Abstract
Meningiomas are the most common intracranial tumors. Yet, only few controlled clinical trials have been conducted to guide clinical decision making, resulting in variations of management approaches across countries and centers. However, recent advances in molecular genetics and clinical trial results help to refine the diagnostic and therapeutic approach to meningioma. Accordingly, the European Association of Neuro-Oncology (EANO) updated its recommendations for the diagnosis and treatment of meningiomas. A provisional diagnosis of meningioma is typically made by neuroimaging, mostly magnetic resonance imaging. Such provisional diagnoses may be made incidentally. Accordingly, a significant proportion of meningiomas, notably in patients that are asymptomatic or elderly or both, may be managed by a watch-and-scan strategy. A surgical intervention with tissue, commonly with the goal of gross total resection, is required for the definitive diagnosis according to the WHO classification. A role for molecular profiling including gene panel sequencing and genomic methylation profiling is emerging. A gross total surgical resection including the involved dura is often curative. Inoperable or recurrent tumors requiring treatment can be treated with radiosurgery, if the size or the vicinity of critical structures allows that, or with fractionated radiotherapy (RT). Treatment concepts combining surgery and radiosurgery or fractionated RT are increasingly used, although there remain controversies regard timing, type, and dosing of the various RT approaches. Radionuclide therapy targeting somatostatin receptors is an experimental approach, as are all approaches of systemic pharmacotherapy. The best albeit modest results with pharmacotherapy have been obtained with bevacizumab or multikinase inhibitors targeting vascular endothelial growth factor receptor, but no standard of care systemic treatment has been yet defined.
Keywords: meningioma, molecular pathology, neuropathology, radiosurgery, surgery
Key Points.
Observation is the first option in incidental, asymptomatic, suspected meningiomas.
Surgical resection is the first option for growing or symptomatic tumors.
Radiosurgery or fractionated radiotherapy may be complementary therapies or even alternative approaches to surgery in certain situations.
Molecular diagnostics are developing rapidly. Tissue asservation for molecular diagnostics and future targeted therapies is highly recommended.
In 2016, the European Association of Neuro-Oncology (EANO) issued its first guideline on the diagnosis and treatment of meningiomas.1 Since then, the level of evidence for diagnostic and therapeutic decisions has increased in various areas. Numerous reports about molecular genetics of meningiomas of different WHO grades provided valuable insight into meningioma biology and clinical behavior. Data from controlled clinical studies have become available and the new WHO classification of 2021 reshapes the diagnostic approach to meningioma. Accordingly, a task force of the EANO was mandated to reevaluate the current literature on meningiomas and to update the guidelines on the diagnosis and therapy of these tumors.
Methods
All clinical disciplines, which are involved in the diagnosis and treatment of meningiomas are represented in the EANO task force. As a first step, representatives of these disciplines in different European countries were evaluated regarding their clinical and scientific activities and guideline expertise by the EANO guidelines committee and invited to join the task force. Specialists in Neuroradiology, Neurosurgery, Neuropathology, Radiation Oncology, and Medical Neurooncology were invited. Next, the focus areas of the updated guideline and sensitive and specific keywords as well as the combination of keywords were defined by the guideline committee. The main keywords were: chemotherapy, clinical presentation, cognition, epidemiology, histopathology, immunotherapy, medical therapy, magnetic resonance imaging (MRI), meningioma, microsurgery, molecular pathology, neurocognition, neuropathology, pharmacotherapy, positron emission tomography (PET), prognosis, radiation therapy, radiosurgery, risk factors, quality of life, radiosurgery, skull base tumor, supportive therapy, surgery.
The focus areas were distributed to single authors or groups of authors respecting their clinical profession and scientific profile. The individual authors searched the Medline database, the Cochrane Library, Embase Ovid, Cancer Net, and Science Citation Index, using the defined keywords, from May 2016 to May 2020. Inclusion of a small number of older references was accepted if necessary to provide evidence. All types of articles in all languages represented by the members of the task force were considered. The references searched by the single authors were evaluated in a consensus process with the participation of all authors and 120 papers were selected for the final guideline. According to a guideline of the European Federation of Neurological Societies,2 scientific evidence was rated into classes I-IV, and references were labeled as levels A-C. If sufficient evidence for a recommendation was not available, advice as a “good practice point” was offered.
Epidemiology and Risk Factors
Meningiomas have the highest incidence rate (37.6%) among all primary intracranial and central nervous system tumors.3 In the United States (US), the annual age-adjusted incidence rate of meningiomas was 8.58 per 100 000 population in 2012-2016 based on the Central Brain Tumor Registry of the US report released in 2019. Incidence increases with age, with a strong increase after the age of 65 years.3
From 2004 to 2010, the incidence of World Health Organization (WHO) grade 2 atypical intracranial meningiomas increased from 0.28 (95% confidence interval [CI]: 0.27-0.29) to 0.30 (95% CI: 0.28-0.32), representing an annual percentage change of 3.6% (95% CI: 0.8%-6.5%). Conversely, from 2000 to 2010, the incidence of WHO grade 3 anaplastic meningiomas decreased from 0.13 (95% CI: 0.11-0.14) to 0.06 (95% CI: 0.06-0.07), representing an annual percentage change of −5.4% (95% CI: −6.8% to −4.0%). From 2004 to 2010, the overall proportion of WHO grades 1, 2, and 3 intracranial meningiomas (based on the WHO classification from 2000) was 94.6%, 4.2%, and 1.2%, respectively.4 Meningiomas of WHO grades 1 and 2 overall are 2.3 times more common in females compared to males and the incidence of meningiomas is higher in originally Africans compared with Caucasians for meningiomas of all grades.3
Ionizing radiation has been linked to an increased risk for the development of meningiomas. This has been observed not only after irradiation for a variety of medical indications but also in atomic bomb survivors.5 Conversely, the risk of secondary tumors including meningiomas after radiosurgery is considered to be low with 6.8 cases per 100 000 patient-years.6
Type 2 neurofibromatosis (NF2) is the most common genetic condition associated with meningioma. Patients with NF2 are also more likely to develop grade 2 and 3 or multiple meningiomas.7
A number of studies have tried to link endogenous and exogenous hormone exposure to meningiomas because of higher incidences in women of reproductive age, tumor expression of hormone receptors, an association with breast cancer, and changes in size of meningiomas during pregnancy, the menstrual cycle, and menopause. A longer exposure of females to exogenous progesterone was associated with lower levels of progesterone receptor (PR) and NF2 mRNA in blood and proposed to be linked to a higher risk of meningioma.8
Diagnostic Procedures
Imaging
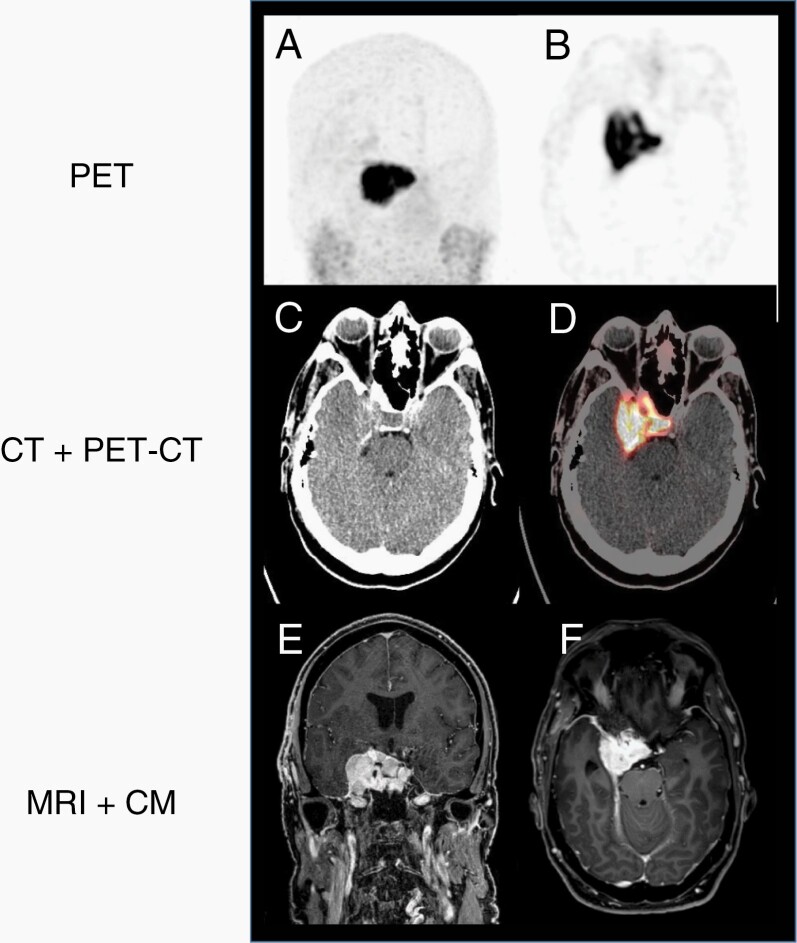
MRI and computed tomography (CT) scans, when used in combination, allow the diagnosis of intracranial meningiomas with high probability in most cases.9 Typically, meningiomas are isointense on T1-weighted sequences and hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences. They show strong contrast enhancement and a dural tail at the perimeter of the tumor. WHO grade 2 or 3 histology might be suspected based on the extent of edema assessed on FLAIR and on heterogeneous contrast enhancement.10 CT with bone window setting is valuable to assess hyperostosis of the adjacent bone and intraosseous tumor growth. Meningiomas express somatostatin receptor 2 and can be delineated by PET after injection of somatostatin analogs such as 68Ga-DOTATATE (DOTA-D-Phe1-Tyr3-octreotate) or 90Y-DOTATOC (DOTA-D-Phe1-Tyr3-octreotide) (see Figure 1). This exploration, not yet available as standard practice, is helpful in distinguishing tumor from healthy tissue and postoperative tissue changes.9,11–13
Fig. 1.
