Abstract
In chronic infections and in cancer, persistent antigen stimulation under suboptimal conditions can lead to the induction of T-cell exhaustion. Exhausted T cells are characterized by an increased expression of inhibitory markers and a progressive and hierarchical loss of function. Although cancer-induced exhaustion in CD8 T cells has been well-characterized and identified as a therapeutic target (i.e., via checkpoint inhibition), in-depth analyses of exhaustion in other immune cell types, including CD4 T cells, is wanting. While perhaps attributable to the contextual discovery of exhaustion amidst chronic viral infection, the lack of thorough inquiry into CD4 T-cell exhaustion is particularly surprising given their important role in orchestrating immune responses through T-helper and direct cytotoxic functions. Current work suggests that CD4 T-cell exhaustion may indeed be prevalent, and as CD4 T cells have been implicated in various disease pathologies, such exhaustion is likely to be clinically relevant. Defining phenotypic exhaustion in the various CD4 T-cell subsets and how it influences immune responses and disease severity will be crucial to understanding collective immune dysfunction in a variety of pathologies. In this review, we will discuss mechanistic and clinical evidence for CD4 T-cell exhaustion in cancer. Further insight into the derivation and manifestation of exhaustive processes in CD4 T cells could reveal novel therapeutic targets to abrogate CD4 T-cell exhaustion in cancer and induce a robust antitumor immune response.
Introduction
T-cell dysfunction can strongly impact both physiologic and pathologic states. Among the known modes of T-cell dysfunction, exhaustion has garnered an increasing degree of recent attention. As exhaustion was initially described as a hyporesponsive T-cell state in chronic lymphocytic choriomeningitis viral (LCMV) infections (1–3), significant effort was initially aimed at characterizing exhaustion in virus-combating CD8 T cells, specifically. A hallmark of mice exposed to chronic infection with LCMV Clone-13, exhaustion has come to encompass a broad state of CD8 T-cell dysfunction resulting from persistent antigen exposure under suboptimal conditions (4), including inadequate CD4 T-cell help (5–7). It has evolved as a transcriptionally programmed and host-adaptive state designed to limit collateral immunologic damage in conditions of failed pathogen clearance and continued antigen exposure, establishing a “stalemate” of sorts between host and pathogen.
More recently, exhaustion has become an acknowledged mode of T-cell dysfunction in cancer as well (8, 9). Importantly, the upregulation of exhaustion-demarcating immune checkpoints by T cells has been associated with the development of tumor resistance to checkpoint blockade therapies (10). Although restoration of exhausted CD8 T-cell function is a primary goal for checkpoint inhibition, CD4 T cells are also liable to suffer exhaustion and contribute to rejuvenation of the antitumor immune response after checkpoint blockade. However, thorough investigations into the definition, prevalence, and mechanisms of CD4 exhaustion remain lacking. This presents a gap in our understanding of the summative immune dysfunction characterizing a number of disease states where CD4 T-cell function is relevant, including cancer.
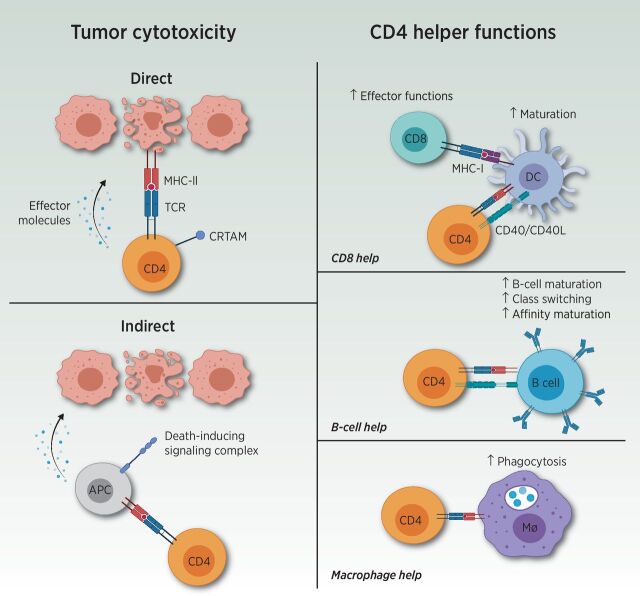
CD4 T cells perform a wide variety of functions within the adaptive immune system and are best known for their role as T helper (Th) cells, including Th1, Th2, Th17, and regulatory T-cell (Treg) subsets. Importantly, CD4 T cells license dendritic cells (DC) to allow optimal priming of CD8 T cells, provide key signals for antibody class switching, promote bactericidal activity of phagocytes, recruit neutrophils, influence angiogenesis, and secrete cytokines, in addition to perhaps possessing direct cytotoxic functions (Fig. 1; refs. 11–13). Likewise, CD4 T cells appear to possess significant plasticity, allowing subsets to transition between one another, broadening their functional impact (14).
Figure 1.
Overview of CD4 T-cell functions. CD4 T cells are most well known for their Th cell functions (displayed on the right). Through recognition of the TCR of the peptide-MHC complex, CD4 T cells mediate increased maturation and activation of DCs. This process allows augmented CD8 T-cell effectors upon interaction with the activated DCs. Furthermore, CD4 T cells increase B-cell maturation, antibody class switching, and affinity maturation, and enhance phagocytosis within macrophages (Mφ). Aside from helper functions, CD4 T cells possess both direct and indirect tumor cytotoxicity capacities (displayed on the left). Direct cytotoxicity was demonstrated by cytotoxic CD4 T expressing class I–restricted T-cell–associated molecule (CRTAM). Indirect cytotoxicity could also be guided by CD4 T cells through interaction with antigen-presenting cells (APC) or natural killer cells. Adapted from an image created with BioRender.com.
CD4 T cells are strongly implicated in the development of antitumor responses (Table 1), as they can enhance tumoricidal activity of other antitumor effector cells, such as CD8 T cells and macrophages (6, 15, 16). Some CD4 subsets, particularly Th2 and Tregs, are known to negatively affect the antitumor response by decreasing antigen presentation and dampening T-cell effector functions, respectively. Furthermore, certain CD4 T cells appear able to directly lyse tumor cells (11, 12), and adoptive transfer of tumor-specific CD4 T cells alone has demonstrated impressive efficacy in some studies (17). Direct tumor cell recognition and killing by CD4 T cells requires class II major histocompatibility complex (MHC), and overexpression of class II MHC transactivator (CIITA) on murine mammary adenocarcinoma cells increased interferon-gamma (IFNγ) and granzyme B production in CD4 T cells and restricted tumor growth (18). These studies remain controversial, however, as many tumor cells will not have the antigen presentation machinery required to properly load peptides onto MHC-II. Nevertheless, CD4 T cells with a cytotoxic transcriptional profile have been found enriched in patients responding to immune checkpoint blockade (19).
Table 1.
