Abstract
Aim of the study
In the context of the worldwide vaccination campaign against COVID-19, France has been deploying multiple sites for mass vaccination. This study aimed to assess the perceived usefulness of a prototype decontamination mobile unit (UMDEO) for COVID-19 vaccination among both the patient and healthcare providers perspectives.
Methods
This was a descriptive cross-sectional study conducted in Toulouse over two days. UMDEO is a fully comprehensive, versatile solution that was deployed as a 5-row vaccination unit. A written questionnaire was distributed from March 6th-7th, 2021 among all patients presenting for vaccination at the mobile center, as well as the team participating in the vaccination campaign.
Results
Among the vaccinated patients (n = 1659), 1409 participants (84.9%) filled out the survey, as well as 68 out of 85 (80%) within the UMDEO team. The maximum patient rate was 98 people per hour. The majority of participants and caregivers (1307 [93.2%] and 67 [98.5%] respectively) agreed that the mobile unit increased access to vaccination. A total of 91.3% patients (n = 1281) and 95.6% caregivers (n = 65) believed that it would speed up the overall vaccination campaign.
Conclusion
The majority of the vaccinated population and of the team participating in the survey were satisfied with the usefulness of UMDEO as a vaccination center. Toulouse is currently the only city to have used such a structure for vaccination, but it could be used as a basis for planning other mobile units to increase vaccination access.
Keywords: Mass vaccination, Mobile unit, COVID-19, Pandemic, Disaster Medicine
1. Introduction
Vaccination is considered ‘‘the most effective medical intervention for mitigating the potentially devastating impact of an evolving pandemic’’[1]. Vaccines represent one of the most successful and cost-effective health interventions in human history [2]. According to the World Health Organization (WHO), global vaccination programs save up to 2–3 million lives each year by priming the immune system to protect the host against potential pathogens [3].
Rapidly organized and conducted mass vaccination campaigns effectively protect susceptible individuals and can often interrupt epidemic transmission within 2 or 3 weeks [4]. Mass vaccinations are usually provided by mobile vaccination teams or fixed vaccination stations at health centers or other community facilities [5]. To date, there is a paucity of studies that have examined local or national operational capacity for mass vaccination in Europe [6] while the current COVID-19 pandemic plainly underscores the need to vastly accelerate mass vaccination programs [7].
Major barriers to implementing rapid mass vaccination operations include vaccine availability [8], the need for a large number of qualified personnel to administer vaccines, logistical challenges to maintaining cold chain for vaccine preservation [9], the financial cost of the program [10], and operational challenges to addressing the whole population, yet maintaining a focus on high-risk populations [11].
The COVID-19 vaccination campaign in France started at the end of December 2020, prioritizing first the elderly population aged ≥ 75 years and health professionals ≥ 50 years [12]. At the beginning of March, 3.2 million people had received a first injection, and more than 1.7 million had received two doses [13].
In this context, the prefecture of Haute-Garonne in France and the Toulouse University Hospital set up a two-day mass vaccination operation using a prototyped mobile decontamination unit (UMDEO) as a mobile vaccination center. This brand-new unit had been designed by physicians, caregivers and decontamination specialists, and had never been used in any drill or real-life event before. Mobile decontamination units are used to decontaminate persons contaminated by toxic substances [14]. A decontamination unit (often colloquially called a “decon unit”) is an area equipped with tools and systems used to remove hazardous and non-hazardous contaminants from people, clothing and equipment [15]. These can be mobile units or permanent installations. They are generally divided into separate areas that the person or element undergoing decontamination must pass through (areas for undressing the exposed person, washing with soap and water and a dressing area after decontamination is completed).
In the context of the COVID-19 pandemic, UMDEO has been redesigned to fit perfectly with the need of a quick and efficient mass vaccination.
This study aims to assess the usefulness of the decontamination mobile unit for COVID-19 vaccination as measured by the experience of those vaccinated, and the medical team who participated in the vaccination operation.
2. Methods
2.1. Design
This descriptive cross-sectional study took place in the mobile decontamination unit, UMDEO located in Toulouse, France.
