Abstract
Objectives:
Increased risk of morbidity and hospitalization has been observed in children who are HIV-exposed uninfected (HEU) compared to HIV-unexposed uninfected (HUU). Studies in the era of universal maternal antiretroviral treatment (ART) are limited.
Design:
Prospective cohort.
Methods:
We investigated hospitalization between 29 days and 12 months of life in a South African cohort of infants born February 2017 - January 2019 (HEU=455; HUU=458). All mothers known with HIV during pregnancy received ART. We reviewed hospital records and classified and graded infectious diagnoses using a standardized tool. We examined factors associated with infectious-cause hospitalization using mixed-effects Poisson regression.
Results:
Infants HEU vs. HUU had higher all-cause and infectious-cause hospitalization (13% vs. 7%, p=0.004 and 10% vs. 6%, p=0.014 respectively). Infectious causes accounted for most hospitalizations (77%). More infants HEU were hospitalized with severe or very severe infections than those HUU (9% vs. 6%, p=0.031). Mortality (<1%) did not differ between groups. HIV exposure was a significant risk factor for infectious-cause hospitalization (aIRR=2.8; 95% CI 1.5-5.4). Although increased incidence of preterm birth (14% vs. 10%; p<0.05) and shorter duration of breastfeeding (44% vs. 68% breastfed for ≥3 months, p<0.001) among infants HEU vs. HUU contributed to increased hospitalization, they did not account for all the increased risk.
Conclusion:
Infectious-cause hospitalization incidence was higher among infants HEU vs. HUU, likely partly due to higher incidence of preterm birth and lower breastfeeding rates among infants HEU. The increased infectious disease burden in HEU infants has important implications for health services in sub-Saharan Africa.
Keywords: HIV, Exposed Uninfected, Infant, Hospitalization, Infectious Morbidity
Introduction
In 2018, ~15 million children were HIV-exposed but uninfected (HEU) globally, of whom 13 million resided in sub-Saharan Africa[1]. In South Africa, where antenatal HIV prevalence is high (31%) and early vertical transmission rates are low (<3%), children HEU form a substantial and growing population[2,3]. Infants HEU are more likely to be preterm, have lower birthweight and be small-for-gestational age than infants HIV-unexposed and uninfected (HUU) [4-10]. Preterm birth in turn is a risk factor for morbidity, hospitalization and infant mortality [11,12].
A meta-analysis found that infants HEU have a 50% and 70% increased risk of diarrhoea and pneumonia, respectively, in the first 6 months of life, compared to infants HUU [13]. Infants HEU have increased risk of hospitalization in the first year of life and higher mortality than infants HUU, predominantly due to infectious causes [8,14-20]. Although causes of morbidity in infants HEU vs. HUU are similar, they occur with greater severity in infants HEU, resulting in more frequent hospitalization and higher mortality [21]. Poorer maternal education, suboptimal vaccination and breastfeeding, lower maternal CD4 counts, ART initiation during pregnancy vs. before conception and elevated maternal viral load (VL) at delivery have been associated with increased risk of infectious morbidity and hospitalization in infants HEU [15-18,22-26].
More accurate evaluation of the health risks and needs of children HEU, particularly in resource-limited settings, is a research priority [27,28]. Much of the existing evidence of infectious morbidity in infants HEU predates the era of universal maternal ART. We previously found similar all-cause and infection-related hospitalization rates in a cohort of neonates HEU vs. HUU, although hospitalized neonates HEU had increased risk of very preterm birth, very low birthweight and intensive care admission [29]. In this additional analysis of the cohort, we describe hospitalization patterns in the post-neonatal period until 1 year of age and we examine maternal and infant factors associated with infectious-cause hospitalization.
Methods
Study population
We conducted a prospective cohort study of pregnant women and their infants at a large primary maternity care facility in Gugulethu, a lower-income area in Cape Town, South Africa. From January 2017-July 2018, consecutive pregnant women aged ≥18 years, with and without HIV, were enrolled if: they were attending their first antenatal visit during the current pregnancy at the facility (before onset of labor); their HIV status was confirmed through routine testing previously or at the current visit; they planned to reside with their infants in Cape Town until at least 1 year postpartum; and they provided informed consent to participate and allow researchers to access medical records.
Study procedures
Data sources included: interview-based measures; clinical assessments; routine electronic data; and medical record reviews of hospitalizations. Study visits occurred around routinely scheduled antenatal and postnatal clinic visits. At the first antenatal visit, baseline maternal demographic and clinical information was recorded. At postnatal visits (at ≤7 days; 10 weeks; 6 months; and 12 months): women were interviewed about infant feeding practices; vaccination data were abstracted from patient-held records; and infants were weighed and measured after removing clothing and diapers.