PET-CT after injection of 68Ga-DOTATATE. (A) PET-gamma-scan in coronar plane, (B) PET-gamma-scan in axial plane, (C) computed tomography in axial plane, (D) fusion of computed tomography and PET-scan in axial plane, (E) MRI T1-weighted with CM in coronar plane, and (F) MRI T1-weighted with CM in axial plane. Abbreviations: 68Ga-DOTATATE, 68-gallium DOTA-D-Phe1-Tyr3-octreotate; CM, contrast medium; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography.
When management by observation is indicated, MRI is the gold standard for follow-up imaging. Meningioma size is usually evaluated by the T1 sequence with gadolinium injection. Regarding measurements, T2-weighted axial MRI provides information comparable to the T1 gadolinium.14 The general recommendation that surveillance should be based on gadolinium-enhanced, T1-weighted sequences (good practice point) remains, but the option to follow small meningiomas by T2-weighted imaging only may be considered. Several MRI-based machine learning models have been used in the last years to improve the accuracy of meningioma diagnosis and may play a role in specific settings in the near future.15
The use of conventional angiography in the diagnosis of meningioma has been steadily declining, but it may provide useful information in particular cases when a major sinus (lateral sinus or superior sagittal sinus) seems invaded by the tumor and MRI angiography provides insufficient information. 3D venous angiography provides accurate information concerning the patency of the sinus and the collateral venous drainage.16
Preoperative embolization is not recommended in current practice and has been associated with increased risk of postoperative cardiovascular complications.17 A recent randomized trial identified as the only potential benefit of embolization a reduction in surgery time.18 Individual decisions for embolization should thus be left to the discretion of the surgical team. It may be useful when the feeding arteries are not accessible to the surgeons such as in petro-clival meningiomas. In this situation, embolization aims to occlude the meningeal branch of the ascending pharyngeal artery or the tentorial branch of the internal carotid artery.
Histopathology and Molecular Pathology
The WHO classification system describes 15 different meningioma subtypes, 9 of which are allotted WHO grade 1, 3 WHO grade 2, and 1 WHO grade 3 (Table 1).19 Grading of meningioma depends on mitotic rate, brain invasion, or specific histological features. While brain invasion has been introduced as new criterion for atypical meningioma WHO grade 2 in the 2016 WHO classification, several recent studies have challenged its prognostic role.20,21 However, brain invasion remains an independent criterion for atypical meningioma WHO grade 2 in the WHO classification 2021. In contrast to previous versions of the classification, molecular markers are now introduced as grading criteria for selected subtypes: besides histological features, secretory meningiomas can also be diagnosed on the basis of detecting KLF4/TRAF7 mutations. Likewise, any meningioma with TERT promoter mutation and/or CDKN2A/B homozygous deletion is allotted to WHO grade 3, irrespective of histological criteria of anaplasia. Further, 2 subtypes formerly associated with WHO grade 3, rhabdoid and papillary meningioma, will no longer be allotted to a specific grade based on the subtype-specific histology alone. For these both subtypes, grading is now determined on basis of the same criteria for atypia and anaplasia as for other meningioma variants.
Table 1.
Molecular Characteristics of Meningioma Subtypes
| Common Mutations | CNVs | MC | |
|---|---|---|---|
| WHO grade 1 | |||
| Meningothelial | AKT1(/TRAF7), SMO | None | ben-2 |
| Fibroblastic | NF2 | del 22q | ben-1 |
| Transitional | NF2 | del 22q | ben-1 |
| Secretory | KLF4/TRAF7 a | None | ben-2 |
| Psammomatous | NF2 | del 22q | ben-1 |
| Metaplastic | NF2 | gain 5 | ben-3 |
| Microcystic | NF2 | gain 5 | ben-3 |
| Angiomatous | NF2 | gain 5 | ben-3 |
| WHO grade 2 | |||
| Atypical | NF2 | del 1p, del 22q | int-A/B |
| Chordoid | (NF2) | del 2p | int-A/B |
| Clear cell | SMARCE1 | None | No specific |
| WHO grade 3 | |||
| Anaplastic | NF2, TERT promotera | del 1p, 10, 22q, homo del CDKN2A/Ba | Malignant |
| Formerly WHO grade 3 | |||
| Rhabdoid | BAP1 | (BAP1 locus) | No specific |
| Papillary | PBRM1 | No specific | No specific |
Abbreviations: ben, benign; CNVs, copy number variations; del, deletion; homo del, homozygous deletion; int, intermediate; MC, methylation class.22
aNovel molecular criterion for subtype, besides histology features, in WHO classification 2021.
Despite adequately predicting outcomes for the majority of patients, the grading scheme has limitations. While patient cohorts with WHO grade 2 meningioma generally exhibit shorter intervals to tumor recurrence, there is a considerable number of individual patients with WHO grade 1 meningiomas with unexpectedly early tumor relapse. Conversely, some patients with WHO grade 2 meningiomas, especially when a complete resection can be achieved, experience a long indolent clinical course even without postsurgical radiotherapy (RT).
Despite the progress in the molecular understanding of meningioma, only few markers of clinical relevance have emerged so far. The most frequent alterations in all WHO grades are chromosome 22q deletions and mutation of the other NF2 allele. With increasing aggressiveness and WHO grade, NF2 mutant meningiomas accumulate copy number alterations, of which deletion of chromosomal arm 1p and chromosome 10 are typically the first events, and with CDKN2A/B homozygous deletion indicating a highly aggressive course.23
In WHO grade 1 NF2 wild-type meningiomas, several other mutations are found with the following overall frequencies: AKT1 (up to 20%), SMO (up to 11%), KLF4 (up to 28%), PIK3CA (up to 7%), and TRAF7 (up to 40%).24–29 AKT1 and KLF4 mutations often occur in combination with TRAF7 mutations, while isolated TRAF7 mutations are rare. AKT1/TRAF7 and SMO mutations are typical for the meningothelial subtype, particularly in skull base localizations. KLF4/TRAF7 mutations constitute the driver alteration in secretory meningiomas and can serve as alternative criterium, besides secretory granula, to identify this subtype.
Due to their association with meningothelial WHO grade 1 meningiomas, mutations in AKT1 and SMO, are discussed as markers for low risk of recurrence.30,31 However, larger studies assessing their independent prognostic value are lacking, and their association with meningothelial meningioma is less strong than that of KLF4 with the secretory subtype, preventing their use as criteria for grading. Similarly, the correlation with morphology suggests defining subtypes by mutations. Most literature on these mutations is, however, confounded, eg, by enrichment for certain subtypes or focusing on NF2 wild-type meningiomas, thus precluding robust conclusions on the distribution across the entire meningioma spectrum.