2.2. Ethical consideration
According to French ethic and regulatory law, article R1121-1 of the public health code, ethical approval for the study was waived by the national ethical committee (CPP, Comité de Protection des Personnes). It was registered at the register of epidemiologic studies of Toulouse University Hospital (RnIPH 2021–33) and has also been submitted to the National Commission of Informatics and Liberty (CNIL number: 2,206,723 v0). The University Hospital signed a commitment of compliance to the reference methodology MR-004. All participants received an informed consent form before filling out the survey.
2.3. Setting
We conducted a written questionnaire from March 6th to March 7th 2021 to the entire population presenting for vaccination at the mobile center in Toulouse and the UMDEO team participating in the vaccination campaign. Following the Prime Minister's announcement on Thursday, March 4, the vaccination operation had been stepped up with significant acceleration in the first weekend of March, in the Toulouse area. The vaccination had been proposed to all the population aged ≥ 75 years with or without comorbidity, as well as the population between 50 and 74 years of age suffering from one or more comorbidities. Participants had to make an appointment by phone on 5th and 6th March. The mobile centers were opened from 8am to 7 pm on Saturday 6th to Sunday 7th. Apart from telephone appointments, any eligible population wishing to be vaccinated could come to the mobile vaccination center independently on both days.
2.4. UMDEO, the mobile unit prototype
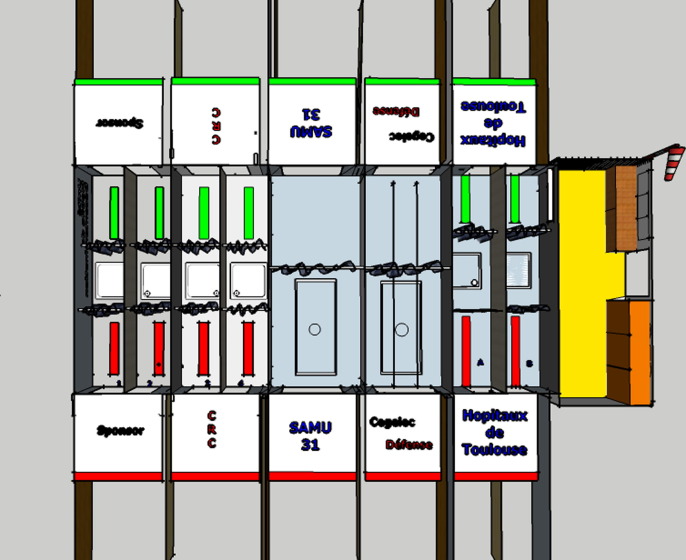
The prototyped mobile decontamination unit, UMDEO has been developed in Toulouse with the help of a French Industrial (Cegelec Défense, Toulouse, France) and is the property of Toulouse University Hospital (Fig. 1 ). It is a fully mobile and versatile equipped (for decon) solution with a surface area of 84.5 m2, divided in 8 rows (6 for ambulatory patients, 2 for injured or otherwise immobile patients on a stretcher), designed to decontaminate up to 100 patients per hour. It is deployable within 45 min by a 5-person team and can adapt to multiple site characteristics and layouts such as parking lots, fields or sand areas. These features make it amenable to use as a temporary mass vaccination center (see Fig. 2 ).
Fig. 1.
Layout of UMDEO.
Fig. 2.
Vaccination in the mobile decontamination unit UMDEO.
2.5. Organization of the vaccination process
The team involved in the vaccination operation were selected amongst the hospital caregivers and local firefighter association on a voluntary basis and according to their availability. It was composed of physicians, nurses, first aid workers, firefighters and medical students.
COVID-19 vaccinations in the mobile unit were carried out following the “front-end” doctrine (16-17). People presenting for vaccination were first greeted at the entrance of the site and were asked to complete a pre-vaccination medical questionnaire listing any allergies or potential medical conditions that may prevent them from taking the vaccine and to sign it.
