Abstract
Background
Chemotherapy improves overall survival after surgery and radiotherapy for newly diagnosed high-risk IDH-mutant low-grade gliomas (LGGs), but a proportion of patients treated with temozolomide (TMZ) will develop recurrent tumors with TMZ-induced hypermutation. We aimed to determine the prevalence of TMZ-induced hypermutation at recurrence and prognostic implications.
Methods
We sequenced recurrent tumors from 82 patients with initially low-grade IDH-mutant gliomas who underwent reoperation and correlated hypermutation status with grade at recurrence and subsequent clinical outcomes.
Results
Hypermutation was associated with high-grade disease at the time of reoperation (OR 12.0 95% CI 2.5-115.5, P = .002) and was identified at transformation in 57% of recurrent LGGs previously exposed to TMZ. After anaplastic (grade III) transformation, hypermutation was associated with shorter survival on univariate and multivariate analysis (HR 3.4, 95% CI 1.2-9.9, P = .024), controlling for tumor grade, subtype, age, and prior radiotherapy. The effect of hypermutation on survival after transformation was validated in an independent, published dataset. Hypermutated (HM) tumors were more likely to develop discontiguous foci of disease in the brain and spine (P = .003). To estimate the overall incidence of high-grade transformation among low-grade IDH-mutant tumors, data from a phase II trial of TMZ for LGG were analyzed. Eight-year transformation-free survival was 53.8% (95% CI 42.8-69.2), and 61% of analyzed transformed cases were HM.
Conclusions
TMZ-induced hypermutation is a common event in transformed LGG previously treated with TMZ and is associated with worse prognosis and development of discontiguous disease after recurrence. These findings impact tumor classification at recurrence, prognostication, and clinical trial design.
Keywords: hypermutation, IDH-mutant, low-grade glioma, temozolomide, tumor mutational burden
Key Points.
TMZ-induced hypermutation is associated with high-grade transformation.
TMZ-induced hypermutation is associated with shorter survival after transformation.
TMZ-induced hypermutation is associated with the development of discontiguous disease.
Importance of the Study.
Chemotherapy is a part of the standard of care for initial management of low-grade IDH-mutant gliomas. A proportion of patients initially treated with TMZ will develop disease that is hypermutated. TMZ-induced hypermutation is associated with high-grade histology at recurrence. After high-grade transformation, TMZ-induced hypermutation is associated with shorter overall survival and increased risk of discontiguous disease in the brain and spine. Next-generation sequencing should be considered for recurrences in this patient population. These data have important implications for the management of recurrent IDH-mutant LGG.
Diffuse IDH-mutant low-grade gliomas (LGGs) are low-grade primary brain tumors that are typically diagnosed in young, otherwise healthy adults. Although most tumors initially follow an indolent clinical course, the natural history of these tumors is punctuated by repeated recurrences. A proportion of patients will eventually develop high-grade transformation, resulting in rapid growth and shortened survival. Median survival after transformation is just 2.4 years.1
Following surgical resection of IDH-mutant LGGs, treatment strategies range from observation to aggressive treatment with radiation plus chemotherapy or chemotherapy alone.2 RTOG 9802 established a survival benefit for the addition of procarbazine, lomustine (CCNU), and vincristine (PCV) to radiotherapy over radiotherapy alone following maximal safe resection.3 Although the initial study did not stratify by molecular subtype, a recent post-hoc molecular analysis confirmed the benefit of PCV in both IDH-mutant 1p/19q co-deleted oligodendrogliomas and IDH-mutant 1p/19q non-co-deleted astrocytomas (IDH-mutant astrocytoma).4 In contemporary practice, temozolomide (TMZ) is frequently used in place of PCV due to a more favorable toxicity profile, extrapolating from trials in higher-grade gliomas that have demonstrated efficacy, but this practice is not based on randomized clinical trials in LGG. The CATNON trial has already established the benefit of adjuvant TMZ after maximal safe resection and radiotherapy for patients with grade III IDH-mutant astrocytomas; the utility of concurrent TMZ has not yet been reported.5 The ongoing CODEL clinical trial (NCT00887146) is currently comparing strategies using radiotherapy and TMZ or PCV for the treatment of WHO grade II and III 1p/19q co-deleted oligodendrogliomas.6
TMZ is a cytotoxic DNA alkylating agent with mutagenic potential.7–9 These agents induce cell death via mismatch repair (MMR)-mediated futile cycling, and loss of MMR is a well-described evolutionary escape mechanism for both low- and high-grade gliomas exposed to alkylators.10 Cells lacking MMR function fail to recognize alkylated bases and can develop thousands of mutations distributed throughout the genome. Because of the base specificity of TMZ, these mutations are characterized by a specific mutational signature. Thus, following exposure to alkylating agents, neoplastic cells can develop defects in DNA repair that lead to a hypermutator phenotype.7,11–14
In a previous study, examining the mutational profile of recurrent IDH-mutant gliomas, we identified TMZ-induced hypermutation in six IDH-mutant astrocytomas that had undergone high-grade transformation. Detailed clinicopathologic dissection of resection specimens suggested high-grade transformation resulted from the clonal expansion of a hypermutated (HM) cell that had acquired thousands of new mutations, including in tumor suppressors and oncogenes. These and subsequent studies13,15–17 have focused on genomic correlates of hypermutation. In this integrated analysis of recurrent IDH-mutant LGGs, we investigate the association between hypermutation and high-grade transformation, estimate the prevalence of hypermutation in transformed gliomas, and define the significant clinical impact of hypermutation on patterns of recurrence and prognosis. We validate the prognostic value of hypermutation status using an independent dataset.13
Methods
Recurrent Glioma Cohort
The institutional Neurosurgery Tissue Biorepository was queried for patients with recurrent initially WHO grade II diffuse gliomas in adult patients (age at diagnosis ≥ 18 years). Patients underwent surgery between October 2001 and February 2018. Samples were examined by an expert neuropathologist (J.J.P.) to confirm histology. Patients with tumors lacking mutations in IDH1 or IDH2 were excluded. Patients were required to have ≥1 clinical visit after their neurosurgical intervention to be included in this study. Similarly, the institutional database of patients sequenced with UCSF500 Cancer Panel was queried for adult patients with initially WHO grade II IDH-mutant diffuse gliomas, who had reoperation for recurrent disease. Recurrent tumors must have been sequenced for inclusion in this study. Of the 82 patients in this cohort, 28 were also within the UCSF Phase II cohort (described in the Supplementary Materials).
