Abstract
Background
Although the incidence of tuberculosis is higher in men than in women, the relationship of sex with tuberculosis treatment outcomes has not been adequately studied.
Methods
We performed a retrospective cohort study and a systematic review and meta-analysis of observational studies during the last 10 years to assess sex differences in clinical and microbiological outcomes in tuberculosis.
Results
In our cohort of 2894 Taiwanese patients with drug-susceptible pulmonary tuberculosis (1975 male and 919 female), male patients had higher adjusted hazards of 9-month mortality due to all causes (hazard ratio, 1.43 [95% confidence interval (CI), 1.03–1.98]) and infections (1.70 [1.09–2.64]) and higher adjusted odds of 2-month sputum culture positivity (odds ratio [OR], 1.56 [95% CI, 1.05–2.33]) compared with female patients. Smear positivity at 2 months did not differ significantly (OR, 1.27 [95% CI, .71–2.27]) between the sexes. Among 7896 articles retrieved, 398 were included in our systematic review describing a total of 3 957 216 patients. The odds of all-cause mortality were higher in men than in women in the pooled unadjusted (OR, 1.26 [95% CI, 1.19–1.34]) and adjusted (1.31 [1.18–1.45]) analyses. Men had higher pooled odds of sputum culture (OR, 1.44 [95% CI, 1.14–1.81]) and sputum smear (1.58 [1.41–1.77]) positivity, both at the end of the intensive phase and on completion of treatment.
Conclusions
Our retrospective cohort showed that male patients with tuberculosis have higher 9-month all-cause and infection-related mortality, with higher 2-month sputum culture positivity after adjustment for confounding factors. In our meta-analysis, male patients showed higher all-cause and tuberculosis-related mortality and higher sputum culture and smear positivity rates during and after tuberculosis treatment.
Keywords: culture, female, HIV, mortality, sputum
Our retrospective cohort of Taiwanese patients with pulmonary tuberculosis and systematic review showed that male sex is associated with higher all-cause and infection-related mortality and higher sputum culture and acid-fast bacilli smear positivity during and after tuberculosis treatment.
The World Health Organization reported nearly 10 million new cases of active tuberculosis in 2019 [1]. Compared with women, men have a 1.8-fold higher incidence of active tuberculosis [1, 2]. The tuberculosis prevalence-to-notification ratio is also much higher in men [3, 4]. These sex disparities exist irrespective of geographic locale [5].
Many potential medical and cultural confounding factors, such as the increased prevalence of diabetes, alcohol use, and smoking among men, and decreased access to healthcare among women, may account for sex differences in tuberculosis [1, 5]. Men have higher sputum bacterial loads and more severe tuberculosis-related lung disease seen at imaging than women [6, 7]. These clinical observations are supported by animal models showing more extensive lung disease, higher lung bacterial burdens, and accelerated mortality in males after Mycobacterium tuberculosis infection [8, 9]. Although prior population-based studies have highlighted sex differences in tuberculosis outcomes [10, 11], the findings are often unclear and inconsistent because of the inherent heterogeneity of study populations and the difference in the level of care between the sexes in low-income countries. In additional, many studies do not report sex-disaggregated data.
We analyzed a retrospective cohort of Taiwanese pulmonary patients with tuberculosis to better understand the role of sex on mortality and sputum microbiological status after tuberculosis treatment initiation, after adjusting for confounders. We also performed a systematic review and meta-analysis, given the lack of generalizability from individual cohort studies, and to assess the impact of other important parameters, including human immunodeficiency virus (HIV) coinfection status, site of tuberculosis involvement, drug resistance, duration of follow-up, and study country on the effect of sex differences.
METHODS
Retrospective Cohort Study
Study Design and Population
Our retrospective cohort consisted of adult patients (aged >18 years) with drug-susceptible pulmonary tuberculosis, treated according to the American Thoracic Society guidelines [12], enrolled at the National Taiwan University Hospital in Taipei from 2000 to 2016 [13]. All patients were sputum culture-positive at baseline by either MGIT-960 or Lowenstein-Jensen medium. There were no exclusion criteria. The institutional review boards at Johns Hopkins University and National Taiwan University Hospital approved the study. The methods are described in greater detail in the Supplementary Materials (Section Ia).
Exposure and Outcomes
To ascertain biological sex differences, we considered male sex as the exposure group and female sex as the comparison group. The primary outcomes were 9-month all-cause and infection-related mortality after initiation of antituberculosis therapy (ATT). Infection-related mortality was a composite outcome of deaths due to pneumonia, sepsis, and tuberculosis. Secondary outcomes were positivity of sputum cultures and sputum smear acid-fast bacilli (AFB) by microscopy at 2 months after ATT initiation.
Statistical Analysis
Participant characteristics stratified by sex were compared using χ 2 tests for categorical variables, and 2-sided t and Mann-Whitney U tests for normally and nonnormally distributed continuous variables, respectively. Kaplan-Meier analysis was performed to evaluate the survival probability of all-cause and infection-related mortality between the sexes. Cox proportional hazards models were used to measure the association between sex and all-cause and infection-related mortality in separate univariable models. Person-time at risk of outcome stratified by sex was calculated from the time of tuberculosis treatment initiation up to 9 months or loss to follow-up or death, whichever occurred first. Separate bivariable models assessed the interaction between sex and major factors, such as age, body mass index, smoking status, and cavitary disease, and AFB smear at baseline was assessed using multiplicative interaction terms. Multivariable Cox regression was designed to adjust for confounding variables identified a priori through literature review, by simultaneously including them in the model. We used Charlson comorbidity index (CCI) as the composite index to account for the comorbid conditions in the multivariable model. Variables that are components of the CCI were not separately adjusted for. The association of sex with sputum culture and smear positivity at 2 months was analyzed using univariable and multivariable logistic regression. Potential confounders for multivariable analyses were identified, as described above.
