Abstract
Background
In patients with melanoma and asymptomatic brain metastases (MBM), nivolumab plus ipilimumab provided an intracranial response rate of 55%. Here, we present the first report for patients who were symptomatic and/or required corticosteroids and updated data for asymptomatic patients.
Methods
Patients with measurable MBM, 0.5-3.0 cm, were enrolled into Cohort A (asymptomatic) or Cohort B (stable neurologic symptoms and/or receiving corticosteroids). Nivolumab, 1 mg/kg, and ipilimumab, 3 mg/kg, were given intravenously every 3 weeks ×4, followed by nivolumab, 3 mg/kg, every 2 weeks until progression, unacceptable toxicity, or 24 months. The primary endpoint was intracranial clinical benefit rate (CBR; complete response [CR], partial response [PR], or stable disease ≥6 months).
Results
Symptomatic patients (N = 18) received a median of one nivolumab and ipilimumab combination dose and had an intracranial CBR of 22.2%. Two of 12 patients on corticosteroids had CR; 2 responded among the 6 not on corticosteroids. Median intracranial progression-free survival (PFS) and overall survival (OS) were 1.2 and 8.7 months, respectively. In contrast, with 20.6 months of follow-up, we confirmed an intracranial CBR of 58.4% in asymptomatic patients (N = 101); median duration of response, PFS, and OS were not reached. No new safety signals were observed.
Conclusions
Nivolumab plus ipilimumab provides durable clinical benefit for asymptomatic patients with MBM and should be considered for first-line therapy. This regimen has limited activity in MBM patients with neurologic symptoms and/or requiring corticosteroids, supporting the need for alternative approaches and methods to reduce the dependency on corticosteroids.
Clinical trial registration. ClinicalTrials.gov, NCT02320058.
Keywords: checkpoint inhibitor, ipilimumab, melanoma, nivolumab, symptomatic brain metastases
Key Points.
Nivolumab + ipilimumab therapy is effective in asymptomatic melanoma brain metastases.
Limited clinical activity was observed in patients with symptomatic brain metastases.
Impact of steroids on checkpoint inhibitor therapy initiation requires more investigation.
Importance of the Study.
Patients with melanoma brain metastases (MBM) who are symptomatic and/or require corticosteroids are challenging to treat and are often excluded from clinical trials, leading to lack of data reported in the literature. Results from our prior CheckMate 204 publication demonstrated impressive efficacy of nivolumab plus ipilimumab for the treatment of patients with asymptomatic brain metastases. Here we report limited clinical activity in patients who are symptomatic and/or require corticosteroids. Specifically, responses were observed in 2 of the 12 patients receiving baseline corticosteroids and in 2 of the 6 patients not receiving baseline corticosteroids. In addition, we present a longer follow-up of patients (N = 101) with asymptomatic brain metastases, with median progression-free survival and overall survival not yet reached with a median follow-up of 20.6 months (minimum follow-up of 11 months). This study highlights the critical need to develop more effective strategies for patients with MBM who are either symptomatic and/or requiring corticosteroids.
In patients with melanoma without brain metastases, the treatment paradigm has been transformed with immune checkpoint inhibitors (ICI) and targeted therapies, resulting in a dramatic decline in melanoma mortality from 2016 onward.1,2 Over one-third of patients with metastatic melanoma have detectable brain metastases at diagnosis, and up to two-thirds can develop them during the course of the disease.3–5 Historically, therapeutic approaches had been limited to craniotomy or radiation, and the median survival of patients with melanoma brain metastases (MBM) was 4-5 months.3,6,7 Although newer treatments improve overall survival (OS),8 patients with active, untreated MBM are usually excluded from clinical trials.
Clinical trials dedicated to studying the intracranial activity of systemic agents have been successfully performed in patients with MBM, but the majority included only asymptomatic patients not receiving corticosteroids. These studies showed durable responses from anti-programmed death (PD)-1 monotherapy with pembrolizumab or nivolumab, and several responses endure ≥2 years after the start of therapy.9,10 In the three single-agent ICI trials, the intracranial objective response rate (ORR) was 16% for ipilimumab,11 26% for pembrolizumab,10 and 20% for nivolumab.9 Combination of nivolumab plus ipilimumab has been investigated in two separate studies for patients with MBM. In the Australian anti-PD-1 brain collaboration (ABC) study (n = 35), an intracranial ORR of 46% was reported (median duration of response [DOR] not reached at a median follow-up of 34 months [minimum, 21.5 months]).9 In CheckMate 204 with a cohort of 94 patients, an ORR of 55% was reported (median DOR not yet reached at a median follow-up of 14 months [minimum, 6 months]).12
Patients with MBM who are symptomatic, either from local mass effect, involvement of critical structures, perilesional edema, and/or midline shift, are more challenging to treat because of their propensity for rapid disease progression and frequent requirement for corticosteroids to control neurologic symptoms. Corticosteroids abrogate the antitumor effects of immunotherapies,13–15 as indicated by the lower activity reported with single-agent cytotoxic T-lymphocyte antigen (CTLA)-4 or PD-1 blockade in patients with symptomatic MBM, who had ORRs of approximately 5% and median OS less than 6 months.9,11,16 It is unclear whether the failure of single-agent ICI to provide benefit in patients with MBMs is due to higher tumor burden and rapid growth, to a more immune-resistant phenotype in tumors that cause symptoms, low/absent tumor-infiltrating lymphocytes, or to immune suppression induced by corticosteroids.11,17
CheckMate 204 is an open-label, phase II trial designed to evaluate the efficacy and safety of nivolumab plus ipilimumab combination treatment in patients with untreated MBM. We previously reported results from 94 patients with asymptomatic MBM who experienced a high rate of intracranial benefit (ORR 55%), with median progression-free survival (PFS) and OS not reached at a median follow-up of 14 months.12 Here, we present the first report of combination ICI (nivolumab plus ipilimumab) in patients who were symptomatic and/or receiving corticosteroids at the time of study therapy initiation. In addition, we present data on the entire asymptomatic cohort (n = 101) with extended follow-up.
Patients and Methods
Patients
In this open-label, non-randomized, phase II trial, eligible patients were aged ≥18 years with histologically confirmed melanoma, able to undergo magnetic resonance imaging (MRI) of the brain, and had evidence of brain metastases including one or more unirradiated, measurable brain metastasis ≥0.5 cm and ≤3 cm. Additional patient criteria and study methods can be found in the Supplemental materials. Patient enrollment started with an asymptomatic cohort in which patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, no neurological symptoms, and no systemic glucocorticoid therapy for >10 days before treatment initiation (Cohort A).12 The protocol was later amended to include a separate cohort of patients who were symptomatic and/or requiring corticosteroids (Cohort B). Patients in Cohort B were allowed to have an ECOG PS of 0-2, stable neurologic signs or symptoms attributable to the metastatic intracranial lesion(s), and could be receiving up to 4 mg dexamethasone or equivalent per day; patients with seizures within 10 days prior to treatment were excluded.