Tumor Classification and Grading of Recurrent Tumors
During the study period, the classification and grading of gliomas evolved from a purely histologic classification to one incorporating molecular classification.18IDH1 and IDH2 mutation status were determined by sequencing in all cases. Chromosomes 1p/19q co-deletion status was determined based on fluorescence in-situ hybridization or genome-wide chromosomal copy number analysis. IDH-mutant diffuse astrocytomas were defined as any diffuse glioma harboring either p.R132 hotspot mutation in IDH1 or p.R172 hotspot mutation in IDH2 without co-deletion of 1p/19q. IDH-mutant 1p/19q co-deleted oligodendrogliomas were defined as any diffuse glioma harboring either p.R132 hotspot mutation in IDH1 or p.R172 hotspot mutation in IDH2 with concomitant 1p/19q co-deletion, involving the entire arms of both chromosomes in those tumors with genome-wide copy number data available. In one case, an initially WHO grade II IDH-mutant astrocytoma was found to have loss of IDH mutation via deletion of the IDH1 genomic locus at the time of recurrence. This tumor was considered an IDH-mutant astrocytoma for this study.
In the 2016 update of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System, all IDH-mutant, 1p/19q co-deleted oligodendrogliomas are designated either grade II or III.18 For our study, IDH-mutant, 1p/19q co-deleted oligodendrogliomas that had been classified as WHO grade IV prior to 2016 were accordingly reclassified as WHO grade III.18
Because histologic tumor grading requires tumor tissue acquisition, the exact date of high-grade biologic transformation cannot be reliably ascertained without surgical intervention. Various imaging surrogates have been developed for transformation; however, these techniques require the consistent use of specialized imaging. Thus, the time of histologically confirmed high-grade transformation was used as the primary landmark for the analysis herein. This provides a robust assessment of transformation and, for IDH-mutant astrocytomas, classification as WHO grade III or grade IV disease, based on the presence of significant mitotic activity for grade III designation, vs palisading necrosis and/or microvascular proliferation for grade IV.
Exome and Targeted Panel Sequencing
DNA from fresh-frozen or formalin-fixed paraffin-embedded (FFPE) tumor samples were extracted and processed as previously described.19 A paired constitutional DNA sample was extracted from whole blood, buccal swab, or other non-tumor tissue. Unstained slides were prepared from either FFPE and or fresh-frozen tumor tissue samples and stained with hematoxylin and eosin for histopathological review by a neuropathologist (J.J.P.) to confirm diagnosis and to identify a corresponding region to isolate for DNA extraction. Whole exome sequencing and mutation calling were performed using a custom mutation calling pipeline.19,20 A subset of our cohort was sequenced using the UCSF500 Cancer Panel as previously described.21
Definition of Hypermutation
The proposed mechanism and mutation spectrum of TMZ-induced hypermutation has been previously described using exome sequencing data.7,11,14,19,22 In the pan-cancer mutational signature analysis, this pattern corresponds to Signature 11,23 which is enriched for mutations in G:C>A:T signature at both CpG and non-CpG sites and also exhibits strong strand bias. This mutational signature appears specific to TMZ exposure in the setting of MMR deficiency.13 To study the impact of TMZ-associated hypermutation, hypermutation was defined as a somatic mutation burden in excess of 10 Mut/Mb and >40% Signature 11.14 Hypermutation on targeted sequencing was determined by a neuropathologist.
Clinical Follow-up
We performed a retrospective analysis of all our recurrent glioma cohorts. Clinical status, pathology, and radiology reports were reviewed to include systemic therapies, surgeries, and radiation. Dates of treatment, radiologic or clinical progression, surgery, and death were recorded.
Imaging Analysis
Available imaging was reviewed for enhancement and pattern of progression by a radiation oncologist (Y.Y.). Discontiguous disease was defined as a distinct focus of enhancing or non-enhancing disease without intervening or connecting T2 signal abnormality, or the presence of leptomeningeal or ependymal disease. Clinical MRIs were independently reviewed by a neuroradiologist (J.E.V.) who was blinded to hypermutation status. Tumor volume at the time of TMZ administration was assessed in patients who received TMZ as initial therapy after surgical resection or biopsy.
Statistics
The date of initial diagnosis was recorded as the date of initial surgery confirming initial IDH-mutant LGG. The date of transformation was recorded as the date of the first surgery, demonstrating transformation to WHO grade III or IV disease. Similarly, the date of confirmed hypermutation was defined as the date of the surgery demonstrating hypermutation.
Many patients had multiple reoperations for recurrence, including patients with initial grade II recurrence who subsequently developed high-grade transformation. The relationship between high-grade transformation (WHO grade III or IV) and hypermutation status was evaluated using the tumor specimen with the highest grade for each patient using Fisher exact test.
The Kaplan-Meier method and the log-rank test were used for univariate analyses of categorical variables. Multivariate analyses were performed using Cox proportional hazards models. Patients without high-grade transformation were censored at date of death or last follow-up if alive. Progression-free survival (PFS) after high-grade transformation was defined from the date of surgery confirming transformation to WHO grade III or IV disease to the date of the first subsequent progression or death. Patients without subsequent progression or death were censored at the date of last follow-up. Overall survival (OS) after high-grade transformation was defined from the date of high-grade transformation to the date of death. Alive patients were censored at the date of last follow-up. The median duration of follow-up from diagnosis and after transformation was determined using the inverse Kaplan-Meier method. The cumulative incidence of discontinguous disease was computed using death as a competing risk. Comparison between dichotomized groups was performed using Gray’s test. Comparison of continuous variables was performed using the method of Fine and Gray. Differences in the time to transformation between groups were evaluated using the Kruskal-Wallis rank sum test. Differences in the distribution of HM tumors were analyzed using the Fisher exact test.
Results
Prevalence of Hypermutation at Transformation
We identified 82 patients with initial WHO grade II IDH-mutant LGGs (ie, integrated diagnosis for initial resection of either “diffuse astrocytoma, IDH-mutant, WHO grade II” or “oligodendroglioma, IDH-mutant and 1p/19q co-deleted, WHO grade II”) who underwent at least one reoperation for recurrent or progressive disease and had sufficient tissue for whole exome or targeted sequencing. Patient characteristics are shown in Table 1. A total of 139 reoperations were performed (median total operations 2, range 2-5). Before reoperation, 63 patients (76.8%) patients were previously treated with TMZ, 22 (26.8%) were previously treated with radiotherapy, and 18 were treated with both (28.6%). Details regarding tumor subtype, IDH1/2 mutation, hypermutation, therapy prior to reoperation, and T2-defined tumor volume at the time of TMZ administration are listed in Supplementary Tables S1 and S2. Mutational burden and proportion of Signature 11 mutations (see methods) are shown in Supplementary Figure S1A, B.
Table 1.
