Abstract
Malignant pleural diseases, comprising metastatic lung and breast cancers and malignant pleural mesothelioma (MPM), are aggressive solid tumors with poor therapeutic response. We developed and conducted a first-in-human, phase I study of regionally delivered, autologous, mesothelin-targeted chimeric antigen receptor (CAR) T-cell therapy. Intrapleural administration of 0.3M-60M CAR T cells/kg in 27 patients (25 with MPM) was safe and well tolerated. CAR T-cells were detected in peripheral blood for >100 days in 39% of patients. Following our demonstration that PD-1 blockade enhances CAR T-cell function in mice, 18 patients with MPM also received pembrolizumab safely. Among those patients, median overall survival from CAR T-cell infusion was 23.9 months (1-year overall survival, 83%). Stable disease was sustained for ≥6 months in 8 patients; 2 exhibited complete metabolic response on PET scan. Combination immunotherapy with CAR T cells and PD-1 blockade agents should be further evaluated in patients with solid tumors.
Keywords: CAR T-cell therapy, PD-1, PD-L1, Regional therapy, Immune checkpoint inhibitor agents, Adoptive cell therapy, Phase I clinical trial
INTRODUCTION
Malignant pleural diseases, comprising metastatic lung and breast cancers and mesothelioma, are aggressive solid tumors. Malignant pleural mesothelioma (MPM) is an aggressive cancer characterized by resistance to treatment and poor survival (1). Median overall survival (OS) for patients with MPM following first-line treatment comprising cisplatin and pemetrexed is 13 to 16 months; the addition of bevacizumab prolongs OS to 18.8 months, albeit at the cost of increased toxicity (2). We and others have shown that immune responses are prognostic in patients with MPM (3-6). Immune checkpoint inhibitor (ICI) therapy has been investigated in MPM, a solid tumor with a low tumor mutational burden (TMB). Programmed death ligand 1 (PD-L1) expression is very low in patients with epithelioid histologic profile, the most common type of MPM (7). This is consistent with the lack of improved survival in patients with MPM with single-agent checkpoint blockade inhibitors (Table S1) (8). The National Comprehensive Cancer Network guidelines for MPM include the use of ICIs as second-line treatment. A recent phase III trial of first-line dual nivolumab and ipilimumab therapy in unresectable MPM reported a median OS of 18.1 months, compared with 14.1 months for standard-of-care chemotherapy (9). This dual-ICI-agent drug regimen was approved by the FDA in October 2020 (https://www.fda.gov/news-events/press-announcements/fda-approves-drug-combination-treating-mesothelioma), 16 years after the previous approval of any systemic therapy for MPM.
Chimeric antigen receptor (CAR) T-cell therapy induces durable and curative responses in patients with hematological malignancies, but attempts to treat solid tumors with this approach have so far met with limited success (10,11). The obstacles to effectively treating solid tumors with T-cell therapy include heterogenous antigen expression, an inability to achieve T-cell infiltration of the tumor, and inhibition of CAR T-cell function by an immunosuppressive microenvironment (12,13). To address these obstacles, we developed a regionally-delivered mesothelin-targeted CAR T-cell therapy (14,15) and translated it for use in clinical trials. We selected mesothelin as a target cell-surface antigen because of its overexpression (14,16) and association with aggressiveness—malignant transformation, cancer cell invasion, proliferation, and metastases (17-21)—in a majority of MPM and other solid tumors, together with its low levels of expression in normal tissues (22). All of the components in our CAR construct are fully human, including the single-chain variable fragment (scFv), an intentional choice to avoid human anti-mouse antibody reactions observed in some trials using CARs that comprised an scFv of murine origin.
As MPM is locoregionally aggressive (23,24), we investigated regional versus systemic CAR T-cell administration in an orthotopic MPM mouse model: regional CAR T-cell therapy achieved superior efficacy at lower doses by avoiding sequestration of CAR T cells in lungs and by augmenting CD4 helper function (15). Regionally activated CAR T cells were able to circulate and establish systemic immunity.
In our clinically relevant mouse model, we observed that even costimulated CAR T cells became functionally exhausted when faced with large tumor burdens, in part because of inhibitory programmed death 1 (PD-1)/PD-L1 signaling. We demonstrated that the administration of anti-PD-1 agents rescues function in exhausted CAR T cells and enhances antitumor efficacy (25,26).
We therefore initiated an open-label, dose-escalating, single-center, first-in-human phase I trial of intrapleural delivery of mesothelin-targeted CAR T cells in patients with previously treated histologically proven pleural cancer from MPM, metastatic lung cancer, or metastatic breast cancer. Patients with mesothelin expression in at least 10% of tumor cells on immunohistochemical analysis and/or serum soluble mesothelin related peptide (SMRP) >1.0 nM/L were eligible (as detailed in the protocol [see Supplementary Material]). We demonstrate the feasibility and safety of this approach and report our experience in a subcohort of patients who received pembrolizumab after CAR T cells.
RESULTS
Patients and Treatment
From November 2015 to April 2019, 71 patients with malignant pleural disease were screened: 36 patients were not eligible, and 35 patients underwent leukapheresis (Figure 1A). Two patients did not undergo cell production; 6 patients who had cells produced did not undergo infusion (Figure 1A). In total, 27 patients were infused: 25 with MPM, 1 with metastatic lung cancer, and 1 with metastatic breast cancer (Figure 1B). All patients had received ≥1 prior line(s) of therapy (median, 2; range, 1-13); 33% of patients had received ≥3 prior lines.
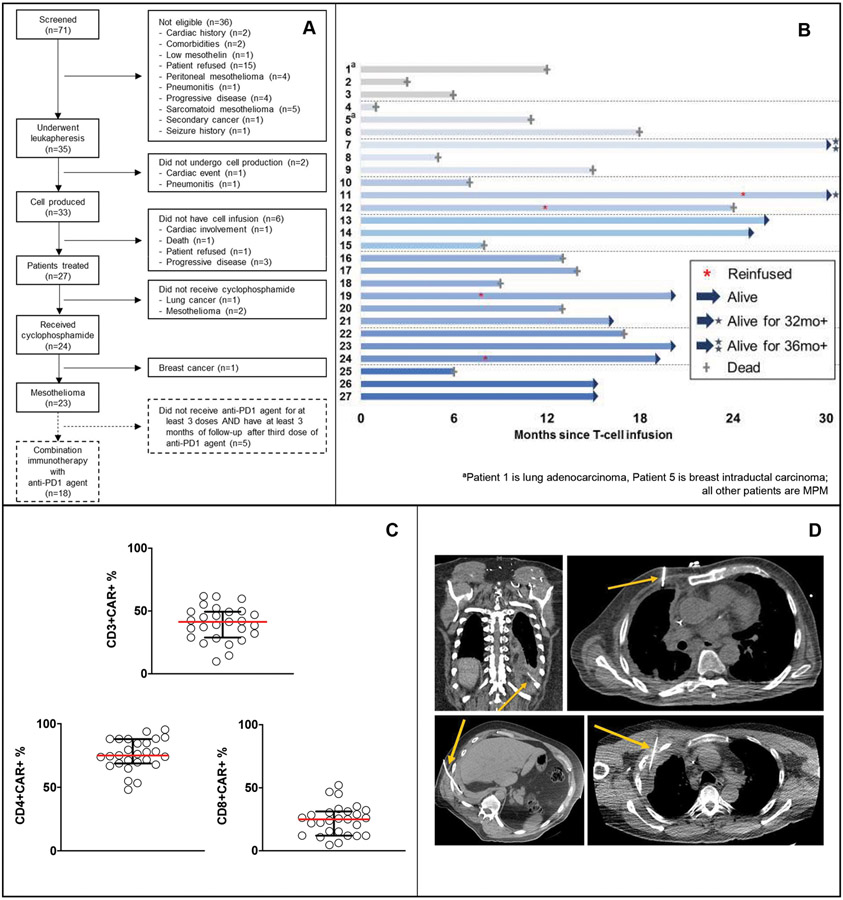
Figure 1. Clinical details of patients with primary or metastatic pleural malignancy treated in the phase I clinical trial (N=27).