Study Design and Treatment
Patients in both cohorts received the US Food and Drug Administration-approved combination regimen for advanced melanoma, consisting of nivolumab, 1 mg/kg, intravenously plus ipilimumab, 3 mg/kg, intravenously every 3 weeks for 4 doses (induction phase), followed by nivolumab monotherapy at 3 mg/kg every 2 weeks (maintenance phase). All patients were treated for at least 6 months before the clinical data cutoff date of May 1, 2018, and treatment continued up to 24 months or until disease progression, unacceptable toxicity, or withdrawal of consent. Patients completing 2 years of treatment or who had discontinued treatment were followed for safety and remission status for up to 3 years from treatment initiation. Minimum follow-up was calculated from the time of last patient’s first dose to the clinical cutoff date.
The study protocol was approved by the institutional review board at each participating center and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent.
Assessments
The primary endpoint was intracranial clinical benefit rate (CBR), defined as the percentage of patients with complete or partial response (CR or PR) confirmed ≥4 weeks after first occurrence, and/or stable disease (SD) for ≥6 months, using modified Response Evaluation Criteria in Solid Tumors (mRECIST; defined below). Secondary endpoints included extracranial (systemic) CBR; intracranial ORR and PFS; extracranial ORR and PFS; global CBR, ORR, and PFS; OS; and safety evaluation.
Treatment response was determined by radiographic assessment every 6 weeks for the first year, and then every 12 weeks for up to 24 months. Responses were investigator-assessed. Intracranial lesions were assessed by gadolinium-enhanced MRI; RECIST v1.1 was modified as described previously to allow up to 5 intracranial target lesions 5-30 mm in their longest diameter.18 Extracranial lesions were assessed by computed tomography according to RECIST, v1.1, with ≤5 baseline target lesions a minimum of 10 mm in diameter. Global responses were assessed using modified RECIST v1.1 and included up to 10 target lesions in the brain (intracranial) and systemic (extracranial) compartments.
Statistical Analysis
Statistical analyses of the primary endpoint were based on a planned sample size of 110 patients for the entire study population (90 patients for Cohort A and 20 for Cohort B). Primary results for the majority of Cohort A have been published previously12 and are further updated herein based on a data cutoff date of May 1, 2018. Based on the same data cutoff date, the primary results for Cohort B are reported here along with patient-level data of the 18 symptomatic patients. Results for Cohorts A and B were reported separately because of the differences in the timing of enrollment and clinical course of the patient populations. Descriptive analyses were provided for Cohort B. Time-to-event analyses were estimated using the Kaplan-Meier method, with medians presented along with 95% CIs based on the Brookmeyer and Crowley method. PFS analyses for intracranial, extracranial, and global disease were based on response criteria explained above. Analyses were performed using SAS software v9.2.
Results
Patient Enrollment
Patients were enrolled at 28 sites in the United States from February 2015 through November 2017. There were 18 patients in Cohort B (symptomatic and/or corticosteroid-treated group) and 101 patients enrolled in Cohort A (asymptomatic, corticosteroid-free group; Supplementary Figure A1). All patients in both cohorts started treatment ≥6 months before the clinical data cutoff date of May 1, 2018.
Cohort B
Summary baseline patient characteristics are provided for both cohorts (Supplementary Table A1) and a detailed description of baseline, treatment, and response characteristics for the Cohort B symptomatic patients shows the variability of the patients in this small cohort (Table 1). Only 1 patient had an ECOG PS status of 2, and 9 had a score of 1; 8 (44%) of patients had a BRAFV600-mutant tumor. Four patients received prior systemic therapy (2 adjuvant and 2 metastatic, all with targeted therapy), and 2 patients received prior stereotactic radiotherapy (SRT) (Table 1). Seven of 18 patients had ≥3 target lesions, and the median sum of diameters for all brain metastases was 26 mm (range 7-86), indicating a larger tumor burden compared with asymptomatic patients, whose medium sum of diameters was only 15 mm (range 5-91) (Supplementary Table A1).
Table 1.
Characteristics of Symptomatic Patients
| Pt. No. | BRAF Status | Prior Cancer Treatment | PD-L1 Expression | ECOG PS | IC Baseline Target Lesions (No.) | Tumor Burden (mm) | Corticosteroid use(baseline; mg/day) | Induction Doses | Maintenance Doses | Best IC Response | Duration of IC Response (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mutant | – | 0 to 1% | 0 | 1 | 8 | D: 2 | 4 | 37 | CR | >12.5 (ongoing) |
| 2 | Wild-type | – | 1 to <5% | 1 | 2 | 5, 5 | 0 | 1 | 23 | CR | ≥9.6 (ongoing) |
| 3 | Mutant | – | 5 to <20% | 1 | 3 | 8, 5, 6 | 0 | 4 | 23 | PR | ≥4.1 (ongoing)a |
| 4 | Not reported | SRT | Not determined | 1 | 1 | 22 | D: 2b | 3 | 0 | PR | >2.7 (off-treatment) |
| 5 | Wild-type | – | Not determined | 1 | 1 | 7 | D: 2 | 1 | 0 | No SD >6 mo | – |
| 6 | Mutant | – | Not determined | 0 | 2 | 24, 25 | D: 4 | 1 | 0 | No SD >6 mo | – |
| 7 | Wild-type | Adjuvant (investigational) | Not determined | 0 | 2 | 14, 15 | D: 4 | 2 | 0 | PD | – |
| 8 | Wild-type | – | 0 to <1% | 0 | 5 | 17, 18, 27, 16, 8 | D: 4 | 2 | 0 | PD | – |
| 9 | Wild-type | SRT | 0 to <1% | 0 | 3 | 16, 6, 6 | D: 2, 1 | 1 | 1 | PD | – |
| 10 | Wild-type | Adjuvant (interferon) | Not evaluable | 1 | 3 | 12, 11, 6 | D: 4, 4 | 1 | 0 | PD | – |
| 11 | Mutant | – | 0 to <1% | 0 | 1 | 19 | D: 2 | 1 | 0 | PD | – |
| 12 | Mutant | Metastatic (dabrafenib + trametinib) | 0 to <1% | 0 | 2 | 9, 5 | 0 | 1 | 0 | PD | – |
| 13 | Mutant | – | Not determined | 1 | 2 | 16, 18 | D: 4 | 1 | 0 | PD | – |
| 14 | Wild-type | – | 1 to <5% | 1 | 2 | 19, 6 | 0 | 2 | 0 | PD | – |
| 15 | Mutant | Metastatic (dabrafenib + trametinib) | 0 to <1% | 0 | 3 | 23, 20, 9 | D: 4 | 2 | 0 | PD | – |
| 16 | Wild-type | – | Not determined | 1 | 3 | 22, 21, 13 | D: 8c, 4, 2, 4 | 1 | 0 | PD | – |
| 17 | Not reported | – | ≥20% | 1 | 3 | 5, 18, 6 | 0 | 2 | 0 | Death | – |
| 18 | Mutant | – | Not evaluable | 2 | 2 | 6, 5 | 0 | 1 | 0 | Death | – |
Abbreviations: CR, complete response; D, dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; IC, intracranial; mo. month; No., number; PD, progressive disease; PD-LI, programmed death ligand 1; PR, partial response; SD, stable disease; SRT, stereotactic radiotherapy.