Independent of classification, in the rare case of more aggressive AKT1 or SMO mutant meningiomas, these alterations may present promising targets for intervention as shown in a single case.32
Among WHO grade 2 meningiomas, virtually all clear cell meningiomas harbor SMARCE1 mutations (97%).26 SMARCE1 germline mutations can pose an alternative to NF2 germline mutations as predisposition to pediatric meningioma, which then mostly occur at a spinal location.33 However, no comprehensive data exist on what fraction of clear cell meningiomas results from SMARCE1 germline compared to somatic mutations. Among the subtypes formerly allotted to WHO grade 3, BAP1 mutations and deletions occur in a subset of cases with rhabdoid morphology. Of 6 cases with BAP1 mutant rhabdoid meningioma, 2 were based on BAP1 germline alterations.34 In papillary meningioma, 1 study identified enrichment for PBRM1 mutations.35 Whether BAP1 and PBRM1 alterations identify those rhabdoid and papillary meningiomas, respectively, which are exhibit the aggressive clinical course of WHO grade 3 meningiomas remains to be determined.
TERT promoter mutations have already been substantiated as a marker of high risk of recurrence,36,37 and thus are an independent criterium for WHO grade 3 in the new WHO classification. TERT promoter mutations can evolve during progression and be limited to focal, more aggressive areas of the tumor, necessitating considerate sampling for DNA extraction. Similarly, homozygous deletions of CDKN2A/B are associated with unfavorable outcome independent of histology, thus also sufficing as a marker for WHO grade 3 in the new WHO classification.36,38,39
For pediatric meningiomas, YAP1 fusions can be an alternative driver to the often germline-associated SMARCE1, BAP1, or NF2 mutations.40
The implications of subjective interpretation of histological criteria, and of spatial and longitudinal heterogeneity of mutations, may be overcome by DNA methylation-based subtyping of meningioma. Different subgroups and classifier algorithms have been proposed which may provide prognostic information beyond the updated WHO classification and candidate gene panel sequencing.22,41,42
These data on molecular characteristics, especially copy number alterations and mutations, suggest that a future molecularly based classification will have the potential to direct individualized meningioma-specific therapy (Table 2). Tumor tissue sampling and storage for future molecular testing should therefore be standard of practice.
Table 2.
Molecular Targets
| Drug Class | Molecular Target/Biomarker |
|---|---|
| AKT inhibitor | AKT1 (pGlu17Lys) mutation24,25 |
| Hedgehog inhibitor | SMO (pTrp535Leu) mutation24,25 |
| FAK inhibitor | NF2 (merlin) loss43,44 |
| Immune checkpoint inhibitor | PD-L1, PD-L2, B7-H3, and CTLA-445–47 |
| VEGF or VEGFR inhibitor | VEGF or VEGFR248–50 |
| PI3K inhibitor | PI3K29 |
| mTOR inhibitor | mTOR51,52 |
| Somatostatin analog | Somatostatin receptors51 |
| Gemcitabine | Cytidin53 |
Abbreviation: AKT, gene coding for protein kinase B; FAK, focal adhesion kinase; mTOR, mammalian target of rapamycin; PI3K, phospho-inositol-3-kinase; VEGF, vascular endothelial growth factor.
Therapeutic Strategies
Observation
The number of incidental meningiomas has increased because of the broad availability of neuroimaging. Incidental meningiomas are present on brain MRI of 0.9% to 1.0% of the general population.54 A 5-year prospective study was conducted to identify risk factors for tumor growth of incidental meningiomas.55 None of the 64 patients with incidental meningioma developed tumor-related symptoms during the study period follow-up of 5 years, although 48 tumors (75%) increased by 15% or more in volume. However, more than 60% of the tumors displayed a self-limiting growth pattern, suggesting that asymptomatic tumors can be safely managed by serial imaging until persistent radiological or symptomatic growth.55 Lee et al reported a series of 232 patients who had been prospectively followed up without treatment from 1997 to 2013. Fifty-nine tumors (25.4%) showed rapid growth. Tumor size (odds ratio per cm3 1.07, P = .000), the absence of calcification (odds ratio 3.87, P = .004), peritumoral edema (odds ratio 2.74, P = .025), and hyperintense or isointense signal on T2-weighted MRI (odds ratio 3.76, P = .049) were predictors of tumor growth rate.56 The authors suggest a weighted scoring system that predicts the specific probability of rapid tumor growth for patients with untreated meningioma. The requirement for long-term follow-up for every patient with an incidental meningioma is debatable. Islim et al developed a prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas.57 By combining data on patient age, performance status, co-morbidities, and MRI features (meningioma hyperintensity, peritumoral edema, proximity to neurovascular structures, size) patients are categorized as low, medium, or high risk for growth and progression, and an individualized monitoring strategy can be developed and the calculator is freely available (https://www.impact-meningioma.com). Moreau et al developed methods and a practical app designed to assist with the diagnosis and prognosis of meningiomas.58 Currently, by consensus, annual MRI scans are recommended in suspected meningiomas or meningiomas of WHO grade 1 for 5 years. Thereafter, intervals can be doubled (good practice point).
Surgery
The primary treatment for the majority of symptomatic or enlarging meningiomas is surgery. There are no randomized trials comparing surgery to other therapies for meningioma. Evidence for the effectiveness of surgery as monotherapy is derived from institutional case series which established that extent of resection (EOR) is an important prognostic factor.59,60 This is commonly still categorized using the Simpson classification.61 However, in clinical trials, EOR is often defined as either gross total resection (ie, no residual solid tumor) or subtotal resection. This definition has been adopted by research organizations such as the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG).62
The fundamental principles of meningioma surgery are maximum safe resection with low morbidity and preservation of neurological function. While the aim is gross total resection (all involved dura and bone), EOR is determined by tumor location, consistency, size, and proximity or involvement of critical neurovascular structures. Although EOR is the only modifiable risk factor for recurrence, striving to achieve a gross total resection should not be at the expense of neurological or cognitive function. When gross total resection is not possible, a planned subtotal resection should be undertaken to preserve neurological function. Residual meningioma can then be monitored or treated with postoperative conformal fractionated RT or stereotactic radiosurgery (SRS).
Successful surgery achieves 2 goals—relief of neurological symptoms and mass effect and provision of tissue for diagnosis. Surgical risks should be fully discussed with the patients prior to surgery including location-specific risks and more general risks such as seizures and hydrocephalus. Careful preoperative planning with attention to neurovascular anatomy will maximize surgical success and minimize morbidity. Special attention has to be paid to neurocognitive impairment, which might be present in a high proportion of patients.63,64 It may be relieved by surgery.65,66 On the other hand, postoperative neurocognitive impairment represents a significant surgical risk.
Image guidance should be used routinely and allows multiple datasets to be integrated into the surgical plan, including DOTATATE PET imaging for intraosseous meningioma.9,12 Intra-operative imaging can be used for emerging approaches such as adaptive hybrid surgery, to intentionally leave small volume residual meningioma that can be treated with postoperative SRS.67 Intra-operative neurophysiological monitoring, eg, facial nerve and brainstem-evoked potentials, may help to minimize neurological deficits. The use of minimally invasive and endoscopic techniques has been assessed. Generally, a superiority of these approaches in the management of anterior skull base tumors could not be shown leaving open craniotomy a valid option for the resection of these tumors.68
Spinal meningiomas are much rarer than intracranial meningiomas, however, the same surgical principles apply. The majority of data supports strategies aiming at gross total resection even in elderly patients.69 Institutional case series support the strategy of striving for gross total resection since the recurrence rate ranges from 1.3% to 14.7%.70–72 The decision to offer surgery for spinal meningioma, rather than observation, should balance the benefit of tumor removal vs surgical risk.73
Radiosurgery
SRS has been established as an alternative therapy to surgery in well-defined cases with small tumors in elderly or critically sick patients. Local control of small-sized intracranial meningiomas of a diameter of 3 cm or less after SRS was comparable to Simpson Grade I resection.74
Two retrospective series found that a reduction of tumor size after SRS or hypofractionated RT was predictive for long-time tumor control after 5 and 10 years. The 10-year recurrence-free survival was 93.4% and 95.7%, respectively, using doses above 13 Gy.75,76
Cranial nerve function is a major concern in the therapy of skull base meningiomas. Therefore the concept of combined treatment using subtotal surgery and SRS is increasingly used. Cranial nerve outcome was addressed in a registry-based analysis of 150 patients who received resection and SRS of skull base meningiomas at different locations. In 19% of patients, cranial nerve function improved after SRS, 10% suffered from deterioration 10 years after SRS. The rate of deterioration increased with time being 3.5% after 1 year, 5.5% after 3 years, and 7% after 5 years.