A, A single dose of mesothelin-targeted CAR T cells was administered by intrapleural administration without (cohort 1, patients 1-3) or with (cohorts 2-8, patients 4-27) cyclophosphamide preconditioning. All patients had histologically proven pleural malignancy (patient 1, non-small cell lung cancer; patient 5, human epidermal growth factor receptor 2–negative breast cancer; all others, MPM). B, The swimmer plot demonstrates each patient as a bar, with alive or dead status indicated as of June 2020. Four patients received reinfusion of CAR T cells (*). C, CAR T-cell transduction (median, 41% [range, 10%-62%]) was successful in both CD4 and CD8 T cells – percentages of CD4+ CAR+ (median, 75%; range, 48%-95%) and CD8+ CAR+ (median, 25%; range, 5%-52%). Circles represent individual data points corresponding to a single patient across all dose cohorts. Horizontal lines indicate median values, with interquartile ranges shown for the group. D, CAR T cells were administered intrapleurally either through a pleural catheter (n=11) or via intervention radiology–guided imaging (n=16).
Patients in cohort 1 (2 MPM, 1 metastatic lung cancer) did not receive cyclophosphamide preconditioning. Of the 24 patients who received cyclophosphamide, 23 presented with MPM, and 1 presented with metastatic breast cancer (Table 1). Of the 23 patients with MPM, 17 (74%) had clinical stage 2 to 4 disease at enrollment, and 6 (26%) had relapsed disease (Table 2). Before CAR T-cell infusion, 11 patients with MPM (48%) had progressive disease (PD), and 12 (52%) had stable disease (SD) from prior lines of therapy (Table 2). Median mesothelin expression in the tumor was 100% (range, 25%-100%), median serum SMRP level at the time of infusion was 2.7 nM (range, 0.9-17.5 nM), median tumor mutational burden (TMB) was 2.6 (range 0-4.9), and median PD-L1 percentage was 0 (range, 0%-80%) (Table S1). Median time from diagnosis to T-cell infusion was 6.1 months (range, 2.9-73.3 months) (Table 2).
Table 1.
Clinical characteristics of patients treated in the phase I trial
| Cohort | Patient No. |
Age | Sex | Diagnosis | Histologic Subtype |
Stage | Route of Administration |
PD-L1 | Mesothelin | ICI Started, Week |
ICI Doses, No. |
CD3+ CAR+, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 3e5/kg (no cyclo) | 1 | 59 | F | Lung Cancer | Adenocarcinoma | R | Catheter | - | 10% | - | - | 37 |
| 2 | 69 | M | Mesothelioma | Epithelioid | R | Catheter | 0 | 100% | 9 | 1 | 23 | |
| 3 | 66 | F | Mesothelioma | Epithelioid | R | Catheter | 0 | 100% | - | - | 28 | |
| 2 3e5/kg | 4 | 56 | M | Mesothelioma | Epithelioid | R | Catheter | 0 | - | - | - | 36 |
| 5 | 70 | F | Breast Cancer | Intraductal carcinoma | IV | IR | 0 | - | 5 | 4 | 43 | |
| 6 | 72 | M | Mesothelioma | Biphasic | IIIA | IR | 1% | 25% | 6 | 21 | 41 | |
| 3 1e6/kg | 7 | 70 | M | Mesothelioma | Epithelioid | IIIA | Catheter | 30% | 100% | - | - | 52 |
| 8 | 73 | M | Mesothelioma | Epithelioid | IIIB | Catheter | 0 | 100% | - | - | 39 | |
| 9 | 66 | M | Mesothelioma | Epithelioid | R | IR | - | - | 17 | 10 | 62 | |
| 4 3e6/kg | 10 | 70 | M | Mesothelioma | Epithelioid | IIIB | Catheter | 0 | 100% | 6 | 5 | 60 |
| 11a | 74 | M | Mesothelioma | Epithelioid | IIIB | Catheter | 10% | 100% | 6 / 4 | 23 | 45 | |
| 12a | 66 | M | Mesothelioma | Epithelioid | IIIB | Catheter | 0 | 100% | 5 / 9 | 19 | 35 | |
| 5 6e6/kg | 13 | 76 | M | Mesothelioma | Epithelioid | IIIA | IR | 0 | 100% | 6 | 29 | 55 |
| 14 | 69 | M | Mesothelioma | Epithelioid | IIIA | IR | 0 | 100% | 7 | 30 | 46 | |
| 15 | 71 | M | Mesothelioma | Epithelioid | IIIB | Catheter | 5% | 95% | 8 | 5 | 27 | |
| 6 1e7/kg | 16 | 77 | F | Mesothelioma | Epithelioid | R | IR | 80% | 95% | 6 | 8 | 32 |
| 17 | 71 | M | Mesothelioma | Biphasic | IIIA | IR | 0 | 99% | 6 | 1 | 10 | |
| 18 | 53 | M | Mesothelioma | Epithelioid | IV | IR | 0 | 80% | 6 | 4 | 42 | |
| 19a | 64 | M | Mesothelioma | Epithelioid | IIIB | IR | 0 | 90% | 6 / 4 | 23 | 29 | |
| 20 | 70 | M | Mesothelioma | Epithelioid | IIIA | Catheter | 0 | 100% | 6 | 7 | 49 | |
| 21 | 61 | F | Mesothelioma | Epithelioid | IIIB | IR | 0 | 99% | 5 | 22 | 39 | |
| 7 3e7/kg | 22 | 73 | M | Mesothelioma | Epithelioid | IIIB | IR | 0 | 80% | 5 | 3 | 36 |
| 23 | 71 | F | Mesothelioma | Epithelioid | IV | IR | 0 | 100% | 8 | 14 | 50 | |
| 24a | 70 | M | Mesothelioma | Epithelioid | R | IR | 0 | 100% | 6 / 4 | 9 | 44 | |
| 8 6e7/kg | 25 | 55 | M | Mesothelioma | Epithelioid | R | IR | 0 | 99% | 5 | 2 | 62 |
| 26 | 61 | M | Mesothelioma | Epithelioid | R | IR | 0 | 100% | 4 | 22 | 24 | |
| 27 | 77 | M | Mesothelioma | Epithelioid | II | IR | 5% | 80% | 7 | 19 | 15 |
ICI, immune checkpoint inhibitor; IR, intervention radiology; MSLN, mesothelin; PD-L1, programmed death ligand 1; PT, patient; R, relapse.
Patients 11, 12, 19, and 24 were administered a second dose of mesothelin-targeted CAR T cells intrapleurally at weeks 110, 51, 34, and 36.
Table 2.
Demographic and clinical characteristics and outcomes of patients with malignant pleural mesothelioma
| Characteristic | Cyclophosphamide (N=23) |
Cyclophosphamide without pembrolizumab (N=5) |
Cyclophosphamide and pembrolizumaba (N=18) |
|---|---|---|---|
| Age, median (range), years | 70 (53–77) | 70 (55–73) | 70 (53–77) |
| Sex | |||
| Male | 20 (87) | 5 (100) | 15 (83) |
| Female | 3 (13) | 0 (0) | 3 (17) |
| ECOG status | |||
| 0 | 15 (65) | 4 (80) | 11 (61) |
| 1 | 8 (35) | 1 (20) | 7 (39) |
| 2 | 0 (0) | 0 (0) | 0 (0) |
| Body mass index, kg/m2 | 27.5 (19.9–40.9) | 26.0 (19.9–28.1) | 27.8 (22.6–40.9) |
| Mesothelioma histological subtype | |||
| Epithelioid | 21 (91) | 4 (80) | 17 (94) |
| Biphasic | 2 (9) | 1 (20) | 1 (6) |
| Clinical stage | |||
| 2 | 1 (4) | 0 (0) | 1 (6) |
| 3 to 4 | 16 (70) | 3 (60) | 13 (72) |
| Relapse | 6 (26) | 2 (40) | 4 (22) |
| Previous anticancer regimens | 1 (1–13) | 4 (1–13) | 1 (1–6) |
| Serum SMRP level, nm | 2.7 (0.9–17.5) | 3.3 (1.2–17.5) | 2.4 (0.9–11.8) |
| Tumor mesothelin expression, % | 100 (25–100)b | 100 (99–100)g | 100 (25–100)c |
| Tumor mutational burden, mt/Mb | 2.6 (0–4.9)d | 2.6 (2.6–3.0)g | 1.9 (0–4.9)e |
| PD-L1, % | 0 (0–80)f | 0 (0–30) | 0 (0–80)c |
| Time from diagnosis to T-cell infusion, months | 6.1 (2.9–73.3) | 18.5 (2.9–73.3) | 5.9 (4–52.1) |
| Overall survival (95% CI) since T-cell infusion, months | 17.7 (13.2–NE) | 6.1 (4.6–NE) | 23.9 (14.7–NE) |
Data are median (range) or no. (%). Patient characteristics were similar between those treated with CAR T cells alone and those in combination with pembrolizumab. ECOG, Eastern Cooperative Oncology Group; NE, not estimable; PD-L1, programmed death-ligand 1; SMRP, soluble mesothelin-related peptide.
