Porto, Portugal.
André Carvalho, Department of Orthopaedic Surgery, Centro Hospitalar do Porto, Porto, Portugal.
Karan Goswami, Department of Orthopaedic Surgery, Rothman Institute at Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA.
Javad Parvizi, Department of Orthopaedic Surgery, Rothman Institute at Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA.
Katherine A Belden, Department of Infectious Diseases, Sydney Kimmel Medical College at Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA.
Marjan Wouthuyzen-Bakker, Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
References
- 1. Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 2013; 95-B:1450–2. [DOI] [PubMed] [Google Scholar]
- 2. Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 3. Lora-Tamayo J, Murillo O, Iribarren JA, et al. ; REIPI Group for the Study of Prosthetic Infection . A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56:182–94. [DOI] [PubMed] [Google Scholar]
- 4. Widmer AF, Frei R, Rajacic Z, Zimmerli W. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis 1990; 162:96–102. [DOI] [PubMed] [Google Scholar]
- 5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 6. Karlsen ØE, Borgen P, Bragnes B, et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: a randomized controlled trial. J Orthop Surg Res 2020; 15:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aydın O, Ergen P, Ozturan B, Ozkan K, Arslan F, Vahaboglu H. Rifampin-accompanied antibiotic regimens in the treatment of prosthetic joint infections: a frequentist and Bayesian meta-analysis of current evidence. Eur J Clin Microbiol Infect Dis 2021; 40:665–71. [DOI] [PubMed] [Google Scholar]
- 8. Becker A, Kreitmann L, Triffaut-Fillit C, et al. Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Jt Infect 2020; 5:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiaux E, Titecat M, Robineau O, et al. ; G4 bone and joint infection study group (G4BJIS) . Outcome of patients with streptococcal prosthetic joint infections with special reference to rifampicin combinations. BMC Infect Dis 2016; 16:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tornero E, Martínez-Pastor JC, Bori G, et al. Risk factors for failure in early prosthetic joint infection treated with debridement. Influence of etiology and antibiotic treatment. J Appl Biomater Funct Mater 2014; 12:129–34. [DOI] [PubMed] [Google Scholar]
- 11. Elkins JM, Kates S, Lange J, et al. General Assembly, Diagnosis, Definitions: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019; 34:181–5. [DOI] [PubMed] [Google Scholar]
- 12. Löwik CAM, Jutte PC, Tornero E, et al. ; Northern Infection Network Joint Arthroplasty (NINJA) . Predicting Failure in Early Acute Prosthetic Joint Infection Treated With Debridement, Antibiotics, and Implant Retention: External Validation of the KLIC Score. J Arthroplasty 2018; 33:2582–7. [DOI] [PubMed] [Google Scholar]
- 13. Tornero E, Morata L, Martínez-Pastor JC, et al. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J Antimicrob Chemother 2016; 71:1395–401. [DOI] [PubMed] [Google Scholar]
- 14. Scheper H, de Boer MGJ. Comment on “Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France” by Becker et al. (2020). J Bone Jt Infect 2020; 6:17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker A, Kreitmann L, Ferry T. Reply to Scheper and de Boer’s comment on “Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France” by Becker et al. (2020). J Bone Jt Infect 2020; 6:19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sendi P, Zimmerli W. The use of rifampin in staphylococcal orthopaedic-device-related infections. Clin Microbiol Infect 2017; 23:349–50. [DOI] [PubMed] [Google Scholar]
- 17. Achermann Y, Eigenmann K, Ledergerber B, et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): A matched case-control study. Infection 2013; 41:431–7. DOI: 10.1007/s15010-012-0325-7. [DOI] [PubMed] [Google Scholar]
- 18. Svensson E, Hanberger H, Nilsson M, Nilsson LE. Factors affecting development of rifampicin resistance in biofilm-producing Staphylococcus epidermidis. J Antimicrob Chemother 1997; 39:817–20. [DOI] [PubMed] [Google Scholar]
- 19. Scheper H, van Hooven D, van de Sande M, et al. Outcome of acute staphylococcal prosthetic joint infection treated with debridement, implant retention and antimicrobial treatment with short duration of rifampicin. J Infect 2018; 76:498–500. [DOI] [PubMed] [Google Scholar]
- 20. Riedel DJ, Weekes E, Forrest GN. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob Agents Chemother 2008; 52:2463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen S, Robineau O, Titecat M, et al. Influence of daily dosage and frequency of administration of rifampicin–levofloxacin therapy on tolerance and effectiveness in 154 patients treated for prosthetic joint infections. European Journal of Clinical Microbiology and Infectious Diseases 2015; 34:1675–82. DOI: 10.1007/s10096-015-2404-z. [DOI] [PubMed] [Google Scholar]
- 22. Tonnelier M, Bouras A, Joseph C, et al. Impact of rifampicin dose in bone and joint prosthetic device infections due to Staphylococcus spp: a retrospective single-center study in France. BMC Infect Dis 2021; 21:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard A, Kermarrec G, Parize P, et al. Dramatic reduction of clindamycin serum concentration in staphylococcal osteoarticular infection patients treated with the oral clindamycin-rifampicin combination. J Infect 2015; 71:200–6. [DOI] [PubMed] [Google Scholar]
- 24. Leijtens B, Elbers JBW, Sturm PD, Kullberg BJ, Schreurs BW. Clindamycin-rifampin combination therapy for staphylococcal periprosthetic joint infections: a retrospective observational study. BMC Infect Dis 2017; 17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czekaj J, Dinh A, Moldovan A, et al. Efficacy of a combined oral clindamycin?rifampicin regimen for therapy of staphylococcal osteoarticular infections. Scand J Infect Dis 2011; 43:962–7. [DOI] [PubMed] [Google Scholar]
- 26. Wouthuyzen-Bakker M, Tornero E, Morata L, et al. Moxifloxacin plus rifampin as an alternative for levofloxacin plus rifampin in the treatment of a prosthetic joint infection with Staphylococcus aureus. Int J Antimicrob Agents 2018; 51:38–42. [DOI] [PubMed] [Google Scholar]
- 27. Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007; 45:1001–7. [DOI] [PubMed] [Google Scholar]
- 28. Ramachandran G, Hemanth Kumar AK, Srinivasan R, et al. Effect of rifampicin & isoniazid on the steady state pharmacokinetics of moxifoxacin. Indian Journal of Medical Research 2012; 136:979–84. [PMC free article] [PubMed] [Google Scholar]