See article by Schneider et al. pp 1885–1897.
In science, technology, and in private life, we are used to the concept of network resilience. Integration into networks, and communication within these networks makes one stronger, smarter, more efficient, and more resistant to outside harm. Unfortunately, malignant cells of incurable brain tumors appear to do just the same. First described in 2015,1 the discovery of connected, communicating, and resistant brain tumor networks has enriched our fundamental understanding of neuro-oncological diseases—and also showed ways how we might better treat them in the future.2
In this issue of Neuro-Oncology, Schneider et al3 extended the current knowledge about tumor cell networks in glioma in multiple ways. They started with a morphological analysis of tumor microtubes (TMs), the thin and long membrane protrusions formed by glioblastoma cells that make contact with other glioblastoma cells, and that are the anatomical backbone of the tumor cell network1 (Figure 1). They found that different patient-derived glioblastoma cell lines which reflect different gene expression subtypes of the disease vary significantly in their ability to form TM networks in vitro. An association between high-connected cell lines and the expression of developmental programs was found. This is in line with the known molecular machinery driving TMs, prominently the neuronal growth-associated protein 43 (GAP-43),1 and Ttyh1 for invasive TMs,4 another neurodevelopmental factor identified so far. Furthermore, the previous work has identified that an intact 1p/19q status is a prerequisite for TM network formation,1 making oligodendrogliomas deficient of various neurodevelopmental factors including GAP-43 and Ttyh1 which are crucial for the extension of functional neurites, but also classical neurotrophins and axon guidance molecules. The study of Schneider et al adds to this knowledge by providing first evidence that molecular subgroups of glioblastomas can differ in the extent of TM network formation, too, and that this is linked to differential expression of neurodevelopmental factors. In line, the authors demonstrate that various axon guidance molecules, ephrins and semaphorins, SLIT, and Netrin, are higher expressed in glioblastoma subgroups with more neurodevelopmental cellular states.
Fig. 1.
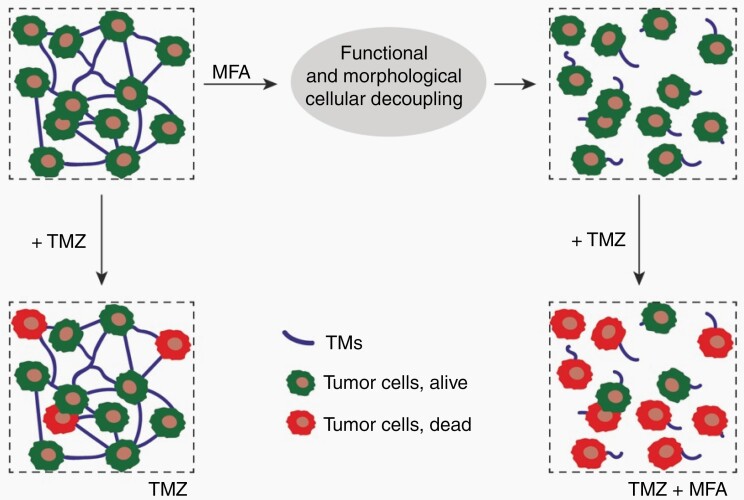
Pharmacological inhibition of glioblastoma networks for improved anticancer therapies. Functional and morphological disconnection of glioblastoma cell networks by the gap junction inhibitor meclofenamate (MFA) can make temozolomide (TMZ) chemotherapy more effective. Tumor microtubes (TMs) that make the network are less functional and also relevantly reduced with MFA treatment. Graphical summary of Schneider et al3.
Next, the authors tested whether meclofenamate (MFA) can silence the glioblastoma cell networks in their models. MFA is a potent gap junction inhibitor that has shown to block the Connexin 43 jap junctions that are located within and at TMs.5,6 Consequently, MFA inhibits the TM network-mediated intercellular communication with calcium waves, finally leading to reduced glioma cell proliferation in vivo.5 In vitro, another Connexin 43 inhibitor even made TMZ-resistant glioblastoma cells sensitive to TMZ.7 Similarly, Schneider et al show that donor cells cannot pass a gap junction-permeable dye efficiently to receiver cells in glioblastoma cell network cultures while under MFA treatment. Furthermore, synchronized calcium activity and overall network functionality in glioblastoma cell cultures were greatly reduced.