Overview of CD4 populations and their contributions to tumor immunity.
| T-cell subset | Master regulator | Cytokine | Functions within the tumor |
|---|---|---|---|
| Th1 | Tbet | IFNγ |
|
| Th2 | GATA3 | IL4, IL5, IL13 |
|
| Th17 | RORγt | IL17 |
|
| Regulatory T-cell | FoxP3 | IL10, TGFβ | • Decrease effector functions of tumor-infiltrating T cells |
| Cytotoxic CD4 T cells | Runx3 | Perforin, granzymes | • Direct tumor cytotoxicity |
Abbreviations: FoxP3, Forkhead Box P3; GATA3, GATA binding protein 3; IFNγ, interferon gamma; IL, interleukin; RORγt, retinoic acid receptor–related orphan nuclear receptor gamma; Runx3, RUNX Family Transcription Factor 3; Tbet, T-box expressed in T cells; TGFβ, transforming growth factor beta.
Given the diverse repertoire of CD4 T-cell capacities, dysfunction in this compartment is assuredly relevant. In this review, we will discuss the current best evidence for the delineation and significance of CD4 T-cell exhaustion in cancer. A brief overview of CD4 T-cell exhaustion in chronic infections, transplantation, and autoimmune diseases will also provide context across pertinent pathologies. Understanding these processes is anticipated to aid in identifying novel therapeutic targets and considerations for improving the antitumor responses.
Overview of Exhaustion
The framework for our current understanding of CD4 T-cell exhaustion is generated from the more extensively studied CD8 T-cell exhaustion, elegantly reviewed by McLane and colleagues (20). When antigen clearance fails and exposure is maintained, as in the setting of chronic infection or cancer, an exhausted T-cell phenotype may emerge. A primary feature of exhausted T cells is the sustained coexpression of multiple inhibitory surface receptors, referred to commonly as immune checkpoints. The function of these checkpoints is to permit protective curbing of T-cell activity following immune activation. The “classical” immune checkpoints include cytotoxic T lymphocyte–associated protein 4 (CTLA4) and programmed cell death protein 1 (PD-1). Newer “alternative” checkpoints include molecules such as T-cell immunoglobulin and mucin-domain containing-3 (TIM3); lymphocyte-activation gene 3 (LAG3); B- and T-lymphocyte attenuator (BTLA); 2B4; T-cell immunoreceptor with Ig and ITIM domains (TIGIT); and SLAM Family Member 6 (SLAMF6; refs. 21, 22). These inhibitory receptors (checkpoints) are known to be expressed on exhausted T cells, with mounting checkpoint expression associated with more severe phenotypes (8, 10). The typical characteristics of CD8 T-cell exhaustion include antigen load–dependent and temporally progressive loss of effector activity (8, 23, 24), loss of proliferative capacity (25, 26), altered expression of transcription factors (27, 28), loss of antigen-independent homeostatic proliferation (29), and modified epigenetic landscapes (30–32) and metabolic requirements (28, 33, 34). In turn, disruption of the PD-1/programmed death ligand 1 (PD-L1) pathway, in particular, has demonstrated the capacity to reverse features of the exhausted phenotype and restore T-cell proliferative and effector function (35).
Recent evidence suggests that the exhausted phenotype in CD8 T cells is not homogeneous and includes lineage spanning, stage-like “progenitor” (SLAMF6+TIM3−) and “terminally-differentiated” (SLAMF6−TIM3+) subtypes (21, 26), with varied capacities for effector function and proliferation dispersed among the subgroups. Terminally exhausted CD8 T cells are further characterized by higher levels of PD-1 on their surface. Whereas progenitor exhausted CD8 T cells remain capable of co-producing multiple cytokines and can proliferate in vivo, terminally exhausted CD8 T cells are limited to single cytokine production and upregulation of granzyme B. Furthermore, only progenitor exhausted subsets are capable of responding to anti–PD-1 treatment (21, 26). SLAMF6-positive CD8 T cells express the transcription factor T-cell factor 1 (TCF1) (21), which has been linked to the preservation of effector functions (36). Loss of TCF1 with concomitant upregulation of multiple coinhibitory receptors is associated with the terminally differentiated exhaustion phenotype and a further decline in effector functions (21) and/or adoption of immunoregulatory function (37). While exhausted CD8 T cells retain the ability to recognize antigen through their T-cell receptor (TCR), antigen exposure fails to elicit a robust, meaningful cytotoxic response (23).
Our current grasp of CD4 T-cell exhaustion is decidedly anemic when compared with the above understanding we have acquired for CD8s. To begin, an accepted definition of CD4 T-cell exhaustion has not yet been established, limiting the capacity to properly assign the term definitively. Much of the research on CD4 T-cell exhaustion to date has focused merely on the expression versus absence of coinhibitory receptors and/or cytokine production, with data being suggestive of an exhausted state (Fig. 2). Further research is required to determine whether additional criteria delineating CD8 T-cell exhaustion, including loss of antigen-independent homeostatic proliferation (29, 38, 39), alterations in metabolic profiles (28, 33, 34), and unique epigenetic features (30–32), also apply to exhausted CD4 T cells. Likewise, it will be crucial to understand whether CD4 exhaustion evolves in a similar stage- and lineage-dependent manner to CD8 T cells (30, 31), and whether or not there are differing relative susceptibilities to and impacts for exhaustion in the various CD4 subsets.
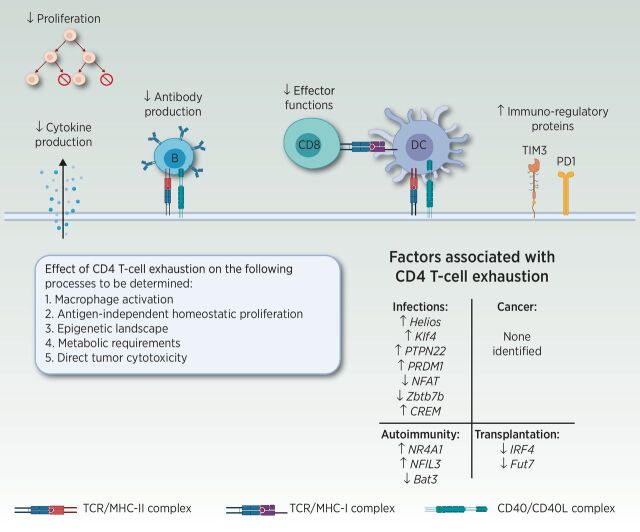
Figure 2.
Consequences of CD4 T-cell exhaustion on CD4 Th functions. Although the details of CD4 T-cell exhaustion remain to be deciphered, negative effects on proliferation, cytokine production, B-cell help, and CD8 effector functions have been reported. In addition, CD4 T cells with reduced effector functions upregulate immune-regulatory proteins, such as T-cell immunoglobulin and mucin domain-3 (TIM3) and PD-1, paralleling phenotypes observed in exhausted CD8 T cells. Whether CD4 T-cell exhaustion negatively impacts macrophage activation and direct tumor cytotoxicity remains to be determined. Further research is required to determine whether loss of antigen independent homeostatic proliferation and alterations in epigenetic and metabolic profiles are features of exhausted CD4 T cells, similar to exhausted CD8 T cells. Abbreviations: Bat3: human leukocyte antigen B (HLA-B)–associated transcript 3; CREM: CAMP responsive element modulator; Fut7: fucosyltransferase 7; IRF4: interferon regulatory factor 4; Klf4: Krüppel-like factor 4; NFAT: nuclear factor of activated T cells; NFIL3: nuclear factor, interleukin 3 regulated; NR4A1: nuclear receptor subfamily 4 group A member 1; PRDM1: PR/SET domain 1 (encodes Blimp1); PTPN22: protein tyrosine phosphatase, non-receptor type 22; Zbtb7b: zinc finger and BTB domain containing 7B (encodes ThPOK). Adapted from an image created with BioRender.com.
