Abstract
Background
There is a lack of knowledge about the real incidence of acute coronary syndrome (ACS) in patients with COVID-19, their clinical characteristics, and their prognoses.
Objective
We investigated the incidence, clinical characteristics, risk factors, and outcomes of ACS in patients with COVID-19 in the emergency department.
Methods
We retrospectively reviewed all COVID-19 patients diagnosed with ACS in 62 Spanish emergency departments between March and April 2020 (the first wave of COVID-19). We formed 2 control groups: COVID-19 patients without ACS (control A) and non–COVID-19 patients with ACS (control B). Unadjusted comparisons between cases and control subjects were performed regarding 58 characteristics and outcomes.
Results
We identified 110 patients with ACS in 74,814 patients with COVID-19 attending the ED (1.48% [95% confidence interval {CI} 1.21–1.78%]). This incidence was lower than that observed in non–COVID-19 patients (3.64% [95% CI 3.54–3.74%]; odds ratio [OR] 0.40 [95% CI 0.33–0.49]). The clinical characteristics of patients with COVID-19 associated with a higher risk of presenting ACS were: previous coronary artery disease, age ≥60 years, hypertension, chest pain, raised troponin, and hypoxemia. The need for hospitalization and admission to intensive care and in-hospital mortality were higher in cases than in control group A (adjusted OR [aOR] 6.36 [95% CI 1.84–22.1], aOR 4.63 [95% CI 1.88–11.4], and aOR 2.46 [95% CI 1.15–5.25]). When comparing cases with control group B, the aOR of admission to intensive care was 0.41 (95% CI 0.21–0.80), while the aOR for in-hospital mortality was 5.94 (95% CI 2.84–12.4).
Conclusions
The incidence of ACS in patients with COVID-19 attending the emergency department was low, around 1.48%, but could be increased in some circumstances. Patients with COVID-19 with ACS had a worse prognosis than control subjects with higher in-hospital mortality.
Keywords: acute coronary syndrome, clinical characteristics, COVID-19, incidence, outcome, risk factors, SARS-Cov-2
Introduction
COVID-19 is a novel disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In March 2020, the World Health Organization declared COVID-19 a pandemic, with >255 million confirmed cases of COVID-19 and 5,127,696 deaths being declared by November 21, 2021 (1).
Symptomatic patients with COVID-19 mainly present with fever and respiratory symptoms, with dyspnea and lung infiltrates being present in >50% of hospitalized patients (2). However, a significant number of other features can also be present, and there is growing concern about cardiovascular system involvement. COVID-19 has been related to acute coronary syndrome (ACS), acute myocardial injury, myocarditis, stress cardiomyopathy and dysrhythmias (2, 3, 4). COVID-19 causes a proinflammatory and prothrombotic state, which can trigger ACS (5). Furthermore, an association has been reported between the severity of COVID-19 infection and several heart conditions, such as coronary artery disease, hypertension, and diabetes (6,7). On the other hand, some studies have found a decline in hospitalization rates for ACS, and admissions for most diagnoses decreased by approximately 50% in the first wave of the COVID-19 pandemic between March and April 2020 (8,9). In this scenario, there is a lack of knowledge about the real incidence of ACS in patients with COVID-19, their clinical characteristics, and their prognoses.
Taking into account all these gaps, we designed the current study with the following specific objectives: 1) to determine the frequency of ACS in patients with COVID-19; 2) to describe whether there is any distinctive clinical characteristic in these patients compared with COVID-19 patients without ACS and ACS patients without COVID-19; and 3) to investigate the outcomes of patients with COVID-19 who also have ACS.
Methods
Study Design and Setting
The present study forms part of the Unusual Manifestations of COVID-19 (UMC-19) project, which was designed to investigate the potential relationships between COVID-19 and 10 different entities that could be influenced by SARS-Cov-2 infection itself because of the publication of ≥1 case with such manifestations at the time of project design, suggesting a potential link with this viral infection. The main object'ives of the UMC-19 project were common for all entities, and consisted in the description of the incidence, clinical characteristics, risk factors, and outcomes for each particular entity (cases), using as comparators patients with COVID-19 who did not develop this entity (control group A) as well as patients without COVID-19 who presented with this entity (control group B). Complete details of the UMC-19 project have been published elsewhere (10,11).
In Spain, the first case of SARS-Cov-2 infection was detected on January 31, 2020, and accordingly the definition of the COVID-19 period for patient inclusion in the present study was set from March 1 to April 30, 2020. During this 61-day period, 213,435 cases of COVID-19 were confirmed by the Spanish Ministry of Health (12). For the recruitment of non-COVID control subjects, the UMC-19 project selected patients from 2 different periods: one corresponding to the same dates as the cases (March 1–April 30, 2020) and the other corresponding to the same period of the previous year (March 1–April 30, 2019).