Patients received at least 3 doses of pembrolizumab and had at least 3 months of follow-up after the third dose of pembrolizumab.
N=21.
N=17.
N=20.
N=16.
N=22.
N=4.
CAR T-Cell Manufacturing
The mesothelin-specific CAR was engineered as a fusion protein encoding a fully human scFv (m912) and the CD28/CD3ζ sequences, inserted in the SFG gamma-retroviral vector. The CAR was linked to iCaspase-9 via an EMCV internal ribosomal entry site (15,27-30). Details of the CAR T-cell manufacturing process (31) and analyses are described in the protocol (provided in Supplementary Material). The median time from apheresis to infusion of the product was 3.2 months (range, 1.1-20.2 months). There were no production failures. Median CD3+ CAR T-cell transduction was 41% (range, 10%-62%) (Figure 1C), without notable differences in CAR T-cell phenotype across cohorts (Figure S1A-D). Patients with clinical benefit and without significant adverse events following the first dose of CAR T cells were considered for reinfusion with a second dose of CAR T cells.
Safety
Following a single dose of preconditioning cyclophosphamide (1500 mg/m2), mesothelin-targeted CAR T cells (0.3M-60M CAR T cells/kg) with the iCaspase-9 safety gene (27) were administered intrapleurally either through a pleural catheter after complete evacuation of pleural effusion in 11 patients, (41%) or via interventional radiology–guided imaging in 16 patients (59%) (Figure 1D, Table 1). Leveraging our interventional radiology expertise, we were able to infuse even in patients with fused pleural cavities, including patients who have undergone thoracotomy or hemithoracic radiation, with no interventional failures (intervention was required at 2 different sites in 3 patients). The site of administration was chosen on the basis of safety and ease of accessibility by either CT- or ultrasound-guided access or a combination of both methods.
Dose escalation is detailed in Table 1. Three patients in cohort 1 were treated without cyclophosphamide preconditioning as a precautionary safety measure. From cohort 2 onwards, 3 patients were treated at each dose level, and dose-escalation continued to dose level 8 (3 additional patients were treated at dose level 6; these patients underwent apheresis and cell manufacturing at a lower dose while on a prior treatment and were treated upon disease progression). There were no dose-limiting toxicities. There were no grade 5 adverse events. Table S2 lists the treatment-emergent adverse events that occurred in ≥15% of the cohort (n=27 patients); all adverse events 1-month postinfusion are detailed in Table S3. All grade 4 adverse events were reversible laboratory abnormalities associated with lymphodepleting chemotherapy. Six patients experienced grade 3 clinical adverse events: constipation (2 patients) and dysphagia, dyspnea following reinfusion, thromboembolic event, and febrile neutropenia (1 patient each). No patients experienced cytokine release syndrome grade >2, neurotoxicity grade >2, or on-target, off-tumor toxicity. The maximum tolerated dose was not reached. Given the absence of toxicities, the iCaspase-9 safety switch was not activated in any patient. Four patients (15%) received a second intrapleural infusion at the time of disease progression, 7 to 24 months after demonstration of safety and clinical benefit from the first dose.
Peak levels of C-reactive protein were observed within 72 h of infusion and during episodes of fever (n=14 patients) (Figure S2A-C). One patient was admitted to the intensive care unit as a precaution for monitoring following reinfusion. Two patients required IL-6 blockade (tocilizumab, maximum of 2 doses), 1 each after infusion and reinfusion. Eleven patients (41%) were readmitted for monitoring, mostly within 2 weeks for fever, fatigue, and malaise. Serum IL-6 levels >50 pg/mL during the first week of treatment were associated with readmission for fever (Figure S2D).
Two patients had grade 1 pleuritic pain, 3 had pleural effusion (grade 1, 2 patients; grade 2, 1 patient), and 1 had pericardial effusion (grade 2), following reinfusion (Figure S3A-H). In 2 patients with pleural metastatic non-small cell lung cancer (Patient #1) or breast cancer (Patient #5), no adverse events were noted. These patients received multiple lines of therapy after CAR T cells and survived 12 and 11 months, respectively.
Outcomes Analyses in Patients with MPM
The outcomes-analysis population included 23 patients with MPM who received cyclophosphamide preconditioning plus CAR T cells (Table 2). Results are presented across dose levels, as no dose-limiting toxicities were observed. At treatment-assessment cutoff (June 2020), median follow-up was 20.3 months (interquartile range, 19.2-26.1 months) (Figure 1B), median OS since T-cell infusion was 17.7 months (95% CI, 13.2 months to not estimable [NE]), and 1-year OS was 74% (95% CI, 58%-94%) (Figure 2A-C).
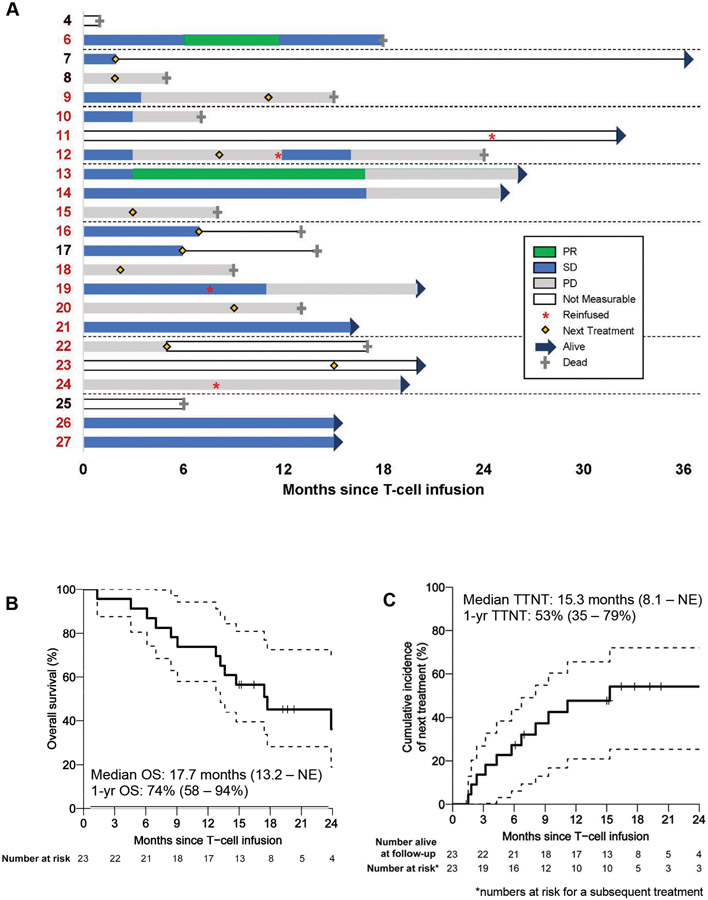
Figure 2. Outcomes of patients with MPM (n=23).
