Abstract
Objective
To update the 2000 American Academy of Neurology (AAN) practice parameter on anticonvulsant prophylaxis in patients with newly diagnosed brain tumors.
Methods
Following the 2017 AAN methodologies, a systematic literature review utilizing PubMed, EMBASE Library, Cochrane, and Web of Science databases was performed. The studies were rated based on the AAN therapeutic or causation classification of evidence (class I-IV).
Results
Thirty-seven articles were selected for final analysis. There were limited high-level, class I studies and mostly class II and III studies. The AAN affirmed the value of these guidelines.
Recommendations
In patients with newly diagnosed brain tumors who have not had a seizure, clinicians should not prescribe antiepileptic drugs (AEDs) to reduce the risk of seizures (level A). In brain tumor patients undergoing surgery, there is insufficient evidence to recommend prescribing AEDs to reduce the risk of seizures in the peri- or postoperative period (level C). There is insufficient evidence to support prescribing valproic acid or levetiracetam with the intent to prolong progression-free or overall survival (level C). Physicians may consider the use of levetiracetam over older AEDs to reduce side effects (level C). There is insufficient evidence to support using tumor location, histology, grade, molecular/imaging features when deciding whether or not to prescribe prophylactic AEDs (level U).
Keywords: antiepileptic drug, GBM, glioma, guideline, seizure
Seizures are a common and potentially devastating complication of both primary and metastatic brain tumors. Precise data are difficult to obtain, but the frequency of epileptic seizures in patients with brain tumors is reported to range from 35% to 70%.1,2 Seizures are described as the first symptom of brain tumors in 20%-40% of all patients, while 10% of patients experience a seizure at some point during the course of their disease.2,3 Seizures in brain tumor patients have a significant impact on long-term disability and are associated with high symptom burden during the end-of-life phase.4,5
Seizures are much more common in patients with lower-grade (WHO II) glioma than patients with higher-grade (WHO III/IV) glioma or brain metastases.6 Since brain metastases affect approximately 10%-30% of patients with systemic cancer,7,8 management of seizures in this population is also a significant issue. While primary brain tumors are overall much rarer than brain metastases with an average annual age-adjusted incidence rate of 23.03 per 100 000, seizures in this population are estimated to cause up to 10% of all epilepsy cases.5
The administration of antiepileptic drugs (AEDs) to patients with brain tumors who have not had seizures is common despite the lack of definitive evidence that the potential benefits might outweigh the side effects of AEDs. Significant side effects include cognitive impairment, neuropsychiatric disorders, fatigue, myelosuppression, liver dysfunction, dermatologic reactions, and interactions with systemic cancer treatment. Therefore, the judicious use of AEDs in the right patient to avoid unnecessary side effects and financial burden on patients is essential.
A prior American Academy of Neurology (AAN) practice parameter report systematically assessed the role of anticonvulsant prophylaxis in patients with newly diagnosed brain tumors in 2000.9 The guideline focused on the question of whether patients with newly diagnosed brain tumors without any history of a seizure should be treated prophylactically with AEDs to prevent first seizures. A total of 4 randomized trials satisfying the criteria of level I evidence at that time,10–12 and 8 papers describing studies of level II evidence13–18 were identified. Two of the abstracts described as level I studies at that time are excluded from this evidentiary assessment due to updated evidence levels. The authors concluded that in patients with newly diagnosed brain tumors, anticonvulsant medications are not effective as primary seizure prophylaxis. Because of a lack of efficacy and potential side effects, they recommended that prophylactic anticonvulsants not be used routinely in patients with newly diagnosed brain tumors. In addition, they concluded that in patients with brain tumors treated surgically who have not had a seizure, tapering and discontinuing anticonvulsants after the first postoperative week is appropriate.
Since then, more modern, nonenzyme-inducing antiepileptic drugs (NEIAEDs, eg, levetiracetam) have been approved and practice patterns in the treatment of seizures in brain tumor patients have evolved. In addition, some AEDs (ex. valproate) have been suggested to have anti-tumor activity. Building on the prior report, a multidisciplinary panel of expert neurologists, epileptologists, neurophysiologists, neurosurgeons, and neuro-oncologists under the guidance of the Society for Neuro-Oncology (SNO) and the European Association of Neuro-Oncology (EANO) was formed to update the practice parameters on anticonvulsant prophylaxis in patients with newly diagnosed brain tumors.
Methods
Description of Analytical Process
No institutional review board approval was obtained as only published data were used for this practice guidelines review.
This practice parameter update follows the methodologies described in the 2017 edition of the AAN’s guideline development process manual.19 Conclusions and recommendations were developed in accordance with the process manual and the updated scheme for classifying therapeutic and causation articles.19 In 2017, after reviewing potential members’ conflict of interest statements and curriculum vitae, a multidisciplinary panel of experts in brain tumors, neurosurgery, and epilepsy were chosen to develop this guideline. The original panel consisted of 9 neuro-oncologists (E.R.G., T.W., P.Y.W., D.S., R.A.H., M.W., E.L.R., G.H.J.S., W.W.), 4 epileptologists (E.K.A., J.W.L., M.C., P.K.), and 1 neurosurgeon (M.A.V.). The panel developed research questions in PICO format: patient, intervention, comparison, and outcome.