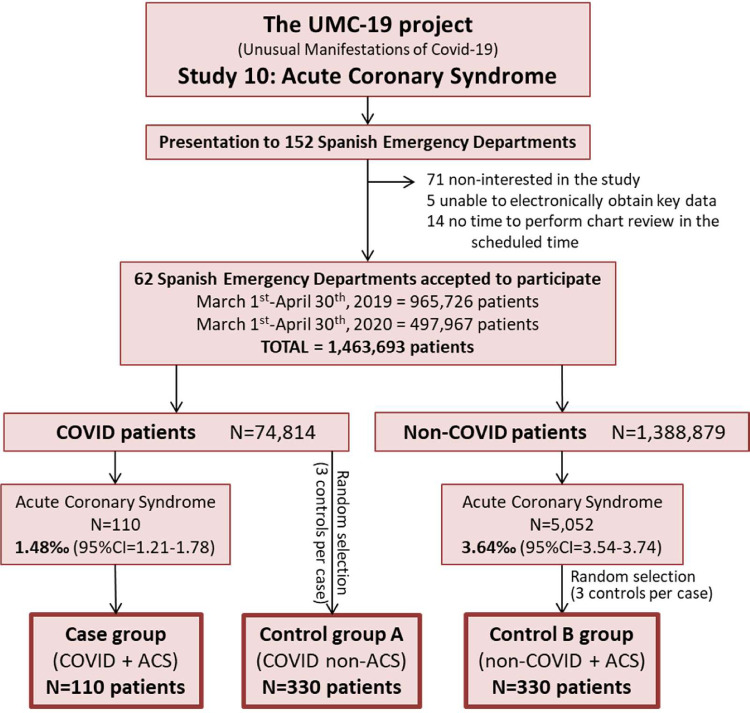
The investigators of the UMC-19 project initially contacted 152 Spanish emergency departments (EDs), which roughly constitute half of the 312 hospital EDs of the Spanish public health network. Of these, 81 were willing to participate and analyzed the protocol, and 62 consented to participate and duly sent all the required data (Figure 1 ). These 62 hospitals provide health coverage to 15.1 million citizens (32% of the population of 46.9 million in Spain) and make up a balanced representation of the Spanish territory (representing 12 of 17 Spanish autonomous communities), type of hospital (community, reference, and high-technology university hospitals were included), and involvement in the pandemic (with EDs attending from 1–47% of the ED census corresponding to COVID-19 patients during the COVID-19 outbreak period).
Figure 1.
Study design and patient inclusion flow chart. ACS = acute coronary syndrome.
The investigation of ACS in patients with COVID-19, one of the entities included in the UMC-19 project, was labeled the UMC-19 Study 10 (UMC-19-S10) and consisted of a retrospective, case-control, ED-based, multicenter study that reviewed the medical reports of patients with COVID-19 who were diagnosed with ACS during ED assessment and managed in Spanish EDs before hospitalization.
Cases and Controls of the UMC-19-S10
The case group was formed by patients with COVID-19 who were diagnosed with ACS at ED presentation based on the medical records and their review by the principal investigator of each center without external review. ACS included patients with suspicion or confirmation of acute myocardial ischemia or infarction (myocardial infarction and unstable angina). The definition of myocardial infarction was according to the 4th Universal Definition of Myocardial Infarction (13). A diagnosis of COVID-19 was accepted on the basis of SARS-Cov-2 antigen detection in nasopharyngeal swab by reverse transcriptase polymerase chain reaction and a clinically compatible clinical picture (including at least malaise, fever, and cough) or the presence of typical lung parenchymal infiltrates on a chest radiograph (bilateral interstitial lung infiltrates and ground-glass infiltrates) in patients with some clinical symptoms attributable to COVID-19.
We defined 2 different control groups. One group was made up of patients with COVID-19 without ACS attending the ED during the same period of the COVID-19 outbreak (March 1–April 30, 2020), hereafter referred to as the non–ACS-COVID-19 or control group A. This group was formed by selecting 3 patients with COVID-19 for every case detected by each center. Selection was performed randomly from the full list of patients with this final diagnosis after complete patient assessment in the ED and by cardiologists. Control group A was specifically designed to uncover the risk factors for ACS development in patients with COVID-19. The second control group was made up of all non–COVID-19 patients with a diagnosis of ACS attending the ED during the same period (March 1–April 30, 2020) and was defined in the same terms as the cases. To avoid the possibility that some of these control cases could eventually have inadvertent infection by SARS-Cov-2, in this group we also included all patients with ACS diagnosed in the ED from March 1 to April 30, 2019, just 1 year before the COVID-19 pandemic. This group is hereafter named the ACS-non–COVID-19 or control group B. Control group B was specifically designed to uncover the specific distinctive clinical characteristics of ACS developed in patients with COVID-19 with respect to ACS developed in the general population. For patients with ACS, we also recorded the diagnostic tests used for diagnosis and the final classification as type I myocardial infarction, type II myocardial infarction, or angina pectoris.
Case and control definitions are summarized in Supplemental Table 1.
Independent Variables
We collected 36 independent variables, which included 2 demographic data (age and sex), 12 comorbidities (chronic obstructive pulmonary disease, asthma, active smoker, hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, obesity [clinically estimated], cerebrovascular disease, chronic kidney disease [creatinine >2 mg/dL], dementia, and active cancer), 16 signs and symptoms recorded at ED arrival (time elapsed from symptom onset to ED attendance, fever, cough, dyspnea, chest pain, syncope, abdominal pain, vomiting, diarrhea, confusion, headache, anosmia or dysgeusia, temperature, systolic blood pressure, heart rate, hypoxemia [pulsioximetry <96%]), and 6 laboratory parameters (cardiac troponin, C-reactive protein, creatinine, hemoglobin, lymphocytes, and D-dimer).
Outcomes
We defined 4 different outcomes for cases and control subjects: 1) the need for hospitalization; 2) the need for admission to the intensive care unit [ICU]; 3) in-hospital mortality; and 4) diagnostic tests (electrocardiogram, echocardiogram, coronary stress test, coronary scan, and invasive cardiac catheterization) performed in patients with COVID-19 and ACS and non–COVID-19 patients with ACS.
Statistical Analysis
Discrete variables were expressed as absolute values and percentages, and continuous variables as means and standard deviations (SDs). Frequencies were expressed per thousand (%) cases or control subjects, with 95% confidence intervals (CIs). The relative frequency of ACS was expressed per thousand (%) of COVID-19 or non–COVID-19 patients coming to the ED, and the incidence was expressed per 100,000 COVID-19 or non–COVID-19 individuals per year. To estimate the COVID-19 and non-COVID-19 population in each ED catchment area, we used the seroprevalence of SARS-CoV-2 in the province where the ED was located. These detailed seroprevalences were determined in a wide Spanish study performed from April 27 to May 11, 2020 (14). Estimations of relative frequencies and annual incidences were made with 95% CIs calculated using the exact method for binomial distributions.