A, Therapy responses in patients with MPM (3 did not have measurable disease, 1 had no subsequent scan available) or disease stabilization or progression until next treatment during a period (0 to 36 months), as monitored by mRECIST on CT scan, are shown—PR (green), SD (blue), and PD (gray) are represented in relation to time in months. Solid line indicates survival post-next treatment. Patients who received combination immunotherapy are represented in red type. B, The Kaplan-Meier curve reports overall survival (OS) of patients with malignant pleural mesothelioma after CAR T-cell infusion (median survival, 17.7 months [95% confidence interval, 13.2 months to not estimable {NE}]). C, The time-to-next-treatment (TTNT) curve shows the proportion of patients receiving next treatment over time (median time to next treatment, 15.3 months [95% confidence interval, 8.1 months to NE]).
Twenty-two of the 23 patients with MPM had a decrease in serum SMRP following CAR T-cell infusion (Figures S4, S5A-B); CAR T cells were detectable in peripheral blood within 4 days in 87% of patients, for >100 days in 39% of patients, and for >200 days in 17% of patients (Figure 3A). In 2 patients, CAR T cells were detected in pleural tumor at weeks 25 and 32 by polymerase chain reaction (PCR).
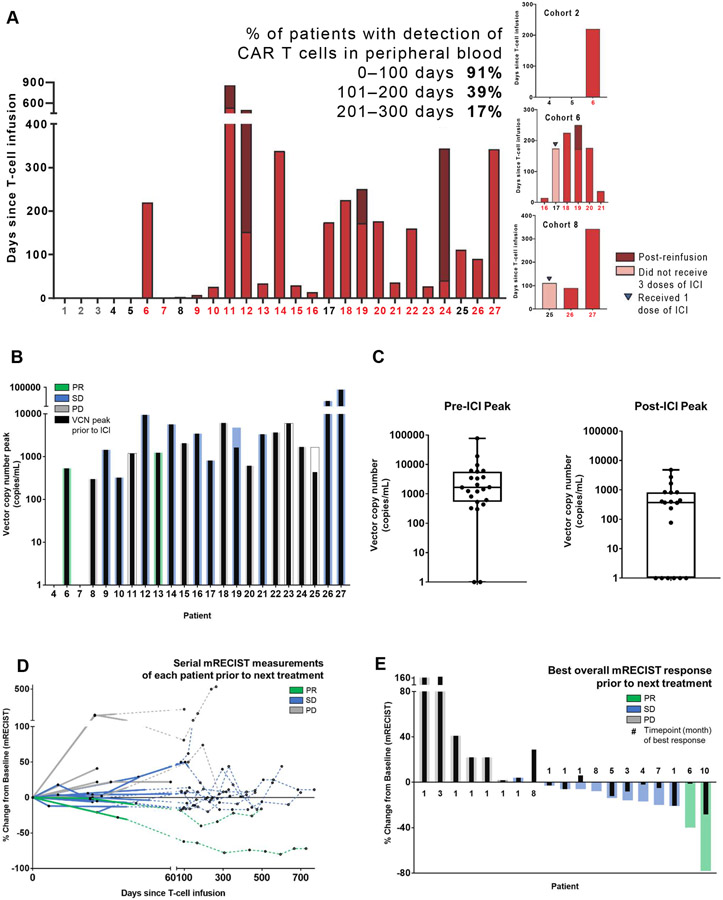
Figure 3. Outcomes analyses of patients with MPM (n=23).
A, Presence of CAR T cells in serially collected peripheral blood samples from each patient was detected and quantified using a multiplex real-time PCR assay. CAR T cells (as quantified by vector copy number per milliliter) can be detected in peripheral blood as early as 1 to 5 days after intrapleural administration and as late as 100 to 300 days in a majority of patients. Duration of detection of CAR T cells by PCR in the peripheral blood is shown. No CAR T cells were detected in the peripheral blood of patients treated in cohort 1. Patients who received combination therapy are represented in red color on the X-axis; patients who received reinfusion are represented by stacked bar graphs. B, Following intrapleural administration, CAR T cells can be detected in peripheral blood as early as 1 to 5 days in a majority of patients and as late as 100 to 300 days in some patients. Peak vector copy number is shown; colored bars indicate best overall response via mRECIST, and black bars indicate best response prior to ICI start. C, Box-plot shows peak vector copy number pre- and post-ICI. D, Therapy response of patients with MPM (3 patients did not have measurable disease, 1 had no subsequent scan available) before starting next treatment, defined by mRECIST measurement on CT scan as partial response (PR), stable disease (SD), or progressive disease (PD) (within 0-770 days of CAR T-cell infusion). Solid line indicates measurements pre-ICI; dotted line indicates measurements post-ICI. E, Best overall response as measured by mRECIST, along with the time point of best response in months, is shown for 23 patients with MPM (3 patients did not have measurable disease, 1 had no subsequent scan available). The median time point of initiation of the best response among patients without progression (PR or SD) was 4 months, compared with 1 month for patients with progression (PD). Colored bars indicate best overall response via mRECIST; black bars indicate best response prior to ICI start.
Combination Immunotherapy
The combination-immunotherapy outcomes analyses population included patients with MPM who received cyclophosphamide plus CAR T cells and subsequent pembrolizumab for a minimum 3 doses, with at least 3 months of follow-up after the third dose, representing 18 of the 23 MPM patients. Seven of these patients (39%) had received ≥2 lines of therapy before CAR T-cell infusion (3 of 7 received ≥3 prior lines) (Tables 1 and 2). The median time to initiation of pembrolizumab after CAR T cell administration was 6 weeks (range, 4-17 weeks) (Figure S6 and Table 1). The adverse events experienced by these 18 patients up to 6 months after T-cell infusion are detailed in Table S4.
Median OS after CAR T-cell treatment was 23.9 months (95% CI, 14.7 months to NE; Figure S7A). One-year OS was 83% (95% CI, 68%-100%); time to next treatment was not reached (Figure S7B). Reduction in serum SMRP level was observed after administration of both CAR T cells and pembrolizumab (Figures S4, S8A-B). In peripheral blood, peak levels of CAR T cells, quantified on the basis of vector copy number (VCN), were higher following intrapleural CAR T-cell infusion (median, 1660 VCN/mL [range, 0-77,300 VCN/mL]), relative to peak levels after pembrolizumab administration (median, 397 VCN/mL [range, 0-48200 VCN/mL]) (Figures 3A-C, S9A-G).
Radiological Evaluation
Radiological evaluation by computed tomography (CT) scan using modified Response Evaluation Criteria in Solid Tumors (mRECIST) was performed at 4 to 6 weeks (not predefined in the protocol). Among combination immunotherapy patients with measurable disease by mRECIST (N=16), the best overall response was partial response (PR) in 2 of 16 (12.5%), SD in 9 of 16 (56.3%), and PD in 5 of 16 (31.3%) (Figure 3D-E). In 8 of 16 patients, SD or better was sustained for ≥6 months (Figures 2A, 3E, S6). Radiological measurements following CAR T-cell infusion before and after pembrolizumab administration are shown in Figure 3E. Most importantly, patients with SD remained functionally well and did not require a next treatment for a prolonged duration (Figures 2A, 3D, S3A-H, S6, S10).
Treatment Responses Examples
A 76-year-old patient with epithelioid MPM had a 28% reduction in the target lesion with CAR T cells alone (Figure 4A), with subsequent complete metabolic response on PET scan and PR on CT scan, with 78% reduction in the target lesion following pembrolizumab treatment (Figure 4A). This response lasted for 26 months. The patient’s serum SMRP level remained at baseline, and the patient remained functionally well (Figure 4B). CAR T cells were detected in peripheral blood up to 12 weeks, with an increase in T-cell Simpson clonality, T-cell clonal expansion (Figure S11), and detection of new and sustained IgG responses (Figure 4C).