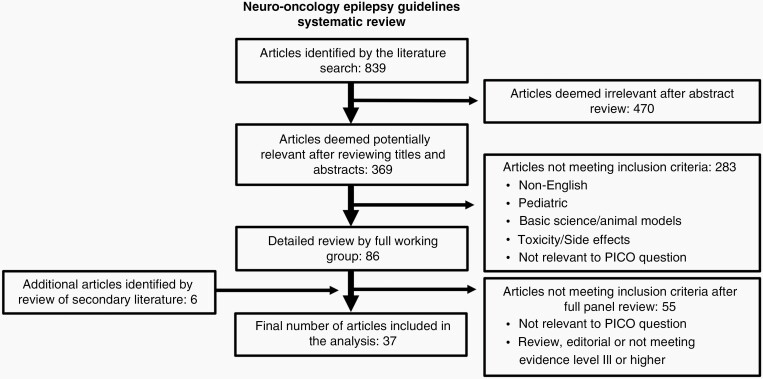
The guideline panel included articles in adult patients with brain tumors related to treatment for seizures or seizure prophylaxis. The panel excluded pharmacologic treatment trials with fewer than 20 participants. The complete search strategy is presented in Supplementary Appendix 1. The panel engaged a medical librarian to search the PubMed/OVID Medline, EMBASE, Cochrane Library, and Web of Science databases from January 1999 to March 31, 2017. An updated literature search was performed prior to starting the analysis on April 16, 2018 and again on May 14, 2021 to identify any newly published high-level evidence that might substantially change the recommendations. A total of 839 titles and abstracts were obtained (Figure 1).
Fig. 1.
Literature search strategy.
Two panel members (E.R.G., T.W.), working independently of each other, reviewed each of the abstracts for basic inclusion criteria: (1) article was relevant to at least one of the clinical questions; (2) article described adult brain tumor patients with or without seizures; (3) study population was greater than or equal to 20 to reduce the likelihood of spurious results due to small samples; and (4) article was not a single-patient case report, review, or editorial. Of the 839 abstracts reviewed, the 2 panelists identified 369 as possibly pertinent, for which they obtained and reviewed the full-text articles. Of the 369 reviewed articles, 86 met inclusion criteria and were reviewed and classified by 2 panel members each. Reviewers, working independently of each other, assessed the quality of evidence on the basis of the AAN therapeutic and causation study classification schemes (Supplementary Appendix 2).19 Discrepancies in article classification between the 2 reviewers were reconciled by 2 other independent reviewers. An additional 6 articles were found by reviewing references and secondary literature.
Class III studies are discussed in the guideline text only when no class I or limited class II studies were identified. Class IV studies were excluded from consideration because of their high risk of bias. The panelists noted that what constituted a seizure was not always clearly defined in each article, potentially limiting the accuracy of seizure occurrence. Table 1 summarizes the literature cited.
Table 1.
Clinical Questions, Conclusions, Recommendations and Evidence
| Pico Questions | Conclusion | Recommendation | Level of Evidence | Rating of Evidence | |
|---|---|---|---|---|---|
| PICO 1: In patients with newly diagnosed primary or metastatic brain tumors who have not already experienced a seizure: Does anticonvulsant prophylaxis compared to no anticonvulsant prophylaxis (a) increase seizure-free survival and (b) reduce the frequency of first seizures at 6 months from diagnosis | For patients with newly diagnosed brain tumors, anticonvulsant prophylaxis compared to no anticonvulsant prophylaxis is unlikely to be effective in increasing seizure-free survival and reducing the frequency of first seizures at 6 months from diagnosis | In patients with newly diagnosed brain tumors who have not had a seizure, clinicians should not prescribe AEDs to reduce the risk of seizures | Level A | II | Forsyth PA, et al20 |
| II | Goldlust SA, et al21 | ||||
| II | Skardelly M, et al22 | ||||
| III | Al-Dorzi HM, et al23 | ||||
| III | Ansari SF, et al24 | ||||
| III | Chaichana KL, et al25 | ||||
| III | de Oliveira JA, et al26 | ||||
| III | Garbossa D, et al27 | ||||
| III | Gokhale S, et al28 | ||||
| III | Lapointe S, et al29 | ||||
| III | Liang SL, et al30 | ||||
| III | Lee MH, et al31 | ||||
| III | Lwu S, et al32 | ||||
| III | Riva M, et al33 | ||||
| III | Wychowski T, et al34 | ||||
| PICO 2a: In patients with newly diagnosed primary or metastatic brain tumors who have not already experienced a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor does perioperative anticonvulsant prophylaxis compared to no perioperative anticonvulsant prophylaxis prolong time to seizure occurrence | For patients with newly diagnosed primary or metastatic brain tumors who never had a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor, perioperative anticonvulsant prophylaxis is possibly not effective in reducing seizures overall | In patients with brain tumors who have never had a seizure and are undergoing surgery, there is insufficient evidence to recommend prescribing AEDs to reduce the risk of seizures in the peri- or postoperative period | Level C | III | Al-Dorzi HM, et al23 |
| III | Lapointe S, et al29 | ||||
| III | Wychoswski T et al34 | ||||
| PICO 2b: In patients with newly diagnosed primary or metastatic brain tumors who have not already experienced a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor does perioperative anticonvulsant prophylaxis compared to no perioperative anticonvulsant prophylaxis reduce the frequency of first seizure at 14 days following surgery | For patients with newly diagnosed primary or metastatic brain tumors who never had a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor, perioperative anticonvulsant prophylaxis is possibly not effective in reducing seizures during the first 14 days following surgery | In patients with brain tumors who have never had a seizure and are undergoing surgery, there is insufficient evidence to recommend prescribing AEDs to reduce the risk of seizures in the peri- or postoperative period | Level C | II | Wu et al35 |
| III | Al-Dorzi HM, et al23 | ||||
| III | Lapointe S, et al29 | ||||
| III | Liang SL, et al30 | ||||
| III | Lockney D et al36 | ||||
| III | Lwu S, et al32 | ||||
| III | Sughrue ME, et al37 | ||||
| III | Zachenhofer I et al38 | ||||