Differences between the case and the control groups were assessed by the Chi-square test (or Fisher exact test if needed) for qualitative variables and the Student t test for quantitative variables. The magnitude of associations was expressed as unadjusted odds ratio (OR) with 95% CI, using logistic regression, with previous dichotomization of the statistically significant continuous variables using clinically meaningful cutoffs. For calculations of adjusted OR (aOR), missing values in the independent variables were replaced using the multiple imputation technique provided by SPSS software (version 24; IBM, Armonk, NY), generating 5 datasets in which there are no misses among all the variables included in the adjustment.
Statistical significance was accepted in all comparisons if the p value was < 0.05 or if the 95% CI of the risk estimations excluded the value 1. The analyses were performed with the SPSS statistical software package.
Ethics
The UMC-19 project was approved by the Ethics Committee of the Hospital Clínic of Barcelona (Spain), which acted as the central ethical committee, with reference number HCB/2020/0534.
Results
A total of 74,814 patients with COVID-19 were attended in the 62 Spanish EDs participating in the UMC-19-S6 (Figure 1) during the 61-day study period. One hundred ten of these patients presented with ACS (frequency 1.48% [95% CI 1.21–1.78%) and constituted the case group. Control group A was formed by 330 randomly selected patients with COVID-19 without ACS during the same period. COVID-19 infection was confirmed by positive reverse transcriptase polymerase chain reaction results in nasopharyngeal swab in 89 cases (80.9%) and 242 control A patients (73.3%; p = 0.11). On the other hand, 1,388,879 non–COVID-19 patients were seen during the 122-day period (962,726 during the 61 days in the 2020 COVID-19 period and 423,153 during the 61 days in the 2019 pre–COVID-19 period), and 5052 diagnoses of ACS were made in non-COVID patients (frequency 3.64% [95% CI 3.54–3.74]), 3388 in 2019, and 1664 in 2020. These patients constituted control group B.
We found a significantly lower prevalence of ACS in the COVID-19 group compared with the non–COVID-19 group (1.48% vs. 3.64%; OR 0.40 [95% CI 0.33–0.49]). On the other hand, the overall annual standardized incidences of ACS were 92.7 per 100,000 patients with COVID-19 and year (95% CI 85.8–100.0) and 102.8 per 100,000 non–COVID-19 individuals and year (95% CI 101.2–104.5, with partial standardized annual incidences of 69.8 in the 2020 COVID-19 period and 134.7 in the 2019 pre–COVID-19 period). Accordingly, the OR for the standardized annual incidence of ACS in patients with COVID-19 compared with non–COVID-19 patients was 0.90 (95% CI 0.83–0.97; OR compared with 2020 COVID-19 period of 1.33 [95% CI 1.23–1.44]; OR compared with 2019 pre–COVID-19 period of 0.69 [95% CI 0.64–0.74]). Otherwise, the OR for the standardized annual incidence of ACS in non–COVID-19 patients during 2020 with respect to 2019 was 0.52 (95% CI 0.51–0.53).
The mean age of patients with COVID-19 with ACS (cases) was 74 years, 70% were male, and the most frequent comorbidities were hypertension (78%), dyslipidemia (55%), previous coronary artery disease (42%), and diabetes mellitus (30%). The remaining baseline characteristics are shown in Table 1. The most frequent symptomatology was dyspnea (66%), chest pain (62%), fever (41%), and cough (38%). However, it should be highlighted that 41 (37%) patients did not have chest pain. The median time from first symptom onset to ED consultation was 3 days. The remaining clinical characteristics, as well as the vitals at ED arrival and the laboratory findings, are shown in Table 2 .
Table 1.
Baseline Characteristics of Patients With COVID-19 With ACS and Comparison With Patients With COVID-19 Without ACS (Control Group A) and With Patients Without COVID-19 With ACS (Control Group B)
| Cases (COVID-19 and ACS), n = 110 | Control Group A (COVID-19 and Non-ACS), n = 330 | Control Group B (Non-COVID-19 and ACS), n = 330 | p Value* | p Value† | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 74 (13) | 63 (18) | 67 (14) | <0.001‡ | <0.001‡ |
| ≥60 years, n (%) | 95 (86.4) | 196 (59.4) | 231 (70.0) | <0.001‡ | 0.001‡ |
| Female, n (%) | 33 (30.00) | 156 (47.27) | 98 (29.70) | 0.002‡ | 0.95 |
| Pulmonary comorbidities, n (%) | |||||
| COPD | 15 (13.64) | 28 (8.48) | 40 (12.12) | 0.12 | 0.68 |
| Asthma | 4 (3.64) | 23 (6.97) | 10 (3.03) | 0.21 | 0.75 |
| Active smoker | 11 (10.00) | 22 (6.67) | 80 (24.61) | <0.001‡ | 0.002‡ |
| Other comorbidities, n (%) | |||||
| Hypertension | 86 (78.18) | 150 (45.45) | 212 (24.24) | <0.001‡ | 0.007‡ |
| Dyslipidemia | 61 (55.45) | 110 (33.33) | 166 (50.30) | <0.001‡ | 0.35 |
| Diabetes mellitus | 33 (30) | 57 (17.27) | 108 (32.73) | 0.004‡ | 0.57 |
| Coronary artery disease | 47 (42.73) | 25 (7.58) | 92 (27.88) | <0.001‡ | 0.004‡ |
| Obesity (clinically estimated) | 19 (17.27) | 51 (15.45) | 74 (22.42) | 0.65 | 0.25 |
| Cerebrovascular disease | 14 (12.73) | 23 (6.97) | 19 (5.79) | 0.06 | 0.016‡ |
| Chronic kidney disease | 17 (15.45) | 21 (6.36) | 38 (11.52) | 0.003‡ | 0.28 |
| Dementia | 10 (9.09) | 29 (8.79) | 17 (5.15) | 0.92 | 0.14 |
| Active cancer | 13 (11.82) | 31 (9.39) | 46 (13.94) | 0.46 | 0.57 |
Values refer to comparison between cases and control group A.