Infant hospitalization was tracked through the provincial hospital admissions system, facilitated by the Western Cape Provincial Health Data Centre (WCPHDC), a health information system which links individuals via unique health identifiers to a range of datasets, including outpatient visits, pharmacy records, laboratory results and hospitalizations [30]. Each unique identifier, referred to as the Patient Master Index (PMI), is issued to infants at birth, linked to the maternal PMI, and enables electronic identification of public-sector hospitalizations within the province. The WCPHDC established an electronic alert system for all enrolled infants, whereby we were notified via daily, automated email alerts of hospitalizations linked to the PMI. Because intention to reside in Cape Town was a study inclusion criterion, we anticipated that most hospitalizations would occur in Cape Town or surrounding areas. After infants were discharged home, the study doctor reviewed medical records related to hospitalizations at provincial hospitals. We excluded hospitalizations commencing during the neonatal period (from birth/day 0-day 28 of life) and infants who died during the neonatal period or during hospitalizations commencing during the neonatal period. We collected detailed data on reasons for hospitalization and severity of illness using standardized case report forms. Study data were managed using REDCap electronic data capture tools hosted at the University of Cape Town [31]. The study was approved by the Western Cape Department of Health Provincial Health Research Committee (REF WC_2016RP6_286) and ethics approval was granted by the Human Research Ethics Committee at the University of Cape Town (REF 541/2015 and 749/2015).
Definitions
For classification of infection-related diagnoses, we used the modified Pediatric Infectious Event Tool for Research (PIETR), previously used in a Cape Town study of infants HEU [32]. The tool includes case definitions for classification and grading (mild/moderate, severe or very severe) of infectious diagnoses, including respiratory tract infections, diarrhoeal disease, skin and mucocutaneous infections, invasive bacterial infections and congenital infections. If criteria for severe infections persisted for ≥2 days after hospitalization, they were graded as very severe. For PIETR case definitions, see Supplemental Digital Content, Table 1.
Preterm and very preterm births were defined as live births at <37 and <32 completed weeks gestation, respectively. We reported congenital anomalies listed by the Centers for Disease Control and Prevention as ‘major congenital defects,’ tracked by the Metropolitan Atlanta Congenital Defects Program [33]. If breastfeeding ceased but duration was not explicitly reported, we used last study visit or hospitalization with a report of breastfeeding as the date of cessation. We censored breastfeeding data at age 12 months. We recorded maternal CD4 counts closest to delivery date (within 12 months before or after delivery date); and maternal VL measurements closest to delivery date (during pregnancy or ≤7 days after delivery). National guidelines during the study period recommended PCR HIV testing of HIV-exposed infants at birth and 10 weeks; and 6 weeks after breastfeeding cessation [34]. We regarded PCRs conducted within the first 7 days of life as birth PCRs; and between 6-14 weeks as 10 week PCRs. Infants with positive PCRs were censored from the study at the last time point known to be HIV-uninfected. In women who test HIV negative during pregnancy, national guidelines recommend repeat HIV testing: in labor or immediately post-delivery; at 6 weeks post-delivery; and every 3 months while breastfeeding [34].
We defined all vaccinations as up-to-date if inspection of vaccination records confirmed that: all vaccinations routinely prescribed [35] up until 14 weeks were administered by 16 weeks; measles vaccine prescribed at 6 months was administered by 28 weeks; and pneumococcal vaccine booster prescribed at 9 months was administered by 41 weeks. We used anthropometric data from postnatal visits to assess prevalence of chronic malnutrition, using stunting as a proxy. Length-for-age Z-scores (LAZ) were calculated using the WHO Anthro Survey Analyser [36]. We excluded values <−6 or >6 as implausible. We used available LAZ at 6 months (183 days ± 42 days) and 12 months (365 days ± 42 days) to describe proportions of infants with stunting at each time point. We defined stunting as LAZ<−2 and severe stunting as LAZ<−3.
Data analysis
We compared characteristics and pregnancy outcomes of women living with vs. without HIV, as well as characteristics of infants HEU vs. HUU, using the Chi-squared test or Fisher’s exact test if expected cell frequencies were <5. We used the two-sample t-test to compare means between groups. We described non-normally distributed data using medians and interquartile ranges (IQR) and used the Wilcoxon sum rank test to compare medians. We calculated crude all-cause and infectious-cause hospitalization rates (per 100 child-years). We used random-effects Poisson regression models to calculate unadjusted and adjusted incidence rate ratios (IRR/aIRR) for infectious-cause hospitalization among infants HEU vs. HUU. We adjusted for potential confounders and clustered by infant. We assigned infant sex, vaccination, maternal age, education and type of housing as potential confounders a priori. Breastfeeding duration and preterm delivery were evaluated as potential mediators in separate models. We also performed stratified analyses conditioned on breastfeeding status at 3 months. Among infants HEU, we examined maternal HIV-related factors (CD4, VL and ART) for associations with infectious-cause hospitalization. We excluded second-born twins and infants with major congenital anomalies regarded as high-risk for infection-related hospitalization from all regression analyses. We allocated twins as first- vs. second-born according to their birth order which is conventionally indicated in their medical and electronic records as “Twin A” vs. “Twin B.”
We aimed to enrol 900 pregnant women, half living with HIV. Based on a test of proportions for independent groups, using a 2-tailed α= 0.05 and allowing for ~10% attrition, we calculated a sample size of 400 infants in each group would provide 85% power to detect a relative increase of 75% in the proportion of infants HEU with ≥1 infectious-cause hospitalization ≤12 months of age, assuming this would be 10% in infants HUU.
We calculated person-time (child-years) from day 29 of life until censoring at either 12 months of age, last date HIV uninfected or date of death (if <12 months). We excluded periods of hospitalization from person-time at-risk for hospitalization. Because it is common practice in South Africa for children to relocate to other provinces for care by relatives for extended periods, we performed sensitivity analyses with person-time adjusted for infants who spent time in other provinces. If exact travel dates were unknown, infants with reports of time spent in other provinces were censored at the date last known to be in the Western Cape province (using the most recent of either last study visit or last known date of any provincial public healthcare system encounter, identified electronically by the WCPHDC). Infants re-entered the study if and when there was evidence of their return to the province <12 months. Analyses were performed using Stata 14 (College Station, TX: StataCorp LP).