Patient Demographics and Baseline Characteristics
| Recurrent Glioma Cohort | |||
|---|---|---|---|
| IDH-Mutant 1p/19q Co-Deleted Oligodendroglioma | IDH-Mutant Astrocytoma | Total | |
| N | 34 | 48 | 82 |
| Sex | |||
| Male | 16 (47.1) | 28 (58.3) | 44 (53.7) |
| Female | 18 (52.9) | 20 (41.7) | 38 (46.3) |
| Age at diagnosis, median (range) | 38 (17-66) | 31 (22-49) | 34 (17-66) |
| Prior treatment | |||
| TMZ | 27 (79.4) | 36 (76.6) | 63 (76.8) |
| RT | 10 (29.4) | 12 (25.5) | 22 (26.8) |
| Transformed | 28 (82.4) | 38 (79.2) | 66 (80.5) |
| Grade III | 28 (82.4) | 20 (41.7) | 48 (58.5) |
| Grade IV | - | 18 (37.5) | 18 (22.0) |
| Hypermutation | 15 (44.1) | 16 (33.3) | 31 (37.8) |
| Transformed Tumors | |||
| IDH-Mutant Co-Deleted Oligodendroglioma | IDH-Mutant Astrocytoma | Total | |
| N | 28 | 38 | 66 |
| Sex | |||
| Male (%) | 13 (46.4) | 22 (57.9) | 35 (53.0) |
| Female (%) | 15 (53.6) | 16 (42.1) | 31 (47.0) |
| Age at diagnosis, median (range) | 38 (17-66) | 32 (22-49) | 35 (17-66) |
| Age at transformation, median (range) | 46 (22-71) | 37 (23-56) | 43 (22-71) |
| Years to transformation, median (range) | 6.9 (2.6-16.4) | 5.6 (1.3-16.1) | 5.9 (1.3-16.4) |
| TMZ months, median (range) | 15.4 (0-27) | 10.4 (0-30) | 12 (0-30) |
| KPS at transformation, median (range) | 90 (10-90) | 90 (70-100) | 90 (10-100) |
| CDKN2A homozygous deletion (%) | 2 (7.1) | 2 (5.3) | 4 (6.1) |
| Extent of resection (%) | |||
| Gross total resection | 2 (7.1) | 5 (13.2) | 7 (10.6) |
| Subtotal resection | 23 (82.1) | 31 (81.6) | 54 (81.8) |
| Biopsy | 0 (0.0) | 1(2.6) | 1 (1.5) |
| Prior treatment (%) | |||
| TMZ | 23 (82.1) | 30 (79.0) | 53 (80.3) |
| RT | 10 (35.7) | 11 (28.9) | 21 (31.8) |
| Transformation (%) | |||
| Grade III | 28 (100) | 20 (52.6) | 48 (72.7) |
| Grade IV | - | 18 (47.4) | 18 (27.3) |
| Hypermutation (%) | 14 (50.0) | 16 (42.1) | 30 (45.4) |
Abbreviations: IDH, isocitrate dehydrogenase; KPS, Karnofsky performance status; RT, radiation therapy; TMZ, temozolomide.
Of these 82 patients, 66 had tumors that transformed to higher-grade disease. Median time from diagnosis to transformation was 5.9 years (range 1.3-16.4) for all IDH-mutant LGGs; 4.8 (range 1.3-16.1) for IDH-mutant astrocytomas and 7.0 (range 2.5-16.4) for IDH-mutant 1p/19q co-deleted oligodendrogliomas. Fifty-three of the 66 (80.3%) patients with transformed tumors were previously treated with TMZ. In this select cohort, 30 of the 53 (57%) patients with transformed tumors previously exposed to TMZ had hypermutation at the time of transformation. One patient had a histologically grade II tumor at first recurrence, which was HM, but a subsequent recurrence was transformed and remained HM.
Association Between Hypermutation and High-Grade Transformation
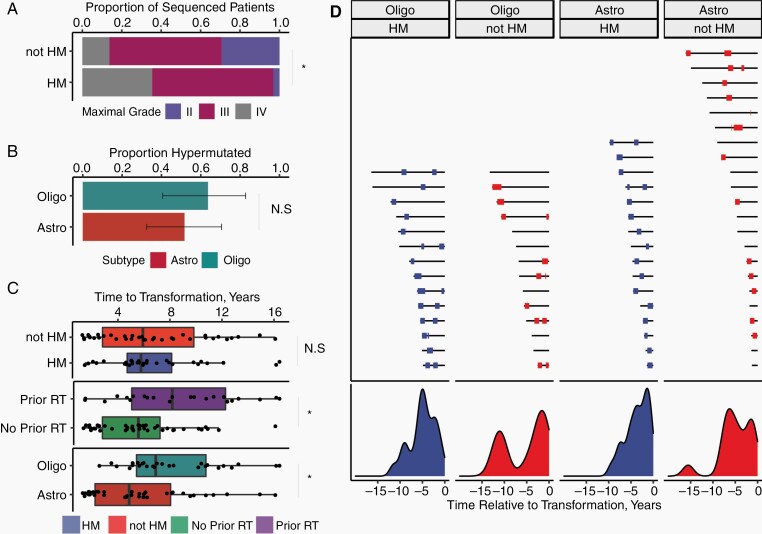
From the cohort of 82 patients, 105 recurrent glioma samples were analyzed for pathologic grade and hypermutation status, including patients with multiple recurrences. The distribution of WHO grade and hypermutation status per tumor specimen is shown in Supplementary Table S3. HM tumors were more likely to be high grade (OR 12.0, 95% CI 2.5-115.5, P = .002, Figure 1A). The prevalence of hypermutation among transformed tumors was similar (P = .78) for IDH-mutant 1p/19q co-deleted oligodendrogliomas 61% (14/23) and IDH-mutant astrocytomas 53% (16/30, Figure 1B). No patients with transformed tumors (0/13) developed hypermutation in the absence of TMZ exposure.
Fig. 1.
The frequency and prognostic significance of TMZ-associated hypermutation in IDH-mutant LGG. Panels A-D describe data from the cohort of gliomas sequenced at recurrence. (A) Among all sequenced tumors, highest histologically confirmed grade at re-resection for HM and non-HM tumors. (B) Proportion of transformed tumors exposed to TMZ that were hypermutated, stratified by subtype. (C) Time from diagnosis to transformation stratified by subtype, hypermutation status, and prior radiotherapy. (D) Timelines of prior TMZ exposure. Black lines represent a time from initial diagnosis to time of transformation (time zero). Red and blue bars represent TMZ exposure for HM vs non-HM tumors. Many patients received multiple courses of TMZ. Density plots of TMZ exposure are shown below the patient timelines. Abbreviations: Astro, IDH-mutant astrocytoma; HM, hypermutated; IDH, isocitrate dehydrogenase; LGG, low-grade glioma; Oligo, IDH-mutant 1p/19q co-deleted oligodendroglioma; N.S., non-significant; TMZ, temozolomide. * indicates significant result with P < .05.