Values refer to comparison between cases and control group B.
Statistically significant (p < 0.05).
ACS = acute coronary syndrome; COPD = chronic obstructive pulmonary disease.
Table 2.
Clinical, Analytical and Radiological Characteristics of the Acute Episode in Patients With ACS and Comparison With Patients With COVID-19 Without ACS (Control Group A) and With Patients Without COVID-19 With ACS(Control Group B)
| Cases (COVID-19 and ACS), n = 110 | Control Group A (COVID-19 and Non-ACS), n = 330 | Control Group B (Non-COVID-19 and ACS), n = 330 | p Value* | p Value† | |
|---|---|---|---|---|---|
| Symptoms at ED arrival, n (%) | |||||
| Duration of symptoms (days), median (IQR) | 3 (1, 2, 3, 4, 5, 6, 7) | 7 (3, 4, 5, 6, 7, 8, 9, 10) | 1 (0–3) | <0.001‡ | <0.001‡ |
| Lasting ≥3 days | 47 (42.7) | 243 (73.6) | 57 (17.3) | <0.001‡ | <0.001‡ |
| Fever | 46 (41.82) | 193 (58.48) | 4 (1.21) | 0.002‡ | <0.001‡ |
| Cough | 42 (38.18) | 191 (57.88) | 9 (2.73) | <0.001‡ | <0.001‡ |
| Dyspnea | 73 (66.36) | 182 (55.15) | 88 (26.67) | 0.039‡ | <0.001‡ |
| Chest pain | 69 (62.73) | 42 (12.73) | 285 (86.36) | <0.001‡ | <0.001‡ |
| Syncope | 8 (7.27) | 14 (4.24) | 21 (6.36) | 0.21 | 0.74 |
| Abdominal pain | 7 (6.36) | 17 (5.15) | 19 (5.76) | 0.63 | 0.82 |
| Vomiting | 8 (7.27) | 24 (7.27) | 31 (9.39) | 1 | 0.49 |
| Diarrhea | 13 (11.82) | 54 (16.36) | 5 (1.52) | 0.25 | <0.001‡ |
| Confusion | 11 (10.00) | 25 (7.58) | 13 (3.94) | 0.42 | 0.015‡ |
| Headache | 8 (7.27) | 39 (11.82) | 4 (1.21) | 0.18 | 0.001‡ |
| Anosmia or dysgeusia | 3 (2.7) | 32 (9.7) | 2 (0.6) | 0.02‡ | 0.07 |
| Signs at ED arrival | |||||
| Fever (>37.3°C) | 29 (26.6) | 76 (23.5) | 4 (1.2) | 0.52 | <0.001‡ |
| Hypotension (<90 mm Hg) | 6 (5.5) | 7 (2.2) | 12 (3.6) | 0.08‡ | 0.40 |
| Tachycardia (>100 beats/min) | 18 (16.4) | 72 (22.3) | 44 (13.4) | 0.19 | 0.44 |
| Hypoxemia (pulse oximetry <96%) | 65 (59.6) | 148 (45.5) | 94 (28.5) | 0.01‡ | <0.001‡ |
| Laboratory findings, mean (SD) | |||||
| Raised troponin (>99th percentile) | 90 (85.7) | 28 (24.1) | 274 (86.4) | <0.001‡ | 0.85 |
| Creatinine >1.3 mg/dL) | 31 (28.4) | 43 (14.2) | 68 (21.3) | 0.001‡ | 0.12 |
| Hemoglobin <120 g/L | 34 (31.8) | 52 (17.2) | 65 (20.0) | 0.001‡ | 0.012‡ |
| Lymphocytes count <1000 cells/μL | 51 (48.6) | 112 (39.0) | 45 (15.0) | 0.09 | <0.001‡ |
| C-reactive protein >5 mg/dL | 49 (53.8) | 157 (55.1) | 32 (19.0) | 0.84 | <0.001‡ |
| D-dimer >500 ng/mL | 60 (72.3) | 150 (60.0) | 19 (20.4) | 0.04 | <0.001‡ |
Values refer to comparison between cases and control group A.
Values refer to comparison between cases and control group B.
Statistically significant (p < 0.05).
ACS = acute coronary syndrome; ED = emergency department; IQR = interquartile range; SD = standard deviation.
When cases were compared with control subjects, some statistically significant differences were found. In summary, cases compared with control group A (non-ACS–COVID-19) were older, predominantly male, and had a higher frequency of cardiovascular risk factors (smoking, hypertension, diabetes, and dyslipidemia), previous coronary disease, and chronic renal failure. Regarding the clinical findings at ED arrival, the symptoms of the cases were shorter lasting, and they less frequently had fever, cough and dyspnea, anosmia or dysgeusia, but more frequently presented with chest pain, hypotension, and hypoxemia. Regarding the laboratory findings, cases more frequently had raised troponin, creatinine, and D-dimer but lower hemoglobin values (Table 2). On the other hand, cases compared with control group B (ACS-non–COVID-19) were older, with a lower frequency of active smokers and a higher frequency of hypertension, coronary artery disease, and cerebrovascular disease. Regarding the clinical findings at ED arrival, the cases had longer lasting symptoms, a higher frequency of respiratory symptoms, diarrhea, confusion, headache, and a lower frequency of chest pain, and more frequently had fever and hypoxemia (Table 2). Some of these statistically significant differences remained in the adjusted analysis (Table 3 ). When cases were compared with control group A, the risk factors of ACS were previous coronary artery disease, age ≥60 years, hypertension, chest pain, raised troponin, and hypoxemia; patients with symptoms lasting <3 days had lower risk. On comparing cases with control group B, the risk factors of presenting ACS were fever, diarrhea, cough, dyspnea, and lymphopenia.
