Abstract
Background
Vismodegib specifically inhibits Sonic Hedgehog (SHH). We report results of a phase I/II evaluating vismodegib + temozolomide (TMZ) in immunohistochemically defined SHH recurrent/refractory adult medulloblastoma.
Methods
TMZ-naïve patients were randomized 2:1 to receive vismodegib + TMZ (arm A) or TMZ (arm B). Patients previously treated with TMZ were enrolled in an exploratory cohort of vismodegib (arm C). If the safety run showed no excessive toxicity, a Simon’s 2-stage phase II design was planned to explore the 6-month progression-free survival (PFS-6). Stage II was to proceed if arm A PFS-6 was ≥3/9 at the end of stage I.
Results
A total of 24 patients were included: arm A (10), arm B (5), and arm C (9). Safety analysis showed no excessive toxicity. At the end of stage I, the PFS-6 of arm A was 20% (2/10 patients, 95% unilateral lower confidence limit: 3.7%) and the study was prematurely terminated. The overall response rates (ORR) were 40% (95% CI, 12.2-73.8) and 20% (95% CI, 0.5-71.6) in arm A and B, respectively. In arm C, PFS-6 was 37.5% (95% CI, 8.8-75.5) and ORR was 22.2% (95% CI, 2.8-60.0). Among 11 patients with an expected sensitivity according to new generation sequencing (NGS), 3 had partial response (PR), 4 remained stable disease (SD) while out of 7 potentially resistant patients, 1 had PR and 1 SD.
Conclusion
The addition of vismodegib to TMZ did not add toxicity but failed to improve PFS-6 in SHH recurrent/refractory medulloblastoma. Prediction of sensitivity to vismodegib needs further refinements.
Keywords: medulloblastoma, SHH pathway, SMO inhibition
Key Points.
The combination of vismodegib and TMZ is feasible in adult SHH medulloblastoma.
Six-month PFS is not significantly increased by the addition of vismodegib to TMZ compared to TMZ alone.
Prediction of sensitivity to vismodegib lacks reliability.
Importance of the Study.
Adult medulloblastoma is an orphan disease requiring specific studies because it differs from its pediatric counterpart on pathology, molecular biology, and tolerance to treatment. Sonic Hedgehog (SHH) pathway is often activated in adult medulloblastoma and may represent an ideal therapeutic target. Vismodegib inhibits SMO and is therefore a specific inhibitor of this pathway. Potential toxicity and efficacy of adding vismodegib to chemotherapy (temozolomide [TMZ]) in relapsing patients deserve further study. The authors report on the final results of a phase I/II study evaluating vismodegib + TMZ vs TMZ in adults with recurrent/refractory medulloblastoma with SHH activation determined by immunohistochemistry. Although the combination did not add significant toxicity and significantly improved radiological response rate, it failed to significantly improve the 6-month progression-free survival. The NGS analysis failed to accurately predict response and resistance to vismodegib. Further studies are required to improve the handling of this targeted therapy.
Adult medulloblastoma is a rare orphan disease that affects about 0.6 cases per million inhabitants. It requires specific studies because it differs from its pediatric counterpart regarding incidence, pathology, molecular biology, and tolerance to treatment. Front-line treatment of adult medulloblastoma has typically been designed based on pediatric protocols.1 It consists of surgical resection followed by radiotherapy and chemotherapy.2–6 At recurrence, the therapeutic options remain limited.
Methylation and transcriptomic profiles have identified subgroups of medulloblastoma based on their activation profile that reflect the cell of origin. Adult medulloblastoma predominantly (about 60% of patients) belongs to the desmoplastic subtype with Sonic Hedgehog (SHH) pathway activation,7 specifically SHH-δ.8 Adult SHH-activated medulloblastoma have an intermediate prognosis with a 5-year survival rate of 50% to 70%,9 provided there is no associated p53 mutation.10 The SHH pathway is a potential candidate for targeted therapy for patients with recurrent or refractory disease.
The SHH pathway is spatially and temporally involved in expansion, migration, and differentiation of immature precursor cells from the external granule cell layer to form the internal granule-cell layer during cerebellar maturation. This pathway becomes normally inactive in most normal adult tissue. Its reactivation is a driving phenomenon in the pathogenesis of medulloblastoma. Adult-type medulloblastoma is enriched for either PTCH1 or SMO (smoothened) mutations and is thus prone to respond to anti-SHH therapies.11 Vismodegib is a ligand-specific inhibitor of the SHH pathway. Even though tumors are sensitive, no long-lasting responses have yet been described.12 Blocking tumor cell proliferation at different molecular levels may generate synergistic/additive effects as reported in various models.13 Temozolomide (TMZ) is an oral chemotherapy that has proved activity in relapsing medulloblastoma and its minimal hematotoxicity allows its use in patients previously treated with craniospinal radiation. It is thus an attractive option both to be tested in association with an anti-SMO compound and to be used as a comparative standard therapy.
The MEVITEM phase I/II trial reported here explored the toxicity and efficacy of adding vismodegib to TMZ in adult patients with recurrent or refractory medulloblastoma with an activation of SHH.