aResponse date was not recorded by the investigator and was derived based on the lesion data.
bAfter the database was locked, this patient was identified as having received dexamethasone 2 mg at baseline by the investigator.
cPatient received 8 mg of dexamethasone outside of the 10-day window that restricted doses to no more than 4 mg/day.
The median follow-up for patients in Cohort B was 5.2 months, with a minimum follow-up of 6 months in patients who did not progress prior to 6 months. Patients received a median of one (range 1-4) combination dose; the 4 patients who started nivolumab maintenance had a median of 23 (range 1-37) doses (Supplementary Figure A1).
Four symptomatic patients had intracranial responses (2 CR and 2 PR; Table 2). All patients who responded intracranially also responded extracranially, with an ORR of 22.2% for intracranial, extracranial, and global disease. Median time to intracranial response was 4.1 months (range 1.0-6.9); median DOR had not been reached, with ongoing response in 3/4 responders at the database lock.
Table 2.
Response to Treatment in Symptomatic Patients
| Patients (n = 18) | |||
|---|---|---|---|
| Intracranial | Extracranial | Global | |
| Best overall response, No. (%)a | |||
| Complete response | 2 (11.1) | 0 | 0 |
| Partial response | 2 (11.1) | 4 (22.2) | 4 (22.2) |
| Stable disease ≥ 6 months | 0 | 0 | 0 |
| Progressive disease | 10 (55.6) | 6 (33.3) | 8 (44.4) |
| Not evaluable for CBR: | 4 (22.2) | 8 (44.4) | 6 (33.3) |
| Death prior to first on-study assessment | 2 (11.1) | 1 (5.6) | 1 (5.6) |
| Early discontinuation due to toxicity | 0 | 0 | 0 |
| Stable disease <6 months | 2 (11.1) | 3 (16.7) | 2 (11.1) |
| No extracranial disease at baseline | NA | 1 (5.6) | NA |
| Otherb | 0 | 3 (16.7) | 3 (16.7) |
| ORR, no./No. (%; 95% CI) | 4/18 (22.2; 6.4-47.6) | 4/18 (22.2; 6.4-47.6) | 4/18 (22.2; 6.4-47.6) |
| CBRc, no./No. (%; 95% CI) | 4/18 (22.2; 6.4-47.6) | 4/18 (22.2; 6.4-47.6) | 4/18 (22.2; 6.4-47.6) |
| Median time to response, months (range) | 4.1 (1.0-6.9) | 1.3 (1.0-2.8) | 2.0 (1.0-4.2) |
| Median duration of response, months (95% CI)d | Not reached (0-not reached) | Not reached | Not reached |
| Ongoing response, no./No. (%) | 3/4 (75.0) | 4/4 (100) | 4/4 (100) |
Abbreviations: CBR, clinical benefit rate; CI, confidence interval; CR, complete response; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
aOne patient had a response preceded by initial progressive disease.
bPD not confirmed (n = 1) and insufficient radiographic scan data (n = 2).
cClinical benefit rate = complete response + partial response + stable disease ≥ 6 months.
dOne patient had a response but was not included in the duration of response because no response date was recorded by the investigator.
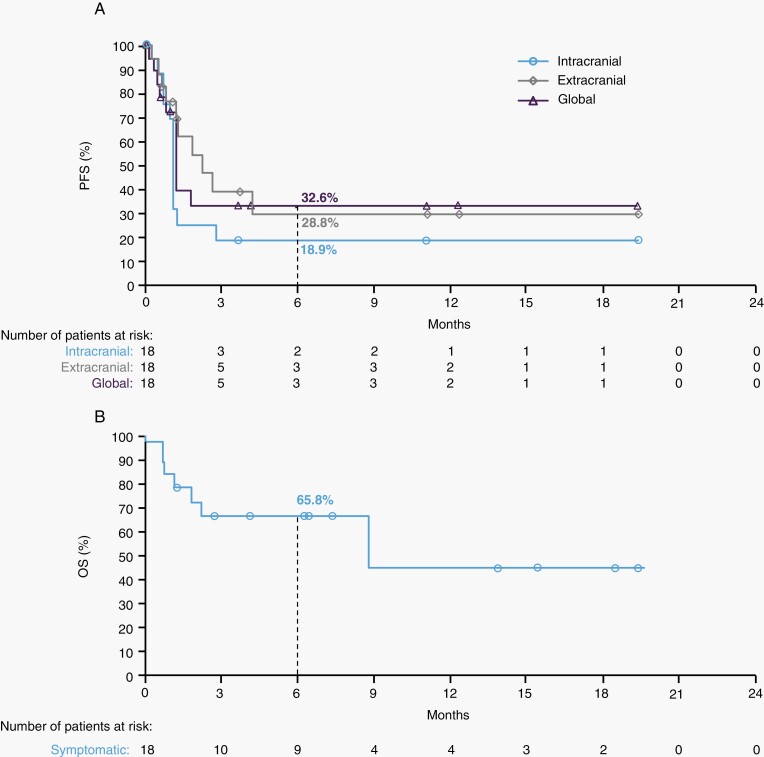
Median PFS in symptomatic patients was 1.2 months (95% CI: 0.7-1.3) for intracranial disease, 2.2 months (95% CI: 0.8-not reached) for extracranial disease, and 1.2 months (95% CI: 0.8-not reached) for global disease, with 6-month rates of 18.9% (95% CI: 4.6-40.5), 28.8% (95% CI: 8.1-54.0), and 32.6% (95% CI: 12.1-55.2), respectively (Figure 1A). Median OS was 8.7 months (95% CI: 1.8-not reached) in symptomatic patients with a 6-month OS rate of 65.8% (95% CI: 39.1-83.0; Figure 1B).