Systematic Review
Search Strategy and Study Selection
The systematic review was conducted according to the PRISMA guidelines [14], using the Covidence platform [15]. We searched PubMed, Embase, and the Web of Science on 15 August 2020, for English-language research articles using the search strategy detailed in the Supplementary Materials (Section Ib) for studies published in the last 10 years.
Studies were required to report sex-disaggregated data on ≥1 of the following outcomes on adult patients with tuberculosis receiving multidrug ATT: all-cause mortality, mortality due to tuberculosis, sputum AFB smear, or culture positivity during or at the end of tuberculosis treatment, or ‘treatment success’ according to the World Health Organization definitions for reporting tuberculosis outcomes [16]. We included prospective and retrospective cohort studies and case-control studies. After removing the duplicates, the titles and abstracts screening followed by full-text screening of the retrieved articles were screened by at least 2 authors (V. C., N. L. T., M. G. M., A. K., or P. N.) independently, and the disagreements were resolved by V. C.
Data Extraction and Quality Assessment
At least 2 authors extracted data from the articles (V. C, N. L. T., S. K. A., R. K. S., E. P. W., E. J. A., S. W., or A. Z.) in the Qualtrics platform [17], and V. C. resolved discrepancies. Data on study country, funding source, patient comorbid conditions, site of tuberculosis involvement, pattern of resistance to ATT, HIV-tuberculosis coinfection, and time points for outcomes were extracted. Data on treatment outcomes were extracted from studies as either raw data or precalculated effect sizes, namely, odds ratio (OR), relative risk, or hazard ratio (HR), along with 95% confidence intervals (CIs). We performed quality assessment using the Newcastle-Ottawa scale (NOS) for observational studies [18].
Data Analysis
For each outcome, we performed a random-effects meta-analysis of the ORs. We pooled HRs separately for each of the outcomes. Differences were considered significant at P <.05 (2 sided0. We assessed heterogeneity using the I2 statistic. When the I2 was >60%, we performed subgroup analyses and meta-regression (detailed in the Supplementary Materials, Section II) with respect to HIV-tuberculosis coinfection status, resistance to ATT, extrapulmonary involvement, time of outcome assessment, study country’s income status classification according to the World Bank [19],and incidence of tuberculosis infection and HIV-tuberculosis coinfection [20]. Publication bias was assessed by means of funnel plot and Egger’s test. We performed statistical analyses using Stata/IC 16.0 software (StataCorp) [21]. The study protocol is registered with PROSPERO (no. CRD42020219050).
RESULTS
Retrospective Cohort
Baseline Characteristics
In our cohort of 2894 patients with culture-confirmed, drug-susceptible pulmonary tuberculosis, 1975 (68.2%) were male and 919 (31.8%) were female (Table 1). Male patients had a higher median age than female patients (68.9 vs 58.2 years; P < .001). Compared with female patients, higher proportions of male patients had comorbid conditions, a history of smoking, and alcohol abuse. Men had a greater proportion of cavitary disease (15.7% vs 11.1%; P < .001) but similar AFB smear positivity at diagnosis (Table 1). Baseline characteristics are described in detail in the Supplementary Materials, Section Ia.
Table 1.
Characteristics of the Study Participants in the Retrospective Cohort From Taiwan, Stratified by Sex (N = 2894)
| Participants, No. (%)a | |||||
|---|---|---|---|---|---|
| Characteristic | Participants With Available Data, No. | Total (N = 2894) | Male (n = 1975) | Female (n = 919) | P Value |
| Age, median (IQR), y | 2894 | 66.6 (49.1–77.8) | 68.9 (53.4–78.8) | 58.2 (38.2–75.4) | <.001 |
| BMI, mean (SD)b | 2178 | 21.3 (4.2) | 21.4 (4.4) | 21.0 (3.6) | .049 |
| Ever smoker | 2296 | 930 (40.5) | 884 (56.1) | 46 (6.4) | <.001 |
| Alcohol abuse | 2894 | 81 (2.8) | 79 (4.0) | 2 (0.2) | <.001 |
| Diabetes mellitus | 2888 | 533 (18.46) | 403 (20.4) | 130 (14.2) | <.001 |
| Hypertension | 2894 | 1052 (36.4) | 759 (38.4) | 293 (31.9) | .001 |
| CVD | 2894 | 352 (12.2) | 265 (13.4) | 87 (9.5) | .002 |
| COPD | 2894 | 451 (15.6) | 374 (18.9) | 77 (8.4) | <.001 |
| Cancer | 2888 | 459 (15.9) | 369 (18.7) | 90 (9.8) | <.001 |
| Cirrhosis | 2894 | 5 (1.7) | 43 (2.2) | 7 (0.8) | .007 |
| Transplant recipient | 2894 | 26 (0.9) | 18 (0.9) | 8 (0.9) | .91 |
| HIV | 2894 | 65 (2.3) | 64 (3.2) | 1 (0.1) | <.001 |
| CCI, median (IQR) | 2894 | 4 (2–6) | 4 (2–6) | 3 (1–5) | <.001 |
| Sputum smear AFB positivity at baseline | 2842 | 1215 (42.75) | 820 (42.40) | 395 (43.50) | .58 |
| Smear grade at baseline, mean (SD) | 2842 | 0.99 (1.36) | 1.00 (1.39) | 0.96 (1.32) | .50 |
| Prior tuberculosis | 1598 | 98 (6.1) | 75 (6.84) | 23 (4.58) | .08 |
| Cavitary disease | 2894 | 413 (14.27) | 311 (15.75) | 102 (11.09) | <.001 |
Abbreviations: AFB, acid-fast bacilli; BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified.
bBMI was calculated as weight in kilograms divided by height in meters squared.