Table 3.
Magnitude of Statistically Significant Association Found in the Adjusted Analysis
| Risk Factors to Develop ACS in Patients With COVID-19 Compared With Control A (COVID-19 Patients Not Developing ACS) | OR (95% CI) |
|---|---|
| Compared with baseline characteristics | |
| Coronary artery disease | 5.86 (3.14–10.95) |
| Age ≥60 years | 2.34 (1.19–4.62) |
| Hypertension | 2.15 (1.16–3.98) |
| Compared with clinical characteristics of the episode | |
| Chest pain | 16.22 (8.49–31.02) |
| Raised troponin (>99th percentile) | 4.93 (2.32–10.46) |
| Hypoxemia (pulse oximetry <96%) | 2.33 (1.19–4.56) |
| Symptoms lasting >3 days | 0.35 (0.19–0.64) |
| Characteristics of ACS in patients with COVID-19 compared with control B (ACS in non–COVID-19 patients) | |
| Compared with baseline characteristics | |
| None achieved statistical significance in the adjusted model) | — |
| Compared with clinical characteristics of the episode | |
| Fever (>37.3°C) | 13.70 (3.87–48.53) |
| Diarrhea | 6.38 (1.45–28.08) |
| Cough | 6.09 (2.25–16.49) |
| Dyspnea | 2.53 (1.31–4.87) |
| Lymphopenia (<1000 µL/mL) | 2.40 (1.20–4.79) |
The number of patients presenting the baseline and current episode conditions in each group can be found in Table 1.
ACS = acute coronary syndrome; CI = confidence interval; OR = odds ratio.
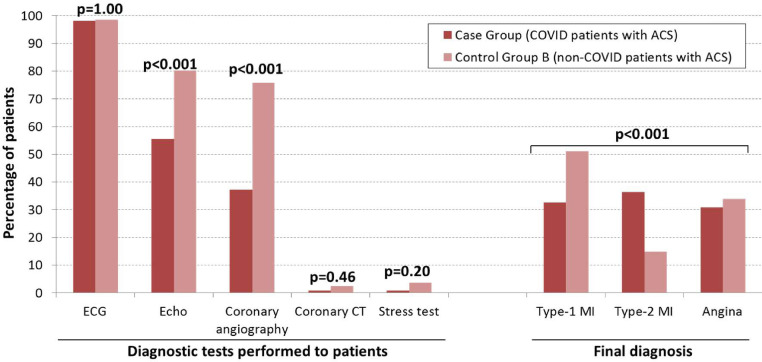
Regarding the diagnostic tests for ACS (Figure 2 ), the cases less frequently underwent echocardiography and invasive coronary angiography compared with control group B patients. Coronariography using computerized tomography and stress tests were seldom performed (1.2% and 1.9%, respectively), with no differences between the 2 groups. The final diagnosis of ACS included a significantly lower proportion of type I myocardial infarction and a higher proportion of type II myocardial infarction in patients with COVID-19, while the proportion of angina at diagnosis was similar (Figure 2).
Figure 2.
Diagnostic tests for acute coronary syndrome and final diagnosis. ACS = acute coronary syndrome; CT = computed tomography; echo = echocardiography; MI = myocardial infarction.
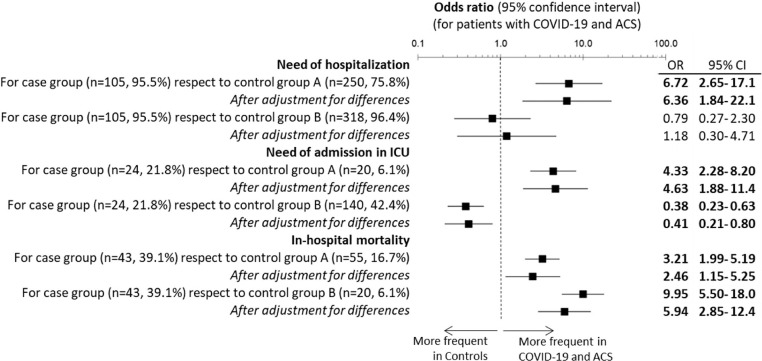
Patients with COVID-19 with ACS were hospitalized in 95.5% of cases; 21.8% were admitted to intensive care at some point during their hospital stay, and 39.1% died during hospitalization. All the outcomes measured were worse in the cases than in control group A (Figure 3 ). Specifically, patients with COVID-19 with ACS had an aOR for need for hospitalization of 6.36 (95% CI 1.84–22.1), an aOR for need for admission to the ICU of 4.63 (95% CI 1.88–11.4), and an aOR for in-hospital mortality of 2.46 (95% CI 1.15–5.25). On the other hand, when comparing cases with control group B, the aOR for admission to the ICU was 0.41 (95% CI 0.21–0.80), while the aOR for in-hospital mortality was 5.94 (95% CI 2.84–12.4).
Figure 3.
Outcomes of patients with COVID-19 and acute coronary syndrome (ACS) compared with controls. Cases were patients with COVID-19 who were diagnosed with ACS at emergency department (ED) presentation. Control group A includes patients with COVID-19 without ACS attending the ED during the same period (March 1–April 30, 2020). Control group B includes non–COVID-19 patients with a diagnosis of ACS during the same period (March 1–April 30, 2020) and also for the same period of the previous year (March 1–April 30, 2019). Numbers denote statistical significance (p < 0.05). The multivariate analysis was adjusted for all significant variables. CI = confidence interval; ICU = intensive care unit; OR = odds ratio.