| PICO 3a: In patients with newly diagnosed primary or metastatic brain tumors, does treatment with valproic acid or other AEDs (either prophylactic or following a seizure) compared to treatment with any other anticonvulsant medication increase progression-free or overall survival | While there is a lack of high-level evidence, in patients with newly diagnosed primary or metastatic brain tumors, treatment with valproic acid or levetiracetam does not appear to increase progression-free or overall survival. Use of valproic acid has also been associated with complications such as thrombocytopenia and hepatotoxicity | In patients with newly diagnosed primary or metastatic brain tumors, there is insufficient evidence to support prescribing valproic acid or levetiracetam with the intent to prolong progression-free or overall survival | Level C | III | Happold C, et al39 |
| III | Kerkhof M, et al40 | ||||
| III | Kim YH, et al41 | ||||
| III | Redjal N, et al42 | ||||
| III | Tsai HC,et al43 | ||||
| III | Weller M, et al44 | ||||
| PICO 3b: In patients with newly diagnosed primary or metastatic brain tumors, does treatment with a NEIAED or more “modern” AED (either prophylactic or following a seizure) compared to treatment with an EIAED (either prophylactic or following a seizure) have a more favorable side effect profile | The use of levetiracetam is well tolerated in patients with brain tumors. The prevention of early postoperative seizures, within 7 days of surgery, is comparable to previous trials with first-generation AEDs. The use of valproic acid in brain tumor patients on chemotherapy may be associated with higher hematologic toxicities | In patients with newly diagnosed primary or metastatic brain tumors, physicians may choose to prescribe levetiracetam rather than older AEDs to reduce side effects | Level C | II | Iuchi T, et al45 |
| III | Gokhale S, et al28 | ||||
| III | Iuchi T, et al46 | ||||
| III | Lee YJ, et al47 | ||||
| III | Merrell RT, et al48 | ||||
| III | Milligan TA, et al49 | ||||
| III | Weller M, et al44 | ||||
| III | Wychoswski T et al34 | ||||
| III | Zachenhofer I et al38 | ||||
| PICO 4: In patients with newly diagnosed supratentorial primary or metastatic brain tumors who have not had a seizure, should aggressive tumor characteristics such as histology (primary vs metastatic), grade, molecular pathology (eg, O6-methylguanine-DNA methyltransferase promoter methylation, IDH mutation, epidermal growth factor receptor amplification) or imaging (eg, tumor location, number of tumors, edema, enhancement, vascularity) compared to tumors considered to be less aggressive, in less epileptogenic regions influence prophylactic anticonvulsant use | The current data do not support extent of resection, histology (primary vs metastatic), grade, molecular pathology, or imaging characteristics as predictors of seizure risk and, thus, these parameters also should not influence prophylactic anticonvulsant use | In patients with brain tumors who have not had seizures, there is insufficient evidence to support using tumor location, histology (primary vs metastatic), grade, molecular pathology, or imaging characteristics when deciding whether or not to prescribe prophylactic AEDs | Level U | II | Cayuela N, et al50 |
| II | Skardelly M, et al22 | ||||
| III | Das RR, et al51 | ||||
| III | Lapointe S, et al29 | ||||
| III | Lee JW, et al52 | ||||
| III | Lee MH, et al31 | ||||
| III | Liang SL, et al30 | ||||
| III | Oushy S, et al53 | ||||
| III | Skardelly M, et al54 | ||||
| III | Wirsching HG, et al55 | ||||
| III | Wychoswski T et al34 |
Abbreviations: AEDs, antiepileptic drugs; EIAED, enzyme-inducing antiepileptic drug; IDH, isocitrate dehydrogenase; NEIAED, nonenzyme-inducing antiepileptic drug; PICO, patient, intervention, comparison, and outcome.
Analysis of Evidence
Clinical Question 1: In patients with newly diagnosed primary or metastatic brain tumors who have not already experienced a seizure, does anticonvulsant prophylaxis compared to no anticonvulsant prophylaxis (a) increase seizure-free survival and (b) reduce the frequency of first seizures at 6 months from diagnosis?
Evidence
The previous AAN practice parameter published in 2000 identified 10 published studies (4 class I and 6 class II studies, excluding the 2 abstracts never published as manuscripts) and the conclusion was that prophylactic anticonvulsant use did not provide a substantial benefit.10–18,20 Subsequent studies not included in the original AAN practice parameter were examined.
There were no class I studies, but 3 class II studies pertained to this issue.20–22 One, examining the role of prophylactic anticonvulsants in newly diagnosed brain tumors, fulfilled all requirements of a class I study but was terminated early.20 The study population, comprised of 60% brain metastasis and 40% glioma patients, was randomized to phenytoin vs no anticonvulsant. Seizure-free survival did not differ between the 2 groups—87% in the phenytoin cohort and 90% in the no anticonvulsant cohort at the primary endpoint of 3 months. The trial closed prematurely based on a feasibility analysis that found an unexpectedly low rate of first seizure in the control arm and a higher mortality rate at the 3-month time point. The authors concluded that it was unlikely that an extension of the study would change the outcomes.
A retrospective institutional chart review study of patients with brain metastases from melanoma found that anticonvulsant prophylaxis was associated with a significantly decreased risk of new-onset seizure, with 3-month rates of 0% vs 17% in those with or without prophylaxis, respectively.21 A second retrospective single-center study examined patients undergoing surgery for meningioma; new-onset seizures within 1 week of craniotomy were not reduced in patients receiving prophylactic levetiracetam or other anticonvulsants compared to those receiving no prophylaxis.22
Twelve class III studies were identified.23–34 Only 1 of 12 class III studies, evaluating different tumor types with different endpoints, suggested benefit from prophylactic AEDs.30 That particular retrospective single-institution study with 141 relevant patients suggested a decrease of seizure frequency during the first 6 months after surgery but not thereafter.