All-Cause and Infection-Related Mortality
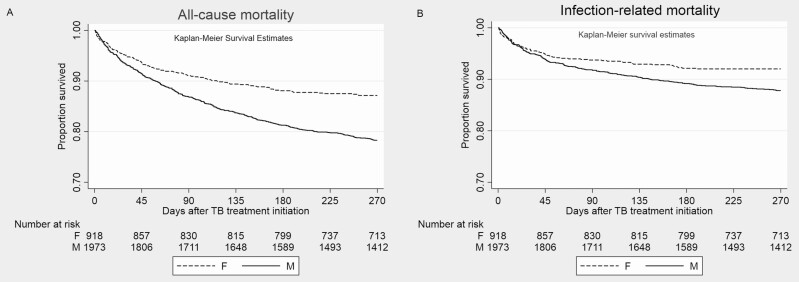
In the univariable Cox regression analysis, male sex was associated with higher hazards of 9-month all-cause mortality (HR, 1.75 [95% CI, 1.42–.2.14]) and infection-related mortality (1.52 [1.17–1.99]). Infection-related causes accounted for 55.7% of all deaths (303 of 544) in the first 9 months (Table 2). Details of the bivariable Cox models are detailed in the Supplementary Materials (Supplementary Table 1A–1E). In the multivariable Cox regression analysis obtained by simultaneously adjusting for the following parameters: body mass index, CCI, hypertension, transplantation status, alcoholism, smoking, cavitary disease, and baseline AFB (Table 2 and Supplementary Table 1F and 1G), male patients had an adjusted HR of 1.53 (95% CI, 1.08–2.17) for all-cause mortality (Figure 1A) and an adjusted HR of 1.81 (95% CI, 1.11–2.93) for infection-related mortality (Figure 1B). Although age is a component of CCI, we also adjusted for age in a separate model, which yielded similar results (Supplementary Materials, Section III, and Supplementary Table 1H).
Table 2.
Association of Male Sex With Tuberculosis Treatment Outcomes in the Retrospective Cohort Using Cox and Logistic Regression Models (N = 2894)
| Mortality, No. of Deaths/Total No. in Population (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total | Men | Women | Estimate | Unadjusted Effect Sizea (95% CI) | P Value | Adjusted Effect Sizeb (95% CI) | P Value |
| All-cause mortality (n = 2667) | 544/2667 (20.4) | 427/1837 (23.2) | 117/830 (14.1) | HR | 1.75 (1.42–2.14) | <.001 | 1.53 (1.08–2.17) | .03 |
| Infection related mortality (n = 2667) | 303/2667 (11.4) | 231/1837 (12.6) | 72/830 (8.7) | HR | 1.52 (1.17–1.99) | .002 | 1.81 (1.11–2.93) | .009 |
| Sputum culture positivity at 2 mo (n = 1640) | 265 (16.2) | 203 (18.4) | 62 (11.6) | OR | 1.72 (1.27–2.34) | .003 | 1.67 (1.06–2.63) | .03 |
| Sputum smear AFB at 2 mo (n = 1640) | 118 (7.2) | 91 (8.3) | 27 (5.0) | OR | 1.70 (1.09–2.63) | .02 | 1.30 (.67–2.55) | .42 |
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aUnadjusted effect size obtained from the univariable model.
bAdjusted for body mass index, Charlson comorbidity index (CCI), chronic obstructive pulmonary disease, alcoholism, smoking, and cavitary disease at baseline. The variables were selected because they differed significantly between male and female participants (see Table 1). The components of the CCI were not adjusted for separately.
Figure 1.
Kaplan-Meier survival graphs for all-cause (A) mortality and infection-related (B) mortality among patients treated for tuberculosis in the retrospective cohort. M = Males, F = Females.
Sputum Culture and Smear AFB Positivity
Male patients had significantly higher sputum culture (18.4% vs 11.6%; P = .003) and AFB smear (8.3% vs 5.0%, P = .02) positivity at 2 months compared with female patients, by χ 2 test, with a considerable difference primarily among individuals <50 years old (Supplementary Figure A). In the univariable logistic regression analysis, male sex was associated with higher odds of 2-month sputum culture (OR, 1.73 [95% CI, 1.27–2.35]) and sputum smear (1.70 [1.09–2.63]) positivity. Details of the bivariable logistic models are detailed in the Supplementary Materials (Supplementary Tables 1A–1E). In the multivariable logistic regression analysis obtained by adjusting for parameters described above (Table 2 and Supplementary Table 1F and 1G), male patients had adjusted ORs of 1.67 (95% CI, 1.06–2.63) for sputum culture positivity and 1.30 (.67–2.55) for sputum smear positivity. Sensitivity analysis also adjusting for age yielded similar results (Supplementary Table 1H).