Every person was invited to one of the five lanes of the unit to get the injection by a health care provider. For the purpose of the vaccination process, UMDEO was reorganized in 6 separate corridors, five of which were used for the vaccination and the last one to prepare each dose of vaccine. To do that, two to four health care providers were present in the 6th compartment, then the doses were transmitted in batches of ten to each vaccinating health care provider. Each vaccination was preceded by local antisepsis and the equipment cleaned after each use (Fig 2).
Vaccinated persons were then asked to wait 10 to 15 min for monitoring by a first aid worker, then exit secretaries registered the patients in the national registration database, which represents the health insurance’s teleservice for tracking and monitoring the vaccination. They provided each patient their vaccination certificate. Participants were asked to make an appointment with their attending physician or a nurse for the second vaccination campaign, between May 9 and May 25.
Finally, before leaving the vaccination area, they were asked to fill out the study questionnaire. It was explained to them that this was anonymous and would be used for research purposes.
2.6. Content of the survey questionnaire
The questionnaire was created by the research staff and validated by the medical coordinator of the operation. It used closed-ended questions with a global descriptive scale, scored from 1 (extremely unsatisfied /disagree) to 6 (extremely satisfied /agree). Two different types of questionnaires were proposed: one for the vaccinated population and the other one for the UMDEO team. In the professional survey, the five questions were about the usefulness of the mobile unit in the vaccination operation, hygiene rules, respect of confidentiality and its accessibility. In the patient survey, items about the quality of reception and information had been added. Each questionnaire began with a demographic section and ended with a global satisfaction evaluation, based on a score rated from 0 (totally unsatisfied) to 10 (extremely satisfied).
2.7. Data analysis
All the collected data were entered into Microsoft excel (Microsoft Corporation, Redmond, WA, USA) and cross-checked for presence of any error to maintain its accuracy. Descriptive statistics were applied to calculate proportions and frequencies as well as means and standard deviations.
3. Results
3.1. The vaccinated population survey
Among the vaccinated population (n = 1659), 1409 participants filled out the survey with a response rate of 84.9%. It is of note that 679 people were vaccinated on the first day (10 h of vaccination campaign) and 980 on the second day. The maximum vaccination rate in UMDEO was 19.6 people per hour per row, with 5 rows.
Demographic characteristics of participants (vaccinated population and UMDEO team) are detailed in Table 1 . The mean age of the respondents was 65.4 ± 11.6 years. The respondents had a near even split by gender (47% female and 53% male). Five hundred and fifty (42%) participants lived more than 10 km from the vaccination site.
Table 1.
Demographics characteristics of study participants (vaccinated population and UMDEO team).
| Vaccinated population n = 1409 |
UMDEO team n = 68 |
|
|---|---|---|
| Gender, n (%) | ||
| Male | 744 (53) | 32 (47) |
| Female | 658 (47) | 36 (53) |
| Mean Age ± SD | 65 ± 11 | 54 ± 12 |
| Distance km | – | |
| (home-vaccination center) | ||
| less than1km | 68 (5) | – |
| 1-5 km | 316 (23) | – |
| 5-10 km | 435 (31) | – |
| 10-20 km | 385 (27) | – |
| greater than20 km | 193 (14) | – |
Concerning the usefulness of UMDEO in the vaccination operation, 93.2% of patients (n = 1307) strongly agreed that the mobile unit increased access to vaccination and 91.3% (n = 1281) strongly believed that it would speed up the vaccination campaign, 1362 (97%) people perceived the vaccination site to be hygienic. 1306 (98.8%) of the respondents considered the mobile unit easily accessible. These results are found in Table 2 . The mean global satisfaction with the service was 9.5 +/- 0.8 out of 10, with a result of 9.5 +/−0.03 for men as for female. The satisfaction score for patients less than 50 years old, between 50 and 74 years old and of 75 years old and more was respectively 9.7+/−0.7, 9.5+/−0.8 and 9.4+/−0.9.
Table 2.