Results
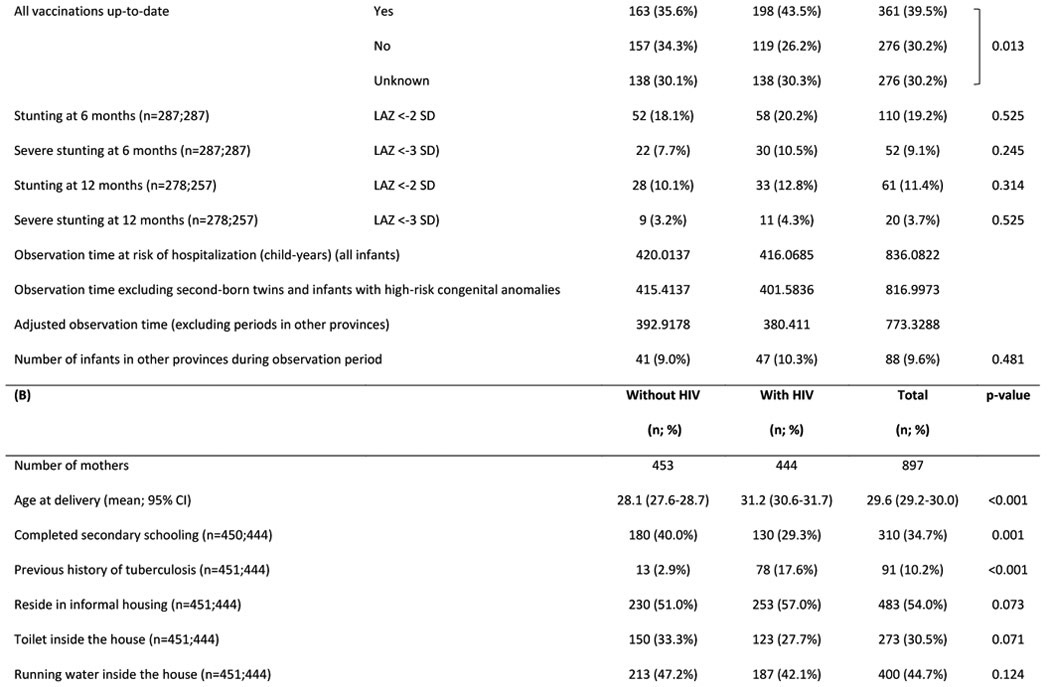
We enrolled 991 pregnant women (487 with HIV; 504 without) (Figure 1). Loss to follow-up (unknown pregnancy outcomes or unknown infant PMIs) occurred in 3% of mother-infant pairs in both groups; relocation to another province was reported in 70% of those lost to follow-up. Characteristics of mothers with included infants (n=897) are shown in Table 1. Women with HIV were older, fewer completed secondary schooling and a larger proportion had previous tuberculosis. Socio-economic conditions (type of housing and access to household amenities) were similar in both groups. Among women with HIV, 53% were on ART at conception; 27% initiated ART for the first time at enrolment (during pregnancy); and 19% had evidence of previous ART and then ‘resumed’ ART during pregnancy. Among women with available data, 88% had VL <400 copies/ml at the measurement closest to delivery and mean CD4 count was 488 cells/μl (95% CI 466-511). The maternal cohort was previously described [29].
Figure 1: Flow diagram of study participants.
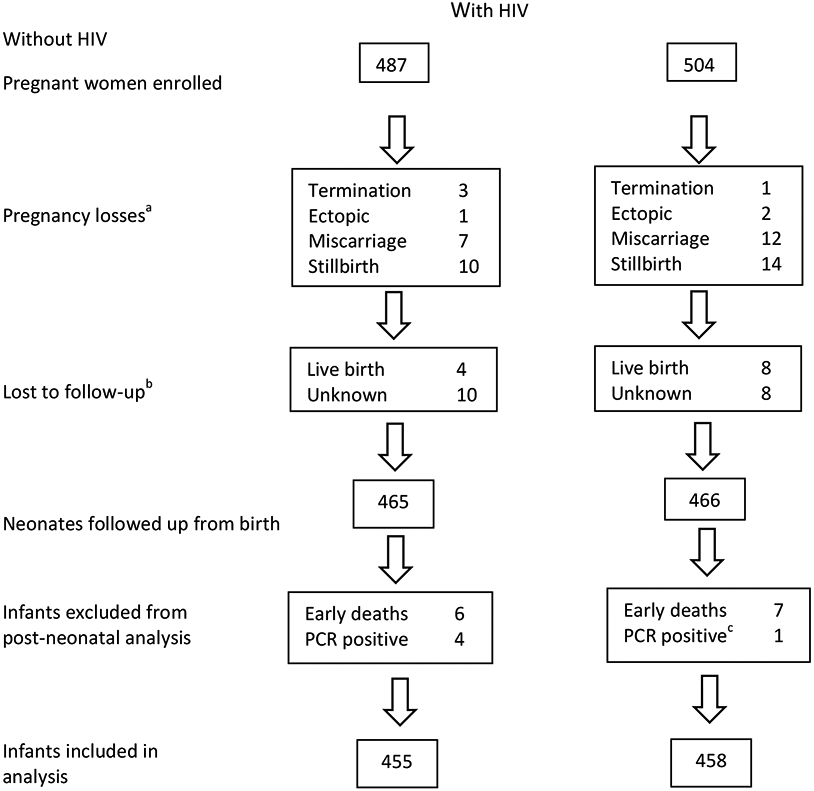