Of 63 patients in the recurrent glioma cohort treated with TMZ, 31 patients were found to have HM recurrences and 93.5% (29/31) had histopathologic evidence of high-grade transformation in the HM specimen. Both patients with grade II HM tumors had clinical courses consistent with high-grade transformation, suggesting possible under-grading due to sampling. One of these patients, who developed a grade II HM IDH-mutant 1p/19q co-deleted oligodendroglioma recurrence, exhibited an aggressive clinical course and developed nodular enhancement within 13 months of reoperation but did not undergo another surgery to confirm histologic transformation. The second patient developed an HM grade II recurrent IDH-mutant astrocytoma 33 months after completion of TMZ. This patient developed enhancing disease after 21 months, and subsequent surgery revealed an HM grade III IDH-mutant astrocytoma. In all other cases, hypermutation coincided with high-grade transformation.
Clinical and Molecular Factors Associated With Hypermutation
To identify other risk factors for hypermutation, we examined the association between hypermutation and clinical-molecular factors among patients treated with TMZ. Our group recently described a higher MGMT promoter methylation level in the initial tumors associated with an increased likelihood of hypermutation upon recurrence.24 We did not identify a difference in either the number of cycles of TMZ received prior to transformation or the volume of tumor at initial TMZ administration between HM and non-HM tumors (Supplementary Figure S1C, D).
Because TMZ-induced hypermutation has been associated with TMZ resistance, we tested the hypothesis that HM tumors would have a shorter time to transformation compared with non-HM tumors. Contrary to this hypothesis, no difference in either time from diagnosis to transformation (mean 6.7 years vs 6.7 years, Kruskal-Wallis P = .38) or time from TMZ exposure to transformation (mean 5.4 years vs 3.1 years, Kruskal-Wallis P = .26) were identified between HM and non-HM tumors (Figure 1C). Receipt of prior radiotherapy (mean 8.6 vs 5.7 years, Kruskal-Wallis P = .010) and IDH-mutant 1p/19q co-deleted oligodendroglioma subtype (mean 8.1 vs 5.6, P = .0037) were associated with increased time from diagnosis to transformation (Figure 1C).
Treatment timelines from initial diagnosis to transformation (Figure 1D) reveal remarkable heterogeneity in the latency period from initial exposure to TMZ to histologically confirmed high-grade transformation.
High Incidence of High-Grade Transformation Following TMZ Monotherapy
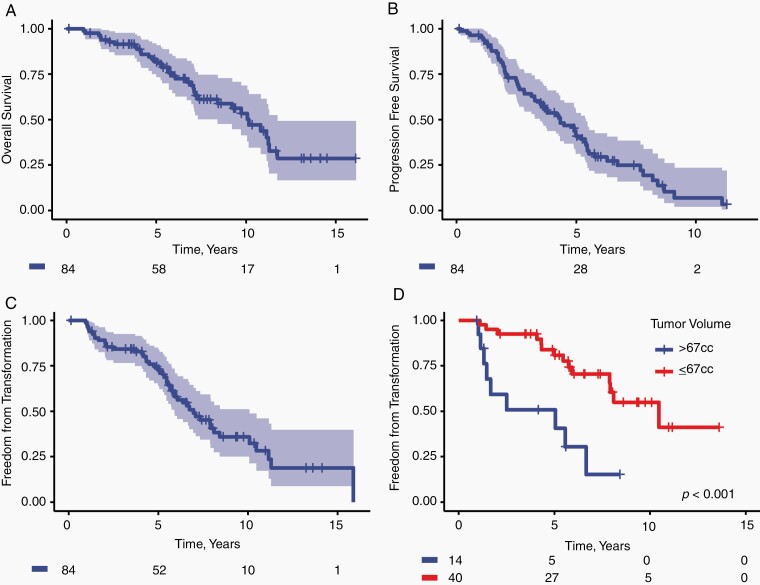
We next sought to determine the risk of transformation in patients treated with TMZ. We previously reported a phase II clinical trial of TMZ monotherapy for sub-totally resected grade II diffuse gliomas.25 Details of the clinical trial and patient characteristics are described in the Supplementary Materials (Supplementary Methods, Supplementary Table S4). Of 84 patients with confirmed IDH-mutant grade II gliomas enrolled, 62 progressed, 48 underwent reoperation, and 32 developed pathologically confirmed high-grade transformation. Eighteen of these patients had sufficient tissue at recurrence for sequencing, of which 61% (11/18) were HM. With median follow-up of 8.7 years, the median OS and PFS were 10.2 years (95% CI, 8.4-11.8, Figure 2A) and 4.3 years (95% CI, 3.5-5.4, Figure 2B), respectively. The median transformation-free survival time was 7.0 years (95% CI, 5.9-10.1, Figure 2C) and was not significantly different between IDH-mutant 1p/19q co-deleted oligodendrogliomas and IDH-mutant astrocytomas. Tumor volume at trial enrollment was associated with shorter time to transformation (HR 1.10/10cc, 1.1-1.2, P = .0002, Figure 2D) and OS (HR 1.09/10cc, 1.0-1.2, P = .0008).
Fig. 2.
Outcomes from the subset of patients with confirmed IDH-mutant LGGs enrolled on a phase II clinical trial of TMZ monotherapy for high-risk LGG. (A) Overall survival and (B) progression-free survival are reported from the time of trial enrollment. Time from trial enrollment to histologic confirmation of high-grade transformation is shown for (C) the subset of confirmed IDH-mutant gliomas and (D) stratified by initial tumor volume on T2-weighted imaging. Abbreviations: IDH, isocitrate dehydrogenase; LGG, low-grade glioma; TMZ, temozolomide.