Evaluation of the mobile decontamination unit by the vaccinated population.
| Questions | Strongly Agree n (%) |
Somewhat Agree n (%) |
Slightly Agree n (%) |
Slightly Disagree n (%) |
Somewhat Disagree n (%) |
Strongly Disagree n (%) |
|---|---|---|---|---|---|---|
| Improved access to vaccination | 1307 (93.2) | 76 (5.4) | 3 (0.2) | 1 (0.1) | 1 (0.1) | 15 (1.1) |
| Acceleration of the vaccination campaign | 1281 (91.3) | 103 (7.3) | 3 (0.2) | 1 (0.1) | 15 (1.1) | 0 |
| Respect of sanitary measures | 109 (78.9) | 253 (18) | 23 (1.6) | 13 (0.9) | 4 (0.3) | 3 (0.2) |
| Respect of confidentiality | 1142 (86.2) | 166 (12.5) | 9 (0.7) | 0 | 4 (0.3) | 3 (0.3) |
| Facility of access | 1142 (86.5) | 163 (12.3) | 8 (0.6) | 1 (0.1) | 2 (0.2) | 4 (0.3) |
| Satisfaction of the reception | 1238 (93.8) | 69 (5.2) | 6 (0.5) | 0 | 1 (0.1) | 6 (0.5) |
| Quality of information | 1189 (90.1) | 110 (8.3) | 10 (0.8) | 0 | 6 (0.4) | 5 (0.4) |
Results are expressed as frequence and percentage.
3.2. The UMDEO team survey
Among the 85 people who made up the UMDEO team, 68 (80%) answered the questionnaire. The mean age of the teammates were 53.6 ± 12.1 years and 52.9% (n = 36) were women. Sixty-seven (98.5%) medical and paramedical staff felt that access to vaccination would be enhanced through the mobile unit (UMDEO) and 65 (95.6%) were convinced that this would speed up the mass vaccination campaign. Concerning the organization of the site and the respect of sanitary measures, 64 (94.4%) respondents found that the hygiene conditions were adhered to. 10 workers (14.7%) found the unit uneasily accessible. Finally, 56 (82.4%) of the team felt that confidentiality was respected for those vaccinated. The average global satisfaction was 8.8 ± 1.1 out of 10 (Table 3 ).
Table 3.
Evaluation of the mobile decontamination unit by the UMDEO staff.
| Questions | Strongly Agree n (%) |
Somewhat Agree n (%) |
Slightly Agree n (%) |
Slightly Disagree n (%) |
Somewhat Disagree n (%) |
Strongly Disagree n (%) |
|---|---|---|---|---|---|---|
| Improved access to vaccination | 55 (80.9) | 12 (17.7) | 1 (1.5) | 0 | 0 | 0 |
| Acceleration of the vaccination campaign | 53 (77.9) | 12 (17.7) | 3 (4.4) | 0 | 0 | 0 |
| Respect of sanitary measures | 36 (52.9) | 28 (41.5) | 2 (2,9) | 1 (1.5) | 1 (1.5) | 0 |
| Respect of confidentiality | 28 (41.2) | 28 (41.5) | 8 (11.8) | 3 (4.4) | 0 | 1 (1.5) |
| Facility of access | 30 (44.1) | 24 (35.3) | 10 (14.7) | 1 (1.5) | 2 (2.9) | 1 (1.5) |
Results are expressed as frequence and percentage.
4. Discussion
To our knowledge, this is the first description of a mobile decontamination unit used as a mass vaccination center. This tool initially planned for decontamination was diverted for a mass vaccination operation. Its functionalities were not designed for a vaccination campaign, but with no modifications to the unit itself, the team was able to vaccinate almost 100 patients per hour in this versatile unit. The vast majority of study participants (93 %) stated that the mobile vaccination unit greatly improved access to vaccination. Over the course of 2 days, more than 1400 people were vaccinated. Moreover, vaccinations were accessible to all eligible volunteers without the need to make an appointment.