In the field of highly precise irradiation, the concept of fractionated radiosurgery has evolved within the last years with a view to preserving cranial nerve function in patients with large tumor volumes. Image-guided, frameless technology enables multisession procedures with stereotactic precision. In several studies, this principle has been used for skull base meningiomas,77 particularly perioptic tumors.78 Two to five fractions with doses of 4-10 Gy per fraction are commonly used, resulting in total doses of 18-25 Gy. A comparison of fractionated stereotactic RT (median 33 sessions) and Cyber Knife-based hypofractionated radiosurgery (median 5 sessions) using pooled data of 3 centers revealed no differences regarding local control and toxicity making short-term hypofractionated radiosurgery a convenient option.77
There are little data for radiosurgery of spinal meningiomas which can be performed as single-dose radiosurgery or in a hypofractionated manner, too.76,79
Fractionated External Beam RT
Fractionated external beam RT remains an important component in the therapeutic armamentarium for the management of meningiomas. For patients with meningiomas not safely amenable to surgery, or after incomplete surgical resection, few large retrospective studies published over the past 3 years have confirmed current EANO guidelines giving class III evidence with recommendations B and C, on the use of fractionated RT.80,81 In a series of 7811 patients with WHO grade 2 and 1936 patients with WHO grade 3 meningiomas obtained from the US National Cancer Database who underwent surgical resection and/or RT from 2004 to 2014, the 5-year overall survival (OS) rate was 75.9% in patients with grade 2, and 55.4% in patients with grade 3 meningiomas (P < .0001). In patients with meningiomas grade 2, gross total resection and postsurgical fractionated RT were independent predictors of improved survival.81
Results of 2 prospective phase II trials have been published by RTOG and EORTC. The first report of NRG Oncology/RTOG 0539 trial showed the initial outcome for patients with intermediate-risk meningiomas, ie, recurrent WHO grade 1 or newly diagnosed WHO grade 2 tumors after gross total resection who were treated with fractionated RT, either intensity-modulated or 3D conformal RT, 54 Gy in 30 fractions.82 With 48 fully evaluable patients for the primary endpoint, 3-year progression-free survival (PFS) was 93.8%. The estimated 3-year OS and local failure rates were 96% and 4.1%. No significant difference in outcome was observed between patients with recurrent WHO grade 1 and patients with WHO grade 2 tumors receiving gross total resection. Adverse events were limited to grade 1 and 2 only. In a second clinical outcomes report from the same trial, Rogers et al83 showed PFS of 58.8%, local control of 68.9%, and OS of 78.6% after 3 years with a median follow-up of 4.0 years in 53 patients who were treated with intensity modified radiotherapy (60 Gy/30 fractions) for high-risk meningioma, defined by new or recurrent WHO grade 3 or recurrent WHO grade 2 meningioma of any resection extent or newly diagnosed WHO grade 2 tumor after subtotal resection. Combined acute and late adverse events occurred in about 40% of patients and were limited to CTCAE (Common Terminology Criteria for Adverse Events) grades 1-3, except for 1 single necrosis-related death.
Using the same primary endpoint of 3-year PFS >70%, 56 patients with newly diagnosed WHO grade 2 meningioma who underwent gross total resection followed by fractionated RT were evaluated in the EORTC 22042-26042 phase II study.84 The estimated 3-year PFS, OS, and local failure were 88.7%, 98%, and 14.3%, respectively, with a late toxicity of grade 3 or more observed in 14% of patients. Noteworthy, patients accrued in this trial were treated with a higher radiation dose, 60 Gy delivered in 2.0 Gy fractions, compared with NRG/RTOG trial.
Both US and European trials suggest potential benefits of fractionated RT for patients with intermediate and high-risk meningiomas with acceptable toxicity; however, the question of whether early adjuvant RT reduces the risk of tumor recurrence after gross total surgical resection of WHO grade 2 meningiomas remains unanswered. Additionally, in the area of DNA methylation22 and molecular profiling85 it may be possible to predict patient’s individual outcome based on clinicopathological features which could predict the probability of recurrence and to identify high-risk tumors who could benefit from adjuvant treatment.86 The use of RT may avoid the need for further surgical procedures but must be balanced against the potential risks of long-term toxicity, which include but are not limited to neurocognitive impairment, hypopituitarism, and secondary, radiation-induced tumors. A phase III intergroup trial (ROAM/EORTC 1308, ISRCTN71502099) was activated in 2016 for these patients, randomizing them between observation and adjuvant RT.87 This active study has an accrual target of 190 patients and over 60% of the patients have been currently accrued in the UK, Ireland, Spain, Switzerland, Italy, Belgium, France, and Australia/New Zealand. The primary outcome measure is PFS (ie, time to MRI evidence of tumor recurrence) and secondary outcome measures include assessing the toxicity of RT, quality of life, neurocognitive function, time to second-line treatment, OS, and incremental cost per quality-adjusted life year (QALY) gained.88 A similar trial, NRG-BN003 (NCT03180268) is currently enrolling in the US.
The role of any type of RT in spinal meningiomas was addressed in a recent review using the US National Cancer Database. Among 10 458 patients with spinal meningioma between 2004 and 2015, 268 patients had received any type of RT; 131 patients had surgery plus R, 137 patients had received radiation alone (in 61% radiosurgery). Large tumor size and “borderline” or “malignant” histology were associated with increased use of RT. There appeared to be no association with OS, but RT was associated with a reduction of mortality in the subset of borderline and “malignant” tumors.89
Pharmacotherapy
The role of pharmacotherapy in meningioma remains ill-defined and there are no positive controlled clinical trials to base sound recommendations on. Still, systemic salvage therapy of meningiomas is commonly considered for patients in whom surgical resection or RT are no longer feasible. Classical cytotoxic agents are commonly not active. This was also seen with trabectedin that was not superior to best physician’s choice in patients with WHO grade 2 or 3 tumors who had their local treatment options exhausted in the EORTC 1320 trial.90 Partial responses of meningiomas to drugs have occasionally been described, notably with multikinase inhibitors.91 Furthermore, a slowing effect on the growth dynamics of meningiomas has been described using bevacizumab, thus indicating that in principle, systemic therapy targeting VEGF (vascular endothelial growth factor) and other kinase-dependent pathways may be useful.92 However, the interpretation of most of the available studies is limited by several factors, in particular small patient numbers, the retrospective design of most studies, the heterogeneity of patient populations with regard to tumor type and prior therapies, the lack of comparator treatment arms or reliable historical benchmark activity parameters and the lack of standardized response criteria. Some recurring molecular aberrations indicate potential sensitivity to specific inhibitors, but no clinical trials on targeted therapy of meningiomas have been completed.93
WHO grade 1 meningiomas
Hydroxyurea, temozolomide, irinotecan, interferon-alpha, Sandostatin LAR, pasireotide LAR, imatinib, erlotinib, and gefitinib have been studied in retrospective and single-arm phase II studies in WHO grade 1 meningiomas that have failed surgical resection and RT, without relevant activity. Mifepristone failed to show an advantage in failure-free or OS in a randomized phase III trial.94 The PFS-6 rates and OS times in the available studies range from 0% to 67% and from 7 to 13 months, respectively. None of the studied drugs showed clear signs of clinically relevant activity sufficient to recommend them for routine clinical use. AKT inhibitors if available may be considered for patients with AKTE17K-mutant meningiomas.32
WHO grade 2 and 3 meningiomas
A variety of drugs including hydroxyurea, cyclophosphamide/adriamycin/vincristine chemotherapy, interferon-alpha, megestrol acetate, medroxyprogesterone acetate, octreotide, Sandostatin LAR, pasireotide LAR, imatinib, erlotinib, gefitinib, vatalanib, sunitinib, and bevacizumab have been evaluated in retrospective studies and small prospective studies in patients with WHO grade 2 and 3 meningiomas. PFS-6 rates ranged from 0% to 64% and median OS times from 6 to 33 months in patients recurring or progressing after surgery and RT. The most promising results have been reported for anti-angiogenic compounds including bevacizumab, vatalanib, and sunitinib.48,95,96 However, these results from uncontrolled studies need to be confirmed in prospective controlled trials, before clinical use of these compounds in patients with WHO grade 2 and 3 meningiomas can be recommended.