Discussion
The first relevant finding of UMC-19-S10 is that the frequency of ACS in patients with COVID-19 coming to the ED was lower than that of ACS in non–COVID-19 patients, with an OR of 0.40. Even taking into account that non-COVID-19 patients less frequently visited the ED than in previous years (the OR for 2019 respect to 2020 was 0.51, in line with previous literature), the extrapolation of these frequencies to standardized annual incidences showed that ACS in patients with COVID-19 compared with non–COVID-19 patients was not increased either (OR 0.90) (15,16). This finding contradicts previous studies that demonstrated an increased incidence of ACS in patients with COVID-19 patients and may be explained by different reasons (17). One explanation is that the relationship between ACS and COVID-19 had not been described at the time of inclusion. Up to 30% of patients with ACS may have had no signs of typical symptomatology and, therefore, an active search for the diagnosis of ACS was not performed in those patients who already had COVID-19 (18). In this sense, some authors advocate for cardiac troponin determination in patients with COVID-19, not only for diagnosing ACS but also for risk stratification (19). It should be noted that cardiac troponin elevation is a common finding in about 10% to 30% of hospitalized patients with COVID-19, and most patients with troponin elevation and COVID-19 do not have a clinical presentation suggestive of ACS and are labeled as having acute myocardial injury and not ACS (20). Furthermore, during the inclusion period, there was a lack of diagnostic tests in Spain, and it is therefore possible that patients with ACS, but who were COVID-19 paucisymptomatic, with scarce respiratory symptoms or absence of fever, were not tested and were considered as non–COVID-19 patients (21). In addition, several studies have found that the incidence of hospitalization for acute myocardial infarction and admissions decreased during the pandemic, which might be explained by patient fear of being infected if hospitalized and health care redistribution (8,9,22).
The second relevant finding was that we identified clinical characteristics that identify patients with COVID-19 with a higher risk of ACS, such as previous coronary artery disease, age >60 years, and hypertension. These risk factors have been previously described and could be used as a red flag to identify patients who would benefit from a targeted cardiac evaluation (4,6). On the other hand, we also identified some clinical characteristics, such as diarrhea, cough, dyspnea, or lymphopenia that could warn of possible COVID-19 infection in a patient with ACS. However, in the current context of mandatory COVID-19 testing in all patients admitted to hospital, this finding is of minor relevance (23,24).
Third, we found some relevant differences in patient outcomes. The smaller number of echocardiographies and coronariographies performed in patients with COVID-19 with ACS could be a direct consequence of the pandemic. Deferring echocardiography studies deemed nonurgent has reduced patient volumes and should be understood as an effort to protect patients and echocardiography laboratory staff members (25). The important reduction in the activity of interventional cardiology has been previously described in Spain during the first wave of COVID-19 and was caused by different factors (26).
COVID-19 infection involves a higher risk for myocardial oxygen supply and demand mismatch (type 2 myocardial infarction) because of responses to acute infection, including the release of inflammatory factors and catecholamines, as well as the consequences of hypoxia and hemodynamic instability (20). Regarding prognosis, not surprisingly, patients with COVID-19 with ACS had a worse prognosis in terms of need for hospitalization, need for admission to the ICU, and in-hospital mortality than patients with COVID-19 without ACS. On the other hand, patients with COVID-19 with ACS had a lower need for ICU admission with higher in-hospital mortality than ACS without COVID-19. Several reasons may explain this result. There was a higher incidence of type 2 acute myocardial infarction in patients with COVID-19, and these patients have a different profile, older age and high comorbidity that could have conditioned their admission to the ICU (27,28). Moreover, in the context of ICU saturation in the first wave of the pandemic, it is possible that some patients spent the first 24 hours of monitoring in the ED with subsequent transfer to a conventional hospital ward. This result coincides with a recent study conducted in 7 Spanish hospitals in which COVID-19 infection was an independent predictor of in-hospital mortality in patients with acute myocardial infarction (29).
Limitations
This study has several limitations. First, ACS was only detected if the diagnosis was performed in the ED, and ACS developing during the hospitalization of patients with COVID-19 was not taken into account. Second, in some cases, especially critically ill patients, type 2 myocardial infarction can be difficult to distinguish from acute myocardial injury. To minimize this possible misclassification, all the investigators reviewed the cases based on the 4th Universal Definition of Myocardial Infarction criteria. Third, in about 1 in 4 of the patients with COVID-19, the diagnosis was based on clinical or radiologic findings, with no microbiological confirmation, and these figures were similar to those in most countries during the first wave of the pandemic because of the shortage of tests. Fourth, as a retrospective study, although the case record form was standardized, there was no monitoring of data collection methods. In addition, outcome adjudication was performed at each hospital level, without external validation. Nonetheless, the outcomes assessed in the present study were objective (hospitalization, ICU admission, or death), and probably no error was committed in this step. Fifth, although the UMC-19-S10 involved 62 EDs, it was carried out in a single country and external validation in other countries is needed before our findings can be generalized. Sixth, as treatments provided during hospitalization were not recorded, the impact of inappropriate management on outcomes, especially in-hospital mortality, was not assessed in the present study. Seventh, the administration of anticoagulation treatment decreased adverse events in patients with COVID-19. However, at the time of the study this treatment was not routinely administered as there was no evidence on this point at the time the study was performed.
Conclusion
Despite the above limitations, we conclude that the incidence of ACS in patients with COVID-19 patients attending the ED is low, about 1.48%. In some circumstances, especially in patients with COVID-19 with previous coronary artery disease, age ≥60 years, hypertension, chest pain, raised troponin, hypoxemia, and symptoms lasting <3 days, this incidence could be increased. Patients with COVID-19 with ACS had a worse prognosis than control groups as well as higher in-hospital mortality.
Article Summary
1. Why is this topic important?
There is a lack of knowledge about acute coronary syndrome (ACS) in patients with COVID-19.