Systematic Review
Characteristics of Included Studies and Quality Assessment
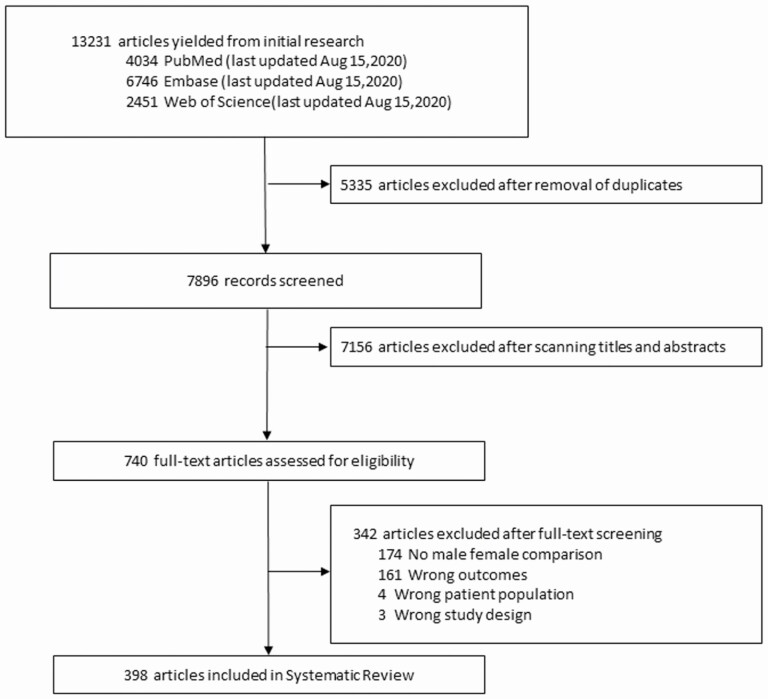
Among the 7896 studies screened, data from 398 studies, reporting on a total of 3 957 216 individuals, were analyzed in our review (Figure 2 and Supplementary Table 2). Characteristics of included studies are described in greater detail in the Supplementary Materials, Section II. Quality assessment using the NOS revealed that 2 studies (0.5%) scored the maximum of 9 points, 190 (47.7%) scored 8, 182 (45.7%) scored 7, and 11 (2.7%) scored 6. A total of 13 studies were of low quality (NOS, ≤5) (Supplementary Table 2).
Figure 2.
Study selection for systematic review.
All-Cause Mortality and Subgroup Analysis
Male patients had higher unadjusted pooled effect sizes for mortality compared with female patients, with a pooled OR of 1.26 (95% CI, 1.19–1.34) from 135 studies (I2 = 77.9%), and a pooled HR of 1.17 (1.07–1.27) from 44 studies (I2 = 64.5%) (Table 3). Eighty-two of the 197 studies reporting all-cause mortality made adjustments for ≥1 confounding variable (Supplementary Table 3). The pooled adjusted OR was 1.26 (95% CI, 1.16–1.37) among 52 studies (I2 = 90.9%), and the pooled adjusted HR was 1.20 (95% CI, 1.05–1.37) among 30 studies (I2 = 82.5%) (Table 3).
Table 3.
Association of Male Sex With All-Cause Mortality Among Patients With Tuberculosis, Using Random Effects Meta-analysis
| All-Cause Mortality | ||||||
|---|---|---|---|---|---|---|
| Characteristic | No. of Studies | OR (95% CI) | I 2 Statistic | No. of Studies | HR (95% CI) | I 2 Statistic |
| Unadjusted | ||||||
| All studies | 135 | 1.26 (1.19–1.34) | 77.90 | 44 | 1.17 (1.07–1.27) | 63.59 |
| During treatment | 122 | 1.26 (1.19–1.34) | 71.30 | 26 | 1.16 (1.06–1.27) | 59.72 |
| General setting | 111 | 1.26 (1.19–1.35) | 77.39 | 25 | 1.18 (1.05–1.30) | 66.87 |
| ICU setting | 11 | 0.92 (.71–1.18) | 0 | 1 | 2.57 (.73–9.08) | … |
| During follow-up | 13 | 1.39 (1.09–1.77) | 78.32 | 18 | 1.14 (1.00–1.30) | 60.27 |
| Adjusted | ||||||
| All studies | 52 | 1.31 (1.18–1.45) | 74.80 | 30 | 1.19 (1.05–1.67) | 82.5 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio.
aDuring follow-up after completion of tuberculosis treatment.
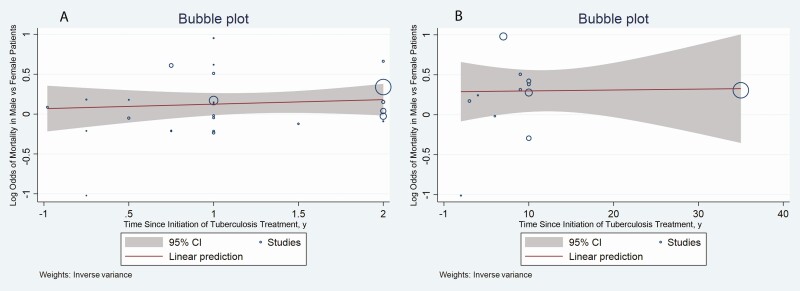
Considerable heterogeneity was present in the meta-analyses of ORs and HRs for all-cause mortality. Studies reporting mortality rates for patients with tuberculosis in intensive care units showed no significant differences in mortality when stratified by sex (Table 3). Further subgroup analyses were performed after excluding studies reporting outcomes in patients receiving intensive care (Table 4). Meta-regression analysis showed that the time point used for assessing mortality did not change the association between male sex and all-cause mortality (Figure 3). Subgroup and meta-regression analyses for all-cause mortality are detailed in the Supplementary Materials (Sections II and III and Supplementary Table 4).
Table 4.