Conclusions
Patients with newly diagnosed brain tumors who have not experienced a seizure do not appear to benefit from AED prophylaxis. Combined with the data found in the earlier AAN practice parameter, there are now 3 randomized trials providing class I evidence,10–12 8 class II studies13–18,20,22 and 11 class III studies23–29,31–34 that suggest that patients do not benefit from primary prophylaxis with AEDs. Only 1 class II21 and 1 class III study30 support a different conclusion. This question deserves further study in patients with brain metastases from melanoma but presently there is insufficient evidence to use prophylactic AEDs in patients with metastatic melanoma to the brain.
For patients with newly diagnosed brain tumors, anticonvulsant prophylaxis compared to no anticonvulsant prophylaxis is unlikely to be effective in increasing seizure-free survival and reducing the frequency of first seizures at 6 months from diagnosis.
Recommendation
In patients with newly diagnosed brain tumors who have not had a seizure, clinicians should not prescribe AEDs to reduce the risk of seizures (level A).
Clinical Question 2: In patients with newly diagnosed primary or metastatic brain tumors who have not already experienced a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor does perioperative anticonvulsant prophylaxis compared to no perioperative anticonvulsant prophylaxis (a) prolong time to seizure occurrence and (b) reduce the frequency of first seizure at 14 days following surgery?
Evidence
There were no class I or class II studies, but 3 relevant class III studies assessed the impact on prolongation of time to seizure occurrence (question 2a).23,29,34 All found that prophylactic AEDs did not prolong postoperative time to seizure occurrence, and none of the studies showed improved overall time to seizure occurrence with prophylaxis.
There were no class I trials, but 1 prospective, randomized class II trial examined the role of 7-day phenytoin prophylaxis in patients undergoing craniotomy for supratentorial brain tumors (question 2b).35 Phenytoin was loaded prior to craniotomy, given for 7 days with dose adjustments for therapeutic levels, and then tapered off. Seizures were determined on clinical grounds or with electroencephalogram (EEG) if an event in question was not felt to be definitive. The study was powered to detect a reduction in clinically significant seizures from 30% to 10%. The incidence of seizures within 30 days of surgery was 8% in the observation group and 10% in the prophylaxis group. The incidence of clinically significant seizures was 2% in the prophylaxis group and 3% in the observation group, while adverse effects of AED were seen in 18% of patients in the prophylaxis group. After enrolling 123 of the planned 142 patients, however, the study was closed early as an interim analysis indicated a probability of 0.003 that prophylaxis would be superior to observation at the end of the study. Given the stringent criteria used for this guideline, this trial is rated as a class II study rather than class I evidence due to early closure.
Two class III studies found reduced seizure frequency with prophylaxis within 14 days of surgery.30,38 Five class III studies found there was no impact of prophylaxis on seizures within this postoperative period.23,29,32,36,37
Conclusion
There are 3 class III studies23,29,34 all showing that postoperative prophylaxis does not result in prolongation of time to seizure occurrence (question 2a). There is 1 prospective randomized class II trial35 and 5 class III studies23,29,32,36,37 indicating that perioperative therapy with an AED has no impact on seizure outcomes within 14 days of surgery. While 2 class III studies30,38 showed reduced seizure activity (question 2b), the collective findings did support perioperative therapy with AEDs.
For patients with newly diagnosed primary or metastatic brain tumors who never had a seizure and who undergo a neurosurgical procedure (craniotomy or biopsy) for initial treatment or diagnosis of their tumor, perioperative anticonvulsant prophylaxis is possibly not effective in reducing seizures overall and during the first 14 days following surgery.
Recommendation
In patients with brain tumors undergoing surgery, there is insufficient evidence to recommend prescribing AEDs to reduce the risk of seizures in the peri- or postoperative period (level C).
Clinical Question 3: In patients with newly diagnosed primary or metastatic brain tumors, does treatment with valproic acid or other AEDs (either prophylactic or following a seizure) compared to treatment with any other anticonvulsant medication increase progression-free or overall survival?
Evidence
There were no class I or class II studies, but 6 class III studies39–44 were identified as pertinent to this question. Five class III studies evaluated the effect of valproic acid on seizure control and survival in patients with glioblastoma (GBM).39,40,42–44 One class III study analyzing a subgroup of patients who received an AED while undergoing treatment in a large randomized clinical chemotherapy trial56 was the first study to find a possible survival benefit when adding valproic acid to the treatment of radiation therapy and temozolomide in GBM patients.44 A positive impact on patient survival was also found in 2 other subsequent single-center retrospective class III studies focusing on valproic acid in GBM treatment40,42 while the same protective effect was not detected in patients with grade II/III gliomas.40
The positive results of valproic acid were not replicated by 2 other class III studies,39,43 1 being a pooled analysis of 4 randomized clinical trials with a total of 1869 patients.39 The findings indicated that there was no improvement in progression-free or overall survival with the use of valproic acid or levetiracetam in patients with GBM. In the second retrospective study with 102 GBM patients treated with valproic acid, a stratified analysis did not show any significant association with overall survival.43
Two retrospective class III studies evaluated the survival benefit of levetiracetam in patients undergoing standard treatment with radiotherapy and temozolomide for newly diagnosed GBM.39,41 While 1 single-center study with 103 patients showed a survival benefit of 2.7 months,41 the above-mentioned analysis of 1869 clinical trial patients did not show any survival benefit associated with levetiracetam39 (Table 1).