(a miscarriage was defined as pregnancy loss <20 weeks gestation; stillbirth as babies born with no signs of life ≥20 weeks' gestation; b includes women in whom pregnancy outcomes were unknown as well as women with pregnancies that resulted in live births but infant Patient Master Index could not be traced (therefore electronic tracking was not possible); c mother was HIV negative at delivery but seroconverted thereafter)
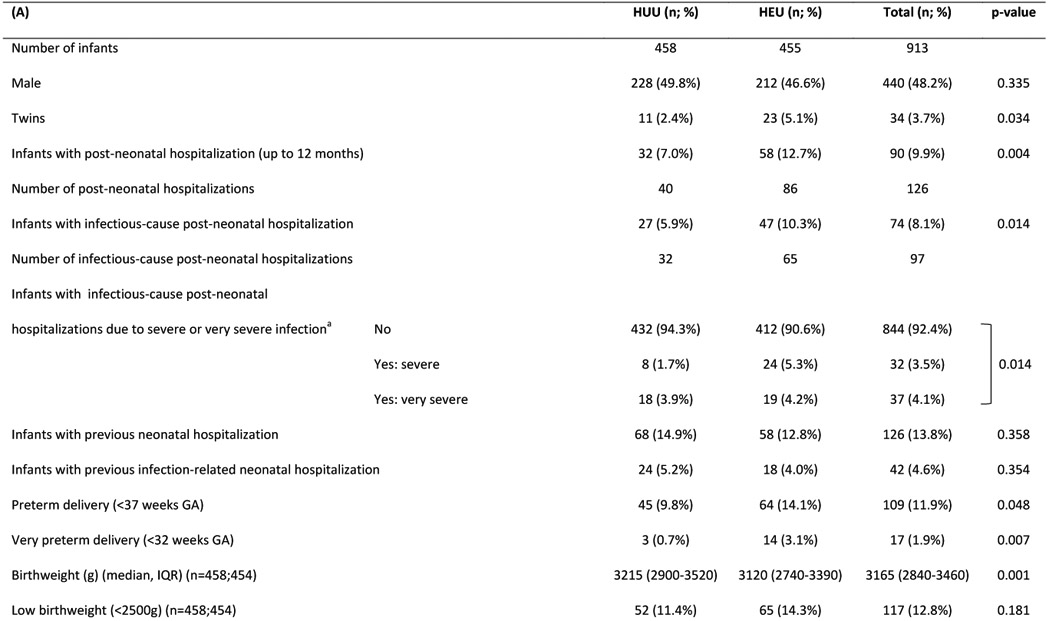
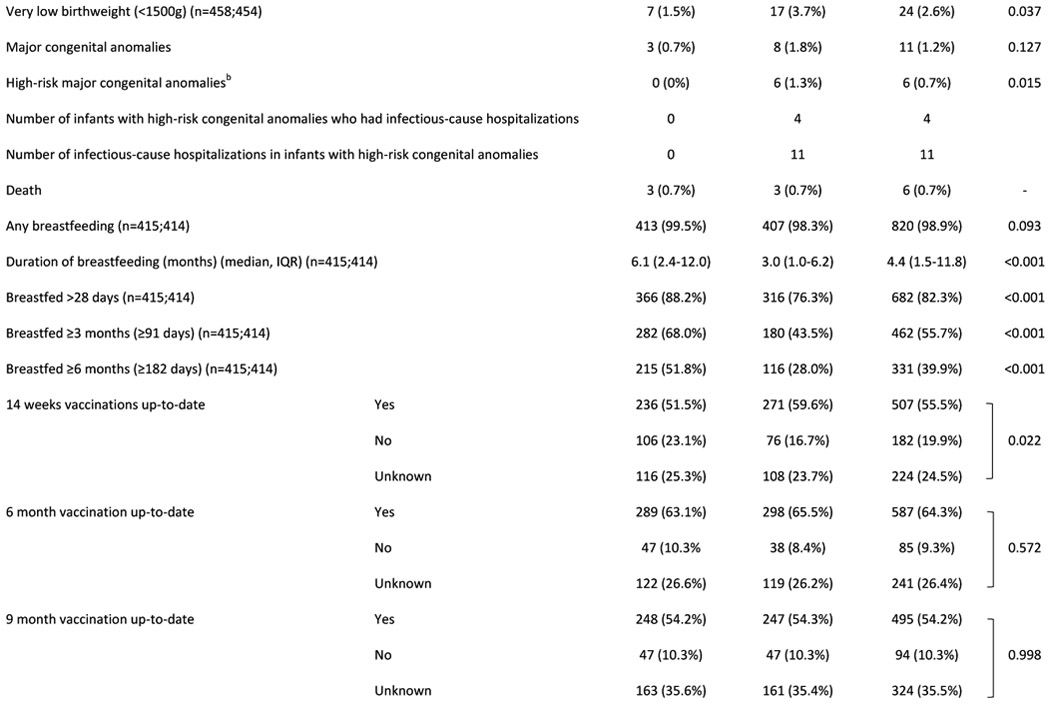
Table 1:
Characteristics of (A) HIV uninfected infants and (B) their mothers
|
|
|
|

|
Abbreviations: HUU – HIV-unexposed uninfected; HEU – HIV-exposed uninfected; GA - gestational age; g - grams; IQR - interquartile range; LAZ - length-for-age Z-score; SD – standard deviation; CI - confidence interval; ART - antiretroviral treatment
Graded with Pediatric Infectious Event Tool for Research; most severe infection grade during any hospitalization was selected
Major congenital anomalies regarded as high-risk for infection-related hospitalization: myelomeningocele (x2); VACTERL syndrome; cleft palate; laryngeal cleft; Trisomy 21 with cardiac defect. (Note: the following major congenital anomalies were not regarded as high-risk: talipes equinovarus (x2); Klinefelter syndrome; polydactyly; thoracic scoliosis.)