Methods
Study Design and Patients
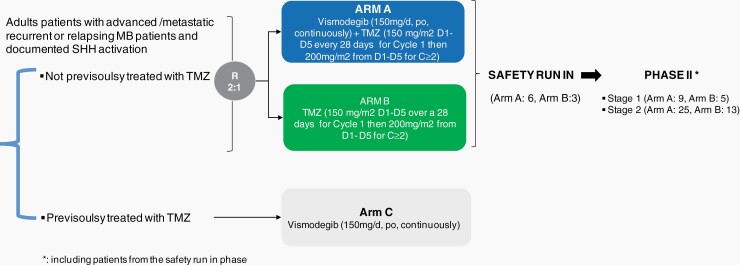
Designed in 2011 and opened in 2012, MEVITEM was a multicentric, randomized (ratio 2:1), open-label, phase I/II trial aiming to evaluate the safety and clinical activity of the association of vismodegib + TMZ in adult patients (>18 years) with recurrent or refractory SHH medulloblastoma. It included (i) a safety run-in, aiming to evaluate the safety of the combination of vismodegib + TMZ and (ii) a phase II part aiming to evaluate the clinical activity of vismodegib + TMZ (Figure 1). An exploratory arm with vismodegib alone was proposed for patients pretreated by TMZ. Main eligibility criteria included: histologically confirmed recurrent or refractory SHH medulloblastoma for which no known curative therapy existed, the absence of previous treatment with TMZ, evidence of measurable disease, and documented activation of the SHH pathway. SHH pathway status was assessed by immunohistochemistry (IHC) and was centrally reviewed. Only patients with cytoplasmic GAB1 and filamin A positive staining, with negative nuclear β-catenin were included.14 Further eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, adequate hematological function (absolute neutrophil count ≥1.5 × 109 cells per L, platelets ≥100 × 109 cells per L, hemoglobin concentration ≥10 g/dL), adequate hepatic function (bilirubin ≤1.5 × ULN, aspartate aminotransferase and alanine aminotransferase ≤2.5 × ULN); adequate renal function (creatinine clearance ≥50 mL/min (calculated by Cockcroft-Gault formula or Modification of Diet in Renal Disease (MDRD) formula for patients older than 65 years) or serum creatinine <1.5 × ULN. Exclusion criteria were the presence of a malabsorption syndrome, uncontrolled hypocalcemia, hypomagnesemia, hyponatremia, or hypokalemia, the absence of any history of congestive heart failure or ventricular arrhythmia requiring medication or congenital long QT syndrome. Of note, there was no limit to the number of previous treatment lines.
Fig. 1.
Study scheme.
Ethics approval was obtained for this study (EudraCT No.: 2011-003372-37, ClinicalTrials.gov identifier NCT01601184) which was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization-Good Clinical Practice, and participating country and institution regulations. All patients provided written informed consent. The sponsor of the study was the Centre Léon Bérard, Lyon, France.
Procedures
Eligible patients naïve of TMZ were randomized 2:1 between arm A and arm B. In arm A, patients received vismodegib (150 mg/day, per os, continuously) plus TMZ (150 mg/m2 from day 1 to day 5 over a 28-day cycle period during cycle 1 and 200mg/m2 from day 1 to day 5 over a 28-day cycle period during subsequent cycles). Patients in arm B received TMZ (same regimen) as a single agent. The protocol was amended to allow on a case-by-case basis, the enrollment of patients previously treated by TMZ in a third independent and parallel arm (arm C, vismodegib single agent, 150 mg/day, per os, continuously; Figure 1).
Study treatments were continued until disease progression, unacceptable toxicity, or withdrawal of consent. Upon disease progression, patients randomized in arm B were allowed to cross over and initiate vismodegib as a single agent treatment. Response assessments according to WHO criteria15 included neurological evaluation and cerebrospinal fluid (CSF) MRI every 2 months. Imaging was reviewed centrally. Patients were followed up for safety through clinical and biological assessments at least monthly (weekly for the first 2 cycles) until treatment discontinuation. Adverse events (AE) were assessed and graded according to the Common Terminology Criteria Adverse Events (CTCAE) version 4.03.
Outcomes
The primary endpoint for the safety run-in (phase I) was the incidence of severe toxicities defined as any of the following AE related to study drugs and occurring during the first 3 months of treatment: any grade ≥4 or any grade 3 AE leading to study treatment interruption for more than 7 days or permanent discontinuation.
The primary endpoint for phase II was the progression-free survival after 6 months of treatment (PFS-6) defined as the proportion of patients without centrally documented disease progression per WHO criteria or death within the first 6 months of study treatment. Secondary endpoints included overall response rate (ORR), duration of response, progression-free survival (PFS), time-to-treatment failure (TTF), and safety.
Statistical Analysis
Sample size.
—Sample size for the safety run-in phase (phase I) was calculated to screen patients for major toxicity events. Based on binomial probabilities, there is a 90% probability of observing 1 or more patients with a toxicity event, if that event occurs in at least 32% of the target population. Upon successful completion of the run-in phase, 6 patients from phase I were set to be carried over to arm A of the phase II part of the trial. Patients from the safety run-in phase were part of phase II.
The phase II study was conducted after the Independent Data Monitoring Committee (IDMC) review of phase I using a minimax Simon’s 2-stage design,16 considering a PFS-6 of 30% being unpromising and a 55% PFS-6 as the lowest response rate considered as effective for the combined treatment. In the absence of data on response rate of relapsing medulloblastoma in adults, the 30% response rate was used as a cutoff for efficacy consideration, derived from pediatric data.17 Assuming a unilateral type I error rate of 5% and a power of 80%, enrollment of 25 patients was planned in arm A (9 for stage I and 16 for stage II).
No formal comparison between arms A and B was scheduled. Arm B was only used to validate the results obtained in arm A. Considering the 2:1 randomization ratio, the sample size in arm B was 3 patients for the safety run-in and 13 patients for phase II (5 for stage I and 8 for stage II).
For arm C, no specific sample size was determined as no formal statistical analysis was performed on this dataset.
Analysis.
—Based on the intention-to-treat principle, efficacy analyses were performed including all randomly assigned patients. All randomly assigned patients who received at least 1 dose of vismodegib were assessed for safety.
An interim safety data analysis was planned after 3 months of follow-up of the first 9 randomized patients (6 in arm A and 3 in arm B). If ≤2/6 patients randomized to the combination arm had experienced severe toxicities, the safety data were considered acceptable and the phase II part could be initiated. A contrario, if >2/6 patients exposed to vismodegib + TMZ experienced severe toxicities related to vismodegib the study would be terminated.
The PFS-6 was defined as the proportion of patients without documented disease progression (complete response [CR], partial response [PR], or stable disease [SD] according to WHO criteria) within the first 6 months of study treatment, based on central review of tumor assessment. At the stage I analysis, if at least 3 out of the first 9 eligible and assessable patients in arm A did not show disease progression after 6 months of treatment, patient enrollment would continue; otherwise, the study would be stopped at this stage for lack of efficacy. In a second stage, if at least 12 successes were observed among the 25 patients of arm A, the treatment would be considered as effective in this indication.
PFS and TTF were estimated as a function of time by the Kaplan-Meier method.
For arm C, all enrolled and treated patients were analyzed.