2. What does this study attempt to show?
We investigated the incidence, clinical characteristics, risk factors and outcomes of ACS in patients with COVID-19 attending the emergency department.
3. What are the key findings?
The incidence of ACS in COVID-19 patients attending the emergency department is low, at around 1.48%. This incidence could be increased in some circumstances, especially in patients with COVID-19 with previous coronary artery disease, age ≥60 years, hypertension, chest pain, raised troponin, hypoxemia, and symptoms lasting <3 days. COVID-19 patients with ACS had a worse prognosis than the control groups as well as a higher in-hospital mortality.
4. How is patient care impacted?
The association of COVID-19 with ACS should be taken into consideration in decision making.
Acknowledgments
All authors discussed the idea and design of study and provided patients. Data analysis and first draft writing was done by OM. All authors read the draft and provided insight for the final version. OM is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. No author declares any conflict of interest directly or indirectly connected with this manuscript. The present work was performed without any direct or indirect financial support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jemermed.2021.10.046.
Appendix
Spanish Investigators on Emergency Situations TeAm (SIESTA) Network
Steering Committee: Òscar Miró, Sònia Jiménez (Hospital Clínic, Barcelona), Juan González del Castillo, Francisco Javier Martín-Sánchez, Eric Jorge García-Lamberechts (Hospital Clínico San Carlos, Madrid), Pere Llorens (Hospital General de Alicante), Guillermo Burillo-Putze (Hospital Universitario de Canarias, Tenerife), Alfonso Martín (Hospital Universitario Severo Ochoa de Leganés, Madrid), Pascual Piñera Salmerón (Hospital General Universitario Reina Sofía, Murcia), Aitor Alquézar-Arbé (Hospital de la Santa Creu i Sant Pau), and Javier Jacob (Hospital Universitari de Bellvitge, Barcelona). Participating Centers: Hospital Universitario Doctor Peset Aleixandre de Valencia (María Luisa López Grima, Mª Ángeles Juan Gómez); Hospital Universitario y Politécnico La Fe de Valencia (Javier Millán, Leticia Serrano Lázaro); Hospital Universitario General de Alicante (Tamara García, Ana Belén Payá); Hospital Clínico Universitario de Valencia (José Noceda); Hospital Arnau de Vilanova de Valencia (María José Cano Cano, Rosa Sorando Serra); Hospital Francesc de Borja de Gandía, Valencia (María José Fortuny Bayarri, Francisco José Salvador Suárez); Hospital General Universitario de Elche, Alicante (Matilde González Tejera); Hospital Marina Baixa de Villajoyosa de Alicante (Eduardo Lorenzo Garrido, Raisa Goretti Afonso Carrillo); Hospital Virgen de los Lirios, Alcoy Alicante (Napoleón Meléndez, Patricia Borrás Albero); Hospital Universitario Vinalopó de Elche (Alicante) (Adelaida Mateo Arenas, Tamara Martin Casquero); Hospital Universitario de Torrevieja de Alicante (Guillermo Moreno Montes, Irene Ruiz Minano); Hospital Lluis Alcanys de Xativa (Carles Pérez García, Pilar Sánchez Amador); Hospital Universitario de La Ribera de Valencia (José Vicente Brasó Aznar, José Luis Ruiz López); Hospital de la Vega Baja Orihuela de Alicante (María Belen Rayos Belda, María Angeles Murcia Herrero); Hospital Universitario Sant Joan Alicante (Elena Díaz Fernández); Hospital General de Requena de Valencia (Maribel Marzo Lambíes, Laura Ejarque Martínez); Hospital de Lliria de Valencia (Ana Peiró Gómez, Elena Gonzalo Bellver); Hospital de la Santa Creu i Sant Pau (Barcelona) (Polo Higa Sansome, Miriam Mateo Roca); Hospital Clinic (Barcelona) (Carlos Cardozo); Hospital Universitari de Bellvitge de Hospitalet de Llobregat (Barcelona) (Alejandro Roset-Rigat, Irene Cabello-Zamora); Hospital Universitari Germans Trias i Pujol de Badalona (Barcelona) (Anna Sales Montufo, Pepe Ferrer Arbaizar); Hospital de Terrassa (Barcelona) (Josep Tost); Hospital del Mar (Barcelona) (Alfons Aguirre Tejedo, Isabel Cirera Lorenzo); Hospital Universitari Joan XXIII (Tarragona) (Anna Palau-Vendrell, Ruth Gaya Tur); Hospital Universitari de Girona Dr. Josep Trueta (Girona) (Maria Adroher Muñoz, Ester Soy Ferrer); Hospital Universitari de Vic (Barcelona) (Lluís LLauger García); Hospital de Sant Pau i Santa Tecla (Tarragona) (Brigitte Silvana Alarcón Jiménez, Silvia Flores Quesada); Clinica Sagrada Familia (Barcelona) (Arturo Huerta); Hospital Clínico San Carlos (Madrid) (Marcos Fragiel); Hospital Universitario La Paz (Madrid) (Susana Martínez Álvarez, Ana María Martinez Virto); Hospital Universitario de la Princesa (Madrid) (Carmen del Arco Galán, Guillermo Fernández Jiménez); Hospital Universitario Severo Ochoa de Leganés (Madrid) (David Martín-Posada Crespo, Belén Sánchez López); Hospital Universitario Rey Juan Carlos (Madrid) (Verónica Prieto Cabezas, Alejandra Sánchez Arias); Hospital Universitario del Henares (Madrid) (María Adalid Moll, María Luisa Pérez Díaz-Guerra); Hospital Universitario de Fuenlabrada (Madrid) (María Eugenia Barrero Ramos, Marta Álvarez Alonso); Hospital Universitario Infanta Cristina de Parla (Madrid) (Guadalupe Pérez Nieto, Paula García Domíngo); Hospital Comarcal El Escorial (Madrid) (Silvia Ortiz Zamorano, Frida Vallejo Somohano); Clínica Universidad Navarra de Madrid (Raquel Piñero Panadero, Nieves López-Laguna); Hospital Universitario de Salamanca (Francisco Diego Robledo, Manuel Ángel Palomero Martín); Complejo Asistencial Universitario de León (Marta Iglesias Vela, Laura Hernando