Evidence for CD4 T-Cell Exhaustion
Original evidence: chronic infections
As CD8 T-cell exhaustion was first defined in chronic LCMV infection, this remains a logical place to begin when analyzing the evidence for a similar exhausted state among CD4 T cells. Compared with acute infections, chronic LCMV infections induce markedly greater expression of exhaustion-suggestive immune checkpoints on CD4 T cells (40, 41). Upregulation of these same inhibitory receptors typical of CD8 exhaustion has also been identified on CD4 T cells in other chronic and recurrent infections, suggesting an analogous CD4 T-cell exhaustion phenotype (42–44). Similar to what is seen with CD8 T cells, antigen-specific CD4 T cells (45–49) and CD4 T cells from infected tissues (50, 51) express higher levels of the relevant coinhibitory receptors, drawing a parallel role for antigen exposure in the induction of the seemingly matched CD4 exhausted state. Accordingly, increased coinhibitory receptor expression is generally associated with more advanced disease (52–56), while successful disease treatment correlates in turn with reduced expression of the same markers (56–58). However, upregulation of inhibitory markers is not sufficient to call a cell exhausted, as some coinhibitory receptors are also activation markers (59).
Importantly, then, functional deficits are also observed amidst the phenotypically exhausted CD4 T-cell compartment following chronic infection (45, 60–63). In LCMV in particular, CD4 T-cell differentiation in the presence of persistent antigen resulted in upregulation of coinhibitory molecules (62), premature contraction of the antigen-specific immune population (41, 61), reduced cytokine production (41, 61), decreased splenic motility (62), and poor recall responses upon a secondary challenge (61). Reduced CD4 effector functions and increased expression of inhibitory molecules could be induced upon exposure of CD4 T cells to TNF (64) and fibrinogen-like 2 (FGL2; ref. 65). A link between decreased performance of CD4 T cells and exhaustion was established through increased motility and cytokine production following anti–PD-1 treatment (41, 62), although recovery of function was inconsistent (46, 59, 63, 66–68).
Exhaustion is also characterized (and confirmed) by prescribed and stereotyped transcriptional programs. Various studies examining transcriptional programs within CD4 T cells have provided mechanistic insight into factors that may be involved in the establishment of CD4 T-cell exhaustion during LCMV or other chronic infection response. Such studies suggest roles for upregulation of IKZF2 (encoding Helios; ref. 42), Klf4 (42), protein tyrosine phosphatase, nonreceptor type 22 (PTPN22; ref. 69), cAMP-responsive element modulator (CREM; ref. 69), and PR/SET domain 1 (PRDM1, encodes Blimp1; ref. 45). Likewise, exhaustion in CD4 T cells has been observed with loss or downregulation of ThPOK (70) and nuclear factor of activated T cells (NFAT; ref. 71).
Not all effector functions of CD4 T cells are necessarily compromised during chronic infections: perforin (72) and granzyme B (73) production in CD4 T cells is increased in patients with HIV when compared with healthy controls, for instance. Interestingly, such regain of cytotoxic function has also been described for “terminally” exhausted CD8 T cells (21). Therefore, the acquisition or retention of these functions might simply indicate varying exhaustion stages, again eliciting similarities with observations made amidst CD8 T-cell exhaustion.
Evidence and significance in cancer
Cytotoxic CD8 T cells promote antitumor immunity that can be correspondingly restricted by their tumor-induced diversion down a pathway toward exhaustion (8, 9, 74–76). Yet, a successful antitumor immune response requires the coordination of a variety of non–T cells constituting the tumor microenvironment (TME), including macrophages, DCs, B cells, and others. Given the role CD4 T cells play in orchestrating the responses by each of these cell types, the potential impact that exhaustion might have amidst the tumor-infiltrating or even systemic CD4 population is substantial. Likewise, the direct cytotoxic role that CD4 T cells can have in mediating antitumor immunity (11–13) makes them a particularly germane population in cancer. As an extension, restoration of exhausted CD4 T-cell function by checkpoint blockade, if feasible, may contribute significant clinical benefit in tumors, either by improving direct CD4 antitumor activity or increasing CD4 helper functions.
Canonical and alternative inhibitory receptors suggestive of exhaustion on CD4 T cells (PD1, CTLA4, LAG3, TIM3, TIGIT) have been identified in multiple solid tumors and hematologic malignancies (77–82) in both humans and mice (Table 2). In many cases, similar to that seen with chronic infection, the expression of such checkpoints has been associated with more advanced disease states and diminished progression-free survival (83–85). Furthermore, successful anticancer therapies have been associated with reductions in the level of these markers on the surface of CD4 T cells (86–88), while failure to achieve complete remission and/or disease relapse has positively correlated with their persistent or enhanced expression (86, 89, 90–92).
Table 2.
Overview of coinhibitory and costimulatory markers assessed in various primary tumors. Markers were either assessed as single positive or double positive.
| Primary tumor | Coinhibitory markers | Costimulatory marker | Main conclusions |
|---|---|---|---|
| Melanoma | PD1, CTLA4, LAG3, TIGIT, TIM3 | 2B4 | • Continuous antigen stimulation decreases antitumor activity (101, 102) |
| • Long-term remission in murine models after αLAG3 + αPDL1 (112, 113) | |||
| Lung cancer | PD1, CTLA4, LAG3, BTLA, CD69, TIM3 | 2B4 | • Patients with a high CD4 PD-1+ frequency had decreased overall and progression-free survival, independent of clinical characteristics (145) |
| GBM | PD1, TIM3, LAG3, CTLA4 |
|
|
| Breast | PD1, TIM3 |
|
|
| Head and neck | TIGIT, LAG3, TIM3, PD1, CD69 | • αTIGIT delayed tumor progression, although direct effect on CD4 T cells not assessed (103) | |
| Gastrointestinal | PD1, CTLA4, TIM3, LAG3 | ICOS |
|
| AML | PD1, CD57, CD69, CTLA4, TIM3, LAG3 | ICOS | • αCD86 and ICOS-ligand prevented the emergence of exhausted CD4 T cells (148) |
| CLL | PD1, TIM3, TIGIT | 2B4, CD226 | • αTIGIT impaired IFNγ and IL10 production in the presence of tumor cells the TIGIT ligand (CD155; ref. 111) |
| Multiple myeloma | PD1, TIM3, CTLA4 | CD40L | • Gradual decrease in CD40L expression with more advanced disease (149) |
As with CD8 exhaustion, a number of coinhibitory receptors may also serve to denote T-cell activation (59). Therefore, their expression alone is not sufficient to signal the true emergence of exhaustion. Functional and transcriptomic correlates are needed. Ultimately, it is a balance between costimulatory and coinhibitory signals that provide a gain adjustment on the immune response. Currently, however, such phenotypic and functional assessments of tumor-infiltrating CD4 T cells remain somewhat lacking. Functional deficits in CD4 proliferation, cytokine production, signaling, and provision of B-cell help have varied according to tumor type and source of CD4 T cells (93–99). Nonetheless, current studies in a variety of cancers indicate a correlative relationship between typical markers of T-cell exhaustion on CD4 T cells and the degree of disease severity. Likewise, at least partial restoration of CD4 effector functions has been observed after treatment with checkpoint inhibitors (Table 2; refs. 84, 93, 97, 100).