Conclusion
There are 3 class III studies42–44 indicating that there is a possible survival benefit of valproic acid and 2 class III studies40,41 showing a positive effect of levetiracetam on overall survival in GBM patients. All of these studies are retrospective or based on post hoc analysis. A larger class III study based on a pooled analysis of participants of multiple clinical trials did not show any survival benefit for patients who were on valproic acid or levetiracetam in addition to chemotherapy. This study included more patients than all other reviewed studies combined and did not reveal any impact on survival.39
While there is a lack of high-level evidence, in patients with newly diagnosed primary or metastatic brain tumors treatment with valproic acid or levetiracetam does not appear to increase progression-free or overall survival. The use of valproic acid has also been associated with complications such as thrombocytopenia and hepatotoxicity.
Recommendation
In patients with newly diagnosed primary or metastatic brain tumors, there is insufficient evidence to support prescribing valproic acid or levetiracetam with the intent to prolong progression-free or overall survival (level C).
Clinical Question 3a: In patients with newly diagnosed primary or metastatic brain tumors, does treatment with a NEIAED or more “modern” AED (either prophylactic or following a seizure) compared to treatment with a enzyme-inducing antiepileptic drug (EIAED, either prophylactic or following a seizure) have a more favorable side effect profile?
Evidence
There were no class I trials, 1 class II,45 and 8 class III studies28,34,38,44,46–49 identified as pertinent to this question.
Of the newer NEIAEDs, levetiracetam was the AED most studied in brain tumor patients with 1 class II45 and 7 class III studies28,34,38,46–49 evaluating the efficacy and side effect profile. Levetiracetam was well tolerated and perioperative seizure frequency was low in the first 7-day postcraniotomy in all studies. One class II study45 and 2 class III46,49 studies compared the efficacy and tolerability of levetiracetam vs phenytoin and carbamazepine after supratentorial brain tumor surgery.49 There was no difference in postoperative seizure outcomes; however, levetiracetam was associated with fewer adverse drug reactions and a higher retention rate in both studies. A second class III study comparing valproic acid with EIAEDs found that GBM patients starting adjuvant temozolomide while taking valproic acid were at significantly higher risk for grade 3 and grade 4 hematologic toxicities.44
There was 1 retrospective class III study evaluating the use of valproic acid or levetiracetam for the prevention of postoperative seizures.47 The study evaluated 282 patients on either levetiracetam or valproic acid and the primary end points were seizure outcome and tolerability. Seizure outcome for the prevention of early postoperative seizures and the development of long-term epilepsy was similar in both groups. However, adverse effects were statistically significantly higher in the valproic acid group leading to changes in AED therapy.
Conclusion
One class II45 and 8 class III studies28,34,38,44,46–49 evaluated the safety and tolerability of newer AEDs, mostly levetiracetam. The use of levetiracetam is well tolerated in patients with brain tumors. The prevention of early postoperative seizures within 7 days of surgery is comparable to previous trials with first-generation AEDs but associated with fewer side effects. The use of valproic acid in brain tumor patients on chemotherapy has also been associated with higher hematologic toxicities.
Recommendation
In patients with newly diagnosed primary or metastatic brain tumors, physicians may choose to prescribe levetiracetam rather than older AEDs to reduce side effects (level C).
Clinical Question 4: In patients with newly diagnosed supratentorial primary or metastatic brain tumors who have not had a seizure, should aggressive tumor characteristics such as histology (primary vs metastatic), grade, molecular pathology (eg, O6-methylguanine-DNA methyltransferase promoter methylation, isocitrate dehydrogenase (IDH) mutation, epidermal growth factor receptor amplification) or imaging (eg, tumor location, number of tumors, edema, enhancement, vascularity) compared to tumors considered to be less aggressive, in less epileptogenic regions, influence prophylactic anticonvulsant use?
Evidence
There were no class I trials identified as pertinent to this question, and 2 class II22,50 and 9 class III studies29–31,34,51–55 were identified as pertinent to this question. While several studies correlated specific brain locations with seizure risk, none of the studies investigated how the use of AEDs should be adjusted according to these associations.
One class II study of GBM patients found that tumors in the superior frontal, inferior occipital, and inferior-posterior temporal regions were associated with higher seizure risk whereas patients with GBM in the medial and inferior-anterior temporal areas had significantly lower risk of developing seizures.50 One class III study found that the incidence of postoperative seizures in patients with GBM was highest with frontal lobe lesions.30 Three other class III studies supported the epileptogenic potential of both low- and high-grade gliomas located in the temporal and insular regions.34,51,52 In meningiomas, 1 class II study and 1 class III study found that a nonskull base tumor location was a significant risk factor for postoperative seizures.22,55
In 2 class III studies, extent of resection had an inconclusive impact on seizure occurrence.53,54 Gross total resection was a risk factor for early postoperative seizures (within the first week after surgery)54 but subtotal resection and biopsy were also associated with seizures within the first 30 days of surgery.53
Only class III studies could be found to suggest an association between seizure occurrence and IDH mutation status,54 tumor size/edema,54 younger age,29 male gender,29 and leptomeningeal dissemination.31
Conclusion
The evidence for tumor location as being an important factor for prescribing AEDs for primary seizure prophylaxis was limited to 2 class II22,50 studies and 4 class III studies.30,34,51,52 Different locations were identified as epileptogenic without answering the question how location should influence the use of prophylactic AEDs. Therefore, interpretation of these studies is limited. Based on 4 class III studies,29,31,53,54 the current data do not support extent of resection, histology (primary vs metastatic), grade, molecular pathology or imaging factors as predictors of seizure risk, and thus, these parameters also should not influence prophylactic anticonvulsant use. Recent updates in the WHO classification of brain tumors are not reflected in these prior studies but should be a focus of future research.
Recommendation
In patients with brain tumors who have not had seizures, there is insufficient evidence to support using tumor location, histology (primary vs metastatic), grade, molecular features, or imaging characteristics when deciding whether or not to prescribe prophylactic AEDs (level U).