Interestingly, the functional disconnection of glioblastoma cell networks with MFA had distinct molecular effects in the cancer cells. The authors compared MFA effects on 3 patient-derived glioblastoma cell lines, and found 163 common genes differentially regulated. Two pathways involved in neurodevelopment and cell-cell connections, prominently NCAM and NETRIN, were downregulated when functional networks were compromised by MFA. This is in line with a previous report demonstrating that interference with intercellular connectivity and communication in glioma cell networks can lead to a similar downregulation of neurogenesis factors.8 The study of Schneider et al demonstrates for the first time that the functional disconnection of glioblastoma cells with a drug can achieve just that: “reprogram” the malignant cells towards a less connectivity-proficient cellular state. Indeed, when followed over a week, cultured glioblastoma cells became less interconnected with TMs when treated with MFA, with TM length significantly reduced over time.
Finally, in light of the previous data demonstrating that TM network integration is key for glioblastoma cell resistance against radiotherapy,1 and also TMZ chemotherapy and surgical lesions,9 the authors tested whether pharmacological network disconnection with MFA makes glioblastoma cells more sensitive towards the cytotoxic effects of TMZ. Indeed, co-treatment with MFA resulted in the failure of glioblastoma cells to strongly upregulate genes involved in DNA repair under TMZ exposure, which was associated with an increased cytotoxicity of TMZ, and a trend towards better survival in tumor-bearing mice. In a brain slice model, TMZ and MFA co-treatment significantly reduced TM length and ultimately tumor volume, when compared with TMZ treatment alone. Together this supports the concept that network integration increases glioblastoma cell resilience by improved cellular homeostasis, like that of intracellular calcium.1 Consequently, disturbance of the ability of glioblastoma cells to integrate in TM-connected networks makes radiotherapy and TMZ chemotherapy more effective.1,9 While network disconnection was achieved genetically in the past (by eg, interfering with GAP-43), the current study now convincingly demonstrates this for the drug MFA.
The study of Schneider et al provides another piece in the puzzle of how TM-connected brain tumor networks function, and how they can be pharmacologically silenced. Most importantly, the study shows a clear road for the introduction of TM network targeting therapies into clinical concepts that aim to overcome the notorious treatment resistance of glioblastomas (Figure 1). Accordingly, the gap junction inhibitor MFA which is an FDA-approved drug (for its NSAID actions against pain, fever, and inflammation), and considerably well tolerated by patients, is currently tested in the German MecMeth trial (EudraCT2021-000708-39) in recurrent glioblastoma in combination with TMZ. It will be exciting to learn whether sufficient drug levels will be reached in resected glioblastoma tissue in the phase I part of the trial, and whether first evidence for biological and clinical activity will be seen in phase I/II. Nevertheless, questions remain. One fundamental challenge is the pharmacological targeting of gap junctions, an inter-cellular communication system that is important for many normal cells in the human body, including normal brain astrocytes. The considerably good tolerability of MFA in humans gives some hope in this respect; however, it needs to be seen whether a therapeutic window towards the treatment of glioblastomas exists. The role of the MGMT promoter methylation status also needs to be better clarified: interestingly, gap junction inhibition can increase lomustine toxicity even in unmethylated glioblastoma cells.10 Taken together, the road to translation for tumor disconnection in glioblastoma is now becoming increasingly clear. It will be exciting to learn which of the concepts and molecular targets will finally succeed to make the current therapy more effective.
Acknowledgments
The text is the sole product of the author, and no third party had input or gave support to its writing.
References
- 1. Osswald M, Jung E, Sahm F, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580): 93–98. [DOI] [PubMed] [Google Scholar]
- 2. Osswald M, Solecki G, Wick W, Winkler F. A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol. 2016;18(4):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schneider M, Vollmer L, Potthoff AL, et al. Meclofenamate causes loss of cellular tethering and decoupling of functional networks in glioblastoma. Neuro Oncol. 2021;23(11):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jung E, Osswald M, Blaes J, et al. Tweety-homolog 1 drives brain colonization of gliomas. J Neurosci. 2017;37(29):6837–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532–538. [DOI] [PubMed] [Google Scholar]
- 7. Murphy SF, Varghese RT, Lamouille S, et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;76(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gritsenko PG, Atlasy N, Dieteren CEJ, et al. p120-catenin-dependent collective brain infiltration by glioma cell networks. Nat Cell Biol. 2020;22(1):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weil S, Osswald M, Solecki G, et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 2017;19(10):1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider M, Potthoff AL, Evert BO, et al. Inhibition of intercellular cytosolic traffic via gap junctions reinforces lomustine-induced toxicity in glioblastoma independent of MGMT promoter methylation status. Pharmaceuticals (Basel). 2021;14(3): 195. [DOI] [PMC free article] [PubMed] [Google Scholar]