To evaluate whether apparent CD4 T-cell exhaustion parallels the development of CD8 T-cell exhaustion, Rausch and colleagues (101) and Malandro and colleagues (102) investigated the role of antigen stimulation on CD4 T-cell function using murine melanoma models. Persistently increased antigen availability reduced CD4 proliferation, cytokine production, and antitumor responses and increased checkpoint expression on CD4 T cells. Checkpoint inhibition induced only a variable recovery of CD4 effector functions in their hands. Furthermore, clinical observations demonstrate that tumor-infiltrating CD4 T cells express higher levels of coinhibitory markers compared with circulatory (98, 103, 104) or adjacent tissue-infiltrating CD4 T cells (100, 105, 106). These data indicate that perpetual antigen encounters can induce a severe, and potentially irreversible, exhaustion phenotype that mimics terminal exhaustion in CD8 T cells (21).
To further investigate parallels between the development of CD4 T-cell and CD8 T-cell exhaustion and differentiation states, Fu and colleagues examined the transition from progenitor exhaustion (SLAMF6+TIM3−) to terminal exhaustion (SLAMF6−TIM3+; ref. 21) occurring among CD4 T cells within a murine melanoma model. They observed a downregulation of TCF1 and SLAMF6 on tumor-infiltrating CD4 T cells compared with CD4 T cells in the spleen, indicating more prevalent differentiation into the terminally exhausted state within tumors. Furthermore, treatment with anti–PD-L1 resulted in an increase in TCF1 and a decrease in TIM3 and LAG3 on CD4 T cells, indicating maintenance of the progenitor exhausted subset (107). These data were corroborated by experiments performed in human samples of head and neck, ovarian, and cervical tumors (108). In contrast to terminally exhausted CD8 T cells, terminal exhaustion in CD4 T cells was represented by the expression of CD39, rather than TIM3. CD39+ cells were found to have higher levels of PD-1, produce fewer cytokines, and were more likely to produce a single cytokine (predominantly IFNγ) rather than coproduce multiple cytokines. Treatment with anti–PD-1 increased cytokine production, upregulated CD40 ligand (CD40L), and increased DC maturation and CD8 proliferation, indicating increased CD4 helper functions (108). Paralleling CD8 T-cell exhaustion, CD39+ CD4 T cells expressed the highest level of thymocyte selection-associated high mobility group box (TOX; ref. 109) and lost expression of TCF1 (21). The parallel of increased TOX expression with that described in CD8 exhaustion is of particular interest, as TOX has recently been found to initiate the epigenetic changes associated with the exhausted phenotype (110). Epigenetic changes are hallmarks of CD8 T-cell exhaustion (30–32), and this similarity should drive further investigation into the epigenetic landscape of CD4 T-cell exhaustion.
Drawing additional similarities to terminally exhausted CD8 T cells, studies suggest that exhausted CD4 T cells may actually gain certain additional functionality, as the acquisition of noncanonical T-cell function was observed among putatively exhausted CD4 T cells in solid tumors. For instance, C-X-C Motif Chemokine ligand 13 (CXCL13) was found to be exclusively produced by PD1hi CD4 T cells in non–small cell lung cancer (106), suggesting a skew toward effector function in what otherwise resembled exhausted CD4 T cells. Conversely, increased effector function might not translate to increased tumoricidal activity. For instance, enhanced IFNγ production was observed in CD4 T cells positive for the inhibitory marker TIGIT in patients with chronic lymphocytic leukemia. In spite of the improved secretion of proinflammatory cytokines, TIGIT expression on CD4 T cells was also associated with more advanced disease, and TIGIT blockade hindered tumor cell viability in vitro, despite also decreasing IFNγ production (111).
Mouse tumor models have been utilized to better evaluate the impact and relevance of CD4 T cells and CD4 T-cell exhaustion for antitumor immunity. Adoptive transfer of melanoma-specific CD4 T cells into a RAG1 knockout recipient resulted in tumor regression. However, a subset of mice presented with tumor relapse. CD4 T cells taken from the recurrent tumors expressed fewer cytokines, increased levels of coinhibitory receptors (112), and were unable to induce tumor regression when transplanted into a secondary tumor-bearing host, suggesting the emergence of response-limiting exhaustion (113). Meanwhile, combination therapy with anti–PD-L1 and anti-LAG3 decreased checkpoint expression, increased CD4 effector functions, and resulted in durable tumor control (112, 113). The lack of CD8 T cells in this model indicates a significant role for CD4 T-cell exhaustion in facilitating tumor escape. Furthermore, these data suggest that checkpoint blockade strategies aimed at CD4 T cells could very well improve tumor control.
Tumor models have likewise been utilized to shed light on important considerations for CD4 T-cell exhaustion, including the relative frequency of exhaustion within the various CD4 subsets. For instance, examining the directly cytotoxic CD4 T-cell subset, we return to an aforementioned study in which tumor cells overexpressing CIITA were generated to permit CD4 recognition of class II MHC-expressing tumors (18). Despite increased cytotoxic CD4 T-cell–mediated tumor control of these tumors, outgrowth eventually occurred, with associated upregulation of coinhibitory markers on tumor-infiltrating CD4 T cells. This expansion was reversed with anti-CTLA4 treatment, highlighting the role of CD4 T-cell exhaustion in tumor progression, as well as the capacity for cytotoxic CD4 T cells, specifically, to undergo an exhaustion program (18).
Interestingly, exhaustion-indicative expression of immune checkpoints has also been observed on the Treg subset of CD4 T cells in the tumors of patients with glioblastoma multiforme (GBM; ref. 114) and hepatocellular carcinoma (115). This suggests that exhaustion among CD4 T cells may not be limited solely to effector CD4 T cells. PD1-expressing Tregs in patients with GBM demonstrate enrichment of exhaustion-related genes and decreased suppressive capacities (114), indicating that suppressive functions may be exhaustion-susceptible as well. Given the substantial role Tregs play limiting cellular immunity in GBM (and other cancers; refs. 116, 117), selective strategies for reversing exhaustion in cytotoxic and Th1-type CD4 T cells while maintaining or enhancing it in Tregs may represent challenging but worthwhile future directions. Accordingly, understanding whether exhaustion-inducing mechanisms are the same in each of these CD4 subsets becomes an important endeavor.
In addition to exhaustion, anergy (118, 119) and senescence (120) have been recognized as modes of T-cell dysfunction that negatively influence antitumor immunity (121, 122). Although these states overlap with regard to various functional and/or phenotypic elements, the mechanisms eliciting the phenotypes are distinct. In contrast to the relatively insidious development of T-cell exhaustion following continuous antigen stimulation, for instance, T-cell senescence is associated with cell-cycle arrest due to shortening of telomeric ends or danger signals, such as oxidative stress (123, 124). Likewise, anergy develops at priming, subsequent to excessive stimulation of the TCR without proper costimulatory signals. Although inhibitory receptors such as PD1, CTLA4, TIM3, and LAG3 are more commonly associated with T-cell exhaustion, other markers, such as CD57 and killer cell lectin-like receptor subfamily G member 1 (KLRG1), are more commonly associated with senescence (124). Unlike senescence T cells, however, progenitor exhausted T cells are capable of responding to checkpoint blockade (21), allowing restoration of function. Further explorations will be required to further delineate these hypo- or unresponsive states and unravel the relative contributions that each of these modes of CD4 dysfunction makes to hindering antitumor immunity.