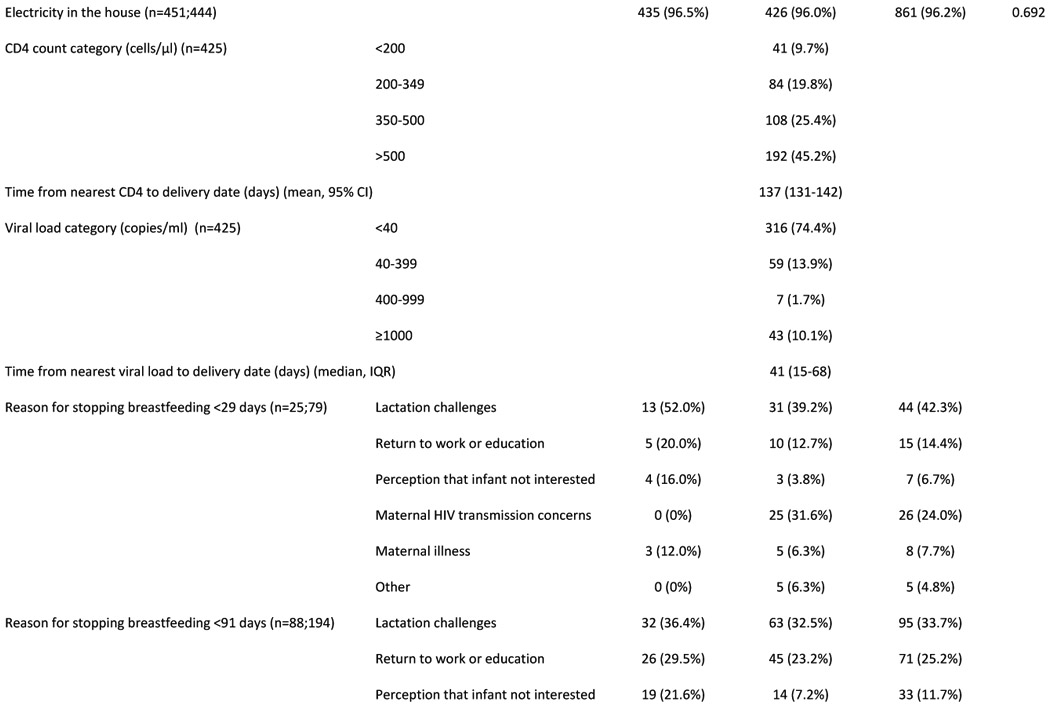
We followed up 913 infants with known PMIs (HUU=458; HEU=455), born February 2017-January 2019 (Table 1). Infants HEU vs. HUU had lower birthweight, more preterm and very preterm births; included more twins and were breastfed for shorter duration (median duration 3 vs 6 months, p<0.001). Infants HEU vs. HUU had higher all-cause and infectious-cause hospitalization between age 29 days-12 months (13% vs. 7%, p=0.004 and 10% vs. 6%, p=0.014 respectively). Mortality did not differ between groups (<1% in each group). No deaths occurred during hospitalization, all were self-reported by mothers and causes of death are unknown. Overall prevalence of severe stunting was 9% at 6 months and 4% at 12 months, with no significant differences between groups. Among infants with full vaccination details available (70%), more infants HEU vs. HUU were up-to-date with all vaccinations (62% vs. 51%; p=0.003). Difficulties with breastfeeding (lactation issues/insufficient milk/mastitis etc.) were reported as the most common reason for stopping breastfeeding <3 months (34%). A substantial proportion of mothers with HIV (22%) reported stopping breastfeeding <3 months due to concerns about HIV transmission.
Birth HIV-PCR was recorded in 96% of all neonates born to women with HIV and in 77% of eligible infants at 10 weeks. Of those not tested at 10 weeks, an additional 8% were tested subsequently but 15% of infants had no record of retesting after birth (up to 12 months). Seven HIV-exposed infants were known to be vertically HIV-infected. Of these, 5 were excluded from analysis: 2 with positive birth PCRs; 1 with negative birth PCR but positive 10 week PCR; 1 with negative birth PCR, no 10 week PCR and positive PCR later; and 1 with no birth or 10 week PCR but positive PCR later. Two infants tested PCR negative at 10 weeks but positive thereafter; they were included in analysis but were censored at 10 weeks.