Fig.1.
Kaplan-Meier plot of (A) progression-free survival (PFS) as assessed by the investigators and (B) overall survival (OS) in symptomatic patients. Symbols indicate censored observations. Median PFS was 1.2 months (95% CI: 0.7-1.3) for intracranial disease, 2.2 months (95% CI: 0.8-not reached) for extracranial disease, and 1.2 months (95% CI: 0.8-not reached) for global disease. Median OS was 8.7 months (95% CI: 8.8-not reached).
Two of the 12 patients receiving dexamethasone (2 mg daily) at study entry responded (17%), and 2 of the 6 patients free of corticosteroids at baseline responded (33%). All of the patients who did not respond received only 1-2 induction doses, mostly due to rapid progression. For the 16 patients who ceased therapy, the majority experienced disease progression or death (9/16, 56.3%) while 4 stopped secondary to toxicity (4/16, 25.0%); reasons for the remaining 3 were patient request, withdrawal of consent, and lost to follow-up.
Patient summaries are provided for the 4 patients with a response, in the order listed in Table 2.
At baseline, patient 1 had an ECOG PS of 0, one intracranial target lesion of 8 mm in diameter, and was receiving dexamethasone 2 mg/day. The patient received 4 induction and 37 maintenance doses and was on therapy for at least 19 months at the time of the database lock. The patient achieved an extracranial and global PR 6 weeks after the start of therapy and an intracranial CR 7 months after the start of therapy. The intracranial CR has continued for at least 12.5 months.
At baseline, patient 2 had an ECOG PS of 1, two intracranial target lesions, both 5 mm in diameter, and was not receiving corticosteroids. The patient received one induction dose and 28 maintenance doses and was on therapy for approximately 12 months. The patient had an extracranial PR at 1 month, a global PR at 2.5 months, and an intracranial CR at 4 months after starting therapy and chose to discontinue treatment after 12 months, owing to the responses. The intracranial CR has continued for at least 9.6 months.
At baseline, patient 3 had an ECOG PS of 1, three intracranial target lesions of 8, 5, and 6 mm in diameter, and was not receiving corticosteroids. The patient received four induction doses and 23 maintenance doses and was on treatment for at least 13 months at the time of the database lock. The patient achieved an extracranial PR at 3 months, a global PR at 4 months, and an intracranial PR at 7 months after the start of therapy. The intracranial PR has continued for at least 4 months.
At baseline, patient 4 had an ECOG PS of 1, one unirradiated intracranial target lesion of 22 mm in diameter, and had prior SRT approximately 6 months before study treatment. This patient was also receiving dexamethasone 2 mg/day at baseline (baseline corticosteroid use was not noted at database lock, but upon further review, it was determined per the investigator that the patient did receive corticosteroids at baseline). The patient received three induction doses and no maintenance doses and was on therapy for 10 weeks. During treatment, the patient had an extracranial, intracranial, and global PR at 4 weeks after the start of therapy. The patient discontinued treatment after 10 weeks because of an adverse event (AE) of pneumonitis and continued with a PR for more than 2.7 months. The patient was alive at the data cutoff date, which was approximately 7 months after starting therapy and 6 months after the PR.
Of note, for all patients in Cohort B, initiation of corticosteroid at any time after the start of study therapy for either immune-related AEs or for new symptoms as a result of progressive disease was administered at the investigator’s discretion and was consistent with standard guidelines for the treatment of such events.
Cohort A Update
Follow-up data of the asymptomatic Cohort A (median follow-up 20.6 months; minimum 11 months) provided response results for these patients similar to the initial report.12 In the updated results, there are seven additional patients included in the analysis who have now reached the required 6-month follow-up. Compared with symptomatic patients, asymptomatic patients had lower tumor burden (Supplementary Table A1): a lower proportion of asymptomatic vs symptomatic patients had ≥3 intracranial target lesions (21.8% vs 38.9%) and asymptomatic patients had a lower median sum of intracranial target lesions (15 [range 5-91] vs 26.0 [range 7-86] mm). Of the 66 patients with a BRAF mutation, 5 patients received targeted therapy as adjuvant treatment and 6 patients received targeted therapy as metastatic treatment. At the time of database lock, 93 (92.1%) patients were off-treatment (Supplementary Figure A1). The most common off-treatment reason was study-related drug toxicity in 32.3% (30/93) of patients, followed by disease progression in 28.0% (26/93).
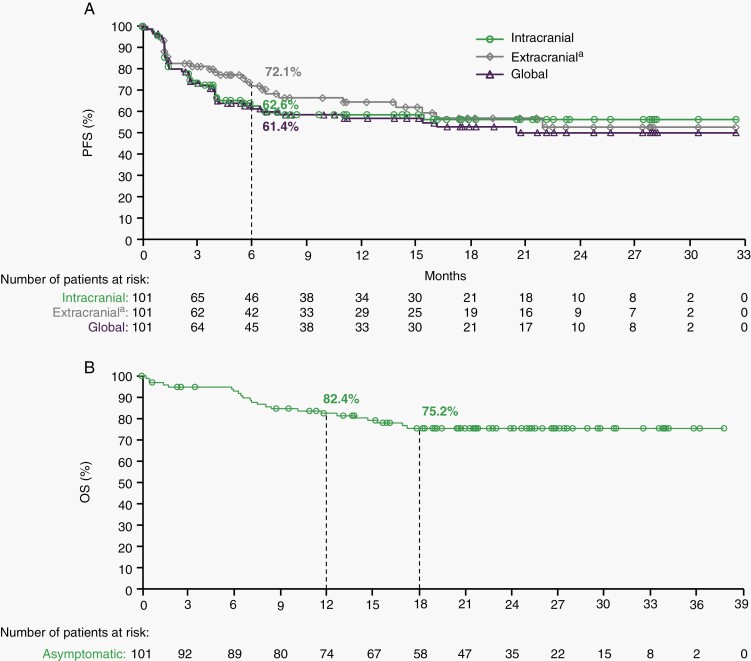
Intracranial, extracranial, and global disease results were similar for both CBR and ORR, although a higher CR rate was observed for intracranial disease at 28.7% vs 10.9% for both extracranial and global disease (Table 3). Median DOR has still not been reached, with a current ongoing response in 48/55 (87.3%) of responders (Supplementary Figure A2) and median tumor burden change of −57.1% (Supplementary Figure A3). For patients with BRAFV600-mutant and wild-type tumors, respectively, intracranial CBR was 63.6% (95% CI: 50.9-75.1) and 48.5% (30.8-66.5) and intracranial ORR was 60.6% (47.8-72.4) and 42.4% (25.5-60.8). With the additional patients with the required follow-up since the last report, 6-month PFS rates were 62.6% (95% CI: 51.7-71.8), 72.1% (60.5-80.8), and 61.4% (50.4-70.6), for intracranial, extracranial, and global disease, respectively (Figure 2A). Projected 12-month PFS rates were 58.5% (47.3-68.1) for intracranial, 64.5% (51.8-74.7) for extracranial, and 56.9% (45.6-66.7) for global. Median OS had not been reached, with 12- and 18-month rates of 82.4% (95% CI: 73.2-88.7) and 75.2% (64.9-82.8), respectively (Figure 2B).