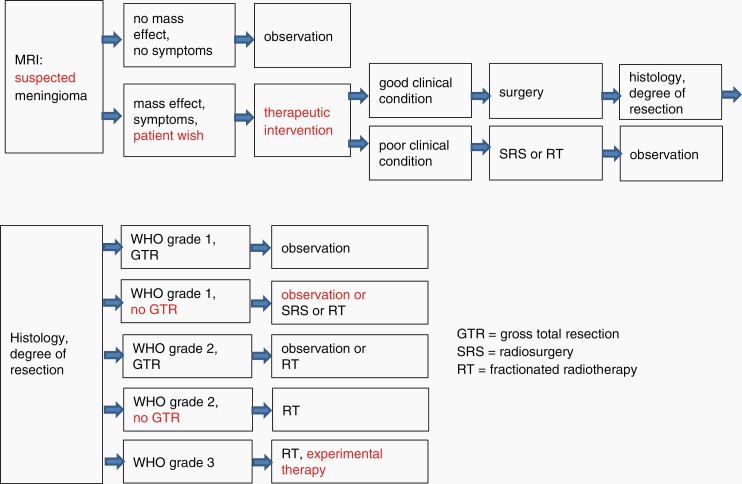
A summary of therapeutic algorithms is displayed in Figure 2.
Fig. 2.
Recommendations for the therapeutic management of WHO grade 1-3 meningiomas.
Cognitive Functioning
Within the last years, a strong focus has been set on neurocognition in meningioma patients. Cognitive functioning may be impaired in meningioma patients pre- and postoperatively.63,64 These impairments are most pronounced in domains of memory, attention, and executive functions. Preoperative cognitive deficits in meningioma patients might be the result of anatomical localization, and elevated intracranial pressure caused by the tumor itself or by tumor-related edema. Notably, frontal or temporal location, tumor size, and edema volume correlated with the decline of cognitive function. However, a clear causality could not be demonstrated.63,97,98 Surgery generally had a beneficial effect on cognitive function.63,66,99–103 Postoperative cognitive deficits might be explained by the use of antiepileptic drugs.104 No clear associations between tumor lateralization and cognitive functioning were found in several studies in postoperative meningioma patients.66,101 Furthermore, meningioma patients with significant preoperative cerebral edema are at risk of experiencing limitations in longer-term postoperative cognitive functioning.63,98 No correlations between RT and cognitive functioning were found.105,106 Other factors that are known to have a relation to cognitive performance such as epilepsy, mood, steroid intake, and quality of life were not systematically investigated.
Quality of Life
Health-related quality of life (HRQoL) is impaired in meningioma patients prior to surgery because of neurological symptoms and signs, or other factors related to illness like use of antiepileptic drugs. However, meningioma patients may have better HRQoL than (brain) cancer patients after surgery.107 Although tumor resection may improve neurological deficits rapidly, a significant decrease of HRQoL is observed after surgery in the long-term108–110 notably with cognitive difficulties but also, emotional and social dysfunction as well as sleep disorders and fatigue.107,109 The number of patients able to drive or going to work decreases over time.109,111 Larger tumor size, higher WHO grade, tumor recurrence, shorter time since diagnosis, age of 50 years or more, more posttraumatic stress, personality changes, confusion, and left hemispheric tumor location, headache, seizures were all associated with lower HRQoL.107,110,111 This long-term impact of the disease on the quality of life should be assessed during follow-up and appropriate interventions should be considered.
Future Directions
Radioimmunotherapy
The expression of receptors for somatostatin has been employed as a target for radionuclide therapy of meningioma using ligands, such as DOTATOC or DOTATATE. Several small studies indicate a limited activity of such treatments, expectedly, local control seems to be better in lower grade tumors that also exhibit higher target gene expression. However, controlled data are missing, and most patients in all series had undergone multiple prior interventions, including RT, rendering major benefit a priori unlikely.112,113
Neuropathology
In order to evaluate the potential impact of therapeutic targets especially in aggressive meningiomas, appropriate in vitro and in vivo models need to be established. Various meningioma cell lines have been characterized in detail including their molecular features, and their different growth characteristics may at least partly reflect the range from slowly growing ordinary meningioma to the highly aggressive atypical or anaplastic variants.114–116 These cells can be used to model somatic mutations, either by using overexpression constructs containing either wild-type or mutant variants31 or by base substitution using Crisp-Cas-mediated gene editing approaches.114–117 Generated cells can be used for drug testing to select candidate drugs with superior efficacy in mutant cells compared to wild-type cells.31
The next step to test in vivo efficacy of selected drugs should be either xenotransplantation of genetically engineered cells into nude mice (xenotransplants), or treatment of genetically engineered mice (GEM) harboring meningioma-relevant mutations with subsequent development of meningeal tumors. Xenotransplants of meningioma cells have been used for several years, and some meningioma cell lines have been confirmed to grow orthotopically in mice.118,119 Recently, xenotransplantation of meningioma cells containing the KLF4K409Q mutation characteristic for secretory meningioma has been demonstrated as a tool to model the sensitivity of mTOR inhibitors based on the presence of the specific mutation.31 In contrast, xenografts derived from patient tumor material have limited feasibility because of slow growth and small fraction of successfully grafted tumors.119 No studies using patient-derived orthotopic xenografts characterized by meningioma-typical mutations have been available so far.
Pharmacotherapy
Future therapeutic approaches in pharmacotherapy are mainly based on the identification of potential therapeutic targets such as NF2/merlin loss, AKT1, SMO, but also PIK3CA, VEGF/VEGFR2, BRAF, telomerase activity, or PD-1/PD-L1.25,93 Yet, molecular testing is not part of routine neuropathology and clinical data on the feasibility of addressing these targets are very limited. A prolonged response (more than 12 months) to AZD5363, currently under development for various cancers including breast cancer, has been reported in a single patient with the AKT1E17K mutation.32 An efficacy of dabrafenib was also noted in a patient with a V600E mutation, CDKN2A/2B loss, and APC I13970K.120 Immune checkpoint inhibition is currently being explored (NCT03279692, NCT02648997). Prospective trials with adequate methodology are mandatory to validate these new potential approaches. A list of candidate targets is stated in Table 2.24,25,29,43–47,49–53
Special recommendations
Key recommendations are summarized in Table 3.
Table 3.
Key Recommendations
| Statement | Evidence Class2 | Recommendation Level |
|---|---|---|
| Diagnosis | ||
| Radiological diagnosis of meningiomas should be made by MRI | 4 | GPP |
| Somatostatin receptor II directed PET offers detection of meningioma with high sensitivity and specificity and should be obtained if tumor extension or the diagnosis of recurrence is uncleara | 3 | C |
| Tissue should be gained for molecular analysis, which has proven to be prognostically relevant and offers potential for future targeted therapyb | 4 | GPP |
| Therapy | ||
| Observation should be selected as the first therapeutic option in asymptomatic patients with newly diagnosed or slow growing meningiomas | 3 | C |
| Neurocognition addressing memory, attention, and executive functions should be assessed and integrated in decision makinga | 3 | C |
| HRQoL might be compromised after therapy and should be respected for any therapeutic indicationa | 4 | GPP |
| Surgery should be considered as the first therapeutic option in tumors of all WHO grades if therapy is indicated | 3 overwhelming | B |
| Goal of surgery is gross total resection according to Simpson Grade I whenever safely feasible | 3 overwhelming | B |
| Radiosurgery should be considered as an alternative to surgery in small tumors, in specific locations, and in specific clinical situations, if tissue collection seems not mandatory | 3 | C |
| WHO grade 1 meningiomas should be treated by radiosurgery or fractionated radiosurgery, if surgery is not possible and treatment is needed | 2 | B |
| Patients with incompletely resected WHO grade 1 meningiomas without neurological deficits may be managed by a watch-and-scan strategya | 3 | C |
| Patients with recurrent or atypical meningiomas should receive fractionated radiotherapy | 2 | B |
| The combination of intended subtotal surgery and radiosurgery or fractionated radiotherapy in WHO grade 1 meningiomas should be considered for comprehensive tumor treatment with reduced risk of tumor progression | 3 | C |
| Radical surgery and fractionated radiotherapy should be performed in WHO grade 3 meningiomas | 3 overwhelming | B |
| Pharmacotherapy using bevacizumab or multikinase inhibitors targeting VEGF receptors should only be considered if no further local treatment option existsb | 3 | C |
| Follow-up | ||
| Follow-up of WHO grade 1 meningiomas should be performed by MRI every 12 months, after 5 years every 2 years | 4 | GPP |
| Follow-up of WHO grade 2 meningiomas should be performed by MRI every 6 months, after 5 years every 12 months | 4 | GPP |
| Follow-up of WHO grade 3 meningiomas depend on clinical progression and should be done at least every 3-6 months | 4 | GPP |
Abbreviations: GPP, good practice point; HRQoL, health-related quality of life; MRI, magnetic resonance imaging; PET, positron emission tomography; VEGF, vascular endothelial growth factor.
aNew key recommendation since 2016.
bStrongly modified key recommendation since 2016.