Summary
This document updates the 2000 AAN guideline “Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors” 9 and extends it to questions related to selection of AEDs and the impact intrinsic tumor characteristics may have on seizure risk. The conclusions from this update confirm that prophylactic AED use in a brain tumor patient who has never had a seizure is not warranted. SNO and EANO approved these guidelines and the AAN affirmed the value of the guidelines.
The data for patients undergoing brain tumor surgery suggested that there may be no need to prescribe prophylactic AED treatment, but the limited data were inconclusive. Several systematic reviews and meta-analyses have attempted to clarify the benefit of perioperative seizure prophylaxis.2,9,57–61 Given the limited number of randomized trials and lack of high-level evidence on this topic, it is not surprising that the majority of these reviews were either unable to answer the underlying question58 or did not see a benefit in prescribing AEDs.2 The exception is one recent meta-analysis that found that perioperative AED use might result in decreased short-term seizure occurrence, but this was not seen in long-term prevention of seizures.57 We noted a lack of literature evaluating newer surgical approaches such as motor mapping where there may be a higher risk of provoking seizures or awake craniotomies in which a seizure might result in urgent intubation and associated risks. Future guidelines would benefit from more neurosurgeon input regarding perioperative seizure management and these novel approaches.
Attempts to identify higher risk subpopulations were fraught with methodological limitations in the published evidence, so we were unable to pinpoint subpopulations based on tumor location or molecular features that might benefit from prophylactic AED treatment. While 1 study6 suggested seizures are more common in low-grade tumors, the data are insufficient to suggest prophylactic AED treatment is needed in these patients.
Based on the studies identified, choice of AED favored the newer generation of agents because of their side effect profile but efficacy seemed equivalent in preventing seizures and convincing data favoring valproic acid or levetiracetam as an anti-tumor agent were lacking. One important factor missing from the studies we identified was the pharmacology of the newer AEDs such as levetiracetam which may have less interaction with other medications. As NEIAEDs, there is limited interaction with other drugs and cancer therapies, in particular where dose is important for efficacy. Also, newer AEDs have less teratogenicity and less long-term impact on bone health.62,63
The panel noted methodological weaknesses across studies that hindered the practice of evidence-based medicine, leading ultimately to a crucial lack of scientific evidence that has persisted since the previous AAN guideline. Very few randomized controlled trials were found and most were closed early before accruing the planned patient population.20,35,64 In addition, data from at least one more recent trial that was also terminated have not yet been published (NCT01432171: Lacosamide in Preventing Seizures in Participants With Malignant Glioma), highlighting the need for more definitive randomized trials. One particular methodical challenge was seizure ascertainment in retrospective studies, particularly the assessment of subclinical and partial seizures, resulting in a significant limitation as correctly identifying a seizure event is critical to understanding seizure frequency.
While substantive progress has been made to define patient populations by combining molecular markers with histopathology, there is no conclusive knowledge about how these new definitions might be applied to managing patients without seizures. Anti-seizure prophylaxis based on molecular findings or histology remains elusive and studies included here often documented contradictory findings.22,29,31,53,54 Thus, important clinical questions such as the impact of molecular markers on the risk of developing seizures cannot be answered at this point and need to be addressed by future studies.
Supplementary Material
Acknowledgments
The authors are grateful for the support of the AAN Neuro-Oncology section during the development of these guidelines. The authors would like to thank Stephanie Stebens for the literature search.
Funding
This practice guideline was developed without financial support.
Conflict of interest statement.
T.W. received honoraria for advisory board from Orbus and Tocagen, consulting work for Novocure, speaker fees from AstraZeneca. D.S. has received honoraria for data safety and monitoring committee work for Orbus and Denovo; he has served on an advisory board for GlaxoSmithKline. J.W.L. is a council member of the American Clinical Neurophysiology Society. He receives research funding as site PI from the NIH (R01NS062092, U01NS098968), and performs contract work for DigiTrace and Advance Medical. He is the co-founder of Soterya Inc. E.L.R. has received honoraria for lectures or advisory board from Tocagen, AbbVie, and Daiichi Sankyo. M.A.V. has indirect equity and royalty interests from Infuseon Therapeutics, Inc., and honoraria from Tocagen, Cellinta, and Celgene. M.W. has received research grants from AbbVie, Adastra, Dracen, Merck, Sharp & Dohme (MSD), Merck (EMD) and Novocure, and honoraria for lectures or advisory board participation or consulting from AbbVie, Basilea, Bristol Myers Squibb (BMS), Celgene, Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Orbus, Roche, and Tocagen. P.Y.W. received research support from Agios, AstraZeneca/MedImmune, Bayer, Beigene, Celgene, Eli Lilly, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Vascular Biogenics, VBI Vaccines; honoraria for participation on advisory boards or consultation from Agios, AstraZeneca, Bayer, Blue Earth Diagnostics, Karyopharm, Vascular Biogenics, VBI Vaccines, Tocagen, Voyager, Novocure, QED, Imvax, Integral Health, and Boston Pharmaceuticals; and speaker fees from Merck and Prime Oncology. No other authors declared any disclosures.
Authorship statement.
Dr. T.W.: study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. R.A.H.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Critical revision of the manuscript for important intellectual content. Dr. D.S.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. E.K.A.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. M.C.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. P.K.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. J.W.L.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. E.L.R.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. G.H.J.S.: analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. M.A.V.: study concept and design, analysis or interpretation of data, drafting/revising the manuscript. Dr. M.W.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. W.W.: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. P.Y.W.: study concept and design, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. E.R.G.: study concept and design, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content.