Table 3.
Response to Treatment in Asymptomatic Patients
| Patients (n = 101) | |||
|---|---|---|---|
| Intracranial | Extracranial | Global | |
| Best overall response, No. (%)a | |||
| Complete response | 29 (28.7) | 11 (10.9) | 11 (10.9) |
| Partial response | 26 (25.7) | 38 (37.6) | 40 (39.6) |
| Stable disease ≥ 6 months | 4 (4.0) | 6 (5.9) | 4 (4.0) |
| Progressive disease | 27 (26.7) | 16 (15.8) | 29 (28.7) |
| Not evaluable for CBR: | 15 (14.9) | 30 (29.7) | 17 (16.8) |
| Death prior to first on-study assessment | 3 (3.0) | 3 (3.0) | 2 (2.0) |
| Early discontinuation due to toxicity | 1 (1.0) | 1 (1.0) | 1 (1.0) |
| Stable disease <6 months | 8 (7.9) | 15 (14.8) | 9 (8.9) |
| No extracranial disease at baseline | NA | 7 (6.9) | NA |
| Otherb | 3 (3.0) | 4 (10.9) | 5 (5.0) |
| ORR, no./No. (%; 95% CI) | 55/101 (54.4; 44.2-64.4) | 49/101 (48.5; 38.4-58.7) | 51/101 (50.5; 40.4-60.6) |
| CBRc, no./No. (%; 95% CI) | 59/101 (58.4; 48.2-68.1) | 55/101 (54.4; 44.2-64.4) | 55/101 (54.4; 44.2-64.4) |
| Median time to response, months (range) | 1.61 (1.1-12.5) | 1.4 (1.1-23.2) | 1.4 (1.1-7.0) |
| Median duration of response, months (95% CI) | Not reached | Not reachedd (18.1-not reached) | Not reached |
| Ongoing response, no./No. (%) | 48/55 (87.3) | 39/48d (81.2) | 43/51 (84.3) |
Abbreviations: CBR, clinical benefit rate; CI, confidence interval; CR, complete response; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
aTwo patients had a response preceded by initial progressive disease.
bTotal of 5 patients for all 3 response categories (intracranial, extracranial, and global): 1 patient withdrew consent (all 3 categories), 2 patients stopped study (all 3 categories), 1 patient for EC lesion procedure not done (just global), 1 patient for gamma knife therapy (global).
cClinical benefit rate = complete response + partial response + stable disease ≥ 6 months.
dOne additional patient had a response, but was not included as no response date was recorded by the investigator.
Fig. 2.
Kaplan-Meier plot of (A) progression-free survival (PFS) as assessed by the investigators and (B) overall survival (OS) in asymptomatic patients. Symbols indicate censored observations. Median PFS has not been reached for intracranial (95% CI: 6.5-not reached), extracranial (95% CI: 13.9-not reached), or global (95% CI: 6.5-not reached) disease. Median OS has not been reached. aIncludes 7 patients without extracranial disease at baseline.
Safety
Any-grade treatment-related AEs (TRAEs) and grade 3/4 TRAEs occurred in 88.9% and 55.6% of patients in the Cohort B symptomatic group (Table 4). The most common any-grade TRAEs in symptomatic patients were diarrhea (27.8%) and fatigue, pyrexia, nausea, pruritus, and maculopapular rash (all 16.7%.). TRAEs leading to treatment discontinuation occurred in 2 patients (11.1%) and were both grade 1-2 (pustular rash [n = 1; patient was not on corticosteroids at the time of the AE] and pneumonitis [n = 1; patient was on corticosteroids at the time of the AE]). Although hard to distinguish from disease-related symptoms, neurologic TRAEs were reported in 3 patients in the symptomatic cohort; grade 3/4 events included headache, amnesia, dysarthria, partial seizures, and syncope (Supplementary Table A2). No treatment-related deaths were reported in the symptomatic patients.
Table 4.
Treatment-Related AEsa
| Symptomatic Patients (n = 18) | Asymptomatic Patientsb (n = 101) | |||
|---|---|---|---|---|
| Any Grade | Grade 3-4 | Any Grade | Grade 3-4 | |
| Treatment-related AEs | 16 (88.9) | 10 (55.6) | 97 (96.0) | 55 (54.4) |
| Diarrahea | 5 (27.8) | 1 (5.6) | 35 (34.7) | 6 (5.9) |
| Fatigue | 3 (16.7) | 1 (5.6) | 45 (44.6) | 4 (4.0) |
| Maculopapular rash | 3 (16.7) | 1 (5.6) | 36 (35.6) | 8 (7.9) |
| Nausea | 3 (16.7) | 0 | 27 (26.7) | 2 (2.0) |
| Pruritus | 3 (16.7) | 0 | 30 (29.7) | 0 |
| Pyrexia | 3 (16.7) | 1 (5.6) | 17 (16.8) | 0 |
| Cough | 2 (11.1) | 0 | 9 (8.9) | 0 |
| Decreased appetite | 2 (11.1) | 0 | 17 (16.8) | 1 (1.0) |
| Dematitis acneiform | 2 (11.1) | 0 | 2 (2.0) | 0 |
| Increased alanine aminotransferase | 2 (11.1) | 0 | 38 (37.6) | 14 (13.9) |
| Increased aspartate aminotransferase | 2 (11.1) | 0 | 35 (34.7) | 14 (13.9) |
| Increased blood creatinine | 2 (11.1) | 0 | 3 (3.0) | 0 |
| Pneumonitis | 2 (11.1) | 0 | 10 (9.9) | 2 (2.0) |
| Pruritus generalized | 2 (11.1) | 0 | 13 (12.9) | 0 |
| Rash | 2 (11.1) | 0 | 12 (11.9) | 2 (2.0) |
| Rash pruritic | 2 (11.1) | 1 (5.6) | 5 (5.0) | 0 |
| Abdominal pain | 1 (5.6) | 0 | 8 (7.9) | 1 (1.0) |
| Colitis | 1 (5.6) | 1 (5.6) | 7 (6.9) | 7 (6.9) |
| Decreased lymphocyte count | 1 (5.6) | 0 | 5 (5.0) | 1 (1.0) |
| Decreased weight | 1 (5.6) | 0 | 6 (5.9) | 0 |
| Headache | 1 (5.6) | 1 (5.6) | 20 (19.8) | 3 (3.0) |
| Hypophysitis | 1 (5.6) | 0 | 12 (11.9) | 6 (5.9) |
| Hypothyroidism | 1 (5.6) | 0 | 23 (22.8) | 1 (1.0) |
| Increased blood alkaline phosphatase | 1 (5.6) | 0 | 10 (9.9) | 0 |
| Increased lipase | 1 (5.6) | 0 | 18 (17.8) | 10 (9.9) |
| Skin hypopigmentation | 1 (5.6) | 0 | 6 (5.9) | 0 |