NGS Analysis
A posteriori, DNA was extracted from archival tumor samples demonstrating a tumor cell fraction above 50%. Using a standard protocol (Qiagen, QIAmp DNA mini Kit) up to 200 ng of DNA was mechanically fragmented using a Covaris instrument (Covaris Inc., Woburn, MA, USA).
Following the manufacturer’s protocol, the Sureselect XTHS kit (Agilent Technologies, Santa Clara, CA, USA) was used for targeted hybrid-capture sequencing on a panel of 66 genes including those implied in the SHH pathway (Supplementary Table 1). Samples were sequenced, on MiSeq platform (Illumina, San Diego, CA, USA). Bioinformatic analyses (alignment, variant calling annotation, and copy number analysis), were performed using the SeqOne (Montpellier, France) online website. Mutation sensitivity was assessed using public databases (clinvar,18 cosmic19) and PubMed search engine.
Results
Patient Characteristics and Treatment
Between 29 October 2012 and 12 December 2016, 10 sites in 2 European countries recruited 36 patients with a diagnosis of medulloblastoma locally assessed. All patients had a centrally confirmed medulloblastoma and 24 patients with documented SHH pathway activation were enrolled: arm A: 10 patients, arm B: 5 patients, and arm C: 9 patients. Their clinical characteristics are described in Table 1. The other 12 patients did not have SHH activation. Of note, 14 patients had CNS relapse and 10 patients developed extra-CNS relapse. Extra-CNS metastasis occurred mainly in lymph nodes and bone (Table 1).
Table 1.
Baseline Characteristics
| Parameters | TMZ + Vismodegib, N = 10 (%) | TMZ, N = 5 (%) | Vismodegib, N = 9 |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 8 (80.0%) | 4 (80.0%) | 6 (66.7%) |
| Female | 2 (20.0%) | 1 (20.0%) | 3 (33.3%) |
| Age (years) | 39.0 (26, 43) | 36.0 (21, 45) | |
| Median (min; max) | 38.0 (21, 55) | ||
| Time since first diagnosis (years) | |||
| Median (min; max) | 6.6 (1.5, 17) | 6.1 (1.8, 10) | 6.3 (1.6, 9) |
| Histological type, n (%) | |||
| Missing | 1 | 0 | |
| Classical | 5 (55.6%) | 1 (20.0%) | 3 (33.3%) |
| Desmoplastic | 3 (33.3%) | 4 (80.0%) | 6 (66.7%) |
| Large cell | 1 (11.1%) | 0 (0.0%) | 0 |
| c-Myc amplification | |||
| No | 2 (20.0%) | 0 (0.0%) | 4 (44.4%) |
| Unknown | 8 (80.0%) | 5 (100.0%) | 5 (55.6%) |
| CNS localization | |||
| Vermis | |||
| Yes | 4 (40.0%) | 1 (20.0%) | 4 (44.4%) |
| No | 6(60.0%) | 4 (80.0%) | 5 (55.6%) |
| Hemisphere | |||
| Yes | 7 (70.0%) | 5 (100.0%) | 5 (55.6%) |
| No | 3 (30.0%) | 0 | 4 (44.4%) |
| Supratentorial metastasis | |||
| Negative | 10 (100.0%) | 4 (80.0%) | 8 (88.9%) |
| Positive | 0 | 1 (20.0%) | 1 (11.1%) |
| Spinal metastasis | |||
| Negative | 9 (90.0%) | 5 (100.0%) | 7 (77.8%) |
| Positive | 1 (10.0%) | 0 (0.0%) | 2 (22.2%) |
| Extra-CNS disease | 4 (40.0%) | 1 (20%) | 5 (55.6%) |
| Lymph node | 1 | 1 | 3 |
| Bone | 4 | 1 | 4 |
| Other site, n = 1 each (breast, mediastinum, lung, pleura liver, pancreas, colon) | 1 | 0 | 1 |
| Cerebrospinal fluid (CSF) | |||
| Negative | 8 (80.0%) | 4 (80.0%) | 8 (88.9%) |
| Unknown | 2 (20.0%) | 1 (20.0%) | 1 (11.1%) |
Abbreviations: CNS, central nervous system; TMZ, temozolomide.
Bold values indicate TMZ-naïve randomized patients.
Safety Endpoints
The safety analysis performed after enrollment of the first 6 patients in arm A was reviewed by an IDMC that allowed the opening of phase II. Overall, the combination of vismodegib + TMZ was safe, with 1/6 patients experiencing severe toxicity during the first 3 months of treatment (of note, another case showed expected hematological toxicities grade 4).
All patients (100%) experienced at least 1 AE and 9/10 (90%) patients randomized in arm A experienced at least 1 AE related to vismodegib (Table 2). In arm B, post-switch, 4 out of 5 patients experienced at least 1 AE related to vismodegib, with 2 of grade ≥3. In arm C, all 9 patients experienced at least 1 AE related to vismodegib that was at least a grade ≥3 in 4 of them. The main AE (ie, >20%) related to vismodegib included alopecia, fatigue, cramps, diarrhea, dysgeusia, hemoglobin decrease, hematological disorders, ionogram abnormalities, hot flush, liver lab abnormality (Table 2).
Table 2.
Safety Endpoints
| Number of Patients With at Least | Arm ATMZ + vismodegib, N = 10 | Arm BTMZ, N = 5 | Arm CVismodegib, N = 9 |
|---|---|---|---|
| 1 AE related to vismodegib | 9 | 4a | 9 |
| 1 grade ≥3 AE related to vismodegib | 2 | 2a | 4 |
| 1 SAE related to vismodegib as per sponsor assessment | 2 | 0 | 3 |
| Main AE related to vismodegib (>20%) per patient | |||
| Alopecia | 3 (33.3%) | 0 | 4 (44.4%) |
| Fatigue | 3 (30%) | 0 | 3 (33.3%) |
| Cramps | 4 (40%) | 1 (20%) | 3 (33.3%) |
| Diarrhea | 1 (10%) | 0 | 2 (22.2%) |
| Dysgeusia | 2 (20%) | 0 | 3 (33.3%) |
| Hemoglobin decreased | 1 (10%) | 3 (60%) | 3 (33.3%) |
| Hematological disorders | 4 (40%) | 3 (60%) | 5 (55.6%) |
| Ionogram abnormalities | 0 | 1 (20%) | 3 (33.3%) |
| Hot flush | 0 | 0 | 2 (22.2%) |
| Liver lab abnormality | 6 (60%) | 2 (40%) | 3 (33.3%) |
Abbreviations: AE, adverse events; SAE, serious adverse events; TMZ, temozolomide.
aIncluding AE post-switch to vismodegib for arm B.