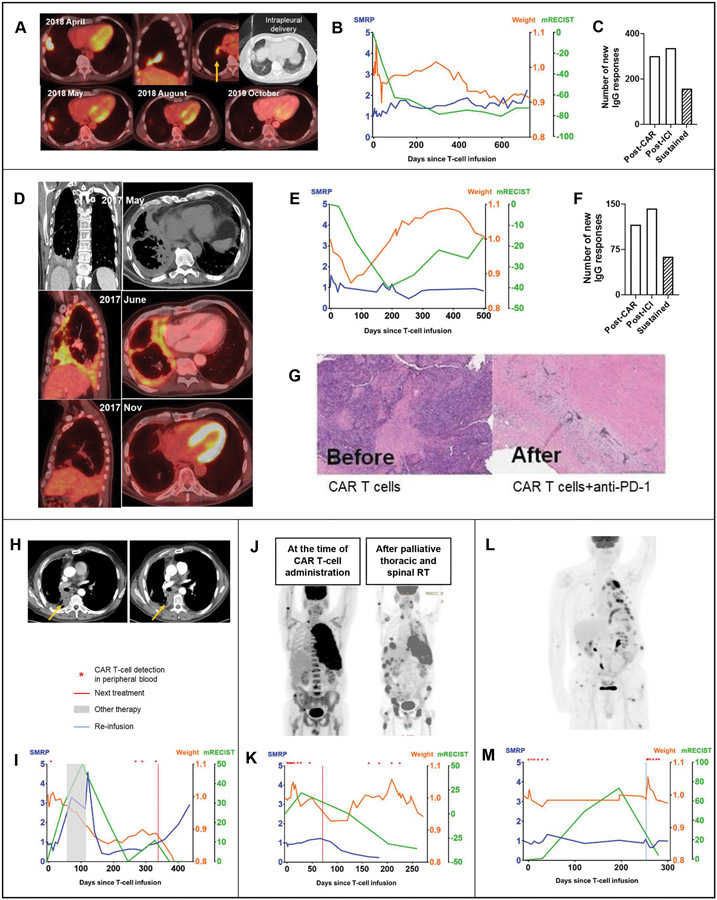
Figure 4. Clinical responses and correlative analyses of representative patients.
A, A 76-year-old patient diagnosed with stage 3A epithelioid MPM (TMB, 2.6 mt/Mb; mesothelin expression, 100%; PD-L1 expression, 0%) following platinum-based chemotherapy with disease burden in the right pleura, fissure, diaphragm, and hilar lymph nodes received a single intrapleural dose of 6M mesothelin-targeted CAR T cells/kg following cyclophosphamide preconditioning. A CT scan performed 4 weeks later showed a 28% reduction in the fissural tumor nodules. The patient received pembrolizumab starting at week 6 after CAR T-cell administration. A CT performed at 3 months showed a 62% reduction in the target disease by mRECIST and complete metabolic response on PET scan. B, At 26 months, the patient remained functionally well, with stable mRECIST, weight, and serum SMRP values (SMRP and weight fold change from baseline, mRECIST percent change from baseline; represented on the Y-axis), and did not require any additional therapies. C, Proteomic analyses showed new IgG responses, compared with baseline, following CAR T cells alone and after initiation of pembrolizumab—total responses sustained at both time points are indicated by the shaded bar. D, A 72-year-old patient with biphasic MPM (stage 3A; TMB, 4.9 mt/Mb; mesothelin expression, 25%; PD-L1 expression, 1%) underwent platinum-based chemotherapy with resulting SD that was unresectable. The patient was administered a single dose of 0.3M mesothelin-targeted CAR T cells/kg intrapleurally after cyclophosphamide (cohort 2). A PET scan showed metabolically active right MPM encircling the pleural cavity. Six weeks after CAR T-cell administration, the patient received pembrolizumab. Three months after pembrolizumab, a PET scan showed complete metabolic response (D), and a CT scan showed 40% PR by mRECIST (E). For 16 months after CAR T-cell administration, the patient remained functionally normal, gained and maintained weight, maintained baseline serum SMRP level (E), and had evidence on peripheral blood evaluation of new IgG responses following CAR T cells and pembrolizumab (some responses sustained) (F)—the patient did not require any additional treatments during this time. Peripheral blood and tumor biopsy evaluation documented presence of CAR T cells at 32 weeks. A repeat biopsy of the suspected tumor relapse showed cancer cells embedded in a bed of stroma (G), compared with abundant tumor noticed before administration of CAR T cells. H, A 66-year-old patient with epithelioid MPM with tumor relapse (TMB, 2 mt/Mb; 3 prior lines of therapy) received a single intrapleural dose of 1M CAR T cells/kg following cyclophosphamide. Reduction in the target lesion can be seen on CT scans after pembrolizumab (right panel), compared with after CAR T-cell therapy (left panel). I, Serial measurements of response by mRECIST (green), serum SMRP level (blue), and patient weight reflecting the general condition (orange) are shown during a period of 500 days. Following CAR T-cell administration, a minimal reduction in serum SMRP level was noted. The patient went on to receive another treatment, with PD indicated by an increase in serum SMRP level and mRECIST measurement associated with weight loss. No CAR T cells were detected in peripheral blood at the time of the patient’s return to the institution. On day 121, the patient started to receive pembrolizumab, with a subsequent decrease in serum SMRP level and mRECIST measurement and with stabilization of weight. More importantly, CAR T cells were redetected in the peripheral blood, as indicated by the red asterisk. J, A patient with epithelioid MPM with advanced, stage 4 disease received hemithoracic and spine palliative radiation therapy for progressive disease 71 days after intrapleural CAR T cells and pembrolizumab. The patient’s further clinical course showed a reduction in the thoracic tumor associated with redetection of CAR T cells in peripheral blood 100 days later. K, Serial tumor measurements (percent change from baseline) by mRECIST, fold change in serum SMRP level, and fold change in weight are shown during a period of 250 days after CAR T-cell infusion. L, A 70-year-old patient with epithelioid MPM with disease relapse with extensive metastases (4 previous lines of therapy) received CAR T cells and subsequent pembrolizumab. M, A second dose of CAR T cells was administered intrapleurally in August 2019. CAR T cells were detectable in peripheral blood for 4 weeks after the first infusion and for 13 weeks after reinfusion, at which time the patient stopped follow-up at our institution. Reduction in the target lesion was seen by mRECIST following reinfusion, although the patient developed new metastatic lesions.
In a 72-year-old patient with biphasic MPM, a similar complete metabolic response on PET scan and PR on CT scan (mRECIST; 40% reduction in target lesion) was observed (Figure 4D). This response persisted, with no other treatments, for 16 months, and the patient remained functionally normal, gained and maintained weight, and maintained baseline serum SMRP level (Figure 4E). Peripheral blood analyses showed new IgG responses following both CAR T cells and pembrolizumab administration (Figure 4F). Tumor biopsy at 32 weeks showed reduction in tumor cell burden, and CAR T cells were detected by PCR (Figure 4G). A patient who received first dose of pembrolizumab 121 days after CAR T-cell infusion had a 50% reduction in the target lesion by mRECIST, with redetection of CAR T cells in the peripheral blood following pembrolizumab (Figure 4H-I). Among 3 patients with MPM, CAR T cells were redetected in peripheral blood up to 100 days after palliative radiation in 1 patient (Figure 4J-K) and after reinfusion in 2 patients (Figures 4L-M, S3A-H). One patient with no measurable disease by mRECIST remained well, with no treatments administered other than pembrolizumab and reinfusion, for 32 months.