PFS and OS After Transformation
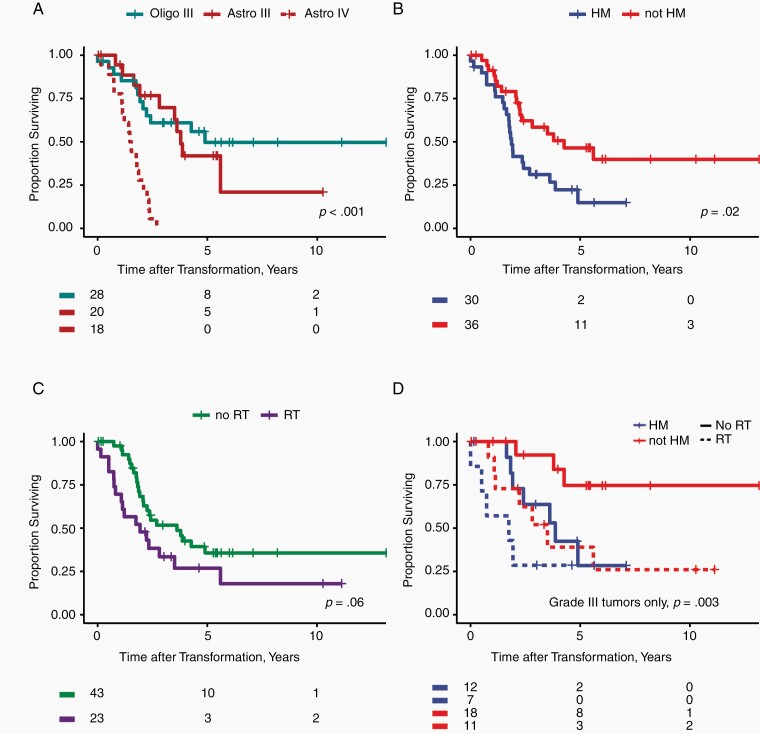
To investigate the prognostic value of hypermutation after transformation, we evaluated clinical outcomes for 66 patients with high-grade transformation and validated our findings using an independent, previously published cohort from Dana-Farber Cancer Institute (DFCI) (Supplementary Figure S2, Supplementary Table S5).13 Baseline characteristics of transformed tumors are shown in Table 1. With median follow-up after high-grade transformation of 5.4 years, median PFS and OS were 1.3 (95% CI, 1.1-1.7) and 2.4 years (95% CI, 1.9-4.9), respectively. Molecular subtype, hypermutation status, and receipt of radiotherapy prior to transformation were associated with OS after transformation (univariate analysis; Figure 3A–C). Karnofsky performance status (KPS) was available for only 33 (50%) of the transformed glioma cohort; higher KPS was associated with better prognosis on univariate analysis (Table 2) but was not included in multivariate modeling due to missing data. Grade and IDH-mutant astrocytoma subtype were associated with shorter survival after transformation, but survival was similar for grade III astrocytomas and grade III IDH-mutant 1p/19q co-deleted oligodendrogliomas (Table 2). Outcomes from diagnosis for the cohort of 82 patients are shown in Supplementary Table S6 and Supplementary Figure S3.
Fig. 3.
Overall survival measured from the time of histologically proven high-grade transformation is shown, (A) stratifying by grade and molecular subtype, (B) hypermutation status, and (C) treatment with radiotherapy prior to transformation. In panel D, we show a bivariate model of survival after transformation, accounting for hypermutation status and receipt of radiotherapy. (D) Multivariate analysis of grade III tumors showing both hypermutation and prior radiation remain significant predictors of survival. Abbreviations: Astro, IDH-mutant astrocytoma; IDH, isocitrate dehydrogenase; Oligo, IDH-mutant 1p/19q co-deleted oligodendroglioma.
Table 2.
Univariate and Multivariate Analyses of Survival After Transformation
| Survival After Transformation, Grade III Tumors Only | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Subtype | ||||||
| IDH-mutant 1p/19q co-deleted oligodendroglioma | 1 | Reference | - | 1 | Reference | - |
| IDH-mutant astrocytoma | 1.27 | 0.55-2.95 | 0.58 | 2.56 | 0.84-7.76 | 0.098 |
| Hypermutation | ||||||
| Non-HM | 1 | Reference | - | 1 | Reference | - |
| HM | 2.32 | 1.00-5.40 | 0.051 | 3.42 | 1.18-9.92 | 0.024 |
| Prior RT | ||||||
| No prior RT | 1 | Reference | - | 1 | - | Reference |
| Prior RT | 2.8 | 1.20-6.54 | 0.018 | 3.33 | 1.36-8.13 | 0.0084 |
| Age at transformation (per year increase) | 1.03 | 0.99-1.08 | 0.16 | 1.03 | 0.98-1.10 | 0.34 |
| Time to transformation (per year increase) | 0.963 | 0.86-1.08 | 0.51 | |||
| KPSa | 0.95 | 0.92-0.99 | 0.024 | |||
| Extent of resection | ||||||
| STR or biopsy | 1 | Reference | - | |||
| GTR | 1.05 | 0.24-4.52 | 0.95 | |||
| CDKN2A status | ||||||
| No homozygous deletion | 1 | Reference | - | |||
| Homozygous deletion | 1.65 | 0.38-7.10 | 0.51 | |||
| Survival After Transformation, All Transformed Tumors | ||||||
| Univariate | ||||||
| Hazard Ratio | 95% CI | P Value | ||||
| Subtype | ||||||
| IDH-mutant 1p/19q co-deleted oligodendroglioma | 1 | Reference | - | |||
| IDH-mutant astrocytoma | 2.45 | 1.23-4.86 | .011 | |||
| Hypermutation | ||||||
| Non-HM | 1 | Reference | - | |||
| HM | 2.15 | 1.14-4.04 | .018 | |||
| Grade III vs IV | ||||||
| Grade III | 1 | Reference | - | |||
| Grade IV | 6.05 | 2.92-12.52 | <.0001 | |||
| Prior RT | ||||||
| No prior RT | 1 | Reference | - | |||
| Prior RT | 1.83 | 0.97-3.43 | .061 | |||
| Age at transformation (per year increase) | 1.03 | 0.70-1.34 | .85 | |||
| Time to transformation (per year increase) | 0.95 | 0.87-1.03 | .21 | |||
| KPSa | 0.96 | 0.93-0.99 | .017 | |||
| Extent of resection | ||||||
| STR or biopsy | 1 | Reference | - | |||
| GTR | 0.75 | 0.23-2.46 | .64 | |||
| CDKN2A status | ||||||
| No homozygous deletion | 1 | Reference | - | |||
| Homozygous deletion | 0.72 | 0.42-4.51 | .59 | |||
Abbreviations: GTR, gross total resection of enhancing and non-enhancing disease; HM, hypermutated; IDH, isocitrate dehydrogenase; KPS, Karnofsky performance status; RT, radiation therapy; STR, subtotal resection of enhancing and non-enhancing disease.
aKPS not included in multivariable models because of missing data (n = 33).