Mass vaccination campaigns offer vaccinations over time-limited periods to provide protection rapidly and efficiently to a maximum numbers of susceptible persons [18], for preventing emerging outbreaks [19], and for accelerating disease control programs [20], [21]. The advantage of using mobile versatile decontamination units such as UMDEO is that they can be set up in a variety of locations, both in cities with a large population needing improved access to vaccinations, and in more remote rural areas where access to vaccination units may be more limited [22]. These mobile units make it possible to vaccinate people with disabilities or reduced mobility safely. In our study, 1306 people found access to and within the mobile unit easy and convenient.
The global satisfaction rating with the decontamination unit for vaccinations was 9.5/10 in the vaccinated population. Patients and caregivers were overall very satisfied with accessibility, hygiene, and confidentiality in the unit. The majority believed that it can serve as a vaccination center during this and future mass vaccination campaigns, enabling rapid mass immunization through a “front-end vertical flow model”[16], [17], while respecting hygiene and distancing measures and facilitating access for people with reduced mobility or living in a non-urban environment. The “front-end” principle that was applied guides the flow gradually from one stage to another; where registration, questionnaire filling, vaccination administration and then monitoring are carried out. This facilitates a constant throughput of patients on an hourly basis promoting efficiency. This model is largely used in emergency departments to decrease delay in care and improves patient satisfaction even in cases of crowding [23]. These mobile units should therefore continue to be deployed in the context of vaccination campaigns, and can form an integral part of the government's planned vaccination strategy.
Furthermore, it should be noted that 86.5% of the staff felt that the mobile unit would increase access to vaccination and 77.4% felt that it would accelerate the vaccination campaign. Although there was room for improvement in hygiene measures, organization, access and equipment, the idea of a mobile vaccination unit using this decontamination unit as a test case could be an important means of encouraging vaccination in this and future pandemics.
5. Conclusion
Toulouse is currently the only city to have used a mobile decontamination unit as a mass vaccination site. However, the success of this deployment could be used as a basis for the use of other mobile units to increase vaccination accessibility and reach the government's planned targets. The majority of the patients vaccinated as well as the team participating in the survey were satisfied with the usefulness of UMDEO as a vaccination center. Moreover, as it can be set up quickly and on many different sites, it allows for the possibility to reach and vaccinate a large and varied population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank all the staff participating in this mass vaccination campaign, the vaccinated population and the Louis Lareng association which financed UMDEO shelter by a donation to the SAMU 31.
References
- 1.D. Hosangadi, M. P. Shearer, K. L. Warmbrod, L. Kan, M. Cantu, et J. B. Nuzzo, Current State of Mass Vaccination Preparedness and Operational Challenges in the United States, 2018-2019, Health Security, vol. 18, no 6, p. 473‑482, oct. 2020, doi: 10.1089/hs.2019.0146. [DOI] [PubMed]
- 2.S. A. Plotkin, Éd., Mass vaccination: global aspects, progress and obstacles ; with 40 figures and 27 tables. Berlin: Springer, 2006.