Correlative Analyses
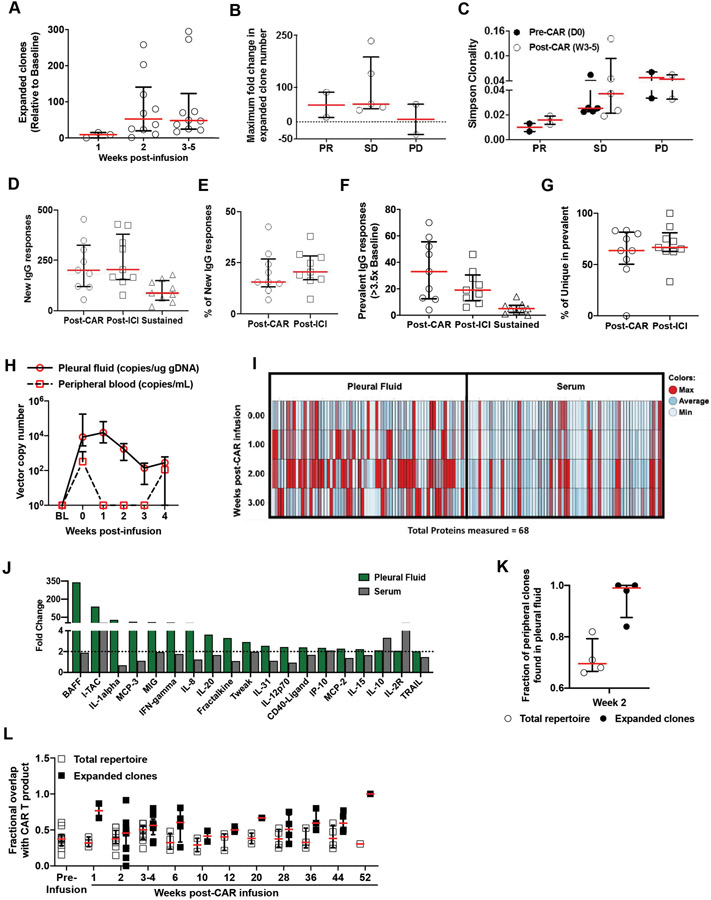
Analysis of peripheral blood T-cell receptor beta clonality (n=10) revealed T-cell clonal expansion at weeks 2 to 5, relative to baseline (Figure 5A), with greater clonal expansion in patients with PR or SD than in patients with PD (Figure 5B). Simpson clonality was lower in patients with PR than in patients with SD or PD (Figure 5C). On ProtoArray analysis of patient serum samples (n=9) (MPM subjects without disease served as controls), new IgG responses were observed after administration of both CAR T cells and pembrolizumab, compared with baseline (pre–CAR T cells) (Figure 5D). New IgG responses accounted for 15% and 20% of total IgG responses at the post-CAR and post pembrolizumab time points, respectively (Figure 5E). Prevalent IgG responses (>3.5-fold over baseline) and the percentage of unique IgG responses among prevalent responses are shown in Figures 5F and 5G, respectively.
Figure 5. Peripheral blood and pleural fluid clonality and protein analyses.
A-C, Peripheral blood mononuclear cells collected from each patient at different time points were subjected to gDNA isolation to study T-cell receptor beta clonality using ImmunoSEQ analysis. A, Shown are the numbers of expanded clones at 1, 2, and 3 to 5 weeks after CAR T-cell infusion followed by (B) maximum fold change in clone number delineated by the response relative to the value at baseline; horizontal lines indicate medians, with interquartile ranges. C, Simpson clonality index (lower value indicates diverse clonal population, an increase indicates clonal expansion) before CAR T-cell infusion and at 3 to 5 weeks after CAR T-cell infusion in the peripheral blood of patients with PR, SD, or PD, as measured by mRECIST, is shown; horizontal lines indicate medians, with interquartile ranges. D-F, Proteomic analyses showed evidence of new IgG responses in the peripheral blood (epitope spreading) after administration of both CAR T cells and pembrolizumab. Serum samples collected from each patient at baseline, after CAR T-cell infusion, and after administration of pembrolizumab were subjected to a ProtoArray assay to identify anti-IgG responses against 24,000 proteins at each time point. Shown are (D) medians (interquartile ranges) of new IgG responses, (E) the percentage of new IgG responses among total IgG responses in each patient, (F) prevalent (>3.5-fold expansion, compared with baseline) IgG responses after administration of CAR T cells and pembrolizumab and sustained IgG responses at both time points, and (G) the percentage of unique IgG responses among total prevalent IgG responses in each patient; horizontal lines indicate medians, with interquartile ranges. H, Presence and expansion of CAR T cells was noted in the pleural effusions collected at early time points (week 0-4), even when the peripheral blood analyses either showed low levels or did not show CAR T cells (n=4 patients with both pleural effusion and peripheral blood samples available). I, Pleural fluid and serum profiles of 68 proteins assessed using Luminex assays calculated as an average at 0, 1, 2, and 3 weeks after CAR T-cell infusion time points are shown as normalized values across time points. The majority of proteins measured were expressed at a maximal level at 2 weeks in the pleural fluid. J, Bar chart of proteins with >2-fold increase at week 2, compared with their baseline values, in pleural fluid (green bars) and their corresponding values in serum (gray bars). K, Clonal expansion was evident in the pleural fluid by week 2. In pleural fluid, there was an 84% to 100% overlap of expanded clones and a 66% to 82% overlap of all clones found in the peripheral blood. L, The peripheral clonality of each patient was determined at several time points and was compared with the infused product clonality to dynamically track changes in the identified clones. White squares show the fraction of clones found in the total repertoire also seen in the infusion product; black squares show the fraction of expanded clones that overlapped with the infusion product at several time points (up to 13 months) after CAR T-cell infusion, including the baseline sample collected before CAR T-cell infusion, is shown. Horizontal lines represent medians and interquartile ranges.
In patients with MPM with pleural effusion available for analysis, CAR T cells and elevated cytokine levels were detected in pleural effusions even in the absence of detection or low levels of these in the peripheral blood (Figure 5H). Luminex analyses of pleural fluid and serum for 68 effector response proteins showed that peak protein levels were seen in pleural fluid at 2 weeks, and much lower levels were seen in serum (Figure 5I-J).
T-cell clonal expansion was evident in the pleural fluid by week 2 (Figure 5A, S11). In pleural fluid, there was an 84% to 100% overlap of expanded clones and a 66% to 82% overlap of all clones found in the peripheral blood (Figure 5K). The peripheral clonality of each patient was determined at several time points and was compared with the infused product clonality to dynamically track changes in the identified clones. Peripheral clonality overlap with infusion product was seen from week 1 to week 52 (Figure 5L).
DISCUSSION
The trial reported herein reflects the clinical translation of preclinical testing and validation performed to overcome obstacles posed by solid tumors (26) by incorporating the following novel features: (a) intrapleural administration of CAR T cells, which is safe and feasible and has the potential to treat solid tumors and establish long-lasting systemic circulation (15); (b) regional administration of CARs adjacent to tumors with high antigen expression without on-target, off-tumor toxicities observed in regional or systemic normal tissues (14,16,17,19-21); (c) the combination of T cells and pembrolizumab to further enhance CAR T-cell persistence and function; (32); and (d) manufacturing of CAR T cells (33), with the ability to produce up to 60M T cells/kg per patient.
Intrapleural administration of mesothelin-targeted CAR T cells in patients with pleural cancer was well-tolerated, without overt toxicity to normal tissues known to express mesothelin. Mesothelin CAR T-cell administration followed by PD-1 blockade has the potential to durably treat solid tumors. Our findings of antitumor efficacy, with no on-target, off-tumor toxicity as observed in other solid tumor T-cell therapy trials (34), in patients with MPM and low TMB and PD-L1 expression (7) (Table 1) are noteworthy. The scFv in our CAR construct was designed with a unique combination of IgM and IgG components, with relatively low-affinity (35). The fully human elements of our CAR, including the scFv (35), possibly contributed to the ability of the CAR T cells to evade immune rejection and to be safely administered a second time, with efficacy (Figures 4L-M, S3A-H) and without adverse reactions, in a small number of patients (n=4). Lack of intense cytokine release syndrome in our trial, compared with patients with hematological malignancy following CAR T-cell infusion, may be indicative of insufficient tumor infiltration or the solid tumor microenvironment limiting CAR T-cell activation.
CAR T-cell engraftment, as measured in blood, required cyclophosphamide conditioning and overall increased with infusion dose (Figure 2A). In our preclinical model, we observed that regionally delivered CAR T cells achieved rapid and efficient tumor infiltration throughout the pleural tumor, followed by systemic circulation within a short period (15,25). These findings are consistent with our observations in this clinical trial (Figures 2, 3, 4, S9, S11, S12A-C). We did not observe any difference in adverse events, circulating CAR T cells (as demonstrated by VCN in peripheral blood), or outcomes between patients who were administered CAR T cells through a pleural catheter or via intervention radiology–guided imaging. The detection of circulating CAR T cells, 100 days after a single infusion underscores the potential of achieving long-lasting T-cell persistence following locoregional infusion (26).