To control for the effect of tumor grade, we examined survival stratifying by tumor grade. Among 48 patients with tumors that transformed to WHO grade III, median survival after transformation was 4.3 years (95% CI 3.5—not reached). Hypermutation and prior radiation therapy (RT) before transformation were associated with shorter OS on univariate analysis. Among grade III IDH-mutant 1p/19q co-deleted oligodendrogliomas and grade III IDH-mutant astrocytomas, hypermutation was a stronger prognostic factor than molecular subtype (Table 2). On multivariate analysis, both hypermutation and prior RT remained significant predictors (Table 2, Figure 3D). This model was validated using data from the DFCI cohort, with Harrell’s C statistic of 0.74 and 0.72 for the training and validation datasets, respectively (Supplementary Figure S2C–F, Supplementary Table S7, Supplementary Results). Among 18 patients with transformation to WHO grade IV IDH-mutant astrocytoma (ie, IDH-mutant glioblastoma), median survival was 1.5 years (95% CI 1.1-2.3), and hypermutation status was not prognostic (P = .78). Patient-specific data on outcomes after transformation are listed in Supplementary Table S8.
Patterns of Failure After Transformation
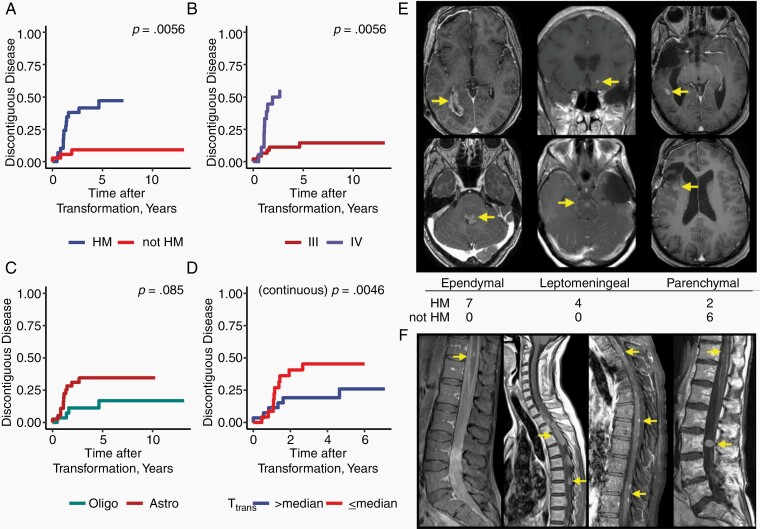
We reviewed the long-term radiologic patterns of failure after recurrence. Nineteen patients developed foci of discontiguous disease within the central nervous system; 16 cases occurred after documented high-grade transformation. Three patients underwent reoperation demonstrating recurrent, non-HM grade II disease, but subsequently developed foci of discontinguous disease. We investigated predictors of discontiguous disease among patients with transformed tumors, including time to transformation. All patients who developed discontiguous disease were exposed to TMZ. Hypermutation (Gray’s test, P = .001, Figure 4A, Supplementary Figure S4A, B) and grade (Gray’s test, P = .0011, Figure 4B, Supplementary Figure S4C, D) were associated with increased risk of discontiguous disease on univariate analysis, and a trend was observed for molecular subtype (Gray’s test P = .08, Figure 4C, Supplementary Figure S4E, F). Time from diagnosis to transformation was associated with decreased risk of discontiguous disease when analyzed as a continuous variable (Fine and Gray, P = .005). The cumulative incidence of discontiguous disease dichotomized by the mean time to transformation (6.7 years) is shown in Figure 4D (Gray’s test P = .05). Competing risks of death are shown in Supplementary Figure S4.
Fig. 4.
Hypermutated tumors are at increased risk of developing discontiguous disease after transformation. Cumulative incidence of discontiguous disease from the time of transformation, stratified by hypermutation status (A), Grade (B), Molecular subtype (C). The cumulative incidence of discontiguous disease dichotomized by the mean time-to-transformation (D). Representative images of ependymal, leptomeningeal, and parenchymal patterns of recurrence on T1 contrast-enhanced MRI and distribution for HM and non-HM tumors (E). Representative spine imaging from all four patients with leptomeningeal disease who were found to have spinal dissemination after high-grade transformation (F). Abbreviations: Astro, IDH-mutant Astrocytoma; Oligo, IDH-mutant 1p/19q co-deleted Oligodendroglioma.
HM tumors that developed discontiguous disease were associated with distinct patterns of failure; seven developed ependymal disease, four developed leptomeningeal disease, and two developed parenchymal disease (Figure 4E). By contrast, all six patients with non-HM tumors that developed discontiguous disease were found to have parenchymal nodules. Four patients with HM tumors developed spinal dissemination (Figure 4F). One patient underwent rapid autopsy and exome sequencing of a spinal deposit, demonstrating a HM tumor derived from a previously identified intracranial HM clone.
Discussion
Our study adds significantly to a growing body of literature suggesting the biological, therapeutic, and clinical relevance of hypermutation status in IDH-mutant LGG.13,17,26 Exome sequencing at recurrence reveals a high prevalence of hypermutation in transformed IDH-mutant LGG previously exposed to TMZ, as well as a strong association between hypermutation and high-grade transformation. Using data from an institutional phase II clinical trial of TMZ monotherapy, we find that high-grade transformation is a common event with long-term follow-up and that hypermutation may be identified in a substantial proportion of transformed tumors. Our longitudinal, blinded review of clinical MRI demonstrated, for the first time, an increased risk of discontiguous disease, including cases of ependymal and leptomeningeal spread.
Two recent papers have investigated the prognostic significance of hypermutation after recurrence with mixed results.13,17 While an initial analysis from the GLASS Consortium did not demonstrate a prognostic effect for hypermutation on univariate or multivariate analysis, these findings were in the context of smaller (42 IDH-mutant low-grade tumors) and heterogeneous cohort containing both initially low and initially high-grade tumors.17 Additionally, the GLASS study analyzed survival from diagnosis, rather than recurrence, which may impact their analysis, since only patients who are alive for reoperation could be included in their analysis. A larger study by Touat and colleagues found hypermutation to be associated with worse prognosis after recurrence, but it controlled only for low- vs high-grade recurrence and did not differentiate between grade III vs grade IV recurrence, and thus could not establish whether hypermutation was prognostic independent of grade at recurrence. Indeed, in our study, HM IDH-mutant astrocytomas were much more likely to be grade IV than grade III. In this study, we find that hypermutation is a strong prognostic factor after anaplastic transformation to grade III disease and develop an externally validated prognostic model for survival after transformation.