Subgroup Analysis of Pooled Effect Sizes for the Association of Male Sex With All-Cause Mortality Among Patients With Tuberculosis
| Pooled ORsa | Pooled HRsa | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup Characteristics | No. of Studies | Unadjusted OR (95% CI) | I 2 Statistic | P Valueb | No. of Studies | Unadjusted HR (95% CI) | I 2 Statistic | P Valueb |
| Income status of country | ||||||||
| Low | 18 | 1.19 (1.03–1.38] | 27.6 | .06 | 7 | 1.17 (1.02–1.35) | 44.7 | .02 |
| Middle | 60 | 1.19 (1.09–1.32) | 75.4 | 21 | 1.06 (.94–1.19) | 55.9 | ||
| High | 41 | 1.39 (1.26–1.52) | 80.1 | 13 | 1.34 (1.20–1.56) | 48.3 | ||
| Tuberculosis burden | ||||||||
| High | 65 | 1.19 (1.09–1.30) | 74.2 | .03 | 23 | 1.08 (.98–1.20) | 57.1 | .031 |
| Low | 54 | 1.37 (1.26–1.49) | 74.9 | 18 | 1.30 (1.14–1.49) | |||
| HIV-tuberculosis burden | ||||||||
| High | 66 | 1.19 (1.09–1.36) | 73.9 | .03 | 24 | 1.10 (.99–1.21) | 58.2 | .08 |
| Low | 53 | 1.37 (1.25–1.50) | 75.3 | 17 | 1.28 (1.12–1.47) | 56.8 | ||
| Continent | ||||||||
| Africa | 44 | 1.19 (1.08–1.30) | 43.1 | <.001 | 17 | 1.14 (1.03–1.25) | 48.9 | .004 |
| Asia | 48 | 1.32 (1.19–1.47) | 76.9 | 17 | 1.19 (1.01–1.41) | 68.5 | ||
| Europe | 11 | 1.41 (1.35–1.47) | 0 | 2 | 2.35 (1.04–5.28) | 22.5 | ||
| North America | 9 | 1.16 (.96–1.40) | 23.6 | 4 | 1.38 (1.17–1.62) | 0 | ||
| South America | 6 | 0.97 (.94–1.01) | 0 | 3 | 0.78 (.61–1.00) | 0 | ||
| Australia/Pacific | 2 | 2.13 (1.72–2.63) | 0 | … | … | … | ||
| Drug resistance pattern of tuberculosis | ||||||||
| Drug sensitive | 24 | 1.31 (1.13–1.52) | 62.6 | .88 | 1 | 1.75 (1.43–2.15) | 0 | .05 |
| Drug resistant | 23 | 1.18 (1.03–1.34) | 46.5 | 9 | 1.23 (1.04–1.61) | 10 | ||
| Multidrug resistant | 11 | 1.26 (1.01–1.57) | 50.2 | 6 | 1.23 (.98–1.62) | 15 | ||
| Extensively drug resistant | 1 | 1.19 (.64–2.21) | … | 1 | 0.86 (.54–1.37) | … | ||
| Tuberculosis-HIV coinfection | ||||||||
| HIV negative | 4 | 1.55 (1.12–2.16) | 32.15 | .03 | 3 | 1.53 (1.22–1.90) | 24.4 | .005 |
| HIV positive | 21 | 0.97 (.94–1.01) | 42.98 | 14 | 1.04 (.89–1.21) | 44.0 | ||
| Tuberculosis site | ||||||||
| Pulmonary | 29 | 1.36 (1.16–1.59) | 76.4 | .29 | 12 | 1.29 (1.07–1.56) | 68.7 | .12 |
| Extrapulmonary | 11 | 1.13 (.94–1.36) | 0 | 2 | 1.00 (.77–1.30) | 0 | ||
| DOTS implementation in study country | ||||||||
| Implemented | 111 | 1.33 (1.15–1.43) | 74.9 | .09 | 35 | 1.15 (1.07–1.26) | 58.7 | .60 |
| Not implemented | 19 | 1.47 (1.21–1.60) | 66.9 | 5 | 1.25 (.93–1.68) | 73.2 | ||
Abbreviations: CI, confidence interval; DOTS, directly observed treatment, short-course; HIV, human immunodeficiency virus; HR, hazard ratio; OR, odds ratio.
aStudies reporting outcomes in intensive care unit settings were excluded from the subgroup analyses.
b P values pertain to the difference in the ORs and HRs for mortality in male patients among the subgroups.
Figure 3.
Bubble plots showing the association between log odds of mortality in male compared with female patients and the time of assessment of mortality, during tuberculosis treatment (A) and during follow-up (B). Abbreviation: CI, confidence interval.
Mortality Due to Tuberculosis
We included 12 studies that reported death due to tuberculosis (Supplementary Table 7). The pooled OR for death due to tuberculosis for male compared with female patients was 1.28 (95% CI, 1.1–1.6) in 10 studies, and the pooled HR was 1.34 (1.09–1.55) in 2 studies.
Microbiological Outcomes
Compared with female patients, male patients had higher pooled unadjusted and adjusted ORs for sputum culture positivity after 2 or 3 months of ATT (Table 5). Among studies reporting HR for sputum culture conversion to negativity, men had a pooled unadjusted HR of 0.83 (95% CI, .70–.97) and a pooled adjusted HR of 0.76 (.59–.97), compared with women (Supplementary Materials, Section IV). With respect to sputum AFB smear positivity, men had higher pooled unadjusted and adjusted ORs compared with women, both at the end of the intensive phase and at treatment completion (Table 5). The odds of treatment success were lower in male patients in the meta-analysis of both adjusted and unadjusted effect sizes (Supplementary Table 5). Data on sputum culture or AFB smear positivity had very low heterogeneity and thus did not warrant further subgroup analysis.
Table 5.