| Vision blurred | 1 (5.6) | 0 | 5 (5.0) | 0 |
| Vomiting | 1 (5.6) | 0 | 13 (12.9) | 2 (2.0) |
| Adrenal insufficiency | 0 | 0 | 8 (7.9) | 1 (1.0) |
| Anemia | 0 | 0 | 8 (7.9) | 1 (1.0) |
| Arthralgia | 0 | 0 | 23 (22.8) | 0 |
| Chills | 0 | 0 | 6 (5.9) | 0 |
| Decreased platelet count | 0 | 0 | 6 (5.9) | 0 |
| Hyperthyroidism | 0 | 0 | 12 (11.9) | 3 (3.0) |
| Hyponatremia | 0 | 0 | 6 (5.9) | 2 (2.0) |
| Increased amylase | 0 | 0 | 13 (12.9) | 7 (6.9) |
| Increased blood bilirubin | 0 | 0 | 7 (6.9) | 1 (1.0) |
| Myalgia | 0 | 0 | 10 (9.9) | 0 |
| Night sweats | 0 | 0 | 6 (5.9) | 0 |
| Vitiligo | 0 | 0 | 7 (6.9) | 0 |
| Peripheral edema | 0 | 0 | 5 (5.0) | 0 |
| Hypokalemia | 0 | 0 | 5 (5.0) | 0 |
| Treatment-related AEs leading to discontinuation | 2 (11.1) | 0 | 29 (28.7) | 19 (18.8) |
Abbreviation: AE, adverse event.
Data provided as No. (%).
aShown are any-grade treatment-related adverse events in ≥5% of patients in the asymptomatic cohort (at least 5 patients) or ≥10% in the symptomatic cohort (at least 2 patients).
bOne death reported: treatment-related grade 5 myocarditis (previously reported).
Safety analysis in the asymptomatic cohort was similar to the prior report, with the most common TRAEs being fatigue (44.6%), increased alanine aminotransferase (37.6%), and maculopapular rash (35.6%) (Table 4).12 Any-grade and grade 3/4 neurologic TRAEs occurred in 34.7% and 6.9% of patients in the asymptomatic cohort with the most common being headache in 19.8% (any-grade) and 3.0% (grade 3/4) of patients (Supplementary Table A2). There was one treatment-related death in the asymptomatic cohort (grade 5 myocarditis), which was previously reported.12,19
Discussion
CheckMate 204 investigated the safety and efficacy of combination immunotherapy with nivolumab plus ipilimumab in MBM and enrolled both asymptomatic patients (Cohort A) and patients requiring corticosteroids and/or with neurologic symptoms (Cohort B). Results in both cohorts demonstrated a safety profile consistent with that seen in patients with advanced melanoma without brain metastases.20,21 Updated results in asymptomatic patients confirm durable efficacy with median PFS and OS not yet reached with a median follow-up of 20.6 months (minimum 11 months). Limited efficacy was observed in patients requiring corticosteroids and/or with neurologic symptoms at baseline. The vast majority of responses observed in all cohorts appeared durable, consistent with immune-mediated clinical activity.
Patients with symptomatic MBM have rarely been treated with systemic therapy without radiation and are poorly represented in clinical trials. Dabrafenib plus trametinib provided an intracranial ORR of 59% with median DOR 4.5 months and median OS 11.5 months in 17 patients with BRAFV600-mutant symptomatic MBM in the COMBI-MB study.18 Ipilimumab alone provided an intracranial ORR of 5% and median OS of 3.7 months among 21 symptomatic patients,11 and nivolumab monotherapy showed 6% intracranial ORR and median OS of 5.1 months in symptomatic patients in the ABC study.9,16 In the current study, we included 18 symptomatic patients and observed intracranial responses in 4 (22%) patients with a median OS of 8.7 months and DOR not reached at 6 months. Despite the use of baseline corticosteroid (≤4 mg/day dexamethasone equivalents) among 12 of the 18 Cohort B patients, their safety profile was similar to that observed in Cohort A. As expected, these patients had higher tumor burden than asymptomatic patients. Inherently, timing of treatment for symptomatic patients may be an issue because of rapid clinical deterioration—the majority of symptomatic patients experienced rapid, early disease progression that may have limited their potential to receive immunotherapy. Of the patients who received baseline corticosteroids and did not respond, 7 of 10 were able to receive only one dose of the combination, commonly due to progressive disease.
Corticosteroids (commonly dexamethasone) are frequently used in patients with brain metastases to help control symptoms related to peritumoral brain edema, often prophylactically, even for symptom-free vasogenic edema. Of symptomatic patients in this study, 2 of 12 who received baseline corticosteroids achieved a response vs 2 of 6 who were not. Of note, both patients received low-dose corticosteroids (2 mg dexamethasone daily), and 1 achieved a CR. These results may be consistent with the principle that corticosteroid therapy that is ongoing at baseline may interfere with the efficacy of ICI, although our sample is too small to investigate disease burden and rapid progression vs corticosteroid use and other factors. Further, corticosteroid immunosuppression mechanisms may differ between CTLA-4 and PD-1 blockade.22,23 Most clinical trials evaluating ICI in cancer prohibit the use of corticosteroids at the start of treatment, highlighting the importance of these results regarding the potential for corticosteroid inhibition of immunotherapy benefit.