Strikingly, we found that patients with HM tumors were more likely to develop discontiguous disease, including several patients that developed ependymal and leptomeningeal dissemination, patterns that are otherwise uncommon in non-HM cases. Four patients with HM tumors developed spinal dissemination, including one patient where the spinal metastasis was sampled at autopsy and found to be HM and clonally related to a previously identified left temporal tumor. The cumulative incidence of discontiguous disease approached 50% among HM tumors, far higher than the 12% crude incidence of discontiguous disease reported by Fukuya and colleagues among transformed LGGs previously treated with nitrosourea-based chemotherapy.27 For patients who are found to have HM disease, this pattern of spread may have implications for follow-up imaging and selection of therapies at recurrence. To limit study bias, a neuroradiologist who was blinded to tumor hypermutation status reviewed all available clinical imaging following transformation and classified discontiguous disease as parenchymal, ependymal, or leptomeningeal. While this finding is striking, it is based upon a limited number of patients, and validation in an external dataset is warranted.
The high frequency of hypermutation among transformed, initially LGGs treated with TMZ has implications for the interpretation of previous clinical trials and management of these patients with transformed LGGs. Because hypermutation is prognostic following anaplastic transformation, randomized trials that do not account for this phenomenon will be confounded if the proportions of tumors that are HM are not uniformly distributed between treatment arms. In current practice, TMZ re-challenge is often considered for patients who are MGMT methylated and who have a long disease-free interval after initial TMZ exposure, but HM tumors are at increased risk of TMZ resistance due to acquired MMR deficiency.13 As our study shows, a long disease-free interval may not preclude the possibility of hypermutation. Furthermore, tumors that are MGMT-hypermethylated are associated with an increased risk of subsequent hypermutation.24 By contrast, HM cell lines remain sensitive to nitrosoureas in vitro.13,28,29 Clinical trials that explicitly consider hypermutation status at recurrence will be needed.
In our analysis of high-risk LGGs treated with 12 months of TMZ without radiotherapy, the median transformation-free survival was 7.0 years, and there was a strong association between residual tumor volume at the time of TMZ administration and the long-term risk of high-grade transformation. This is similar to the 10-year transformation-free survival of 60% reported by investigators from the Cleveland Clinic.1 Because our trial included only patients with residual disease, the risk of transformation we observed is likely higher than for unselected IDH-mutant gliomas. Interestingly, Tom et al. also reported an association between adjuvant treatment strategy and subsequent risk of transformation; 10-year transformation-free survival was approximately 80% for patients treated with observation, radiotherapy alone, or chemoradiotherapy.1 Although estimates of the transformation-free survival are biased by the rate of reoperation and the variable latency between initial treatment and transformation, the 20% risk of transformation observed in patients treated with observation, radiotherapy, or chemoradiotherapy observed by Tom et al. is similar to the crude risk of transformation observed on EORTC 22845 (18.9% vs 16.6% crude risk for radiotherapy vs observation arms).30 Ultimately, because of the biases associated with this analysis and the limited sample size, this study cannot definitively address whether TMZ monotherapy is associated with increased risk of transformation compared with other adjuvant therapy strategies. Long-term follow-up of clinical trials, such as EORTC 22033 and CODEL31 will better address this question.
The patients in the sequencing cohort were identified from a single institution with a uniform treatment strategy, where a large proportion of patients were treated with TMZ monotherapy. Many of these patients were treated as part of a prospective clinical trial. As such, this study evaluates hypermutation in TMZ-treated patients and cannot address the risk of hypermutation in patients treated with other standard alkylating chemotherapy regimens, including CCNU and PCV. Previous reports have demonstrated that CCNU can also lead to hypermutation,11 but HM cell lines appear to retain sensitivity to CCNU.13 Furthermore, the previous phase II clinical trial25 at our institution enrolled patients with subtotal resection, enriching our cohort for patients with large volumes of residual disease who were exposed to TMZ monotherapy. The prevalence of hypermutation in cohorts with small volumes of residual disease or differing adjuvant therapy strategies may be reduced. Finally, tumors were selected for resection and tissue banking over a period of two decades, limiting the generalizability of our study.
Hypermutation is strongly associated with alterations in the MMR pathway,7,10,14,19 which is thought to contribute to resistance to TMZ and survival of HM, clonal populations. Whether HM tumors have differing susceptibility to chemotherapy, PD-1/PD-L1-based immunotherapy, or therapies targeting novel immune checkpoints are questions under active investigation.
This study supports an association between hypermutation and shorter survival after transformation, but the mechanism for this association remains unclear. HM tumors uniformly harbor MMR mutations, most of which are G to A, and carry thousands of mutations in exons, likely tens of thousands across the whole genome. In our companion genomic analysis, we identified frequent deleterious mutations in cell cycle regulatory genes, bearing the hallmark of TMZ mutagenesis. These same genes and pathways are altered by other genetic mechanisms during malignant transformation in non-HM tumors, suggesting shared mechanisms via different types of genetic alteration. Alternatively, an unmeasured clinical or molecular factor could be driving both the risk of hypermutation and poor prognosis after transformation. In our analysis of the phase II trial of TMZ monotherapy, we found residual tumor volume at the time of TMZ administration to be strongly associated with PFS, time to transformation, and death; thus, if HM tumors were larger than non-HM tumors, this could explain the association between hypermutation and poor prognosis. However, we could not identify a significant difference in tumor volume between HM and non-HM transformed tumors in the primary sequencing cohort, suggesting that tumor volume may be a risk factor for transformation regardless of hypermutation status. In a recent analysis of MGMT status and hypermutation, our group found MGMT-hypermethylated tumors were at increased risk of hypermutation. While MGMT-hypermethylated tumors are typically associated with favorable prognosis after treatment with TMZ, they are also subject to strong evolutionary selection pressure.10,24 This may in part contribute to the high incidence of MMR mutations, which are associated with TMZ resistance.
The results and conclusions of this study are subject to selection bias since all patients necessarily underwent reoperation in order to undergo sequencing. At our institution, reoperation is typically reserved for patients who have a single focus of progressive disease that can be completely resected. Patients with inoperable or multifocal recurrences are excluded. In the phase II clinical trial of TMZ monotherapy, 60% of patients ultimately underwent reoperation. This likely impacts the analysis of discontiguous disease, as patients who had developed multifocal recurrences at progression would not have been captured in this analysis. In addition, our cohort was biased toward patients with tumors that transformed to grade III or IV disease. Because this may impact the overall prevalence of hypermutation among all sequenced recurrences, we have reported the prevalence of hypermutation among transformed tumors that were previously exposed to TMZ. This metric should be robust, even if grade II recurrences were not sequenced. In future studies, noninvasive methods of genotyping tumor recurrences, such as with sequencing of CSF, may help to avoid these biases and facilitate longitudinal sampling that may not be possible with invasive testing.32
This study should not be used to change treatment recommendations for newly diagnosed IDH-mutant LGG. Additionally, these data cannot be used to evaluate the overall benefit of TMZ for newly diagnosed LGGs, since only patients who have surgical resection of the recurrence are captured in our analysis. Furthermore, there is emerging randomized evidence which may provide better guidance on TMZ use in this population. The CATNON clinical trial has shown an OS benefit for adjuvant TMZ in IDH-mutant grade III gliomas without 1p/19q co-deletion treated with surgery and postoperative radiotherapy.5 The exploratory molecular subgroup analysis of the EORTC 22033 clinical trial found TMZ monotherapy was associated with shorter PFS compared with radiotherapy alone.31 Similarly, the CODEL clinical trial found TMZ monotherapy was associated with inferior outcomes in grade II and grade III IDH-mutant 1p/19q co-deleted gliomas.6 Ultimately, long-term follow-up results from these clinical trials will determine the overall risk and benefit of TMZ in the up-front management of low-grade IDH-mutant gliomas.