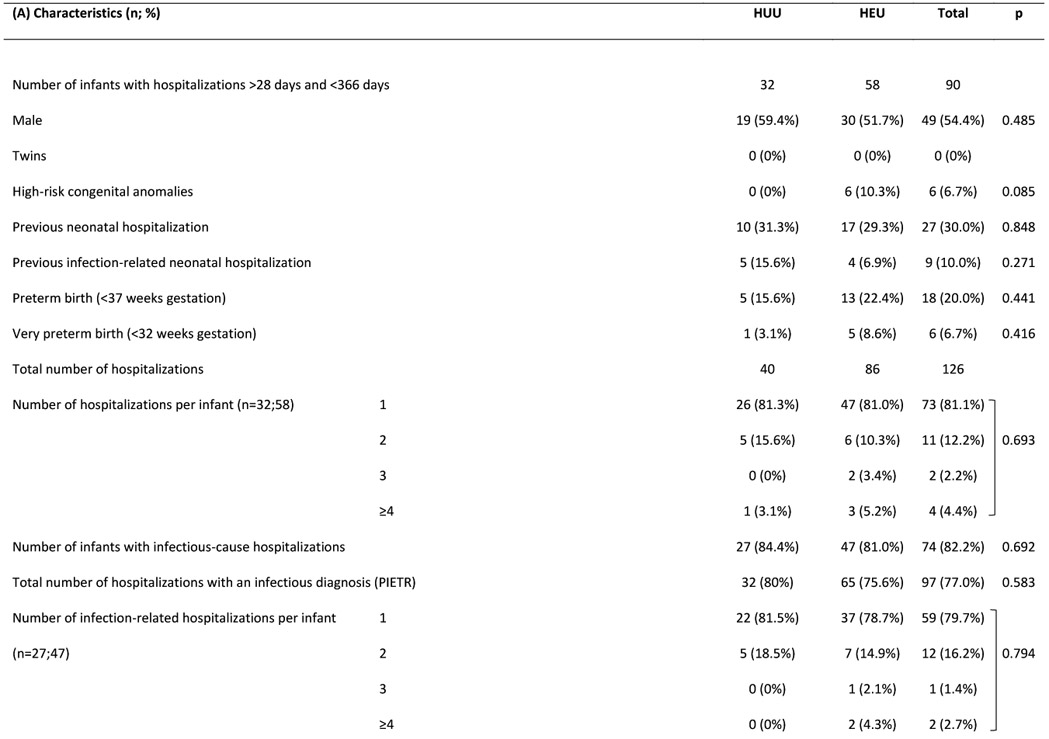
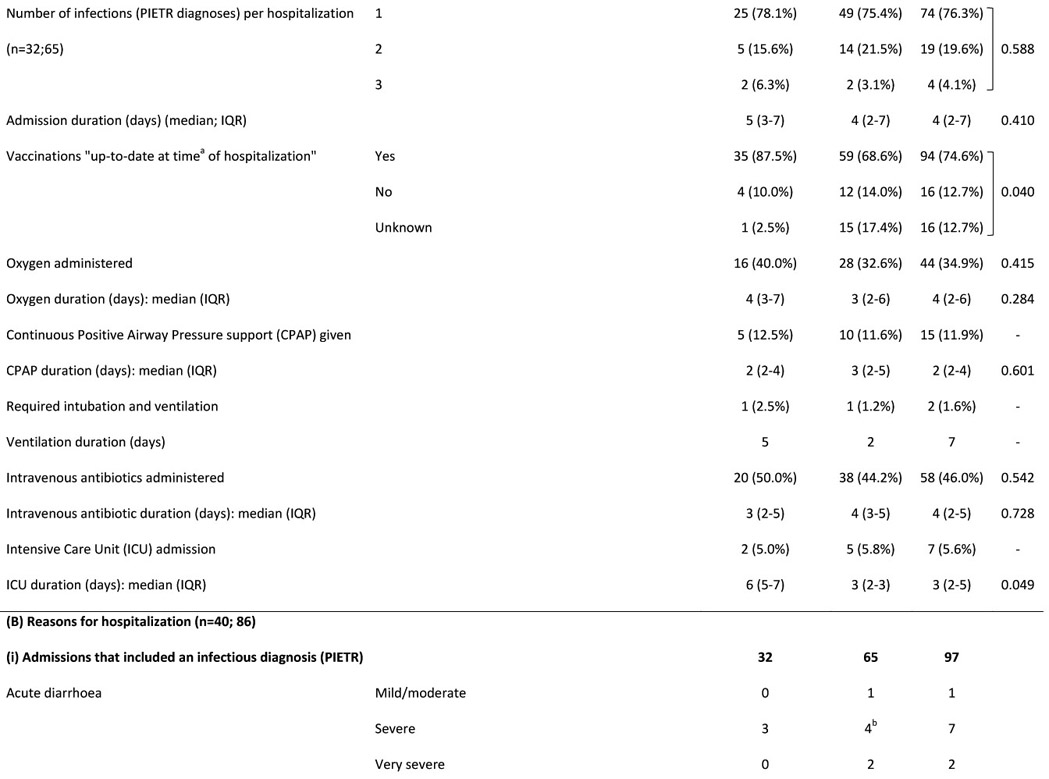
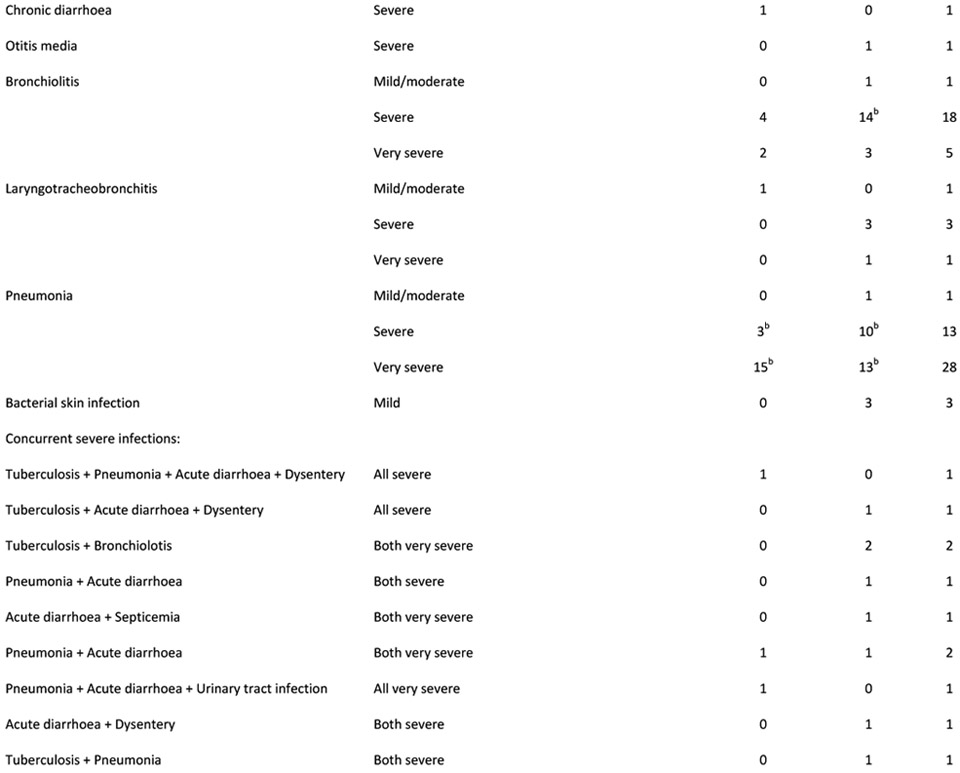
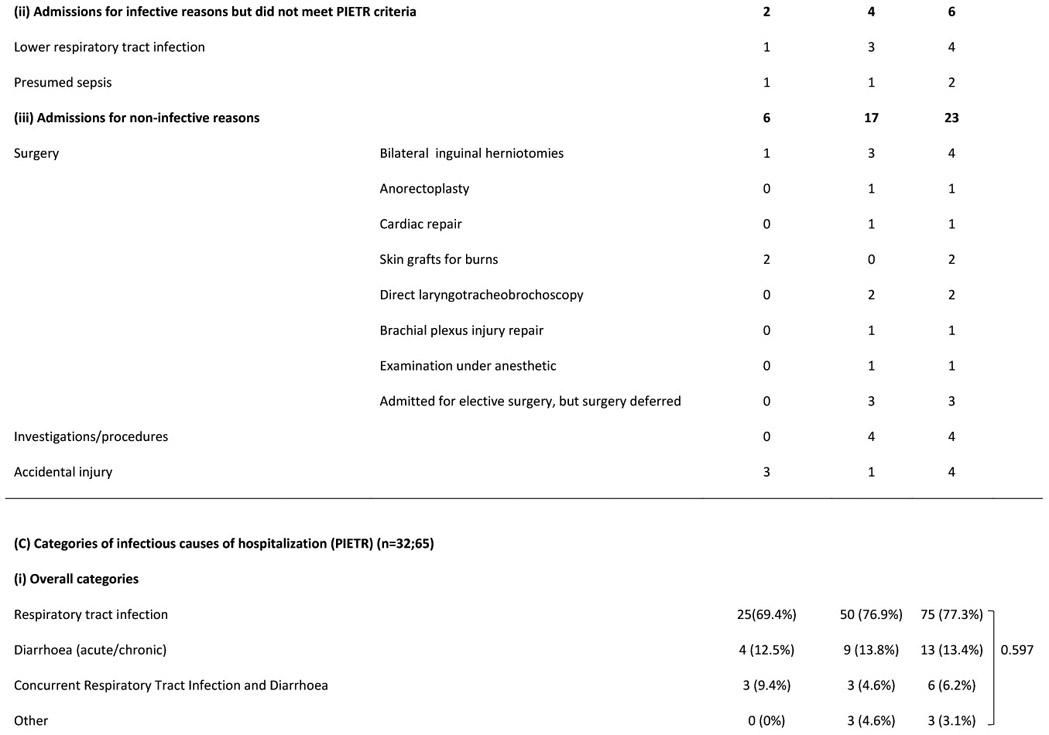
Figure 2A shows number of infection-related hospitalizations per infant, stratified by prior (all-cause) neonatal hospitalization and HIV exposure status. Among infants hospitalized in the postnatal period for infection-related causes, 26% of infants HEU (n=12/47) vs. 30% of infants HUU (n=8/27) were previously hospitalized (all causes) in the neonatal period (p=0.702). Figure 2B shows age at infectious-cause hospitalization in infants HEU vs. HUU. In infants HUU, the number of hospitalizations per month peaked at 4-6 months, whereas in infants HEU, monthly hospitalizations reached twice the number and peaked at 8 months. Table 2 shows characteristics of all hospitalized infants and reasons for hospitalization. A notable proportion were previously hospitalized as neonates (30%) and were born preterm (20%), with no significant differences between HEU vs. HUU groups. Infections were diagnosed in 77% of all hospitalizations, with respiratory tract infections present in 84% of infectious-cause hospitalizations. Although the proportions of hospitalizations associated with different categories or different severity of infections did not differ significantly between infants HEU vs. HUU, among the whole infant cohort, more infants HEU were hospitalized with severe or very severe infections during the study period (9% vs. 6%, p=0.031). Among all infants, crude incidence rate for all-cause hospitalization was 20.7 (HEU) vs. 9.5 (HUU) per 100 child-years (IRR=2.2; 95% CI 1.5-3.2), and for infectious-cause hospitalization was 15.6 (HEU) vs. 7.6 (HUU) per 100 child-years (IRR=2.1; 95% CI 1.3-3.2).