While the results of our asymptomatic cohort patients show high rate of durable responses among patients who can be weaned off initial corticosteroid therapy, patients with symptomatic disease have lower responses and require further study. This suggests that strategies to maximize the potential of withdrawing corticosteroids prior to immunotherapy, such as initial SRT or craniotomy or upfront treatment with other systemic treatments (eg, MAPK inhibitors), may be considered to improve the outcomes in this population. These strategies may also give patients more time to be able to receive more doses of combination nivolumab plus ipilimumab. However, retrospective reports have shown that corticosteroid therapy started after treatment initiation for immune-related toxicities resulting from ICI does not seem to abrogate the antitumor effects (presumably because the response is already established and maintained by steroid-resistant effector cells), an observation consistent with the associations between immune-related AEs and favorable therapeutic outcomes.24,25 It must be noted that other patient factors with baseline corticosteroids, such as increased intracranial tumor burden, could also impact the efficacy results in these patients. The small patient numbers in Cohort B and trial design do not allow firm conclusions to be drawn about the impact of baseline corticosteroid use, but it is a treatment factor that warrants continued investigation. Our results highlight the need to investigate the incorporation of corticosteroid-sparing approaches and to evaluate the optimal use and timing of SRT in patients with MBM who are also being considered for immunotherapy. The use of SRT for MBM may synergize with immunotherapy and enhance the benefit, but has also been associated with increased radionecrosis.26–28 Our study also emphasizes the critical need to shorten the timeframe for screening and simplify the entry criteria for patients with MBM to enable rapid initiation of therapy for patients at risk of precipitous clinical deterioration.
These results confirm those previously reported for the nivolumab plus ipilimumab combination in patients with asymptomatic MBM, with an expanded patient population and with longer follow-up for this population at high risk for progression. At a median of 20.6 months of follow-up for asymptomatic MBM patients, ORR was 54.4% with nivolumab plus ipilimumab (28.7% intracranial CR and 25.7% intracranial PR) with median OS, PFS, and DOR not yet reached. While it is challenging to compare across trials, the ORR in all patients was similar to the 58% observed in COMBI-MB with dabrafenib plus trametinib in patients with asymptomatic MBM and BRAFV600-mutant tumors.18 However, durability of responses with combined dabrafenib + trametinib therapy is limited, as the median DOR in those patients was 6.5 months, with a median OS of 10.8 months.18 The CheckMate 204 nivolumab plus ipilimumab results demonstrated here are similar to those of the ABC trial in a smaller cohort of 35 patients in which median OS was 32.8 months and median DOR was not reached with a median follow-up of 34 months (minimum 21.5 months).9 Additional analyses from that study suggest that patients who received prior BRAF/MEK inhibitors had a lower ORR with nivolumab plus ipilimumab compared with treatment-naive patients (28% vs 59%), and PFS was shorter in the total treated population (5.4 months) compared with treatment-naive patients (26.3 months).9 However, the results from our current study suggest that the efficacy of nivolumab plus ipilimumab regardless of BRAFV600-mutation status is preserved, even with prior MAPK inhibitor-directed therapy.
In this study, we acknowledge the lack of an independent review of the imaging data, which would strengthen the conclusions. In addition, we acknowledge the small population in the symptomatic Cohort B, which limits the ability to draw firm conclusions to the data. Lastly, although this cohort comprised patients who were symptomatic or receiving corticosteroids, the exclusion of patients with unstable neurologic symptoms, recent seizures, or a higher corticosteroid dose requirement still excluded an important population. These patients facing more severe symptoms are at a much higher risk of imminent neurological deterioration and are not deemed stable enough to treat with systemic therapy unaided by the use of other modalities such as surgery and/or radiation. Although this limits the generalizability of our results, we do believe that future protocols can build on our findings to allow for combined modality approaches to improve the outcomes of this population in dire need of therapeutic options.
In conclusion, although patients with symptomatic MBM remain relatively resistant to systemic therapy, some responses were observed with nivolumab plus ipilimumab combination. There is a need to develop novel therapies and treatment strategies for these patients. Successful outcomes will likely involve multimodality expertise and sequential strategies incorporating radiotherapy, surgery, or targeted therapy, with one goal to reduce or eliminate corticosteroid use. Novel combinations of immunotherapy with targeted agents are also under investigation for patients with untreated BRAFV600-mutant MBM (eg, encorafenib plus binimetinib plus nivolumab vs ipilimumab plus nivolumab [NCT04511013; SWOG S2000]). Based on our long-term follow-up data of Cohort A, we believe the standard treatment for patients with advanced melanoma with asymptomatic brain metastases who are candidates for immunotherapy should be 1 mg/kg nivolumab plus 3 mg/kg ipilimumab.
Supplementary Material
Acknowledgments
We thank the patients and investigators who participated in the CheckMate 204 trial. This research was supported in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 (to Dr. Postow). We acknowledge Ono Pharmaceutical Company, Ltd. (Osaka, Japan) for contributions to nivolumab development and Dako, an Agilent Technologies, Inc. company (Santa Clara, CA, USA) for collaborative development of the PD-L1 immunohistochemistry 28-8 pharmDx assay. We acknowledge Agnes Balogh, from Bristol Myers Squibb, for statistical support and Caroline Chung, M.D., University of Texas M.D. Anderson Cancer Center, for contributions to the study. Professional medical writing and editorial assistance were provided by Melissa Kirk, Ph.D., and Michele Salernitano at Ashfield Healthcare Communications, funded by Bristol Myers Squibb.