WHO grade 1 meningiomas
Treatment of WHO grade 1 meningiomas should be stratified by the major prognostic factors and clinical constellations summarized above (Figure 2). In case of incidentally diagnosed and asymptomatic tumors observation by annual MRI initially is the management strategy of choice (evidence level III, recommendation level C). Beyond routine neurological investigation, special attention should be directed to cognitive impairment because its presence argues in favor of intervention. Therapy is indicated in symptomatic or growing meningiomas with surgery being the first option for the following reasons: the patient can often be cured by Simpson grade I resection, neurological and cognitive symptoms and signs may be reversed, tissue-based diagnosis can be made, tissue is gained for molecular pathologic testing. Tissue should be stored for the option of future targeted therapies (good practice point). Conversely, possible short- and long-term effects of surgery on cognition and HRQoL should be considered. Radiosurgery may be an alternative in patients with relative or absolute contraindications for surgery and with small tumors without mass effect, although a higher grade meningioma or a different histology cannot be entirely ruled out. It offers long-term local control in the range of 90% after 10 years. Moreover, radiosurgery can be used in a combination approach consisting of subtotal resection of large skull base meningiomas with a high surgical risk profile and consecutive radiosurgery of intentionally left residual tumor. If the tumor cannot be treated by a single fraction, fractionated radiosurgery or standard fractionated external beam RT can be applied. In WHO grade 1 tumors, there are only data for recurrent, “high-risk” meningiomas to support this recommendation. Patients with incompletely resected WHO grade 1 meningiomas may not require immediate postsurgical RT, notably if no neurological symptoms or signs persist.
Up to date, there is no evidence for effective pharmacological treatment of WHO grade 1 meningiomas. For follow-up, annual MRI in suspected meningiomas or after treatment is recommended for 5 years. Thereafter, follow-up intervals can be prolonged according to the age and clinical condition (good practice point). Somatostatin receptor II-directed PET helps detecting meningioma tissue with high sensitivity and specificity.
WHO grade 2 meningiomas
If a radiologically assumed meningioma shows rapid growth during observation, a higher grade meningioma (or metastasis in particular cases) has to be suspected. In meningioma WHO grade 2, therapy is mandatory. To gain tissue for the diagnosis and remove mass effect, surgery is the first option. Resection according to Simpson grade I should be achieved. Because of increased risk for recurrence, the follow-up interval should be 6 months for 5 years; thereafter, 1-year intervals are recommended. There are increasing data on fractionated RT in WHO grade 2 meningiomas. However, no randomized trials have been completed, allowing only level IV evidence regarding the question whether WHO grade 2 meningiomas should be irradiated after resection Simpson I-III. For WHO grade 2 meningioma with a Simpson IV-V resection, RT is recommended. There is no established pharmacotherapy for these tumors.
WHO grade 3 meningiomas
These tumors are characterized by rapid growth, early recurrence, risk of systemic metastasis, and particular molecular features on genetic and epigenetic levels. Radical surgery as feasible is recommended, followed by fractionated RT. No role for pharmacotherapy has been defined.
Spinal meningioma
Surgical resection is the therapy of choice for patients with spinal meningiomas. Resection should be according to Simpson grade I or II to decompress the spinal cord and remove the tumor. Gross total resection should also be attempted in elderly patients. There are little data about RT in spinal meningiomas.
Funding
None received.
Conflict of interest statement.
There is no COI for all authors as a task force or consortium. M.D.J.—no commercial COI. National Institute for Health Research Health Technology Assessment program for the Radiation vs Observation for Atypical Meningioma trial [NIHR HTA: 12/173/14 and Surgeon’s Trial of Prophylaxis for Epilepsy in Meningioma trial [NIHR HTA: 129748]. F.S.—honoraria (speaker and/or advisory): Agilent, Illumina, Medac, Roche, and AbbVie. M.P.—honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, and AbbVie. E.L.R.—honoraria for lectures or advisory board from AbbVie, Adastra, Daiichi Sankyo, Leo Pharmaceuticals, Seattle Genetics, and Tocagen. G.T.—personal fees (advisory board, speaker’s fees) from AbbVie, Bayer, Bristol-Myers-Squibb, Medac, Novocure, travel grants from Bristol-Myers-Squibb, educational and travel grants from Novocure, research grants from Roche Diagnostics, research and travel grants from Medac. M.W.—research grants from Abbvie, Adastra, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, and Quercis, and honoraria for lectures or advisory board participation or consulting from Abbvie, Bristol Meyer Squibb (BMS), Celgene, Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen, Roche, and Tocagen. All other authors have no conflicts to report.
Authorship statement.
Each author contributed to the text according to his/her specialty and each author reviewed and approved the final version of the manuscript.
References
- 1. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 2. Brainin M, Barnes M, Baron JC, et al. ; Guideline Standards Subcommittee of the EFNS Scientific Committee . Guidance for the preparation of neurological management guidelines by EFNS scientific task forces – revised recommendations 2004. Eur J Neurol. 2004;11(9):577–581. [DOI] [PubMed] [Google Scholar]
- 3. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015;17(8):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner AV, Sugiyama H, Preston DL, et al. Radiation risk of central nervous system tumors in the Life Span Study of atomic bomb survivors, 1958–2009. Eur J Epidemiol. 2020;35(6):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf A, Naylor K, Tam M, et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 2019;20(1):159–164. [DOI] [PubMed] [Google Scholar]
- 7. Abi Jaoude S, Peyre M, Degos V, Goutagny S, Parfait B, Kalamarides M. Validation of a scoring system to evaluate the risk of rapid growth of intracranial meningiomas in neurofibromatosis type 2 patients. J Neurosurg. 2020;22:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Supartoto A, Sasongko MB, Respatika D, et al. Relationships between neurofibromatosis-2, progesterone receptor expression, the use of exogenous progesterone, and risk of orbitocranial meningioma in females. Front Oncol. 2018;8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galldiks N, Albert NL, Sommerauer M, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spille DC, Adeli A, Sporns PB, et al. Predicting the risk of postoperative recurrence and high-grade histology in patients with intracranial meningiomas using routine preoperative MRI. Neurosurg Rev. 2021;44(2):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivanidze J, Roytman M, Lin E, et al. Gallium-68 DOTATATE PET in the evaluation of intracranial meningiomas. J Neuroimaging. 2019;29(5):650–656. [DOI] [PubMed] [Google Scholar]
- 12. Kunz WG, Jungblut LM, Kazmierczak PM, et al. Improved detection of transosseous meningiomas using 68Ga-DOTATATE PET/CT compared with contrast-enhanced MRI. J Nucl Med. 2017;58(10):1580–1587. [DOI] [PubMed] [Google Scholar]
- 13. Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen KJ. Current status of PET imaging in neuro-oncology. Neurooncol Adv. 2019;1(1):vdz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahatli FK, Donmez FY, Kesim C, Haberal KM, Turnaoglu H, Agildere AM. Can unenhanced brain magnetic resonance imaging be used in routine follow up of meningiomas to avoid gadolinium deposition in brain? Clin Imaging. 2019;53:155–161. [DOI] [PubMed] [Google Scholar]
- 15. Neromyliotis E, Kalamatianos T, Paschalis A, et al. Machine learning in meningioma MRI: past to present. A narrative review [published online ahead of print October 2, 2020]. J Magn Reson Imaging. doi: 10.1002/jmri.27378. [DOI] [PubMed] [Google Scholar]
- 16. Lenck S, Bresson D, Bernat AL, et al. 3D digital subtracted CT angiography to evaluate the venous anatomy in extra-axial tumors invading the major dural venous sinuses. Interv Neuroradiol. 2017;23(4):346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wirsching HG, Richter JK, Sahm F, et al. Post-operative cardiovascular complications and time to recurrence in meningioma patients treated with versus without pre-operative embolization: a retrospective cohort study of 741 patients. J Neurooncol. 2018;140(3):659–667. [DOI] [PubMed] [Google Scholar]