Ultimately, the data reviewed above begin to establish CD4 T-cell exhaustion as a unique differentiation state impacting antitumor immunity, paralleling exhaustion in CD8 T cells. Further comparisons, including epigenetic and metabolic profiles between exhausted and nonexhausted CD4 T-cell states, will be essential. These experiments will increase our understanding of the complex immune responses generated to cancer and persistent infections and could provide novel therapeutic targets. Additional insights should be gathered by evaluating other pathologies where CD4 exhaustion has been identified. In addition to cancer and chronic infections, these can include transplantation and autoimmune diseases.
Salient studies in transplantation and autoimmune diseases
Studies into transplantation and autoimmune diseases can help shed light on the possibility of modulating CD4 T-cell exhaustion as a therapeutic strategy. In contrast to cancer and chronic infections, CD4 T cells in transplantation and autoimmunity are a source of undesirable activity and collateral host tissue damage. In the case of hematopoietic stem cell (HSC) and solid organ transplantation, CD4 T cells have been demonstrated to play a significant role in allograft rejection (125, 126) by providing help to the two major cell subsets responsible for tissue damage: cytotoxic donor-specific CD8 T cells and B cells (127). Therefore, inducing specific CD4 T-cell tolerance (128–130) or exhaustion could potentially mitigate the need for systemic immunosuppression by selectively restraining graft-specific effector cells while leaving the remainder of the immune system capable of responding to foreign antigens, reducing the risk of infections and malignancy.
Upregulation of coinhibitory molecules on CD4 T cells is observed following HSC (131, 132) and solid organ transplantation (133). Transgenic overexpression of TIM3 on CD4 T cells resulted in decreased proinflammatory cytokine production and prevented immune-mediated graft pathology (134), indicating a direct role for TIM3 on CD4 T cells in the prevention of rejection. Mechanistically, loss of IFN regulatory factor 4 (IRF4) or Fucosyltransferase 7 (Fut7) in CD4 T cells induced graft tolerance through the establishment of exhaustion in these cells. Graft rejection could be initiated in the early phases after transplant upon treatment with monoclonal antibodies interfering with the PD-1–PD-L1 pathway (135, 136). However, irreversible dysfunction was established in IRF4 knockout (KO) CD4 T cells if anti–PD-1 treatment was delayed until 30 days posttransplant (135).
Much like transplantation, the management of autoimmune diseases frequently involves systemic immunosuppression, and the role of CD4 T cells in autoimmune pathology has long been recognized (137, 138). Conversely, a negative correlation between CD4 inhibitory receptor expression and disease severity has been observed in a rheumatoid arthritis population (139), although this has not been a consistent finding (140). Insights into the role of CD4 T-cell exhaustion and its influence on disease severity in autoimmunity comes from mechanistic studies evaluating Nuclear Receptor Subfamily 4 Group A Member 1 (NR4A1), Nuclear Factor Interleukin 3 Regulation (NFIL3), and human leukocyte antigen B (HLA-B)–associated transcript 3 (Bat3). Upregulation of NR4A1 and NFIL3 and downregulation of Bat3 increased expression of exhaustion markers on CD4 T cells and decreased cytokine production and disease severity (141–143). In addition, persistent stimulation of CD4 T cells with endogenous peptides resulted in loss of cytokine production and proliferation, upregulation of inhibitory markers, and delayed onset of autoimmune diabetes (144).
Conclusion and Future Directions
Compared with CD8 T-cell exhaustion, the impact of CD4 T-cell exhaustion in cancer and other disease states has remained relatively underappreciated. Current studies have provided mostly phenotypic data, and investigations into additional criteria established for CD8 T-cell exhaustion, such as metabolic profiles and epigenetic landscapes, will be required to determine whether CD4 exhaustion likewise comprises a distinct and progressing T-cell differentiation state. Our current mechanistic understanding of factors involved in CD4 T-cell exhaustion is summarized in Fig. 2. Further evaluation of CD4 T-cell–specific factors will be essential to increase our understanding of the mechanistic derivations of exhaustion in this population. Analysis of factors associated with CD4 T-cell exhaustion in other pathologies should be extended into tumor models to evaluate similarities and differences in mechanistic determinants. It remains to be seen whether the processes underlying CD4 T-cell exhaustion are similar across different disease pathologies and CD4 subsets, or whether different convergent transcriptional programs happen to result in the same terminally differentiated fate. Furthermore, in-depth assessment of the potentially differential susceptibility of various CD4 T-cell subsets to exhaustion will be required to increase our understanding of its consequences, as well as the relative contribution of each CD4 T-cell subset to antitumor immunity. Finally, examination of the dynamics of the initiation and progression of CD4 T-cell exhaustion and assessment of the role of tumor cells and the TME in the process will be invaluable to understanding parallels and differences between CD4 and CD8 T-cell exhaustion. Crystallizing these insights will be vital to increase our understanding of CD4 T-cell exhaustion and its therapeutic implications.
Authors' Disclosures
No disclosures were reported.
Acknowledgments
Figures were generated with BioRender.com.