AE were classified as follows: (“Fatigue,” “Asthenia”) = ‘Fatigue’; (“Cramps,” “Leg cramps,” “Muscle cramps”) = ‘Cramps’; creatinine increased, ALT (SGPT) increased, AST (SGOT) increased, alkaline phosphatase increased, GGT increased = ‘Liver lab value abnormality’; (“Leucocytes decreased,” “Leucocytes increased,” “Lymphocytes decreased,” “Neutrophils decreased,” “Neutrophils increased,” “Platelets decreased”) = ‘Hematological disorders’; (“Phosphorus decreased,” “Calcium decreased,” “Magnesium increased”) = ‘Ionogram abnormalities.’
A total of 5 patients exposed to vismodegib (arm A: 2, arm C: 3) experienced at least 1 serious AE related to vismodegib: a grade 3 muscle spasm, a grade 3 E. coli urinary tract infection, a grade 2 erectile dysfunction/hair loss/muscle spasms, a grade 3 sinus tachycardia, and a grade 3 thrombocytopenia/menorrhagia.
Efficacy Endpoints
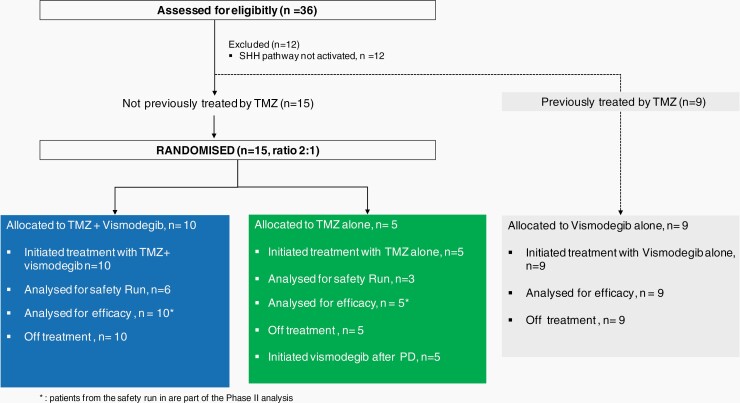
After completion of the safety analysis, 6 additional patients were randomized. At the end of stage I of Simon’s design, recruitment was suspended according to stopping rules defined in the protocol (Figure 2).
Fig. 2.
Consort graph.
The median treatment exposure to vismodegib was 6.67 months (min, max: 0.8-26.2) in arm A and 3.71 months (min, max: 1.7-11.7) in arm C. Median exposure duration to TMZ was 5.81 (min, max: 0.2-26.2) and 1.97 (min, max: 0.2-4.3) months in arm A and arm B, respectively.
All patients (n = 5) randomized in arm B crossed over to vismodegib as compassionate use following disease progression under TMZ single agent.
In all arms, the main reason for ending the treatment was progression (90% in arm A, 100% in both arm B and C).
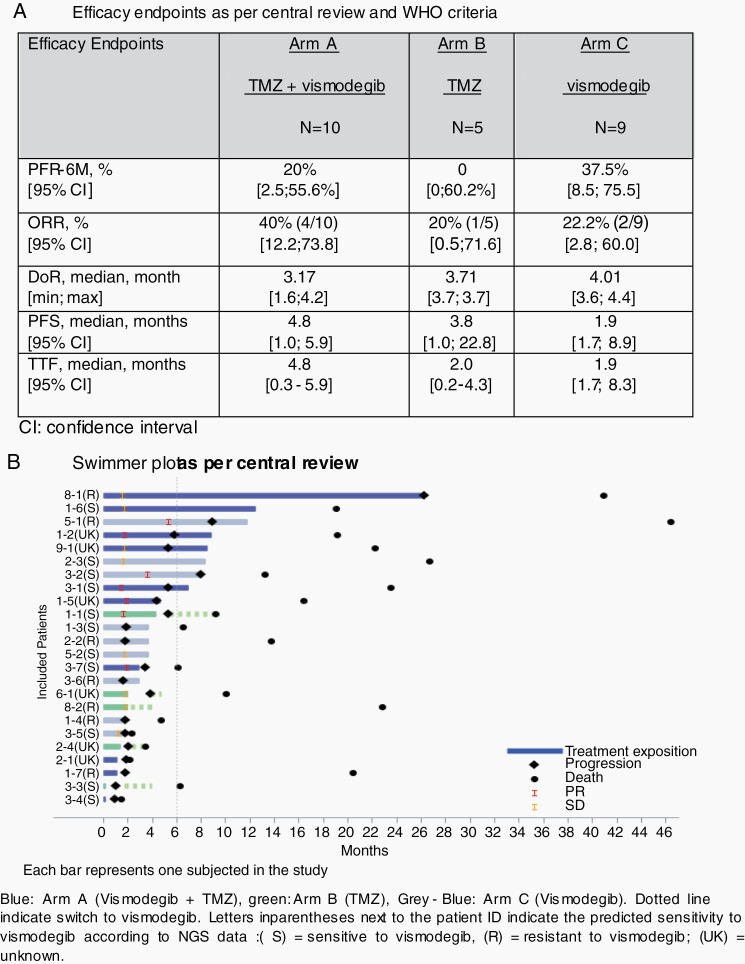
According to central review, at the end of stage I, PFS-6 was 20% (95% CI, 2.5-55.6) in arm A, 0% (95% CI, 0-60.2) in arm B (Figure 3A). The proportion of successes required to initiate stage II (ie, 3 patients without progression at 6 months out of 9 in arm A) was not reached, thus the study was terminated. The primary endpoint was analyzed according to central review assessment, the decision rule for the endpoint was based on this review. We also reviewed the PFS data based on investigator-based assessment. This evaluation reached the same conclusion of futility.
Fig. 3.
Efficacy endpoints.