- 18. Iacobucci M, Danieli L, Visconti E, et al. Preoperative embolization of meningiomas with polyvinyl alcohol particles: the benefits are not outweighed by risks. Diagn Interv Imaging. 2017;98(4):307–314. [DOI] [PubMed] [Google Scholar]
- 19. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 20. Spille DC, Heß K, Sauerland C, et al. Brain invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg. 2016;93:346–354. [DOI] [PubMed] [Google Scholar]
- 21. Baumgarten P, Gessler F, Schittenhelm J, et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–481. [DOI] [PubMed] [Google Scholar]
- 22. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 23. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 24. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youngblood MW, Miyagishima DF, Jin L, et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. 2020;23(5):783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams SR, Juratli TA, Castro BA, et al. Genomic analysis of posterior fossa meningioma demonstrates frequent AKT1 E17K mutations in foramen magnum meningiomas. J Neurol Surg B Skull Base. 2019;80(6):562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams EA, Santagata S, Wakimoto H, et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol Commun. 2020;8(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuzawa S, Nishihara H, Yamaguchi S, et al. Clinical impact of targeted amplicon sequencing for meningioma as a practical clinical-sequencing system. Mod Pathol. 2016;29(7):708–716. [DOI] [PubMed] [Google Scholar]
- 31. von Spreckelsen N, Waldt N, Poetschke R, et al. KLF4 K409Q -mutated meningiomas show enhanced hypoxia signaling and respond to mTORC1 inhibitor treatment. Acta Neuropathol Commun. 2020;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weller M, Roth P, Sahm F, et al. Durable control of metastatic AKT1-mutant WHO grade 1 meningothelial meningioma by the AKT inhibitor, AZD5363. J Natl Cancer Inst. 2017;109(3):1–4. [DOI] [PubMed] [Google Scholar]
- 33. Smith MJ, O’Sullivan J, Bhaskar SS, et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45(3):295–298. [DOI] [PubMed] [Google Scholar]
- 34. Shankar GM, Abedalthagafi M, Vaubel RA, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19(4):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams EA, Wakimoto H, Shankar GM, et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. 2020;140(1):89–93. [DOI] [PubMed] [Google Scholar]
- 36. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(4):378–387. [DOI] [PubMed] [Google Scholar]
- 38. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sievers P, Chiang J, Schrimpf D, et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol. 2020;139(1):215–218. [DOI] [PubMed] [Google Scholar]
- 41. Nassiri F, Mamatjan Y, Suppiah S, et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olar A, Wani KM, Wilson CD, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah NR, Tancioni I, Ward KK, et al. Analyses of merlin/NF2 connection to FAK inhibitor responsiveness in serous ovarian cancer. Gynecol Oncol. 2014;134(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Domingues P, González-Tablas M, Otero Á, et al. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6(13):10671–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han SJ, Reis G, Kohanbash G, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130(3):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berghoff AS, Kresl P, Rajky O, et al. Analysis of the inflammatory tumor microenvironment in meningeal neoplasms. Clin Neuropathol. 2020;39(6):256–262. [DOI] [PubMed] [Google Scholar]
- 47. Proctor DT, Patel Z, Lama S, Resch L, van Marle G, Sutherland GR. Identification of PD-L2, B7-H3 and CTLA-4 immune checkpoint proteins in genetic subtypes of meningioma. Oncoimmunology. 2019;8(1):e1512943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baumgarten P, Brokinkel B, Zinke J, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors VEGFR1 and VEGFR2 in primary and recurrent WHO grade III meningiomas. Histol Histopathol. 2013;28(9):1157–1166. [DOI] [PubMed] [Google Scholar]
- 50. Preusser M, Hassler M, Birner P, et al. Microvascularization and expression of VEGF and its receptors in recurring meningiomas: pathobiological data in favor of anti-angiogenic therapy approaches. Clin Neuropathol. 2012;31(5):352–360. [DOI] [PubMed] [Google Scholar]
- 51. Graillon T, Sanson M, Campello C, et al. Everolimus and octreotide for patients with recurrent Meningioma: results from the Phase II CEVOREM trial. Clin Cancer Res. 2020;26(3):552–557. [DOI] [PubMed] [Google Scholar]
- 52. Barresi V, Lionti S, La Rocca L, Caliri S, Caffo M. High p-mTOR expression is associated with recurrence and shorter disease-free survival in atypical meningiomas. Neuropathology. 2019;39(1):22–29. [DOI] [PubMed] [Google Scholar]
- 53. Takeda H, Okada M, Kuramoto K, et al. Antitumor activity of gemcitabine against high-grade meningioma in vitro and in vivo. Oncotarget. 2017;8(53):90996–91008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Håberg AK, Hammer TA, Kvistad KA, et al. Incidental intracranial findings and their clinical impact; the HUNT MRI study in a general population of 1006 participants between 50-66 years. PLoS One. 2016;11(3):e0151080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Behbahani M, Skeie GO, Eide GE, Hausken A, Lund-Johansen M, Skeie BS. A prospective study of the natural history of incidental meningioma—hold your horses! Neurooncol Pract. 2019;6(6):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee EJ, Kim JH, Park ES, et al. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017;127(5):971–980. [DOI] [PubMed] [Google Scholar]
- 57. Islim AI, Kolamunnage-Dona R, Mohan M, et al. A prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro Oncol. 2020;22(2):278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moreau JT, Hankinson TC, Baillet S, Dudley RWR. Individual-patient prediction of meningioma malignancy and survival using the Surveillance, Epidemiology, and End Results database. NPJ Digit Med. 2020;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nanda A, Maiti TK, Bir SC, Konar SK, Guthikonda B. Olfactory groove meningiomas: comparison of extent of frontal lobe changes after lateral and bifrontal approaches. World Neurosurg. 2016;94:211–221. [DOI] [PubMed] [Google Scholar]
- 60. Paldor I, Awad M, Sufaro YZ, Kaye AH, Shoshan Y. Review of controversies in management of non-benign meningioma. J Clin Neurosci. 2016;31:37–46. [DOI] [PubMed] [Google Scholar]
- 61. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vogelbaum MA, Leland Rogers C, Linskey MA, Mehta MP. Opportunities for clinical research in meningioma. J Neurooncol. 2010;99(3):417–422. [DOI] [PubMed] [Google Scholar]
- 63. Bommakanti K, Somayajula S, Suvarna A, et al. Pre-operative and post-operative cognitive deficits in patients with supratentorial meningiomas. Clin Neurol Neurosurg. 2016;143:150–158. [DOI] [PubMed] [Google Scholar]
- 64. Meskal I, Gehring K, Rutten GJ, Sitskoorn MM. Cognitive functioning in meningioma patients: a systematic review. J Neurooncol. 2016;128(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hendrix P, Hans E, Griessenauer CJ, Simgen A, Oertel J, Karbach J. Neurocognitive function surrounding the resection of frontal WHO grade I meningiomas: a prospective matched-control study. World Neurosurg. 2017;98:203–210. [DOI] [PubMed] [Google Scholar]
- 66. Meskal I, Gehring K, van der Linden SD, Rutten GJ, Sitskoorn MM. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. J Neurooncol. 2015;121(3):617–625. [DOI] [PubMed] [Google Scholar]
- 67. Barani IJ, Parsa AT. Adaptive hybrid surgery: feasibility of planned subtotal resection of benign skull base tumors followed by radiosurgery to minimize morbidity without compromising tumor control. Int J Radiat Oncol Biol Phys. 2012;84(3):S278–S279. [Google Scholar]
- 68. Muskens IS, Briceno V, Ouwehand TL, et al. The endoscopic endonasal approach is not superior to the microscopic transcranial approach for anterior skull base meningiomas—a meta-analysis. Acta Neurochir (Wien). 2018;160(1):59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Engel DC, Gawellek L, Peraio S, Stanojevic M, Tatagiba M, Ebner FH. Spinal meningioma surgery in the elderly: who benefits? [published online ahead of print]. J Neurosurg Sci. 2018. doi: 10.23736/S0390-5616.18.04582-4. [DOI] [PubMed] [Google Scholar]
- 70. Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14(6):e2. [DOI] [PubMed] [Google Scholar]
- 71. Kim CH, Chung CK, Lee SH, et al. Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur Spine J. 2016;25(12):4025–4032. [DOI] [PubMed] [Google Scholar]
- 72. Klekamp J, Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999;52(6):552–562. [DOI] [PubMed] [Google Scholar]