- 3.N. Lurie, M. Saville, R. Hatchett, et J. Halton, Developing Covid-19 Vaccines at Pandemic Speed, N Engl J Med, vol. 382, no 21, p. 1969‑1973, mai 2020, doi: 10.1056/NEJMp2005630. [DOI] [PubMed]
- 4.D. L. Heymann et R. B. Aylward, Mass vaccination: when and why Curr Top Microbiol Immunol, vol. 304, p. 1‑16, 2006, doi: 10.1007/3-540-36583-4_1. [DOI] [PubMed]
- 5.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asgary A., Najafabadi M.M., Karsseboom R., Wu J. A Drive-through Simulation Tool for Mass Vaccination during COVID-19 Pandemic. Healthcare (Basel) 2020;8(4) doi: 10.3390/healthcare8040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D. Hosangadi et al., Enabling emergency mass vaccination: Innovations in manufacturing and administration during a pandemic, Vaccine, vol. 38, no 26, p. 4167‑4169, mai 2020, doi: 10.1016/j.vaccine.2020.04.037. [DOI] [PMC free article] [PubMed]
- 8.E. Goralnick, C. Kaufmann, et A. A. Gawande, Mass-Vaccination Sites — An Essential Innovation to Curb the Covid-19 Pandemic, New England Journal of Medicine, vol. 0, no 0, p. null, mars 2021, doi: 10.1056/NEJMp2102535. [DOI] [PubMed]
- 9.M. Rahi et A. Sharma, Mass vaccination against COVID-19 may require replays of the polio vaccination drives, EClinicalMedicine, vol. 25, p. 100501, août 2020, doi: 10.1016/j.eclinm.2020.100501. [DOI] [PMC free article] [PubMed]
- 10.S. Bagcchi, The world’s largest COVID-19 vaccination campaign, Lancet Infect Dis, vol. 21, no 3, p. 323, mars 2021, doi: 10.1016/S1473-3099(21)00081-5. [DOI] [PMC free article] [PubMed]
- 11.Mills Melinda C., Salisbury David. The challenges of distributing COVID-19 vaccinations. E Clin Med. 2021;31:100674. doi: 10.1016/j.eclinm.2020.100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.info coronavirus COVID 19 - Vaccins, Gouvernement.fr. https://www.gouvernement.fr/info-coronavirus/vaccins (consulté le mars 22, 2021).
- 13.Covid: la campagne de vaccination en France, Les Echos, mars 05, 2021. https://www.lesechos.fr/politique-societe/societe/covid-la-campagne-de-vaccination-en-france-1266158 (consulté le mars 22, 2021).
- 14.P. Ribordy et al., Mobile decontamination units-room for improvement?, Prehosp Disaster Med, vol. 27, no 5, p. 425‑431, oct. 2012, doi: 10.1017/S1049023X12001033. [DOI] [PubMed]
- 15.T. L. Hudson, K. Reilly, et J. Dulaigh, Considerations for chemical decontamination shelters, Disaster Manag Response, vol. 1, no 4, p. 110‑113, déc. 2003, doi: 10.1016/j.dmr.2003.10.001. [DOI] [PubMed]
- 16.D. Pateron, Une organisation des flux au sein des urgences, Ann. Fr. Med. Urgence, vol. 3, no 2, p. 69‑70, mars 2013, doi: 10.1007/s13341-013-0294-1.
- 17.M. D. Repplinger et al., The Impact of an Emergency Department Front-End Redesign on Patient-Reported Satisfaction Survey Results, West J Emerg Med, vol. 18, no 6, p. 1068‑1074, oct. 2017, doi: 10.5811/westjem.2017.7.33664. [DOI] [PMC free article] [PubMed]
- 18.Frederiksen L.S.F., Zhang Y., Foged C., Thakur A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front Immunol. 2020;11:juill. doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J. D. Grabenstein et R. L. Nevin, Mass immunization programs: principles and standards, Curr Top Microbiol Immunol, vol. 304, p. 31‑51, 2006, doi: 10.1007/3-540-36583-4_3. [DOI] [PubMed]
- 20.P. Cavailler et al., Feasibility of a mass vaccination campaign using a two-dose oral cholera vaccine in an urban cholera-endemic setting in Mozambique, Vaccine, vol. 24, no 22, p. 4890‑4895, mai 2006, doi: 10.1016/j.vaccine.2005.10.006. [DOI] [PubMed]
- 21.G. Deceuninck, B. Lefebvre, R. Tsang, J. F. Betala-Belinga, G. De Serres, et P. De Wals, Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch, Vaccine, vol. 37, no 31, p. 4243‑4245, juill. 2019, doi: 10.1016/j.vaccine.2019.06.021. [DOI] [PubMed]
- 22.S. Chen et al., Fangcang shelter hospitals: a novel concept for responding to public health emergencies, Lancet, vol. 395, no 10232, p. 1305‑1314, avr. 2020, doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed]
- 23.J. L. Wiler et al., Optimizing emergency department front-end operations, Ann Emerg Med, vol. 55, no 2, p. 142-160.e1, févr. 2010, doi: 10.1016/j.annemergmed.2009.05.021. [DOI] [PubMed]