Evidence of CAR T-cell activity following infusion is reflected in T-cell and CAR T-cell clonal expansion (Figures 3A, 5A, 5H) and new IgG responses (Figures 4C, 4F, 5D-G) before administration of pembrolizumab. The clonal expansion of endogenous T-cell clones not overlapping with the CAR T-cell product (Figure 5) demonstrates that PD-1 blockade expanded endogenous T cells as well. The finding of responses in patients with biphasic MPM with mesothelin expression as low as 25% (Figure 4D-G) is consistent with the recruitment of endogenous T cells against mesothelin-negative tumor cells. The expansion of preexisting IgG responses, and the development of new IgG responses, further suggest that CAR T-cell infusion followed by PD-1 blockade promotes endogenous immunity and antigen spreading (Figures 4, 5, S3A-H, S11). In murine models, we found that PD-1 blockade sustained mesothelin CAR T-cell therapy, which we attributed to a direct effect on CAR T cells, given the human specificity of pembrolizumab and the absence of endogenous T cells in NSG mice (26). In our patients with MPM, baseline PD-L1 expression was low but may have increased following infusion of CAR T cells. Our study was not funded to support iterative biopsies to investigate this question, but in a single instance where tissue was available before and after CAR T-cell infusion (Patient #7), we found 30% PD-L1 expression before CAR T-cell infusion and 70% 8 weeks thereafter. It is thus possible that PD-1 blockade rescues both CAR T cells and endogenous T cells that are recruited to the pleura following CAR T-cell infusion. The overlap of peripheral T-cell clones and expanded clones with infused CAR T cells (Figure 5L) before the initiation of pembrolizumab raises an intriguing possibility that potent preexisting T-cell immunity may have a predisposing effect, resulting in better efficacy of CAR T-cell therapy. Together, these observations suggest the potential importance of combined regional CAR T cells and PD-1 blockade to recruit polyclonal endogenous immunity to overcome tumor antigen heterogeneity and decrease the likelihood of antigen escape.
Radiographic assessment of response in patients with MPM is challenging for multiple reasons: tumor distribution as a pleural rind, invasion into the chest wall and organs that cannot be assessed, interobserver variability in quantitating diffuse tumor, presence of pleural effusion masking underlying disease as well as fissural disease, false-positive PET avidity due to talc, and pseudoprogression, which is seen with immunotherapies (36-38). A consensus report from the NCI Thoracic Malignancy Steering Committee and the International Association for the Study of Lung Cancer attests to these challenges in this uniquely pleural-based cancer and notes that assessment using mRECIST, with the same measurement parameters and the same observer, can be useful (38). The mRECIST responses reported here were measured by an independent radiologist without knowledge of clinical data, and sustained complete metabolic responses were noted on PET scan. The best responses (PR by mRECIST and complete metabolic response on PET) were observed in 2 patients who received CAR T cells and pembrolizumab, lasting for 18 to 26 months, and a third patient had CAR T cells detected in peripheral blood for 18 months and did not require further treatment for 25 months. Four patients received reinfusion of a second dose of CAR T cells at 25, 12, 8, and 8 months after the first infusion and were alive at 32, 24, 20, and 19 months. No adverse events grade >3 were observed in these patients; CAR T cells, as monitored by VCN in the peripheral blood, were detected 90, 146, 7, and 91 days after the second infusion, suggesting they were not immunologically rejected by the recipient.
Although we provide mRECIST measurements as well as outcomes analyses, these results should be interpreted with caution, as they were not a prespecified part of the phase I study design, and pembrolizumab treatment was not initiated in a uniform fashion. With the known difficulties in assessing response by imaging, OS is the only reliable parameter to date to assess response to therapy in patients with MPM (38); OS was used as an endpoint in the approval of pemetrexed as well as recent dual-agent checkpoint blockade for MPM where there are no improvements in progression-free survivals (Table S5) (1,9). Median OS among patients who received combination immunotherapy in our study (39% of patients received ≥2 prior lines of therapy; Table S6) was 23.9 months after CAR T-cell infusion. Several additional measurements of antitumor activity were presented (Figures 2, 3, 4, 5, S3A-H, S9, S10, S12A-C). However, as the patients in this phase I study designed to assess safety were selected for reasonable life expectancy and functional status to be able to await CAR T-cell manufacturing and treatment, the survival results cannot be directly compared with outcomes of other immunotherapy trials.
Our data strongly support the investigation of combination immunotherapy with CAR T cells and PD-1 blockade agents in solid tumors. Owing to safety concerns following regional administration of CAR T cells at a higher dose, we initially administered pembrolizumab several weeks after administration of CAR T cells. With confirmation of safety, we moved pembrolizumab closer to and more consistently following CAR T-cell administration. On the basis of the results of this trial, we are now conducting a phase II study with a fixed dose of mesothelin-targeted CAR T cells (6×107 CAR T cells/kg) followed by initiation of pembrolizumab 4 weeks after CAR T-cell administration.
METHODS
Trial Design And Patients
This is an open-label, dose-escalating, single-center, phase I study of mesothelin-targeted CAR T cells in patients with previously treated histologically proven pleural cancer from MPM, metastatic lung cancer, or metastatic breast cancer (Trial registration number: NCT02414269). Patients with mesothelin expression of at least 10% of tumor cells on immunohistochemical analysis (Figure S13A-D) and/or patients with epithelioid mesothelioma with serum SMRP >1.0 nm/L were eligible (as detailed in the study protocol, see Supplementary Material). Following a single dose of preconditioning cyclophosphamide (1500 mg/m2), mesothelin-targeted CAR T cells (0.3M-60M CAR T cells/kg) with the iCaspase-9 safety gene (27) were administered intrapleurally either through a pleural catheter or via interventional radiology–guided imaging.
Study Oversight
The study protocol and amendments were approved by our institutional review board. All patients provided written informed consent. Response and toxicity outcomes were reviewed by an independent committee established by the institutional Clinical Research Oversight Committee to manage potential conflicts of interest in the interpretation of responses. No one who is not an author contributed to the writing of the manuscript.
CAR T-Cell Manufacturing
Details of the CAR T-cell manufacturing process (31) and analyses are described in the protocol (provided in the Supplementary Material).
Endpoints and Assessment
Our primary objective was to assess the safety, dose requirement, and targeting efficiency of CAR T cells. Secondary objectives included evaluation of changes in serum soluble mesothelin-related peptide (SMRP) level and assessment of CAR T-cell persistence in peripheral blood. All patients, including those who received a second dose of CAR T cells and/or pembrolizumab (detailed in amended protocol [see Supplementary Material]), were included in the reporting of adverse events.
Preliminary Outcomes
The outcomes-analysis population included patients with MPM who received cyclophosphamide preconditioning plus T cells and those who received cyclophosphamide plus combination immunotherapy (CAR T cells and subsequent pembrolizumab for a minimum 3 doses, with at least 3 months of follow-up after the third dose).
Statistical Analyses
The sample size was based on a standard dose-escalation design. Descriptive statistics are presented as medians, with minimums and maximums (or interquartile range where specified) for continuous variables and with counts and percentages for categorical variables. Safety data are described as the number and proportion of patients who had treatment-related adverse events. Exact methods (Clopper-Pearson 95% confidence intervals [CIs]) were used for categorical variables. In the preliminary outcomes analysis, the time of the first CAR T-cell infusion was used as the time of origin in all time-to-event analyses. The analysis of OS used death as the event and was summarized using the Kaplan-Meier method. Time to next treatment after combination immunotherapy was summarized and displayed graphically using the cumulative incidence function. The administrative cutoff date was June 2020; no patient was lost to follow-up before this date. Analyses were performed using R software (3.6.1, R Foundation for Statistical Computing).
Assessment of Toxicity
Cytokine release syndrome was graded in accordance with the Memorial Sloan Kettering cytokine release syndrome grading system (available on request). All patients were evaluated by the neurology team before and after chimeric antigen receptor (CAR) T-cell infusion. Neurological and other toxicities were assessed in accordance with version 4.0 of the National Cancer Institute Common Terminology Criteria for Adverse Events.