Association of Male Sex With Sputum Smear and Sputum Culture Positivity Among Patients With Tuberculosis, Using Random Effects Meta-analysis
| Unadjusted ORs | Adjusted ORs | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome and Time of Assessment | No. of studies | Effect Size (95% CI) | I 2 Statistic | P Valuea | No. of studies | Effect Size (95% CI) | I 2 Statistic | P Valuea |
| Sputum culture for AFB | ||||||||
| All studies | 13 | 1.60 (1.28–1.99) | 24.6 | … | 4 | 1.81 (1.39–2.34) | 0 | … |
| End of intensive phase | 13 | 1.60 (1.28–1.99) | 24.6 | 4 | 1.81 (1.39–2.34) | 0 | ||
| End of treatment | … | … | … | … | … | … | ||
| Sputum smear for AFB | ||||||||
| All studies | 29 | 1.40 (1.24–1.58) | 21.06 | .06 | 6 | 1.45 (1.31–1.61) | 0 | .48 |
| End of intensive phase | 6 | 1.58 (1.41–1.77) | 0 | 3 | 1.42 (1.26–1.60) | 0 | ||
| End of treatment | 22 | 1.23 (1.02–1.48) | 28.27 | 3 | 1.51 (1.16–1.98) | 26.06 | ||
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; OR, odds ratio.
a P values pertain to the difference in the ORs for male patients between the subgroups (end of intensive phase and end of treatment).
Meta-Regression Analysis
In the meta-regression analysis, the mean age of the population and the proportions of patients with diabetes mellitus, hypertension, alcohol abuse, smoking, cardiovascular events, chronic obstructive pulmonary disease, and HIV did not significantly modify the association between male sex and any of the outcomes assessed (Supplementary Table 6). Publication bias measured by Egger’s test was not present for any of the outcomes (Supplementary Materials, Section IV). The forest, funnel, and bubble plots from the analysis are available in Supplementary Figures 1–19.
DISCUSSION
Findings from our retrospective cohort and systematic review, comprising a total of 398 peer-reviewed publications from more than 80 high-, middle-, and low-income countries, suggest that male sex is associated with higher all-cause mortality and death due to tuberculosis, both during treatment and during follow-up after ATT. This association held true even after adjusting for confounders in the cohort study and during meta-regression analysis in the systematic review. Subgroup analysis revealed no impact by the drug susceptibility or the site of tuberculosis disease on the higher mortality rates in male patients. Male patients also had higher positivity for M. tuberculosis by both AFB smear microscopy and culture at the end of the intensive phase, as well as at ATT completion.
Women in low-and middle-income countries tend to have greater patient-related and healthcare system delays in tuberculosis diagnosis and treatment initiation [22–24], owing due to financial dependence, fear of social isolation, and initial access to less qualified providers [25–27]. Although our cohort did not include information on patients’ economic status and healthcare delays the patients, we expect these factors to have minimal impact on our study, because Taiwan is a high-income country with universal health insurance. This was supported by the subgroup analysis in our systematic review, which showed higher ORs for mortality in men compared with women in high-income than in low-or middle-income countries (Table 4). This suggests that the observed sex differences are clearer in high-income countries owing to minimal differences in healthcare access between the sexes.
Despite evidence of earlier diagnostic testing and treatment initiation in male compared with female patients [7, 28], male patients have significantly higher rates of hemoptysis, abnormal chest radiographs, and more severe lung damage at computed tomography and higher odds of tuberculosis-related and all-cause mortality [7, 28]. In addition, compared with women, men show delayed radiological response with computed tomography after ATT [7]. These observations are consistent with the hypothesis that men exhibit more severe tuberculosis disease at presentation and have worse outcomes despite better healthcare access and prompt initiation of treatment.
In our cohort, men had disproportionately high rates of smoking and cavitary disease than women. Prior studies have shown higher proportions of cavitary disease in men compared with women with similar smoking histories [6, 29], which may be explained by increased expression of matrix metalloproteinase in the lungs in men [30]. In our cohort, men had higher odds of 2-month sputum culture positivity, after adjustment for smoking and presence of cavitary disease at baseline. Our systematic review also showed higher pooled unadjusted and adjusted odds of sputum AFB smear and culture positivity in male patients after tuberculosis treatment initiation. These findings highlight the potential biological role of male sex in unfavorable microbiological outcomes.
Male sex has been shown to predispose to other infectious diseases [31–36]. Although tuberculosis risk factors causing immune dysregulation, such as HIV [37] and malnutrition [38], have been well characterized, sex as a risk factor for tuberculosis disease has been understudied. Studies have shown more severe disease in male mice after infection with M. tuberculosis and nontuberculous mycobacteria, manifested by higher lung bacillary burdens and increased mortality rates [8, 9, 39]. However, the molecular basis for these phenotypic differences remains to be elucidated.
Recently, sex hormones were shown to differentially regulate female and male immune responses to various pathogens [40]. Testosterone was shown to reduce antimicrobial activity and promote mycobacterial growth [41, 42], reduce the expression of Toll-like receptor (TLR) 4 in mouse macrophages [43] and to have immunosuppressive effects by increasing inhibitory cytokines, reducing immunoglobulin production, and inhibiting T-cell and B-cell maturation [44–47]. Androgens are also known to induce an interleukin 4–driven M2 alveolar macrophage differentiation [48]. Signaling through the androgen receptor can increase monocyte recruitment by CCL2 and CXCL1 [49], potentially driving more extensive cavitary disease in men. In contrast, estradiol has been shown to enhance macrophage activation [50]. Sex-based differences in baseline activation of mammalian target of rapamycin complex have been reported [51, 52], and dysregulation of this pathway may be postulated to result in up-regulation of lipid accumulation [53], inhibition of autophagy [54], and increased cell necrosis, leading to a proinflammatory environment and increased cavitation in the lungs of men with tuberculosis. The human X chromosome–linked genes that encode effectors in immune responses, including TLR7, TLR8, interleukin 1 receptor–associated kinase, CD40 ligand, and Forkhead box protein P3 (FOXP3), have been implicated in sex differences in immune responses and tissue damage after infection [55]. It is possible that X-linked genes in females escape silencing, leading to higher expression of host-protective immune-related genes [56].