According to central review, the ORR were 40% (4 PR/10; 95% CI, 12.2-73.8) and 20% (1 PR/5, 95% CI, 0.5-71.6) in arm A and B, respectively. The median duration of odds ratios (OR) was 3.17 and 3.71 months in the TMZ + vismodegib and TMZ arms, respectively. Median PFS was 4.8 (95% CI, 1.0-5.9) and 3.8 (1.0-22.8) months in arm A and B, respectively (Figure 3A and Supplementary Figure 2A). Median OS were and 19.1 (1.4-22.2) and 9.3 (3.5-22.8) months in arm A and B, respectively (Supplementary Figure 2B).
The median TTF was 4.8 (0.3-5.9) and 2.0 months (0.2-4.3) in arm A and B, respectively. The median PFS and TTF from switch in arm B patients were 0.92 (0.5-2.2) and 0.89 (0.5-2.2), respectively.
In arm C, PFS-6 was 37.5% (95% CI, 8.8-75.5) and ORR was 22.2% (95% CI, 2.8-60.0). The median PFS was 1.9 (95% CI, 1.7-8.9).
Molecular Profiles
Twenty-one patients who received vismodegib (8 in arm A, 4 in arm B after failure of TMZ, and 9 in arm C) could be analyzed by new generation sequencing (NGS). Inactivating PTCH1 mutations were found in 12 patients, SMO mutations in 6 and 3 patients showed no SHH pathway alterations (PTCH1, SMO, SHH mutation, or GLI2 amplification). An activating TERT promoter mutation was found in 19 of the 21 analyzed patients. The two others were non-informative due to insufficient NGS depth coverage of the region (Table 3).
Table 3.
Predicted Sensitivity According to NGS
| Predicted Sensitivity | Best Response | Arm | Tumor Location | PTCH1 | SMO | hTERT Promoter | Patient ID |
|---|---|---|---|---|---|---|---|
| under Vismodegib | (AF%) | (AF%) | (AF%) | ||||
| S | PR | A | Extra | p.Leu744fs (95%) | WT | c.-124C>T (69%) | 3.1 |
| CNS | p.Lys163* (73%) | WT | c.-124C>T (60%) | 3.7 | |||
| C | CNS | p.Asn386fs (93%) | WT | c.-146C>T (61%) | 3.2 | ||
| SD | A | Extra | p.Tyr446* (95%) | WT | c.-124C>T (52%) | 1.6 | |
| C | Extra | p.Gln160* (82%) | WT | c.-124C>T (69%) | 2.3 | ||
| Extra | p.Arg135* (95%) | WT | c.-124C>T (50%) | 3.5 | |||
| Extra | p.Pro643fs (49%); c.-60 + 2-60 + 3insTT (43%) | WT | c.-124C>T (100%) | 5.2 | |||
| PD | A | CNS | p.Tyr381fs (41%); p.Glu539fs (45%) | WT | c.-124C>T (63%) | 3.4 | |
| B | CNS | p.Asp635fs (24%); p.Gln242fs (62%) | WT | c.-124C>T (49%) | 1.1 | ||
| Extra | p.Arg770* (40%); p.Glu1095* (50%) | WT | NI | 3.3 | |||
| C | CNS | p.Trp926* (95%) | WT | c.-124C>T (70%) | 1.3 | ||
| R | PR | C | Extra | WT | p.Leu412Phe (44%) | c.-124C>T (56%) | 5.1 |
| SD | A | Extra | WT | p.Leu412Phe (37%) | NI | 8.1 | |
| PD | A | CNS | WT | WT | c.-124C>T (29%) | 1.7 | |
| B | CNS | WT | WT | c.-124C>T (73%) | 8.2 | ||
| C | Extra | WT | WT | c.-146C>T (67%) | 1.4 | ||
| CNS | WT | p.Leu412Phe (25%) | c.-124C>T (60%) | 2.2 | |||
| CNS | WT | WT | c.-146C>T (100%) | 3.6 | |||
| UK | PR | A | CNS | WT | p.Gly416Asp (33%) | c.-124C>T (51%) | 1.2 |
| CNS | WT | p.Ile408Val (17%); p.Ser278Ile (15%) | c.-124C>T (69%) | 1.5 | |||
| SD | A | CNS | N/A | N/A | N/A | 9.1 | |
| PD | A | Extra | N/A | N/A | N/A | 2.1 | |
| B | CNS | p.Asp879fs (86%) | p.Ala524Pro (47%) | c.-124C>T (86%) | 2.4 | ||
| CNS | N/A | N/A | N/A | 6.1 |
Abbreviations: AF%, allelic frequency; CNS, central nervous system; N/A, not available; NI, not informative; PD, progressive disease; PR, partial response; R, resistant; S, sensitive; SD, stable disease; WT, wild type.
*The protein coding sequence ends at a translation termination codon (stop codon).
Out of the 12 patients with PTCH1 mutations, 3 harbored 2 concomitant inactivating mutations. The allele frequencies of the PTCH1 mutations were above 70% in the 9 patients with 1 mutation and the sum above 80% in the 3 patients with 2 concomitant mutations. The allele frequencies were consistent with the tumor cell fraction. They were in favor of either a biallelic inactivation of PTCH1 or an inactivating mutation associated with a loss of heterozygosity in the tumor cells.
The 6 SMO mutations identified with the exception of the p.A524P mutation have been previously described as pathogenic, activating, confirmed as somatic and/or resistant to targeted SMO therapy.20–26 The most frequent p.L412F SMO mutation, was identified in 3 patients.
In arm A, 4 out of the 8 patients had PTCH1 inactivating mutations, 1 had a SMO resistance mutation, 2 had SMO activating mutations with unknown vismodegib sensitivity and 1 had no SHH pathway alteration. In arm B, 2 out of the 4 patients had PTCH1 inactivating mutations alone, one had a concomitant PTCH1 inactivating mutation and a SMO variant of unknown significance (VUS) and 1 had no SHH pathway alterations. In arm C, 5 out of the 9 patients had PTCH1 inactivating mutations, 2 had a SMO resistance mutation and 2 had no SHH pathway alterations. Altogether, only 11 of 21 presented an expected molecular sensitivity according to NGS analysis: 3 had a PR 4 remained stable as best response and 4 had progression while out of 7 patients classified as potentially resistant, 1 had a PR, 1 a SD, and 5 had progression.