This work was supported by the National Institutes of Health (T32 AI052077 to S.J. Lorrey) and by CRI Lloyd J Old STAR (CRI3922 to P.E. Fecci).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 1998;187:1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993;362:758–61. [DOI] [PubMed] [Google Scholar]
- 3. Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998;188:2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wherry E, Blattman J, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell Immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003;77:4911–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J Virol 2012;86:2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahrends T, Spanjaard A, Pilzecker B, Bąbała N, Bovens A, Xiao Y, et al. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 2017;47:848–61. [DOI] [PubMed] [Google Scholar]
- 7. Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol 1994;68:8056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woroniecka K, Chongsathidkiet P, Rhodin KE, Kemeny HR, Dechant CA, Farber SH, et al. T cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res 2018;24:4175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest 2011;121:2350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EKM, et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010;207:651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010;207:637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takeuchi A, Badr MESG, Miyauchi K, Ishihara C, Onishi R, Guo Z, et al. CRT AM determines the CD4+ cytotoxic T lymphocyte lineage. J Exp Med 2016;213:123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caza T, Landas S. Functional and phenotypic plasticity of CD4+ T cell subsets. Biomed Res Int 2015;2015:521957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Busselaar J, Tian S, van Eenennaam H, Borst J. Helpless priming sends CD8+ T cells on the road to exhaustion. Front Immunol 2020;11:2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther 2021;28:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez-Diez A, Joncker NT, Choi K, Chan WFN, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007;109:5346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCaw TR, Li M, Starenki D, Cooper SJ, Liu M, Meza-Perez S, et al. The expression of MHC class II molecules on murine breast tumors delays T-cell exhaustion, expands the T-cell repertoire, and slows tumor growth. Cancer Immunol Immunother 2019;68:175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 2020;181:1612–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019;37:457–95. [DOI] [PubMed] [Google Scholar]
- 21. Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019;20:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 2020;52:825–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol 2003;170:477–86. [DOI] [PubMed] [Google Scholar]
- 24. Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol 2004;172:4204–14. [DOI] [PubMed] [Google Scholar]
- 25. Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012;338:1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. PNAS 2008;105:15016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007;27:670–84. [DOI] [PubMed] [Google Scholar]
- 29. Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med 2007;204:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science 2016;354:1165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017;545:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott-Browne JP, Ló Pez-Moyado IF, Trifari S, Wong V, Chavez L, Rao A, et al. Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 2016;45:1327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 2016;45:358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Romero P. Metabolic control of CD8 + T cell fate decisions and antitumor immunity. Trends Mol Med 2018;24:30–48. [DOI] [PubMed] [Google Scholar]
- 35. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Hu J, Li Y, Xiao M, Wang H, Tian Q, et al. The transcription factor TCF1 preserves the effector function of exhausted CD8 T cells during chronic viral infection. Front Immunol 2019;10:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin H-T, Anderson AC, Tan WG, West EE, Ha S-J, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. PNAS 2010;107:14733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, et al. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat Commun 2017;8:15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A 2004;101:16004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dow C, Henderson R, Sette A, Mothé BR. CD4+ T-cell inhibitory ligands: a tool for characterizing dysfunctional CD4+ T cells during chronic infection. Immunology 2013;140:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. PNAS 2011;108:21182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 2014;40:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kasprowicz V, Schulze zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, et al. High Level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol 2008;82:3154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noyan K, Nguyen S, Betts MR, Sönnerborg A, Buggert M. Human immunodeficiency virus type-1 elite controllers maintain low co-expression of inhibitory receptors on CD4+ T cells. Front Immunol 2018;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hwang SJ, Cobb DA, Bhadra R, Youngblood B, Khan IA. Blimp-1-mediated CD4 T cell exhaustion causes CD8 T cell dysfunction during chronic toxoplasmosis. J Exp Med 2016;213:1799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raziorrouh B, Ulsenheimer A, Schraut W, Heeg M, Kurktschiev P, Zachoval R, et al. Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology 2011;141:1422–31. [DOI] [PubMed] [Google Scholar]
- 47. Xiao W, Jiang LF, Deng XZ, Zhu DY, Pei JP, Xu ML, et al. PD-1/PD-L1 signal pathway participates in HCV F protein-induced T cell dysfunction in chronic HCV infection. Immunol Res 2016;64:412–23. [DOI] [PubMed] [Google Scholar]
- 48. Jacobi FJ, Wild K, Smits M, Zoldan K, Csernalabics B, Flecken T, et al. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J Hepatol 2019;70:1103–13. [DOI] [PubMed] [Google Scholar]
- 49. Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 2014;9:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barathan M, Gopal K, Mohamed R, Ellegård R, Saeidi A, Vadivelu J, et al. Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis 2015;20:466–80. [DOI] [PubMed] [Google Scholar]
- 51. Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/Galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One 2012;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Antoine P, Olislagers V, Huygens A, Lecomte S, Liesnard C, Donner C, et al. Functional exhaustion of CD4 + T Lymphocytes during primary cytomegalovirus infection. J Immunol 2012;189:2665–72. [DOI] [PubMed] [Google Scholar]
- 53. Vingert B, Benati D, Lambotte O, de Truchis P, Slama L, Jeannin P, et al. HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term. J Virol 2012;86:10661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007;8:1246–54. [DOI] [PubMed] [Google Scholar]
- 55. Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008;205:2763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J 2011;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rallón N, García M, García-Samaniego J, Cabello A, Álvarez B, Restrepo C, et al. Expression of PD-1 and tim-3 markers of T-cell exhaustion is associated with CD4 dynamics during the course of untreated and treated HIV infection. PLoS One 2018;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Popescu I, Drummond MB, Gama L, Lambert A, Hoji A, Coon T, et al. HIV suppression restores the lung mucosal CD4+ T-cell viral immune response and resolves CD8+ T-cell alveolitis in patients at risk for HIV-associated chronic obstructive pulmonary disease. J Infect Dis 2016;214:1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, et al. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 2010;107:19408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clouthier DL, Zhou AC, Wortzman ME, Luft O, Levy GA, Watts TH. GITR intrinsically sustains early type 1 and late follicular helper CD4 T cell accumulation to control a chronic viral infection. PLoS Pathog 2015;11;1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brooks DG, Teyton L, Oldstone MBA, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol 2005;79;10514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zinselmeyer BH, Heydari S, Sacristán C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med 2013;210:757–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perreau M, Vigano S, Bellanger F, Pellaton C, Buss G, Comte D, et al. Exhaustion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders. J Exp Med 2014;211:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, et al. Tumor-necrosis factor impairs CD4+ T cell-mediated immunological control in chronic viral infection. Nat Immunol 2016;17:593–603. [DOI] [PubMed] [Google Scholar]