In our meta-analysis, male patients had a higher pooled OR for mortality among studies including exclusively HIV-negative patients with tuberculosis than in studies including patients with HIV-tuberculosis coinfection. Prior literature has shown that among HIV-negative patients, men had worse outcomes than women, while among tuberculosis-HIV–coinfected patients, women had worse outcomes [57, 58]. Thus, HIV coinfection appears to reduce the potential immunomodulatory protective effect of female sex on tuberculosis treatment outcomes.
Our study has several strengths. The large sample size of our cohort aided in the analysis of various confounding factors. Because the cohort data were obtained from a single institution, heterogeneity in treatment decisions were minimized. Our systematic review of studies from 81 countries with variable healthcare access, allowed for generalizability of our results. The large number of studies on all-cause mortality in our systematic review enabled us to perform subgroup analyses and to arrive at various inferences.
Several limitations are noted. We were unable to assess parameters such as socioeconomic status, occupational history, and delay in diagnosis in our cohort. We did not have access to additional radiographic information, such as the volume of lung affected as a measure of severity for comparison between the sexes. In our systematic review, antibiotic susceptibilities, study setting, and timing of outcome assessment were not reported consistently among the included studies. Several studies in our review, performed from program registries, did not report data on baseline characteristics stratified by sex. During meta-analysis of adjusted effect sizes, individual studies were adjusted for comparable but different parameters. The treatment regimens followed may not be uniform across the included studies.
Our results have several important therapeutic implications. Recognizing the association between male sex and adverse treatment outcomes should inform medical decision making in tuberculosis treatment programs and motivate further focused research into the biological (immunological and genetic) basis of these sex-based differences, and, in turn, novel host-directed therapies to improve clinical, pathological, and microbiological outcomes during tuberculosis treatment in both sexes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. V. C. and P. C. K. conceived the idea for the review. V. C., N. L. T., M. G. M., J. B., and P. C. K. designed and undertook the literature review. V. C., N. L. T., M. G. M., S. K. A., A. K., P. N., E. P. W., and E. J. A. screened the articles and extracted the data (with help from S. W. and A. Z.). V. C, N. L. T., and M. G. M. performed the statistical analysis and prepared the figures and appendix. V. C., M. G. M., A. K., and R. K. S. analyzed and interpreted the data. V. C., M. G. M., J. R. C., A. K., R. K. S., and P. C. K. wrote the first draft of the manuscript, and V. C., A. G., J. Y. W., and P. C. K. revised the subsequent drafts. All authors reviewed the final draft of the manuscript.
Financial support. The cohort study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants UH3AI122309 and K24AI143447 to P. C. K.) and the Taiwan Centers for Disease Control (grant MOHW-105-CDC-C-114-000103 to J. Y. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Global tuberculosis report 2020. Available at: https://www.who.int/publications/i/item/9789240013131. Accessed 22 April 2021.
- 2. Global tuberculosis report 2019. Available at: https://www.who.int/teams/globaltuberculosis-%0Aprogramme/tb-reports/global-report-2019. Accessed 22 April 2021.
- 3. Horton KC, Macpherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low-and middle-income countries: a systematic review and meta-analysis. PLoS Med 2016; 13. Available at: https://pubmed.ncbi.nlm.nih.gov/27598345/. Accessed 22 June 2021. [DOI] [PMC free article] [PubMed]
- 4. Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis 2000; 4:123–32. [PubMed] [Google Scholar]
- 5. Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009; 6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu Y, Soodeen-Lalloo AK, Huang J, et al. Sex Disparity in Severity of Lung Lesions in Newly Identified Tuberculosis Is Age-Associated. Front Med 2019; 6. Available at: https://pubmed.ncbi.nlm.nih.gov/31380378/. Accessed 22 June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan W, Soodeen-Lalloo AK, Chu Y, et al. Sex influences the association between haemostasis and the extent of lung lesions in tuberculosis. Biol Sex Differ 2018; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dibbern J, Eggers L, Schneider BE. Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Sci Rep 2017; 7:10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bini EI, Mata Espinosa D, Marquina Castillo B, et al. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PLoS One 2014; 9:e93831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiménez-Corona ME, García-García L, Deriemer K, et al. Gender differentials of pulmonary tuberculosis transmission and reactivation in an endemic area. Thorax 2006; 61:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National tuberculosis prevalence survey 2016: Philippines.TB data hub. Available at: https://www.tbdiah.org/resources/publications/national-tuberculosis-prevalence-survey-2016-philippines/. Accessed 22 April 2021.
- 12. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chidambaram V, Gupte A, Wang JY, Golub JE, Karakousis PC. The impact of hypertension and use of calcium channel blockers on tuberculosis treatment outcomes. Clin Infect Dis 2020. Available at: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1446/5911212. Accessed 22 June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P) 2015 statement. Rev Esp Nutr Humana y Diet 2016; 20:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. Available at: http://www.covidence.org. Accessed 22 June 2021. [Google Scholar]
- 16. Definitions and reporting framework for tuberculosis—2013 revision. Available at: http://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf;jsessionid=271AAB60BC6304A676FCB894E8AEADCD?sequence=1. Accessed 21 May 2021.
- 17. Qualtrics XM. The leading experience management software. Available at: https://www.qualtrics.com/au/?rid=ip&prevsite=en&newsite=au&geo=IN&geomatch=au. Accessed 21 May 2021.
- 18. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, Ottawa Hospital Research Institute . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 21 May 2021.
- 19. World Bank. World Bank country and lending groups. World Bank data help desk. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 21 May 2021.