Radiological Measurement
All CT scans were reviewed by two radiologists after the period of data collection had ended. Measurable disease and response were defined per modified Response Evaluation Criteria in Solid Tumors (mRECIST) (37); details are provided in the protocol (available on request). Per mRECIST, measurable disease was defined as disease with a pleural thickness of 1.0 cm. Tumor thickness perpendicular to the chest wall or mediastinum was measured in two positions at three separate levels on transverse cuts of a CT scan. The sum of the six measurements defined a pleural unidimensional measure. Transverse cuts of at least 1 cm apart were selected, when possible, in the upper, middle, and lower chest. At the time of reassessment, pleural thickness was measured at the same position and level. Nodal, subcutaneous, and other bidimensionally measurable lesions were measured unidimensionally. Unidimensional measurements were added to obtain the total tumor measurement. Complete response was defined as the disappearance of all target lesions, with no evidence of tumor elsewhere. Partial response was defined as a reduction in the total tumor measurement of at least 30%. Progressive disease was defined as an increase in the total tumor measurement of at least 20%, compared with the nadir measurement, or the appearance of one or more new lesions. Stable disease was defined as disease that did not fulfill the criteria for partial response or progressive disease.
Correlative Analysis
Measurements of serum soluble mesothelin-related peptide, C-reactive protein, and IL-6 were performed in a Memorial Sloan Kettering chemistry laboratory. The apheresis and infusion product obtained from each patient was characterized by flow cytometric analysis to determine its cellular composition. Flow cytometric quantification of the immune cell markers CD45, CD14, CD3, CD4, CD8, CD28, and CD62L and CAR T-cell expression by protein L assay were used to identify different cellular subsets. A quantitative real-time polymerase chain reaction assay was used to identify and quantify the presence of CAR T cells in the peripheral blood (vector copies per milliliter) and pleural fluid (vector copies per microgram of genomic DNA). Peripheral blood mononuclear cells and pleural effusion cells collected from each patient at different time points were subjected to genomic DNA isolation to study T-cell receptor beta clonality by use of ImmunoSEQ analysis (Adaptive Biotechnologies). Serum samples collected from each patient at baseline, 3 to 4 weeks after CAR T-cell therapy, and after anti–programmed cell death protein 1 treatment were assessed using the HuProt ProtoArray assay (CDI Laboratories) to identify anti-IgG responses against 24,000 proteins at each time point. Pleural fluid and serum profiles of 68 proteins were assessed using Luminex-based assays (65-plex Human ProcartaPlex panel, ThermoFisher Scientific) and the 3-plex Millipore panel (Millipore Sigma).
Supplementary Material
Statement of Significance.
Regional delivery of mesothelin-targeted CAR T-cell therapy followed by pembrolizumab administration is feasible, safe and demonstrates evidence of anti-tumor efficacy in patients with malignant pleural diseases. Our data support the investigation of combination immunotherapy with CAR T cells and PD-1 blockade agents in solid tumors.
Acknowledgments
David B. Sewell of the Department of Surgery, Memorial Sloan Kettering Cancer Center, provided editorial assistance.
Financial Support:
P.S.A.’s laboratory work is supported by grants from the National Institutes of Health (P30 CA008748, R01 CA236615-01, and R01 CA235667), the U.S. Department of Defense (BC132124, LC160212, CA170630, and CA180889), the Baker Street Foundation, the Batishwa Fellowship, the Comedy vs Cancer Award, the Derfner Foundation, the Dalle Pezze Foundation, the Esophageal Cancer Education Fund, the Geoffrey Beene Foundation, the Memorial Sloan Kettering Technology Development Fund, the Miner Fund for Mesothelioma Research, the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnotes
Conflicts of Interest: P.S.A. has received research funding from ATARA Biotherapeutics, has served on the Scientific Advisory Board or as consultant to ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, ImmPACT Bio, and Takeda Therapeutics, and has patents, royalties, and intellectual property on mesothelin-targeted CARs and T-cell therapies, method for detection of cancer cells using virus, and pending patent applications on T-cell therapies. D.R.J. serves as a consultant for AstraZeneca and Merck.
REFERENCES
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21(14):2636–44. [DOI] [PubMed] [Google Scholar]
- 2.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387(10026):1405–14. [DOI] [PubMed] [Google Scholar]
- 3.Ujiie H, Kadota K, Nitadori JI, Aerts JG, Woo KM, Sima CS, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015;4(6):e1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bograd AJ, Suzuki K, Vertes E, Colovos C, Morales EA, Sadelain M, et al. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother 2011;60(11):1509–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klampatsa A, O'Brien SM, Thompson JC, Rao AS, Stadanlick JE, Martinez MC, et al. Phenotypic and functional analysis of malignant mesothelioma tumor-infiltrating lymphocytes. Oncoimmunology 2019;8(9):e1638211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad MM, Jones RE, Liu H, Lizotte PH, Ivanova EV, Kulkarni M, et al. Cytotoxic T cells in PD-L1-positive malignant pleural mesotheliomas are counterbalanced by distinct immunosuppressive factors. Cancer Immunol Res 2016;4(12):1038–48. [DOI] [PubMed] [Google Scholar]
- 7.Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019;4(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantini L, Hassan R, Sterman DH, Aerts J. Emerging treatments for malignant pleural mesothelioma: where are we heading? Front Oncol 2020;10:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397(10272):375–86. [DOI] [PubMed] [Google Scholar]
- 10.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 2016;3:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Li X, Chintala NK, Tano ZE, Adusumilli PS. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr Opin Immunol 2018;51:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiesgen S, Chicaybam L, Chintala NK, Adusumilli PS. Chimeric antigen receptor (CAR) T-cell therapy for thoracic malignancies. J Thorac Oncol 2018;13(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: Driving T cells to solid tumors. Cancer Discov 2016;6(2):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6(261):261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguchi T, Kadota K, Mayor M, Zauderer MG, Rimner A, Rusch VW, et al. Cancer antigen profiling for malignant pleural mesothelioma immunotherapy: expression and coexpression of mesothelin, cancer antigen 125, and Wilms tumor 1. Oncotarget 2017;8(44):77872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res 2014;20(4):1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas A, Chen YB, Steinberg SM, Luo J, Pack S, Raffeld M, et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget 2015;6(13):11694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tozbikian G, Brogi E, Kadota K, Catalano J, Akram M, Patil S, et al. Mesothelin expression in triple negative breast carcinomas correlates significantly with basal-like phenotype, distant metastases and decreased survival. PloS One 2014;9(12):e114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012;18(9):2478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizk NP, Servais EL, Tang LH, Sima CS, Gerdes H, Fleisher M, et al. Tissue and serum mesothelin are potential markers of neoplastic progression in Barrett's associated esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2012;21(3):482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014;74(11):2907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone M, Adusumilli PS, Alexander HR Jr., Baas P, Bardelli F, Bononi A, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69(5):402–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2(10):957–65. [DOI] [PubMed] [Google Scholar]
- 25.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126(8):3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 2019;36(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365(18):1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallardo HF, Tan C, Sadelain M. The internal ribosomal entry site of the encephalomyocarditis virus enables reliable coexpression of two transgenes in human primary T lymphocytes. Gene Ther 1997;4(10):1115–9. [DOI] [PubMed] [Google Scholar]
- 29.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20(1):70–5. [DOI] [PubMed] [Google Scholar]
- 30.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A 1995;92(15):6733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 2009;32(2):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126(8):3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18(4):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther 2009;8(5):1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall DO, Hooper CE, Searle J, Darby M, White P, Harvey JE, et al. 18F-Fluorodeoxyglucose PET/CT and dynamic contrast-enhanced MRI as imaging biomarkers in malignant pleural mesothelioma. Nucl Med Commun 2018;39(2):161–70. [DOI] [PubMed] [Google Scholar]
- 37.Armato SG 3rd, Nowak AK. Revised Modified Response Evaluation Criteria in Solid Tumors for assessment of response in malignant pleural mesothelioma (Version 1.1). J Thorac Oncol 2018;13(7):1012–21. [DOI] [PubMed] [Google Scholar]
- 38.Tsao AS, Lindwasser OW, Adjei AA, Adusumilli PS, Beyers ML, Blumenthal GM, et al. Current and future management of malignant mesothelioma: A consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol 2018;13(11):1655–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.