- 65. Luft O, Khattar R, Farrokhi K, Ferri D, Yavorska N, Zhang J, et al. Inhibition of the fibrinogen-like protein 2:FcγRIIB/RIII immunosuppressive pathway enhances antiviral T-cell and B-cell responses leading to clearance of lymphocytic choriomeningitis virus clone 13. Immunology 2018;154:476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Snell LM, Osokine I, Yamada DH, De la Fuente JR, Elsaesser HJ, Brooks DG. Overcoming CD4 Th1 cell fate restrictions to sustain antiviral CD8 T cells and control persistent virus infection. Cell Rep 2016;16:3286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Foldi J, Kozhaya L, McCarty B, Mwamzuka M, Marshed F, Ilmet T, et al. HIV-infected children have elevated levels of PD-1+ memory CD4 T cells with low proliferative capacity and high inflammatory cytokine effector functions. J Infect Dis 2017;216:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1–mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol 2013;191:5542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maine CJ, Teijaro JR, Marquardt K, Sherman LA. PTPN22 contributes to exhaustion of T lymphocytes during chronic viral infection. Proc Natl Acad Sci U S A 2016;113:E7231–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ciucci T, Vacchio MS, Gao Y, Tomassoni Ardori F, Candia J, Mehta M, et al. The emergence and functional fitness of memory CD4 + T cells require the transcription factor Thpok. Immunity 2019;50:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ames RY, Ting LM, Gendlina I, Kim K, Macian F. The transcription factor NFAT1 participates in the induction of CD4+ T cell functional exhaustion during Plasmodium yoelii infection. Infect Immun 2017;85:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stone SF, Price P, French MA. Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy. HIV Med 2005;6:278–83. [DOI] [PubMed] [Google Scholar]
- 73. Salwe S, Singh A, Padwal V, Velhal S, Nagar V, Patil P, et al. Immune signatures for HIV-1 and HIV-2 induced CD4 + T cell dysregulation in an Indian cohort. BMC Infect Dis 2019;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Woroniecka KI, Rhodin KE, Dechant C, Cui X, Chongsathidkiet P, Wilkinson D, et al. 4–1BB agonism averts Til exhaustion and licenses PD-1 blockade in glioblastoma and other intracranial cancers. Clin Cancer Res 2020;26:1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim K, Park S, Park SY, Kim G, Park SM, Cho J-W, et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med 2020;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shi F, Chang H, Zhou Q, Zhao Y-J, Wu G-J, Song Q-K. Distribution of CD4+ and CD8+ exhausted tumor-infiltrating lymphocytes in molecular subtypes of Chinese breast cancer patients. Onco Targets Ther 2018;11:6139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ryan N, Anderson K, Volpedo G, Hamza O, Varikuti S, Satoskar AR, et al. STAT1 inhibits T-cell exhaustion and myeloid derived suppressor cell accumulation to promote antitumor immune responses in head and neck squamous cell carcinoma. Int J Cancer 2020;146:1717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nair VS, Toor SM, Taha RZ, Ahmed AA, Kurer MA, Murshed K, et al. Transcriptomic profiling of tumor-infiltrating CD4+ TIM-3+ T cells reveals their suppressive, exhausted, and metastatic characteristics in colorectal cancer patients. Vaccines 2020;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mittal R, Chen CW, Lyons JD, Margoles LM, Liang Z, Coopersmith CM, et al. Murine lung cancer induces generalized T-cell exhaustion. J Surg Res 2015;195:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prado-Garcia H, Romero-Garcia S, Puerto-Aquino A, Rumbo-Nava U. The PD-L1/PD-1 pathway promotes dysfunction, but not “exhaustion”, in tumor-responding T cells from pleural effusions in lung cancer patients. Cancer Immunol Immunother 2017;66:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rad Pour S, Morikawa H, Kiani NA, Yang M, Azimi A, Shafi G, et al. Exhaustion of CD4+ T-cells mediated by the kynurenine pathway in melanoma. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tan J, Chen S, Huang J, Chen Y, Yang L, Wang C, et al. Increased exhausted CD8 + T cells with programmed death-1, T-cell immunoglobulin and mucin-domain-containing-3 phenotype in patients with multiple myeloma. Asia Pac J Clin Oncol 2018;14:e266–e74. [DOI] [PubMed] [Google Scholar]
- 83. Nakano M, Ito M, Tanaka R, Yamaguchi K, Ariyama H, Mitsugi K, et al. PD-1+ TIM-3+ T cells in malignant ascites predict prognosis of gastrointestinal cancer. Cancer Sci 2018;109:2986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yuan L, Xu B, Yuan P, Zhou J, Qin P, Han L, et al. Tumor-infiltrating CD4+ T cells in patients with gastric cancer. Cancer Cell Int 2017;17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Allahmoradi E, Taghiloo S, Tehrani M, Hossein-Nattaj H, Janbabaei G, Shekarriz R, et al. CD4+ T cells are exhausted and show functional defects in chronic lymphocytic leukemia. Iran J Immunol 2017;14:257–69. [PubMed] [Google Scholar]
- 86. Tang L, Wu J, Li CG, Jiang HW, Xu M, Du M, et al. Characterization of immune dysfunction and identification of prognostic immune-related risk factors in acute myeloid leukemia. Clin Cancer Res 2020;26:1763–72. [DOI] [PubMed] [Google Scholar]
- 87. Tan J, Huang S, Huang J, Yu Z, Chen Y, Lu Y, et al. Increasing Tim-3+CD244+, Tim-3+CD57+, and Tim-3+PD-1+ T cells in patients with acute myeloid leukemia. Asia Pac J Clin Oncol 2020;16:137–41. [DOI] [PubMed] [Google Scholar]
- 88. Solman IG, Blum LK, Hoh HY, Kipps TJ, Burger JA, Barrientos JC, et al. Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leuk Res 2020;97:106432. [DOI] [PubMed] [Google Scholar]
- 89. Rusak M, Eljaszewicz A, Bołkun Ł, Łuksza E, Łapuć I, Piszcz J, et al. Prognostic significance of PD-1 expression on peripheral blood CD4+ T cells in patients with newly diagnosed chronic lymphocytic leukemia. Pol Arch Med Wewn 2015;125:553–9. [DOI] [PubMed] [Google Scholar]
- 90. Blaeschke F, Willier S, Stenger D, Lepenies M, Horstmann MA, Escherich G, et al. Leukemia-induced dysfunctional TIM-3+CD4+ bone marrow T cells increase risk of relapse in pediatric B-precursor ALL patients. Leukemia 2020;34:2607–20. [DOI] [PubMed] [Google Scholar]
- 91. Dama P, Tang M, Fulton N, Kline J, Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer 2019;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tan J, Yu Z, Huang J, Chen Y, Huang S, Yao D, et al. Increased PD-1+Tim-3+ exhausted T cells in bone marrow may influence the clinical outcome of patients with AML. Biomark Res 2020;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shi W, Yang B, Sun Q, Meng J, Zhao X, Du S, et al. PD-1 regulates CXCR5+ CD4 T cell-mediated proinflammatory functions in non-small cell lung cancer patients. Int Immunopharmacol 2020;82:106295. [DOI] [PubMed] [Google Scholar]
- 94. Zhou ZQ, Tong DN, Guan J, Tan HW, Zhao LD, Zhu Y, et al. Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and B cell help, and is associated with advanced tumor stages. Am J Transl Res 2016;8:2926–36. [PMC free article] [PubMed] [Google Scholar]
- 95. Goods BA, Hernandez AL, Lowther DE, Lucca LE, Lerner BA, Gunel M, et al. Functional differences between PD-1+ and PD-1- CD4+ effector T cells in healthy donors and patients with glioblastoma multiforme. PLoS One 2017;12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pirozyan MR, McGuire HM, Emran AA, Tseng H-Y, Tiffen JC, Lee JH, et al. Pretreatment innate cell populations and CD4 T cells in blood are associated with response to immune checkpoint blockade in melanoma patients. Front Immunol 2020;11:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Formenti SC, Hawtin RE, Dixit N, Evensen E, Lee P, Goldberg JD, et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J Immunother Cancer 2019;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhu S, Lin J, Qiao G, Wang X, Xu Y. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology 2016;221:986–93. [DOI] [PubMed] [Google Scholar]
- 99. Blanco G, Puiggros A, Sherry B, Nonell L, Calvo X, Puigdecanet E, et al. Chronic lymphocytic leukemia–like monoclonal B-cell lymphocytosis exhibits an increased inflammatory signature that is reduced in early-stage chronic lymphocytic leukemia. Exp Hematol 2021;95:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56:1342–51. [DOI] [PubMed] [Google Scholar]