TMZ-associated hypermutation is associated with shorter survival after transformation and unique risk of tumor cell dissemination, and therefore hypermutation status may be clinically useful for the management of patients with tumors that have transformed to higher grades. The presence of hypermutation should be considered in patient eligibility for and interpretation of future clinical trials that enroll patients with recurrent gliomas.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the UCSF Brain Tumor Center SPORE Biorepository and Pathology Core (NIH/NCI P50CA097257 to J.J.P.). The UCSF Cancer Registry was used to confirm clinical histories obtained through a review of the medical record.
Funding
This study was supported by NIH/NCI P50CA097257 (J.F.C. and N.A.O.B.), NIH R01CA169316 (J.F.C.), ABC2, and the Dabbiere Family.
Conflict of interest statement. The authors declare no potential conflicts of interest.
Author contributions. Y.Y.: design and performance of experiments listed, data analysis, and writing of the manuscript; J.V.-M.: blinded imaging analysis, editing of the manuscript; M.R.G.: bioinformatic analysis of exome sequencing data, editing of the manuscript; S.H.: bioinformatic analysis of exome sequencing data, editing of the manuscript; D.A.S.: next-generation sequencing of tumor tissue, editing of the manuscript; S.C.: editing of the manuscript; M.W.: data analysis and writing of the manuscript; T.M.: bioinformatic analysis of exome sequencing data, editing of the manuscript; C.H.: bioinformatic analysis of exome sequencing data, editing of the manuscript; A.S.: bioinformatic analysis of exome sequencing data, editing of the manuscript; J.J.P.: review of pathology slides, editing of the manuscript; B.H.W.: performed rapid autopsy; M.M.: performed neurosurgical resections, editing of the manuscript; D.H.-K.: editing of the manuscript; J.W.T.: neuro-oncologic care of patients, editing of the manuscript; N.B.: neuro-oncologic care of patients, editing of the manuscript; J.L.C.: neuro-oncologic care of patients, editing of the manuscript; M.S.B.: performed neurosurgical resections; A.M.M.: performed statistical analysis; S.M.C.: neuro-oncologic care of patients, editing of the manuscript; J.F.C.: designed experiments, interpretation of data, writing of the manuscript; N.A.O.B.: designed experiments, interpretation of data, writing of the manuscript.
References
- 1. Tom MC, Park DYJ, Yang K, et al. Malignant transformation of molecularly classified adult low-grade glioma. Int J Radiat Oncol. 2019;105(5):1106–1112. [DOI] [PubMed] [Google Scholar]
- 2. Oberheim Bush NA, Chang S. Treatment strategies for low-grade glioma in adults. J Oncol Pract. 2016;12(12):1235–1241. [DOI] [PubMed] [Google Scholar]
- 3. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG Oncology/RTOG 9802: a Phase III trial of radiation versus radiation plus procarbazine, Lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020;38(29):3407–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaeckle K, Vogelbaum M, Ballman K, et al. CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): phase III randomized study of RT vs. RT+TMZ vs. TMZ for newly diagnosed 1p/19q-codeleted anaplastic oligodendroglial tumors. analysis of patients treated on the original protocol design (PL02.005). Neurology. 2016;86(16 Supplement). http://n.neurology.org/content/86/16_Supplement/PL02.005.abstract [Google Scholar]
- 7. Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margison GP, Santibáñez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17(6):483–487. [DOI] [PubMed] [Google Scholar]
- 9. Bodell WJ, Gaikwad NW, Miller D, Berger MS. Formation of DNA adducts and induction of lacI mutations in Big Blue Rat-2 cells treated with temozolomide: implications for the treatment of low-grade adult and pediatric brain tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(6):545–551. [PubMed] [Google Scholar]
- 10. Choi S, Yu Y, Grimmer MR, Wahl M, Chang SM, Costello JF. Temozolomide-associated hypermutation in gliomas. Neuro Oncol. 2018;20(10):1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cahill DP, Codd PJ, Batchelor TT, Curry WT, Louis DN. MSH6 inactivation and emergent temozolomide resistance in human glioblastomas. Clin Neurosurg. 2008;55:165–171. [PubMed] [Google Scholar]
- 13. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell BB, Light N, Fabrizio D, et al. Comprehensive analysis of hypermutation in human cancer. Cell. 2017;171(5):1042–1056.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai H, Harmancı AS, Erson-Omay EZ, et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet. 2016;48(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 17. Barthel FP, Johnson KC, Varn FS, et al. ; GLASS Consortium . Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louis DN, Antonescu CR, Huse JT, Rosenblum MK, International Agency for Research on Cancer, Memorial Sloan Kettering Cancer Center (MSKCC) . WHO Classification of Tumours of the Central Nervous System. Revised 4th ed. Lyon: International Agency For Research On Cancer; 2016. [Google Scholar]
- 19. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129(4):597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kline CN, Joseph NM, Grenert JP, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol. 2017;19(5):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain . Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathur R, Zhang Y, Grimmer MR, et al. MGMT promoter methylation level in newly diagnosed low-grade glioma is a predictor of hypermutation at recurrence. Neuro Oncol. 2020;22(11):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahl M, Phillips JJ, Molinaro AM, et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 2017;19(2):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019;25(18):5537–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukuya Y, Ikuta S, Maruyama T, et al. Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J Neurooncol. 2019;144(3):519–528. [DOI] [PubMed] [Google Scholar]
- 28. Aquilina G, Ceccotti S, Martinelli S, Hampson R, Bignami M. N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea sensitivity in mismatch repair-defective human cells. Cancer Res. 1998;58(1):135–141. [PubMed] [Google Scholar]
- 29. Tong WP, Kirk MC, Ludlum DB. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N′-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982;42(8):3102–3105. [PubMed] [Google Scholar]
- 30. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council . Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 31. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.