Conflict of interest statement. H.A.T.—Consulting/Advisory Role: Bristol Myers Squibb (BMS), Genentech/Roche, Merck, Novartis, Eisai, Iovance; Research Funding: BMS, Celgene, Genentech/Roche, GlaxoSmithKline, Merck, Novartis; Honoraria: Eisai. P.A.F.—Consulting/Advisory Role: AbbVie, Bayer, BTG, Novellus, Novocure, Tocagen, Ziopharm; Honoraria: BTG, Tocagen, Ziopharm; Research Funding: Pfizer. F.S.H.—Consulting/Advisory Role: 7 Hills Pharma, Aduro, Apricity, Bicara, Biocentre, BMS, Checkpoint, Compass Therapeutics, Corner Therapeutics, Eisai, EMD Serono, Genentech/Roche, Gossamer, Idera, Kairos, Merck, Novartis, Pieris, Pionyr, PsiOxus, Rheos, Sanofi, Surface Pharmaceuticals, Takeda, Torque, Zumutor; Research Funding and Patents/Royalties/Intellectual Property: BMS, Novartis; Stock/Other Ownership: Apricity, Bicara, Pionyr, Torque. C.D.L.—Consulting/Advisory Role: Immunocore; Research Funding: BMS, Dynavax, GlaxoSmithKline, Merck; Travel/Accommodations/Expenses: BMS, Immunocore. O.H.—Consulting/Advisory Role: Aduro, Akeso, Amgen, Beigene, BMS, GlaxoSmithKline, Immunocore, Incyte, Janssen, Merck, NextCure, Novartis, Sanofi/Regeneron, Seattle Genetics, Tempus, Zelluna; Honoraria: Array, BMS, Novartis, Sanofi/Regeneron; Research Funding: Arcus, Aduro, Akeso, Amgen, CytomX, Exelixis, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance, Merck, Merck Serono, Moderna, NextCure, Novartis, Pfizer, Sanofi/Regeneron, Seattle Genetics, Zelluna; Speakers’ Bureau: BMS, Novartis, Pfizer, Sanofi/Regeneron. M.B.A.—Consulting/Advisory Role: Adagene, Agenus, Apexigen, AstraZeneca, Aveo, BMS, Calithera, Eisai, Elpis, Exelixis, Genentech-Roche, Idera, Immunocore, Iovance, Leads Biolabs, Merck, Neoleukin, Novartis, Pfizer, PACT, Pyxis, Werewolf Therapeutics. K.L.—Research Funding: BMS. S.J.—Consulting/Advisory Role: Array, BMS, EMD Serono, Genentech, Novartis, Sanofi, Sun Biopharma. A.P.A.—Consulting/Advisory Role: Array, OncoSec, Regeneron, Sensei, Valitor; Research Funding: Acerta, Amgen, AstraZeneca, BMS, Dynavax, Genentech, Idera, Incyte, ISA, Loxo, Merck, Novartis, OncoSec, Regeneron, Sensei, Tessa; Stock/Other Ownership: OncoSec, Valitor; Travel/Accommodations/Expenses: OncoSec. N.I.K.—Consulting/Advisory Role: Array, BMS, EMD Serono, Genentech, HUYA Bioscience, Regeneron, Immunocore, Iovance, Merck, Jounce Therapeutics; Honoraria: BMS, Sanofi; Research Funding: Amgen, BMS, Celgene, GlaxoSmithKline, HUYA Bioscience, Merck, Novartis, Regeneron, Replimune; Stock/Other Ownership: Amarin, Bellicum, Mazor Robotics, TransEnterix. M.A.P.—Consulting/Advisory Role: Array, Aduro, BMS, Eisai, Incyte, Merck, NewLink, Novartis; Honoraria: BMS, Merck; Research Funding: Array, AstraZeneca, BMS, Infinity, Merck, Novartis, Rgenix. A.C.P.—Consulting/Advisory Role: Array, BMS, Merck, Novartis, Regeneron, Sanofi; Research Funding: BMS, Celldex, Merck, Millennium, Regeneron; Travel/Accommodations/Expenses: Array, Regeneron, Sanofi. D.A.R.—Consulting/Advisory Role: AbbVie, Advantagene, Agenus, Amgen, Bayer, Boston Biomedical, BMS, Celldex, DelMar, EMD Serono, Genentech/Roche, Inovio, Juno, Medicenna, Merck, Merck KGaA, Midatech, Monteris Medical, Novartis, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Taiho; Research Funding: Tragara. I.P.—Consulting/Advisory Role: Amgen, Merck, Nouscom. R.R.K.—Consulting/Advisory Role: Array, BMS, Immunocore, Merck, Novartis, Regeneron; Honoraria: Array, BMS; Research Funding: BMS, Merck, Regeneron; Travel/Accommodations/Expenses: BMS. A.A.T.—Consulting/Advisory Role: Array, BioNTech, BMS, Clinigen, Genentech/Roche, Immunocore, Merck, NewLink, Novartis, OncoSec, Partner Therapeutics, Pfizer, EMD Serono, Sanofi-Genzyme/Regeneron; Research Funding: Genentech/Roche, Merck, OncoSec. A.S.—Employment: BMS. J.I.R.—Employment and Stock Ownership: BMS; Intellectual Property/Patents/Copyrights: Pending for BMS. All other authors have no conflicts to report.
Authorship statement. Conception and design: H.A.T. and K.A.M. Provision of study material or patients: H.A.T., P.A.F., F.S.H., C.D.L., S.J.M., O.H., M.B.A., K.L., R.P.T., J.A.G., S.J., A.P.A., N.I.K., M.A.P., A.C.P., M.S.E., D.A.R., I.P., R.R.K., A.A.T., and K.A.M. Collection and assembly of data: H.A.T., P.A.F., F.S.H., C.D.L., S.J.M., O.H., M.B.A., K.L., R.P.T., J.A.G., S.J., A.P.A., N.I.K., M.A.P., A.C.P., M.S.E., D.A.R., I.P., R.R.K., A.A.T., J.I.R., and K.A.M. Data analysis and interpretation, manuscript writing, final approval of manuscript, accountable for all aspects of the work: All authors.
Data sharing. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Prior presentation. Presented in part at the American Society of Clinical Oncology 2019 Congress, Chicago, IL, USA, May 31-June 4, 2019.
Research support. Bristol Myers Squibb (Princeton, NJ).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Iorgulescu JB, Harary M, Zogg CK, et al. Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: results from a national cohort. Cancer Immunol Res. 2018;6(9):1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 4. Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fidler IJ, Schackert G, Zhang R, et al. The biology of brain metastatases. Cancer Metastasis Rev. 1999;18(3):387–400. [DOI] [PubMed] [Google Scholar]
- 6. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 7. Zhang D, Wang Z, Shang D, Yu J, Yuan S. Incidence and prognosis of brain metastases in cutaneous melanoma patients: a population-based study. Melanoma Res. 2019;29(1):77–84. [DOI] [PubMed] [Google Scholar]
- 8. Sloot S, Chen YA, Zhao X, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. 2018;124(2):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long GV, Atkinson VG, Lo S, et al. Long-term outcomes from the randomized Ph 2 study of nivolumab (nivo) or nivolumab + ipilimumab (ipi) in patients (pts) with melanoma brain metastases: anti-PD1 Brain Collaboration (The ABC Trial). Paper presented at: the European Society for Medical Oncology (ESMO) Congress 2019; September 27–October 1, 2019; Barcelona, Spain; Presentation 1311O.
- 10. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol. 2019;37(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 12. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 14. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37(22):1927–1934. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalo JA, González-García A, Martínez C, Kroemer G. Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med. 1993;177(5):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 17. Brastianos HC, Cahill DP, Brastianos PK. Systemic therapy of brain metastases. Curr Neurol Neurosci Rep. 2015;15(2):518. [DOI] [PubMed] [Google Scholar]
- 18. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 22. Maxwell R, Luksik AS, Garzon-Muvdi T, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. [DOI] [PubMed] [Google Scholar]
- 25. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pires da Silva I, Glitza IC, Haydu LE, et al. Incidence, features and management of radionecrosis in melanoma patients treated with cerebral radiotherapy and anti-PD-1 antibodies. Pigment Cell Melanoma Res. 2019;32(4):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol. 2017;133(3):595–602. [DOI] [PubMed] [Google Scholar]
- 28. Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4(8):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.