Discussion
The initial development of anti-SHH started with the isolation of cyclopamine. This steroidal alkaloid is a constituent of the corn lily. If pregnant sheep ingested their flowers, they gave birth to offspring with only 1 eye and brain malformations. Vismodegib and sonidegib were then discovered by high-throughput screening of a library of small-molecule compounds and subsequent chemical optimization. Other chemical semi-synthetic derivatives of the alkaloid cyclopamine are currently developed such as saridegib, taladegib, BMS-833923, CUR-61414, or glasdegib.27 Second-generation triazole antifungal drugs such as itraconazole or posaconazole are another alternative to inhibit the Hedgehog signaling pathway.28
In animal models, mice with a heterozygous deletion of PTCH1+/− develop basal cell carcinoma (BCC) and medulloblastoma that are highly responsive to inhibition by SMO antagonists, strongly suggesting that these tumors are “addicted” to SMO activity. Anti-SMO has been developed for use in hematological malignancies and is approved for the treatment of BCC that show similar pathway activation as SHH medulloblastomas. The role of anti-SHH therapies was explored in pediatric and adult relapsing or refractory medulloblastoma.29,30 The underlying mechanisms of activation of this pathway are different in adults and children, where downstream mutations are often responsible for SHH activation and are resistant to anti-SMO. In the PBTC-032 and PBTC-025B phase II trials, out of 12 children and 31 adults with relapsing/resistant histologically confirmed medulloblastoma treated by vismodegib, 1/12 and 3/31 showed objective responses, respectively. All responders were classified as SHH subgroup based on IHC. The median PFS in adults was significantly longer in the SHH-MB as compared with non-SHH subgroup, though it was consistently less than 4 months.29 Out of 39 children and 16 adults with relapsing/resistant histologically confirmed medulloblastoma treated by sonidegib, 2/39 and 3/16 showed an objective response, respectively. The duration of response in adults was however short-lived (1.6 and 8.7 months, respectively, for patients with CR and 4.8 months for the PR). These 5 responses all occurred among the 10 patients who tested positive with a 5-gene Hh signature reverse transcriptase-polymerase chain reaction (RT-PCR) assay, while no response was documented in any patient with Hh-negative signature.30,31 A meta-analysis of all patients with medulloblastoma treated with anti-SMO suggests an ORR of 55% for sonidegib and 17% for vismodegib. Sonidegib shows significantly more responses than vismodegib in the pediatric population, but similar activities in adults.32 These rates are worse than those observed in phase I for locally advanced or metastatic BCC.33 In our series, of 19 patients selected by positive IHC, 2/9 treated with vismodegib alone and 4/10 treated in combination with TMZ showed a PR. The median duration of response did, however, not exceed 4 months.
Of 24 patients exposed to vismodegib in our series (19 in arm A and C plus 6 in arm B treated with vismodegib after progressive disease under TMZ), 6 objective responses were documented, however with a short duration. The incidence of extra CNS of relapses in this series is unusually high as 10 out of 24 patients showed extra-CNS relapse: this is more than the usual 20% reported in prospective trials and might be linked to a recruitment bias, as it is possible that many patients with neurological deficits linked to CNS recurrence were not referred for an experimental treatment. Further, intuitively, one might expect that recurrences outside of the CNS would be more prone to respond as drugs are not required to cross the blood-brain barrier. However, we observed no difference in ORR between CNS and extra-CNS relapses, suggesting that CSF penetration is not a key factor explaining our disappointing results.34
The response to anti-SHH may be spectacular,12 but always transient, suggesting the need for combined therapy to enhance and extend the duration of the response. As anti-SHH therapies only rarely show hematological toxicities, combination therapy with classical chemotherapeutics such as TMZ may be considered. TMZ has demonstrated some efficacy in pediatric medulloblastomas: As a single agent, the ORR ranges from 16% (4/25)35 to 43% (17/40)36 in pediatric relapsing/refractory medulloblastomas. It is 28% at 2 cycles when associated with topotecan37 and 32% at 4 cycles when associated with irinotecan.38 Moreover, TMZ presents a minimal risk of neutropenia, which is an important consideration in recurrent medulloblastoma, where all patients have previously received craniospinal irradiation. It was thus an attractive drug to be used as a control, and in association with anti-SHH, especially if maintenance therapy is envisaged in further development. Our series confirms that the combination of vismodegib and TMZ does not increase toxicity and results in a significant response rate. Unfortunately, it also shows no advantage on PFR-6M, strongly suggesting that the addition of TMZ fails to extend the duration of response of vismodegib in recurrent SHH medulloblastoma. This observation may be compounded by a number of factors:
First, we intentionally selected relatively lax inclusion criteria and allowed for inclusion of patients with heterogeneous presentations and that could have been heavily pretreated to reflect the real-life situation experience of recurrent/progressive medulloblastoma and allow recruitment in a reasonable timeline according to the epidemiology in potential referring centers.39 It is therefore possible, that these results may not apply to naïve medulloblastoma patients.
Second, our results reported here with vismodegib either alone or associated with TMZ might be due to a bias in SHH selection: Several methods can be used to confirm the subgrouping of formalin-fixed and paraffin-embedded (FFPE) samples in a clinical setting. The simplest and fastest way to screen SHH activation is the use of IHC combination of 4 markers GAB1, β-catenin, filamin A, and YAP1.14 SHH medulloblastoma are typically and specifically stained with antibodies against GAB1. They share YAP1 and filamin A with the Wnt subgroup. A recent study confirmed the excellent specificity (99.5%) and sensitivity (91.5%) of IHC to detect SHH-activated medulloblastomas.40 Expression profiling using nanoString41 or methylation profiling42 offer reliable methods for FFPE samples molecular grouping and/or prognosis classification43 but require costly equipment and bioinformatics skills. However, none of these methods identifies the molecular alteration responsible for the activation of the SHH pathway, and a complementary NGS analysis is required to further specify whether the tumor may be sensitive to targeted SMO inhibitors.44 Indeed, whole-genome and transcriptome sequencing, would have made it possible to add more information and might have revealed other alterations (rearrangements, copy number variations, loss of heterozygosity, fusion transcripts, splicing variants, etc.) that could have helped to better understand the lack of correlation between NGS-based prediction and actual clinical response. However, these technologies were not available at the time of study conception.