- 73. van der Vossen S, Schepers VP, Berkelbach van der Sprenkel JW, Visser-Meily JM, Post MW. Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med. 2014;46(5):430–437. [DOI] [PubMed] [Google Scholar]
- 74. Bir SC, Patra DP, Maiti TK, Bollam P, Minagar A, Nanda A. Direct comparison of gamma knife radiosurgery and microsurgery for small size meningiomas. World Neurosurg. 2017;101:170–179. [DOI] [PubMed] [Google Scholar]
- 75. Patibandla MR, Lee CC, Tata A, Addagada GC, Sheehan JP. Stereotactic radiosurgery for WHO grade I posterior fossa meningiomas: long-term outcomes with volumetric evaluation. J Neurosurg. 2018;129(5):1249–1259. [DOI] [PubMed] [Google Scholar]
- 76. Cohen-Inbar O, Tata A, Moosa S, Lee CC, Sheehan JP. Stereotactic radiosurgery in the treatment of parasellar meningiomas: long-term volumetric evaluation. J Neurosurg. 2018;128(2):362–372. [DOI] [PubMed] [Google Scholar]
- 77. Alfredo C, Carolin S, Güliz A, et al. Normofractionated stereotactic radiotherapy versus CyberKnife-based hypofractionation in skull base meningioma: a German and Italian pooled cohort analysis. Radiat Oncol. 2019;14(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marchetti M, Conti A, Beltramo G, et al. Multisession radiosurgery for perioptic meningiomas: medium-to-long term results from a CyberKnife cooperative study. J Neurooncol. 2019;143(3):597–604. [DOI] [PubMed] [Google Scholar]
- 79. Meola A, Soltys S, Schmitt A, Gerszten PC, Chang SD. Stereotactic radiosurgery for benign spinal tumors. Neurosurg Clin N Am. 2020; 31(2):231–235. [DOI] [PubMed] [Google Scholar]
- 80. Combs SE, Farzin M, Boehmer J, et al. Clinical outcome after high-precision radiotherapy for skull base meningiomas: pooled data from three large German centers for radiation oncology. Radiother Oncol. 2018;127(2):274–279. [DOI] [PubMed] [Google Scholar]
- 81. Rydzewski NR, Lesniak MS, Chandler JP, et al. Gross total resection and adjuvant radiotherapy most significant predictors of improved survival in patients with atypical meningioma. Cancer. 2018;124(4):734–742. [DOI] [PubMed] [Google Scholar]
- 82. Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rogers CL, Won M, Vogelbaum MA, et al. High-risk meningioma: initial outcomes from NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106(4):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weber DC, Ares C, Villa S, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042–26042). Radiother Oncol. 2018;128(2):260–265. [DOI] [PubMed] [Google Scholar]
- 85. Vasudevan HN, Braunstein SE, Phillips JJ, et al. Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep. 2018;22(13):3672–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fioravanzo A, Caffo M, Di Bonaventura R, et al. A risk score based on 5 clinico-pathological variables predicts recurrence of atypical meningiomas. J Neuropathol Exp Neurol. 2020;79(5):500–507. [DOI] [PubMed] [Google Scholar]
- 87. Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical Meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jenkinson MD, Weber DC, Haylock BJ, et al. Letter to the Editor. Phase III randomized controlled trials are essential to properly evaluate the role of radiotherapy in WHO grade II meningioma. J Neurosurg. 2018;129(4):1104–1105. [DOI] [PubMed] [Google Scholar]
- 89. Yolcu YU, Goyal A, Alvi MA, Moinuddin FM, Bydon M. Trends in the utilization of radiotherapy for spinal meningiomas: insights from the 2004–2015 National Cancer Database. Neurosurg Focus. 2019;46(6):E6. [DOI] [PubMed] [Google Scholar]
- 90. Preusser M, Silvani A, Rhun EL, et al. PL3.2 Trabectedin for recurrent WHO grade II or III meningioma: a randomized phase II study of the EORTC Brain Tumor Group (EORTC-1320-BTG). Neuro Oncol. 2019;21(suppl_3):iii2–iii3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Furtner J, Schöpf V, Seystahl K, et al. Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol. 2016;18(3):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. 2018;14(2):106–115. [DOI] [PubMed] [Google Scholar]
- 94. Ji Y, Rankin C, Grunberg S, et al. Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol. 2015;33(34):4093–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lou E, Sumrall AL, Turner S, et al. Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol. 2012;109(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. [DOI] [PubMed] [Google Scholar]
- 97. van Nieuwenhuizen D, Ambachtsheer N, Heimans JJ, Reijneveld JC, Peerdeman SM, Klein M. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113(3):433–440. [DOI] [PubMed] [Google Scholar]
- 98. van Nieuwenhuizen D, Slot KM, Klein M, et al. The association between preoperative edema and postoperative cognitive functioning and health-related quality of life in WHO grade I meningioma patients. Acta Neurochir (Wien). 2019;161(3):579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yoshii Y, Tominaga D, Sugimoto K, et al. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol. 2008;69(1):51–61; discussion 61. [DOI] [PubMed] [Google Scholar]
- 100. Tucha O, Smely C, Lange KW. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. Br J Neurosurg. 2001;15(2):184–188. [DOI] [PubMed] [Google Scholar]
- 101. Tucha O, Smely C, Preier M, Becker G, Paul GM, Lange KW. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg. 2003;98(1):21–31. [DOI] [PubMed] [Google Scholar]
- 102. Koizumi H, Ideguchi M, Iwanaga H, et al. Cognitive dysfunction might be improved in association with recovered neuronal viability after intracranial meningioma resection. Brain Res. 2014;1574:50–59. [DOI] [PubMed] [Google Scholar]
- 103. Rijnen SJM, Meskal I, Bakker M, et al. Cognitive outcomes in meningioma patients undergoing surgery: individual changes over time and predictors of late cognitive functioning. Neuro Oncol. 2019;21(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80(8):910–915. [DOI] [PubMed] [Google Scholar]
- 105. Steinvorth S, Welzel G, Fuss M, et al. Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: a prospective 1-year follow-up. Radiother Oncol. 2003;69(2):177–182. [DOI] [PubMed] [Google Scholar]
- 106. van Nieuwenhuizen D, Klein M, Stalpers LJ, Leenstra S, Heimans JJ, Reijneveld JC. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84(3):271–278. [DOI] [PubMed] [Google Scholar]
- 107. Zamanipoor Najafabadi AH, Peeters MCM, Lobatto DJ, et al. Health-related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir (Wien). 2017;159(11):2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Benz LS, Wrensch MR, Schildkraut JM, et al. Quality of life after surgery for intracranial meningioma. Cancer. 2018;124(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nassiri F, Price B, Shehab A, et al. Life after surgical resection of a meningioma: a prospective cross-sectional study evaluating health-related quality of life. Neuro Oncol. 2019;21(Suppl 1):i32–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Timmer M, Seibl-Leven M, Wittenstein K, et al. Long-term outcome and health-related quality of life of elderly patients after meningioma surgery. World Neurosurg. 2019;125:e697–e710. [DOI] [PubMed] [Google Scholar]
- 111. Wirsching HG, Morel C, Roth P, Weller M. Socioeconomic burden and quality of life in meningioma patients. Qual Life Res. 2020;29(7):1801–1808. [DOI] [PubMed] [Google Scholar]
- 112. Seystahl K, Stoecklein V, Schüller U, et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hartrampf PE, Hänscheid H, Kertels O, et al. Long-term results of multimodal peptide receptor radionuclide therapy and fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Clin Transl Radiat Oncol. 2020;22:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27(3):389–395; discussion 396. [PubMed] [Google Scholar]
- 115. Mei Y, Bi WL, Greenwald NF, et al. Genomic profile of human meningioma cell lines. PLoS One. 2017;12(5):e0178322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Püttmann S, Senner V, Braune S, et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85(9):1163–1171. [DOI] [PubMed] [Google Scholar]
- 117. Riesenberg S, Chintalapati M, Macak D, Kanis P, Maricic T, Pääbo S. Simultaneous precise editing of multiple genes in human cells. Nucleic Acids Res. 2019;47(19):e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Baia GS, Dinca EB, Ozawa T, et al. An orthotopic skull base model of malignant meningioma. Brain Pathol. 2008;18(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mawrin C. Animal models of meningiomas. Chin Clin Oncol. 2017;6(Suppl 1):S6. [DOI] [PubMed] [Google Scholar]
- 120. Mordechai O, Postovsky S, Vlodavsky E, et al. Metastatic rhabdoid meningioma with BRAF V600E mutation and good response to personalized therapy: case report and review of the literature. Pediatr Hematol Oncol. 2015;32(3):207–211. [DOI] [PubMed] [Google Scholar]