References
- 1. Vecht CJ, Wilms EB. Seizures in low- and high-grade gliomas: current management and future outlook. Expert Rev Anticancer Ther. 2010;10(5):663–669. [DOI] [PubMed] [Google Scholar]
- 2. Rudà R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(Suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossetti AO, Stupp R. Epilepsy in brain tumor patients. Curr Opin Neurol. 2010;23(6):603–609. [DOI] [PubMed] [Google Scholar]
- 4. Koekkoek JAF, Dirven L, Reijneveld JC, et al. Epilepsy in the end of life phase of brain tumor patients: a systematic review. Neurooncol Pract. 2014;1(3):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maschio M, Sperati F, Dinapoli L, et al. Weight of epilepsy in brain tumor patients. J Neurooncol. 2014;118(2):385–393. [DOI] [PubMed] [Google Scholar]
- 6. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 7. Wen PY, Loeffler JS. Management of brain metastases. Oncology (Williston Park). 1999;13(7):941–954, 957–961; discussion 961-942, 949. [PubMed] [Google Scholar]
- 8. Rudà R, Mo F, Pellerino A. Epilepsy in brain metastasis: an emerging entity. Curr Treat Options Neurol. 2020;22(2):6. [DOI] [PubMed] [Google Scholar]
- 9. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 10. Franceschetti S, Binelli S, Casazza M, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien). 1990;103(1-2):47–51. [DOI] [PubMed] [Google Scholar]
- 11. Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46(4):985–991. [DOI] [PubMed] [Google Scholar]
- 12. North JB, Penhall RK, Hanieh A, Frewin DB, Taylor WB. Phenytoin and postoperative epilepsy. A double-blind study. J Neurosurg. 1983;58(5):672–677. [DOI] [PubMed] [Google Scholar]
- 13. Boarini DJ, Beck DW, VanGilder JC. Postoperative prophylactic anticonvulsant therapy in cerebral gliomas. Neurosurgery. 1985;16(3):290–292. [DOI] [PubMed] [Google Scholar]
- 14. Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1(4):313–317. [DOI] [PubMed] [Google Scholar]
- 15. Cohen N, Strauss G, Lew R, Silver D, Recht L. Should prophylactic anticonvulsants be administered to patients with newly-diagnosed cerebral metastases? A retrospective analysis. J Clin Oncol. 1988;6(10):1621–1624. [DOI] [PubMed] [Google Scholar]
- 16. Hagen NA, Cirrincione C, Thaler HT, DeAngelis LM. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40(1):158–160. [DOI] [PubMed] [Google Scholar]
- 17. Mahaley MS Jr, Dudka L. The role of anticonvulsant medications in the management of patients with anaplastic gliomas. Surg Neurol. 1981;16(6):399–401. [DOI] [PubMed] [Google Scholar]
- 18. Moots PL, Maciunas RJ, Eisert DR, Parker RA, Laporte K, Abou-Khalil B. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 1995;52(7):717–724. [DOI] [PubMed] [Google Scholar]
- 19. Gronseth GS, Cox J, Gloss D, et al. Clinical Practice Guideline Process Manual. 2017 ed. Minneapolis, MN: American Academy of Neurology; 2017. [Google Scholar]
- 20. Forsyth PA, Weaver S, Fulton D, et al. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003;30(2):106–112. [DOI] [PubMed] [Google Scholar]
- 21. Goldlust SA, Hsu M, Lassman AB, Panageas KS, Avila EK. Seizure prophylaxis and melanoma brain metastases. J Neurooncol. 2012;108(1):109–114. [DOI] [PubMed] [Google Scholar]
- 22. Skardelly M, Rother C, Noell S, et al. Risk factors of preoperative and early postoperative seizures in patients with Meningioma: a retrospective single-center cohort study. World Neurosurg. 2017;97:538–546. [DOI] [PubMed] [Google Scholar]
- 23. Al-Dorzi HM, Alruwaita AA, Marae BO, et al. Incidence, risk factors and outcomes of seizures occurring after craniotomy for primary brain tumor resection. Neurosciences (Riyadh). 2017;22(2):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ansari SF, Bohnstedt BN, Perkins SM, Althouse SK, Miller JC. Efficacy of postoperative seizure prophylaxis in intra-axial brain tumor resections. J Neurooncol. 2014;118(1):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaichana KL, Parker SL, Olivi A, Quiñones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111(2):282–292. [DOI] [PubMed] [Google Scholar]
- 26. de Oliveira JA, Santana IA, Caires IQ, et al. Antiepileptic drug prophylaxis in primary brain tumor patients: is current practice in agreement to the consensus? J Neurooncol. 2014;120(2):399–403. [DOI] [PubMed] [Google Scholar]
- 27. Garbossa D, Panciani PP, Angeleri R, et al. A retrospective two-center study of antiepileptic prophylaxis in patients with surgically treated high-grade gliomas. Neurol India. 2013;61(2):131–137. [DOI] [PubMed] [Google Scholar]
- 28. Gokhale S, Khan SA, Agrawal A, Friedman AH, McDonagh DL. Levetiracetam seizure prophylaxis in craniotomy patients at high risk for postoperative seizures. Asian J Neurosurg. 2013;8(4):169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lapointe S, Florescu M, Nguyen DK, Djeffal C, Bélanger K. Prophylactic anticonvulsants for gliomas: a seven-year retrospective analysis. Neurooncol Pract. 2015;2(4):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang S, Zhang J, Zhang S, Fu X. Epilepsy in adults with supratentorial glioblastoma: incidence and influence factors and prophylaxis in 184 patients. PLoS One. 2016;11(7):e0158206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee MH, Kong DS, Seol HJ, Nam DH, Lee JI. Risk of seizure and its clinical implication in the patients with cerebral metastasis from lung cancer. Acta Neurochir (Wien). 2013;155(10):1833–1837. [DOI] [PubMed] [Google Scholar]