- 101. Rausch MP, Hastings KT. An exhaustion-like phenotype constrains the activity of CD4+ T cells specific for a self and melanoma antigen. PLoS One 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Malandro N, Budhu S, Kuhn NF, Liu C, Murphy JT, Cortez C, et al. Clonal abundance of tumor-specific CD4+ T cells potentiates efficacy and alters susceptibility to exhaustion. Immunity 2016;44:179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, et al. Blockade of TIGIT/CD155 signaling reverses t-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res 2019;7:1700–13. [DOI] [PubMed] [Google Scholar]
- 104. Dubinski D, Wölfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, et al. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol 2016;18:807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhou H, Liu T, Wang Z. Analysis of non-small cell lung cancer microenvironment indicates preponderance of T cell exhaustion marker expression. Exp Cell Res 2017;360:205–9. [DOI] [PubMed] [Google Scholar]
- 106. Oja AE, Piet B, Van Der Zwan D, Blaauwgeers H, Mensink M, De Kivit S, et al. Functional heterogeneity of CD4+ tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front Immunol 2018;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fu J, Yu A, Xiao X, Tang J, Zu X, Chen W, et al. CD4+ T cell exhaustion leads to adoptive transfer therapy failure which can be prevented by immune checkpoint blockade. Am J Cancer Res 2020;10:4234–50. [PMC free article] [PubMed] [Google Scholar]
- 108. Balança C-C, Salvioni A, Scarlata C-M, Michelas M, Martinez-Gomez C, Gomez-Roca C, et al. PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1 hi CD39 + CD4 T cells. JCI Insight 2021;6:e142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sekine T, Perez-Potti A, Nguyen S, Gorin JB, Wu VH, Gostick E, et al. TOX is expressed by exhausted and polyfunctional human effector memory CD8+ T cells. Science Immunology 2020;5:eaba7918. [DOI] [PubMed] [Google Scholar]
- 110. Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 2019;571:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Catakovic K, Gassner FJ, Ratswohl C, Zaborsky N, Rebhandl S, Schubert M, et al. TIGIT expressing CD4+T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. OncoImmunology 2017;7:e1371399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, et al. Restoring immune function of tumor-specific CD4 + T cells during recurrence of melanoma. J Immunol 2013;190:4899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Goding SR, Wilson KA, Rosinsky C, Antony PA. PD-L1–independent mechanisms control the resistance of melanoma to CD4 + T cell adoptive immunotherapy. J Immunol 2018;200:3304–11. [DOI] [PubMed] [Google Scholar]
- 114. Lowther DE, Goods BA, Lucca LE, Lerner BA, Raddassi K, van Dijk D, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 2016;1:e85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kalathil SG, Wang K, Hutson A, Iyer R, Thanavala Y. Tivozanib mediated inhibition of c-Kit/SCF signaling on Tregs and MDSCs and reversal of tumor induced immune suppression correlates with survival of HCC patients. OncoImmunology 2020;9:1824863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 2006;66:3294–302. [DOI] [PubMed] [Google Scholar]
- 117. Andaloussi AE, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme1. Neuro-oncol 2006;8:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Alonso R, Flament H, Lemoine S, Sedlik C, Bottasso E, Péguillet I, et al. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat Commun 2018;9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abe BT, Shin DS, Mocholi E, Macian F. NFAT1 supports tumor-induced anergy of CD4+ T cells. Cancer Res 2012;72:4642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fornara O, Odeberg J, Wolmer Solberg N, Tammik C, Skarman P, Peredo I, et al. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. OncoImmunology 2015;4:e1036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schietinger A, Greenberg PD. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol 2014;35:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T cell dysfunction in cancer immunity and immunotherapy. Front Immunol 2019;10:1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol 2018;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol 2020;17:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Porrett PM, Lee MK, Lian MM, Wang J, Caton AJ, Deng S, et al. A direct comparison of rejection by CD8 and CD4 T cells in a transgenic model of allotransplantation. Arch Immunol Ther Exp 2008;56:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol 2001;167:1891–9. [DOI] [PubMed] [Google Scholar]
- 127. Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4 + T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol 2004;172:5456–66. [DOI] [PubMed] [Google Scholar]
- 128. Mcdonald-Hyman C, Flynn R, Panoskaltsis-Mortari A, Peterson N, Macdonald KPA, Hill GR, et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood 2016;128:1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003;9:1144–50. [DOI] [PubMed] [Google Scholar]
- 130. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002;99:3493–9. [DOI] [PubMed] [Google Scholar]
- 131. Lucas F, Pennell M, Huang Y, Benson DM, Efebera YA, Chaudhry M, et al. T cell transcriptional profiling and immunophenotyping uncover LAG3 as a potential significant target of immune modulation in multiple myeloma. Biol Blood Marrow Transplant 2020;26:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Simonetta F, Pradier A, Bosshard C, Masouridi-Levrat S, Dantin C, Koutsi A, et al. Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front Immunol 2019;10:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bettonville M, D'aria S, Weatherly K, Porporato PE, Zhang J, Bousbata S, et al. Long-term antigen exposure irreversibly modifies metabolic requirements for T cell function. eLife 2018;7:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu Y, Ji H, Zhang Y, Shen XD, Gao F, Nguyen TT, et al. Negative CD4+TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation. Am J Transplant 2015;15:954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wu J, Zhang H, Shi X, Xiao X, Fan Y, Minze LJ, et al. Ablation of transcription factor IRF4 promotes transplant acceptance by driving allogenic CD4+ T cell dysfunction. Immunity 2017;47:1114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sarraj B, Ye J, Akl AI, Chen G, Wang JJ, Zhang Z, et al. Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection. Proc Natl Acad Sci U S A 2014;111:12145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Raphael I, Joern RR, Forsthuber TG. Memory CD4+ T cells in immunity and autoimmune diseases. Cells 2020;9:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chemin K, Gerstner C, Malmström V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation—lessons from rheumatoid arthritis. Front Immunol 2019;10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Koohini Z, Hossein-Nataj H, Mobini M, Hosseinian-Amiri A, Rafiei A, Asgarian-Omran H. Analysis of PD-1 and Tim-3 expression on CD4+ T cells of patients with rheumatoid arthritis; negative association with DAS28. Clin Rheumatol 2018;37:2063–71. [DOI] [PubMed] [Google Scholar]
- 140. Frenz T, Grabski E, Buschjäger D, Vaas LAI, Burgdorf N, Schmidt RE, et al. CD4+ T cells in patients with chronic inflammatory rheumatic disorders show distinct levels of exhaustion. J Allergy Clin Immunol 2016;138:586–9. [DOI] [PubMed] [Google Scholar]
- 141. Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med 2012;18:1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 2019;567:525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun 2015;6:6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Swainson LA, Mold JE, Bajpai UD, McCune JM. Expression of the autoimmune susceptibility gene FcRL3 on human regulatory T cells is associated with dysfunction and high levels of programmed cell death-1. J Immunol 2010;184:3639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zheng H, Liu X, Zhang J, Rice SJ, Wagman M, Kong Y, et al. Expression of PD-1 on CD4+ T cells in peripheral blood associates with poor clinical outcome in non-small cell lung cancer. Oncotarget 2016;7:56233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Satpathy AT, Granja JM, Yost KE, Qi Y, Meschi F, McDermott GP, et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat Biotechnol 2019;37:925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ozkazanc D, Yoyen-Ermis D, Tavukcuoglu E, Buyukasik Y, Esendagli G. Functional exhaustion of CD4+ T cells induced by co-stimulatory signals from myeloid leukaemia cells. Immunology 2016;149:460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mozaffari F, Hansson L, Kiaii S, Ju X, Rossmann ED, Rabbani H, et al. Signalling molecules and cytokine production in T cells of multiple myeloma-increased abnormalities with advancing stage. Br J Haematol 2004;124:315–24. [DOI] [PubMed] [Google Scholar]