Another hypothesis for the low response rate reported here is a selection of patients that were potentially primarily resistant to SMO antagonists. Similar to other reports,11 our NGS identified 50% (N = 12) patients with inactivating PTCH1 mutations that should lead to vismodegib sensitivity. One patient had both a PTCH1 inactivating and a yet unreported p.A524P SMO variant. As this patient had not received previous anti-SMO therapy, an acquired SMO-activating resistance mutation is very unlikely. It suggests that the SHH pathway activation in this patient was linked to the PTCH1 inactivating mutation and not the SMO p.A524P variant SMO. This variant probably represents a polymorphism. Another patient harbored 2 SMO mutations which are both considered pathogenic with variant alleles frequencies of 15% compared to the 80% tumor cell content. It is, to our knowledge, the first biallelic activating SMO mutation described, suggesting a clonal heterogeneity in this medulloblastoma. Apart from initial resistance due to downstream mutations, secondary resistance may have appeared in some of our patients.45 Hot spot mutations of SMO (such as G497W and D473Y)46 may appear during vismodegib therapy leading to secondary resistance. Compounds that associate vismodegib with histone deacetylase inhibitors are active in vitro against this type of secondary resistant cells.47 Alternative mechanisms of resistance include up-regulation of AKT, PIP3, and IGF-1R pathways.48 Associating a PI3K inhibitor or dual PI3K-mTOR inhibitor with an anti-SMO delays the apparition of resistance, though these drugs are not active per se.49 Therefore, combined targeting of HH/PI3K-mTOR pathways may be necessary. Furthermore, this association significantly enhances chemosensitivity to platinum derivatives in vitro.13 The association of an anti CXCR4 with a SHH inhibitor may prevent resistance apparition through trimethylation of H3 histone. This highlights the potential role of epigenetic modifiers in preventing resistance to anti-SMO. Anti-glioma-associated oncogene family zinc finger (GLI) compounds are currently developed with the aim to block both canonical and non-canonical pathways to interfere with the downwards pathway.50
Furthermore, we could not detect any SHH pathway alterations, SUFU mutations, or GLI2 amplifications in 4 (P1.7; P8.2; P1.4; P3.6) out of the 21 analyzed patients. Several hypotheses could explain the absence of SHH pathway alteration. The tumor cell content of these samples may have been too low, but in these 4 patients, a TERT promoter mutation was found with a minimal allelic frequency of 29%. About 12% of SHH-activated medulloblastoma remain without an identified molecular alteration that can explain their activation.11 In our series, this proportion reaches 19%. In our series as well as in the published cases, these molecular analyses were performed mostly on samples obtained during the initial surgery. Though SHH grouping never changes between initial diagnosis and relapse, clonal evolution and inter-metastatic molecular heterogeneity may appear and explain unexpected resistance.45 Performing RNA expression profiling or DNA sequencing on fresh frozen samples is considered today as the most reliable tool to classify and analyze medulloblastoma. However, fresh-frozen material was only available for 3 samples, and we were faced with the presence of paraffin-included material only for 18 and a total absence of material for 3 patients. Some of the paraffin-included materials were more than 6 years old and for many, information on the nature of the solvents used for fixation was lacking. Despite these suboptimal conditions, we were able to identify molecular alterations of the SHH pathway in most patients. If higher quality samples would have been available, whole-genome and transcriptome sequencing could have been considered to a better understanding of the lack of correlation of NGS-based prediction and actual clinical response.
Conclusion
Our study demonstrates that the addition of TMZ to vismodegib does not result in significant additional toxicity but fails to extend the duration of responses of vismodegib in SHH-activated relapsing/resistant medulloblastoma. Prediction of potential resistance using NGS lacks sensitivity. Further studies are thus required to explore the association of anti-SMO with other compounds. The use of anti-SMO earlier in the course of the disease also deserves further studies.
Supplementary Material
Acknowledgments
The MEVITEM trial was sponsored by Centre Léon Bérard and supported by Roche. We thank the involved patients and their families for participating in this study. We thank the investigators, nurses, and other study staff for their contributions to this trial. We also want to thank the trial steering committee members (Dr Rutkowski, Dr Massimino, Dr Kramar). The authors would like to thank the Clinical Research Platform (DRCI) and the Biosample Management Platform of CLB for logistic and laboratory support (PGEB). The authors would also like to thank the “Association Cassandra” for financial support.
Funding
This work was an investigator-initiated trial (no grant number is applicable). CLB was the legal sponsor. Roche provided the investigational agent and funding but had no role in the study design, data collection, analysis, interpretation, writing of the report, or decision to publish this report. The database is held by CLB, and CLB statisticians carried out the analysis.
Conflict of interest statement. D.P.: personal fees: training and expertise.
Author contribution. Conceptualization and design: D.F., G.G., L.M., and D.P. Acquisition and control of data: D.F., M.B., F.L.D., O.C., E.L.R., A.B.L., A.F.H., D.M., and A.S.B. Analysis of data: D.F., L.M., G.G., and D.P. Manuscript writing: D.F., M.B., G.G., and A.F.H. Final editing and approval of the manuscript: all authors.