- 20. Use of high burden country lists for TB by WHO in the post-2015 era meeting of WHO’s Strategic and Technical Advisory Group for TB (STAG-TB), 2015. Available at: https://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf. Accessed 21 May 2021. [Google Scholar]
- 21. StataCorp. Stata statistical software: release 16. College Station TSL, 2019. Available at: https://www.stata.com/company/. [Google Scholar]
- 22. Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008; 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward J, Siskind V, Konstantinos A. Patient and health care system delays in Queensland tuberculosis patients, 1985–1998. Int J Tuberc Lung Dis 2001; 5:1021–7. [PubMed] [Google Scholar]
- 24. Cai J, Wang X, Ma A, Wang Q, Han X, Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS One 2015; 10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang WT, Gounder CR, Akande T, et al. Barriers and delays in tuberculosis diagnosis and treatment services: does gender matter? Tuberc Res Treat 2014; 2014:461935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson E, Long NH, Diwan VK, Winkvist A. Gender and tuberculosis control: perspectives on health seeking behaviour among men and women in Vietnam. Health Policy 2000; 52:33–51. [DOI] [PubMed] [Google Scholar]
- 27. Van Den Hof S, Najlis CA, Bloss E, Straetemans M.. A systematic review on the role of gender in tuberculosis control, 2010. Available at: https://www.kncvtbc.org/uploaded/2015/09/Role_of_Gender_in_TB_Control.pdf. Accessed 21 May 2021. [Google Scholar]
- 28. Dale K, Tay E, Trauer JM, Trevan P, Denholm J. Gender differences in tuberculosis diagnosis, treatment and outcomes in Victoria, Australia, 2002–2015. Int J Tuberc Lung Dis 2017; 21:1264–71. [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto K, Arima K, Komukai J, et al. The association between smoking and sputum smear-positive pulmonary tuberculosis in Osaka City [in Japanese]. Kekkaku 2012; 87:541–7. [PubMed] [Google Scholar]
- 30. Sathyamoorthy T, Sandhu G, Tezera LB, et al. Gender-dependent differences in plasma matrix metalloproteinase-8 elevated in pulmonary tuberculosis. PLoS One 2015; 10:e0117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blessmann J, Van Linh P, Nu PA, et al. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am J Trop Med Hyg 2002; 66:578–83. [DOI] [PubMed] [Google Scholar]
- 32. Acuna-Soto R, Maguire JH, Wirth DF. Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol 2000; 95:1277–83. [DOI] [PubMed] [Google Scholar]
- 33. Kadioglu A, Cuppone AM, Trappetti C, et al. Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J Infect Dis 2011; 204:1971–9. [DOI] [PubMed] [Google Scholar]
- 34. Addressing sex and gender in epidemic-prone infectious diseases. 2007. Available at: https://www.who.int/csr/resources/publications/SexGenderInfectDis.pdf. Accessed 21 May 2021.
- 35. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ 2020; 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One 2020; 15:e0241541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hertoghe T, Wajja A, Ntambi L, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin Exp Immunol 2000; 122:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin SJ, Sabina EP. Malnutrition and associated disorders in tuberculosis and its therapy. J Diet Suppl 2019; 16:602–10. [DOI] [PubMed] [Google Scholar]
- 39. Cadena AM, Flynn JL, Fortune SM. The importance of first impressions: early events in Mycobacterium tuberculosis infection influence outcome. mBio 2016; 7:e00342–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto Y, Tomioka H, Sato K, Saito H, Yamada Y, Setogawa T. Sex differences in the susceptibility of mice to infection induced by Mycobacterium intracellulare. Am Rev Respir Dis 1990; 142:430–3. [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun 1991; 59:4089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008; 78:432–7. [DOI] [PubMed] [Google Scholar]
- 44. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005; 11:411–23. [DOI] [PubMed] [Google Scholar]
- 45. Angele MK, Knöferl MW, Schwacha MG, et al. Sex steroids regulate pro-and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol 1999; 277:C35–42. [DOI] [PubMed] [Google Scholar]
- 46. Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 2001; 167:2060–7. [DOI] [PubMed] [Google Scholar]
- 47. Zhao Y, Ying H, Demei J, Xie J. Tuberculosis and sexual inequality: the role of sex hormones in immunity. Crit Rev Eukaryot Gene Expr 2012; 22:233–41. [DOI] [PubMed] [Google Scholar]
- 48. Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol 2018; 201:2923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Volpato S, Palmieri E, Fellin R, Zuliani G. Acute phase markers are associated with reduced plasma lipid levels in a population of hospitalized elderly patients. Gerontology 2000; 46:22–7. [DOI] [PubMed] [Google Scholar]
- 50. Calippe B, Douin-Echinard V, Laffargue M, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol 2008; 180:7980–8. [DOI] [PubMed] [Google Scholar]
- 51. De Mello NP, Andreotti DZ, Orellana AM, Scavone C, Kawamoto EM. Inverse sex-based expression profiles of PTEN and Klotho in mice. Sci Rep 2020; 10. Available at: https://pubmed.ncbi.nlm.nih.gov/33214645/. Accessed 22 May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Congdon EE. Sex differences in autophagy contribute to female vulnerability in Alzheimer’s disease. Front Neurosci 2018; 12:372. Available at: https://pubmed.ncbi.nlm.nih.gov/29988365/. Accessed 22 May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guerrini V, Prideaux B, Blanc L, et al. Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog 2018; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 2010; 584:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 2019; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR Jr. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis 2013; 19:416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oshi SN, Alobu I, Ukwaja KN, Oshi DC. Investigating gender disparities in the profile and treatment outcomes of tuberculosis in Ebonyi state, Nigeria. Epidemiol Infect 2015; 143:932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.