- 32. Lwu S, Hamilton MG, Forsyth PA, Cairncross JG, Parney IF. Use of peri-operative anti-epileptic drugs in patients with newly diagnosed high grade malignant glioma: a single center experience. J Neurooncol. 2010;96(3):403–408. [DOI] [PubMed] [Google Scholar]
- 33. Riva M, Salmaggi A, Marchioni E, et al. Tumour-associated epilepsy: clinical impact and the role of referring centres in a cohort of glioblastoma patients. A multicentre study from the Lombardia Neurooncology Group. Neurol Sci. 2006;27(5):345–351. [DOI] [PubMed] [Google Scholar]
- 34. Wychowski T, Wang H, Buniak L, Henry JC, Mohile N. Considerations in prophylaxis for tumor-associated epilepsy: prevention of status epilepticus and tolerability of newer generation AEDs. Clin Neurol Neurosurg. 2013;115(11):2365–2369. [DOI] [PubMed] [Google Scholar]
- 35. Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013;118(4):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lockney DT, Vaziri S, Walch F, et al. Prophylactic antiepileptic drug use in patients with brain tumors undergoing craniotomy. World Neurosurg. 2017;98:28–33. [DOI] [PubMed] [Google Scholar]
- 37. Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: are prophylactic anticonvulsants indicated? Clinical article. J Neurosurg. 2011;114(3):705–709. [DOI] [PubMed] [Google Scholar]
- 38. Zachenhofer I, Donat M, Oberndorfer S, Roessler K. Perioperative levetiracetam for prevention of seizures in supratentorial brain tumor surgery. J Neurooncol. 2011;101(1):101–106. [DOI] [PubMed] [Google Scholar]
- 39. Happold C, Gorlia T, Chinot O, et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol. 2016;34(7):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerkhof M, Dielemans JC, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15(7):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim YH, Kim T, Joo JD, et al. Survival benefit of levetiracetam in patients treated with concomitant chemoradiotherapy and adjuvant chemotherapy with temozolomide for glioblastoma multiforme. Cancer. 2015;121(17):2926–2932. [DOI] [PubMed] [Google Scholar]
- 42. Redjal N, Reinshagen C, Le A, et al. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J Neurooncol. 2016;127(3):505–514. [DOI] [PubMed] [Google Scholar]
- 43. Tsai HC, Wei KC, Tsai CN, et al. Effect of valproic acid on the outcome of glioblastoma multiforme. Br J Neurosurg. 2012;26(3):347–354. [DOI] [PubMed] [Google Scholar]
- 44. Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77(12):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry. 2015;86(10):1158–1162. [DOI] [PubMed] [Google Scholar]
- 46. Iuchi T, Hasegawa Y, Kawasaki K, Sakaida T. Epilepsy in patients with gliomas: incidence and control of seizures. J Clin Neurosci. 2015;22(1):87–91. [DOI] [PubMed] [Google Scholar]
- 47. Lee YJ, Kim T, Bae SH, et al. Levetiracetam compared with valproic acid for the prevention of postoperative seizures after supratentorial tumor surgery: a retrospective chart review. CNS Drugs. 2013;27(9):753–759. [DOI] [PubMed] [Google Scholar]
- 48. Merrell RT, Anderson SK, Meyer FB, Lachance DH. Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg. 2010;113(6):1176–1181. [DOI] [PubMed] [Google Scholar]
- 49. Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71(9):665–669. [DOI] [PubMed] [Google Scholar]
- 50. Cayuela N, Simó M, Majós C, et al. Seizure-susceptible brain regions in glioblastoma: identification of patients at risk. Eur J Neurol. 2018;25(2):387–394. [DOI] [PubMed] [Google Scholar]
- 51. Das RR, Artsy E, Hurwitz S, et al. Outcomes after discontinuation of antiepileptic drugs after surgery in patients with low grade brain tumors and meningiomas. J Neurooncol. 2012;107(3):565–570. [DOI] [PubMed] [Google Scholar]
- 52. Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67(3):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oushy S, Sillau SH, Ney DE, et al. New-onset seizure during and after brain tumor excision: a risk assessment analysis. J Neurosurg. 2018;128(6):1713–1718. [DOI] [PubMed] [Google Scholar]
- 54. Skardelly M, Brendle E, Noell S, et al. Predictors of preoperative and early postoperative seizures in patients with intra-axial primary and metastatic brain tumors: a retrospective observational single center study. Ann Neurol. 2015;78(6):917–928. [DOI] [PubMed] [Google Scholar]
- 55. Wirsching HG, Morel C, Gmür C, et al. Predicting outcome of epilepsy after meningioma resection. Neuro Oncol. 2016;18(7):1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 57. Joiner EF, Youngerman BE, Hudson TS, et al. Effectiveness of perioperative antiepileptic drug prophylaxis for early and late seizures following oncologic neurosurgery: a meta-analysis. J Neurosurg. 2018:1–9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58. Greenhalgh J, Weston J, Dundar Y, Nevitt SJ, Marson AG. Antiepileptic drugs as prophylaxis for postcraniotomy seizures. Cochrane Database Syst Rev. 2020;4:CD007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perry J, Zinman L, Chambers A, et al. The use of prophylactic anticonvulsants in patients with brain tumours – a systematic review. Curr Oncol. 2006;13(6):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79(12):1489–1494. [DOI] [PubMed] [Google Scholar]
- 61. Tremont-Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008;2:CD004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62(8):1252–1260. [DOI] [PubMed] [Google Scholar]
- 63. Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1237–1246. [DOI] [PubMed] [Google Scholar]
- 64. Armstrong TS, Grant R, Gilbert MR, Lee JW, Norden AD. Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol. 2016;18(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.