López); Hospital Universitario de Burgos (María Pilar López Díez); Hospital Universitario Rio Hortega (Valladolid) (Virginia Carbajosa, Laura Fernández Concellón); Complejo Asistencial de Soria (Fahd Beddar Chaib, Laura Tejada de los Santos); Hospital Universitario Regional de Málaga (Miguel Moreno Fernández, Iván Villar Mena); Hospital Universitario Juan Ramón Jiménez (Eissa Jaloud Saavedra, María Ángeles Garrido López); Hospital Costa del Sol de Marbella (Carmen Agüera Urbano, Ana Belen Garcia Soto); Hospital Valle de los Pedroches de Pozoblanco (Córdoba) (Jorge Pedraza García); Hospital Virgen del Rocío de Sevilla (Amparo Fernández de Simón Almela); Complejo Hospitalario Universitario de A Coruña (Ricardo Calvo López); Hospital Universitario Lucus Augusti Lugo (Juan José López Díaz); Complejo Hospitalario Universitario de Vigo. Hospital Álvaro Cunqueiro (María Teresa Maza Vera, Raquel Rodríguez Calveiro); Hospital Universitario General de Albacete (Francisco Javier Lucas-Galan, María Ruiperez Moreno); Hospital Virgen de la Luz (Cuenca) (Félix González Martínez, Diana Moya Olmeda); Hospital Nuestra Señora del Prado de Talavera de la Reina (Toledo) (Ricardo Juárez); Hospital Universitario de Canarias (Tenerife) (Patricia Eiroa Hernandez, Jose Francisco Fernandez Rodriguez); Hospital Universitario de Gran Canaria Dr. Negrín (José Pavón Monzo, Nayra Cabrera González); Hospital Universitario Central Asturias (Desire Maria Velarde Herrera, Beatriz María Martínez Bautista); Hospital Universitario de Cabueñes (Gijón) (Mª del Rosario Carrió Hevia, Carmen Elvira Menéndez); Hospital Clínico Universitario Virgen de la Arrixaca (Eva Quero Motto, Nuria Tomas García); Hospital General Universitario Reina Sofía de Murcia (Ines Garcia Rosa, Maria Encarnacion Sanchez Canovas); Hospital San Pedro de Logroño (Noemí Ruiz de Lobera); and Hospital Clínico Universitario Lozano Blesa (José María Ferreras Amez, Belen Arribas Entrala).
Appendix B. Supplementary materials
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/?gclid=EAIaIQobChMI9sDNu–k7QIVFxkGAB0V-wB5EAAYASAAEgIZTvD_BwE. Accessed November 21, 2021.
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 4.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. COVID-19: who is at increased risk for severe illness? People with certain medical conditions. Available at: http://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed November 21, 2021.
- 8.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 10.Miró O, González Del Castillo J. Collaboration among Spanish emergency departments to promote research: on the creation of the SIESTA (Spanish Investigators in Emergency Situations TeAm) network and the coordination of the UMC-19 (Unusual Manifestations of COVID-19) macroproject. Emergencias. 2020;32:269–277. [PubMed] [Google Scholar]
- 11.Gil-Rodrigo A, Miró O, Piñera P, et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias. 2020;32:233–241. [PubMed] [Google Scholar]
- 12.Spanish Government Ministry of Health. COVID-19 geographical distribution. Available at: https://cnecovid.isciii.es/covid19/#niveles-de-gravedad. Accessed November 21, 2021.
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 14.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartnett KP, Kite-Powell A, DeVies J, et al. National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:699–704. doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzler B, Siostrzonek P, Binder RK, et al. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with acute myocardial infarction presenting with chest pain. JAMA. 2000;283:3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AR, Bularga A, Mills NL. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141:1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe AS, Cleland JGF, Katus HA. Myocardial injury in severe COVID-19 infection. Eur Heart J. 2020;41:2080–2082. doi: 10.1093/eurheartj/ehaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alquezar-Arbé A, Piñera P, Jacob J, et al. Impact of the COVID-19 pandemic on hospital emergency departments: results of a survey of departments in 2020- The Spanish ENCOVIR study. Emergencias. 2020;32:320–331. [PubMed] [Google Scholar]
- 22.Mahfam MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed March 7, 2021.
- 24.European Center Disease Control. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/TestingStrategy_Objective-Sept-2020.pdf. Accessed March 7, 2021.
- 25.Drake DH, De Bonis M, Covella M, et al. Echocardiography in pandemic: front-line perspective, expanding role of ultrasound, and ethics of resource allocation. J Am Soc Echocardiogr. 2020;33:683–689. doi: 10.1016/j.echo.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Leor O, Cid-Álvarez B, Pérez de Prado A, et al. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Esp Cardiol. 2020;73:994–1002. doi: 10.1016/j.rec.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Miró O, Alquézar-Arbé A, Llorens P, et al. Comparison of the demographic characteristics and comorbidities of patients with COVID-19 who died in Spanish hospitals based on whether they were or were not admitted to an intensive care unit. Med Intensiva (Engl Ed) 2021;45:14–26. doi: 10.1016/j.medin.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solano-López J, Zamorano JL, Pardo Sanz A, et al. Risk factors for in-hospital mortality in patients with acute myocardial infarction during the COVID-19 outbreak. Rev Esp Cardiol. 2020;73:985–993. doi: 10.1016/j.rec.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.