References
- 1. Frappaz D, Faure-Conter C, Bonneville Levard A, Barritault M, Meyronet D, Sunyach MP. Medulloblastomas in adolescents and adults—can the pediatric experience be extrapolated? Neurochirurgie. 2021;67(1):76–82. [DOI] [PubMed] [Google Scholar]
- 2. Beier D, Proescholdt M, Reinert C, et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018;20(3):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Bueren AO, Friedrich C, von Hoff K, et al. Metastatic medulloblastoma in adults: outcome of patients treated according to the HIT2000 protocol. Eur J Cancer. 2015;51(16):2434–2443. [DOI] [PubMed] [Google Scholar]
- 4. Franceschi E, Bartolotti M, Paccapelo A, et al. Adjuvant chemotherapy in adult medulloblastoma: is it an option for average-risk patients? J Neurooncol. 2016;128(2):235–240. [DOI] [PubMed] [Google Scholar]
- 5. Carrie C, Lasset C, Alapetite C, et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74(8):2352–2360. [DOI] [PubMed] [Google Scholar]
- 6. Spreafico F, Massimino M, Gandola L, et al. Survival of adults treated for medulloblastoma using paediatric protocols. Eur J Cancer. 2005;41(9):1304–1310. [DOI] [PubMed] [Google Scholar]
- 7. Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6):737–754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kool M, Jones DT, Jäger N, et al. ; ICGC PedBrain Tumor Project . Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaturvedi NK, Kling MJ, Coulter DW, et al. Improved therapy for medulloblastoma: targeting hedgehog and PI3K-mTOR signaling pathways in combination with chemotherapy. Oncotarget. 2018;9(24):16619–16633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 16. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 17. Massimino M, Casanova M, Polastri D, et al. Relapse in medulloblastoma: what can be done after abandoning high-dose chemotherapy? A mono-institutional experience. Childs Nerv Syst. 2013;29(7):1107–1112. [DOI] [PubMed] [Google Scholar]
- 18. ClinVar: public archive of interpretations of clinically relevant variants. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/?term=10.1093%2Fnar%2Fgkv1222. Accessed May 11, 2020. [DOI] [PMC free article] [PubMed]
- 19. COSMIC: somatic cancer genetics at high-resolution. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/?term=10.1093%2Fnar%2Fgkw1121. Accessed May 11, 2020.
- 20. Pietrobono S, Stecca B. Targeting the oncoprotein smoothened by small molecules: focus on novel acylguanidine derivatives as potent smoothened inhibitors. Cells. 2018;7(12):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korshunov A, Okonechnikov K, Sahm F, et al. Molecular progression of SHH-activated medulloblastomas. Acta Neuropathol. 2019;138(2):327–330. [DOI] [PubMed] [Google Scholar]
- 22. Gröbner SN, Worst BC, Weischenfeldt J, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555(7696):321–327. [DOI] [PubMed] [Google Scholar]
- 23. Fomchenko EI, Erson-Omay EZ, Moliterno J. A novel finding of an IDH2 mutation in an interesting adult Sonic Hedgehog mutated medulloblastoma. J Neurooncol. 2019;144(1):231–233. [DOI] [PubMed] [Google Scholar]
- 24. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharpe HJ, Pau G, Dijkgraaf GJ, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015;27(3):327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharpe HJ, Wang W, Hannoush RN, de Sauvage FJ. Regulation of the oncoprotein smoothened by small molecules. Nat Chem Biol. 2015;11(4):246–255. [DOI] [PubMed] [Google Scholar]
- 27. Hoy SM. Glasdegib: first global approval. Drugs. 2019;79(2):207–213. [DOI] [PubMed] [Google Scholar]
- 28. Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15(5):866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson GW, Orr BA, Wu G, et al. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase ii pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kieran MW, Chisholm J, Casanova M, et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro Oncol. 2017;19(11):1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shou Y, Robinson DM, Amakye DD, et al. A five-gene hedgehog signature developed as a patient preselection tool for hedgehog inhibitor therapy in medulloblastoma. Clin Cancer Res. 2015;21(3):585–593. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Song Q, Day BW. Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis. Acta Neuropathol Commun. 2019;7(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172. [DOI] [PubMed] [Google Scholar]
- 34. Gajjar A, Stewart CF, Ellison DW, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res. 2013;19(22):6305–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110(7):1542–1550. [DOI] [PubMed] [Google Scholar]
- 36. Cefalo G, Massimino M, Ruggiero A, et al. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi-institutional phase II trial. Neuro Oncol. 2014;16(5):748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Teuff G, Castaneda-Heredia A, Dufour C, et al. ; European consortium Innovative Therapies for Children with Cancer (ITCC) . Phase II study of temozolomide and topotecan (TOTEM) in children with relapsed or refractory extracranial and central nervous system tumors including medulloblastoma with post hoc Bayesian analysis: a European ITCC study. Pediatr Blood Cancer. 2020;67(1):e28032. [DOI] [PubMed] [Google Scholar]
- 38. Grill J, Geoerger B, Gesner L, et al. ; European Consortium Innovative Therapies for Children with Cancer (ITCC) and the European Society for Paediatric Oncology (SIOPE) Brain Tumor Group . Phase II study of irinotecan in combination with temozolomide (TEMIRI) in children with recurrent or refractory medulloblastoma: a joint ITCC and SIOPE brain tumor study. Neuro Oncol. 2013;15(9):1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frappaz D, Sunyach MP, Le Rhun E, et al. Adolescent and young adults (AYAS) brain tumor national Web conference. On behalf of ANOCEF, GO-AJA and SFCE societies. Bull Cancer. 2016;103(12):1050–1056. [DOI] [PubMed] [Google Scholar]
- 40. D’Arcy CE, Nobre LF, Arnaldo A, et al. Immunohistochemical and nanoString-Based Subgrouping of Clinical Medulloblastoma Samples. J Neuropathol Exp Neurol. 2020;79(4):437–447. [DOI] [PubMed] [Google Scholar]
- 41. Northcott PA, Shih DJH, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwalbe EC, Williamson D, Lindsey JC, et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol. 2013;125(3):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. da Silva LS, Mançano BM, de Paula FE, et al. Expression of GNAS, TP53, and PTEN Improves the Patient Prognostication in Sonic Hedgehog (SHH) Medulloblastoma Subgroup. J Mol Diagnostics. 2020;22(7):957–966. [DOI] [PubMed] [Google Scholar]
- 44. Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petrirena GJ, Masliah-Planchon J, Sala Q, et al. Recurrent extraneural sonic hedgehog medulloblastoma exhibiting sustained response to vismodegib and temozolomide monotherapies and inter-metastatic molecular heterogeneity at progression. Oncotarget. 2018;9(11):10175–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pricl S, Cortelazzi B, Dal Col V, et al. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2015;9(2):389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gruber W, Hutzinger M, Elmer DP, et al. DYRK1B as therapeutic target in Hedgehog/GLI-dependent cancer cells with smoothened inhibitor resistance. Oncotarget. 2016;7(6):7134–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riobó NA, Lu K, Ai X, Haines GM, Emerson CP Jr. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103(12):4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Infante P, Alfonsi R, Botta B, Mori M, Di Marcotullio L. Targeting GLI factors to inhibit the Hedgehog pathway. Trends Pharmacol Sci. 2015;36(8):547–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.