Figure 2: Number of infection-related hospitalizations in infants aged 29 to 365 days, stratified by HIV exposure status. (A) Total number per infant, stratified by whether the infant had any prior (all-cause) neonatal hospitalization and (B) Number per month of age, stratified by whether first or recurrent hospitalization. n=47/455 (10%) HIV-exposed uninfected (HEU) infants and n=27/458 (6%) HIV-unexposed uninfected (HUU) infants with at least one hospitalization; <1% infants censored during the study period. Hospitalization counts exclude neonatal period hospitalizations; none of the recurrent hospitalizations were readmissions within 7 days for the same condition.
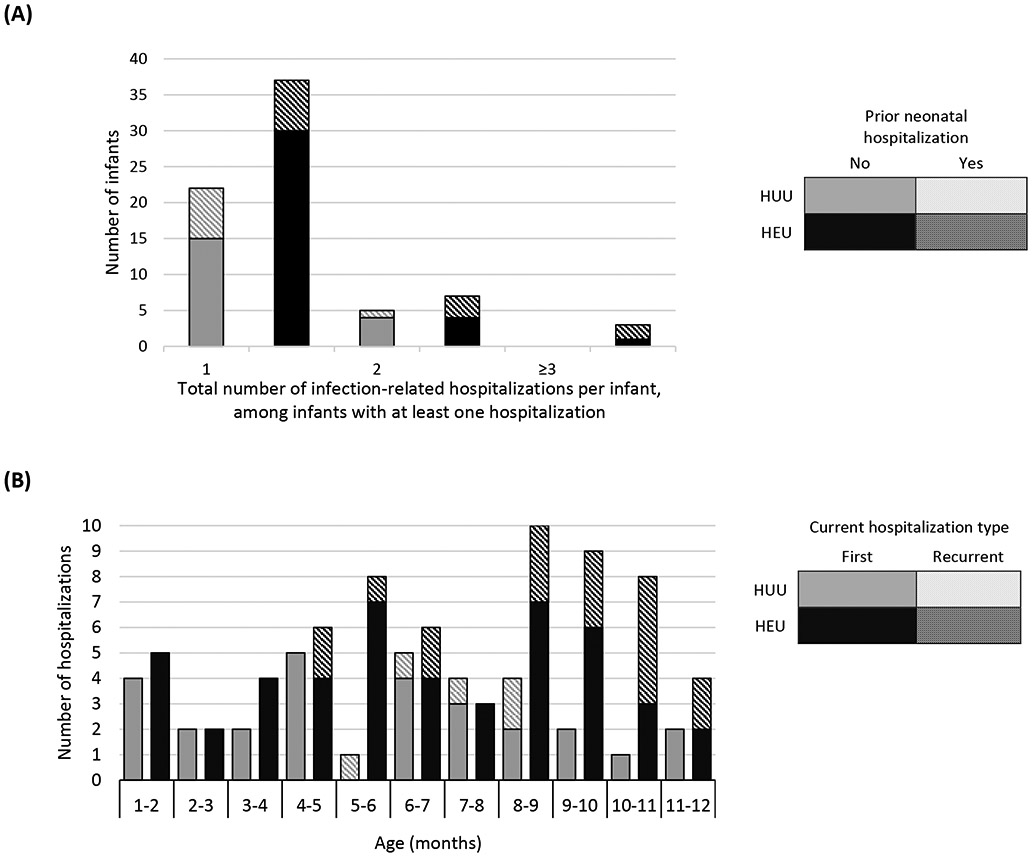
Table 2:
(A) Characteristics of hospitalized infants, (B) detailed reasons for hospitalization and (C) categories of infectious diagnoses
|
|
|
|

|
Abbreviations: HUU – HIV-unexposed uninfected; HEU – HIV-exposed uninfected; PIETR - Pediatric Infectious Event Tool for Research; IQR - interquartile range
as noted by doctors in hospital records only, not confirmed by inspection of vaccination records by study staff
2 infants had additional concurrent mild/moderate infection/s
Table 3 shows random-effects Poisson regression results. Unadjusted analyses showed higher infectious-cause hospitalization rates associated with preterm/very preterm birth, low/very low birthweight and previous neonatal all-cause/infection-related hospitalization. Lower rates were associated with up-to-date vaccination and longer duration of breastfeeding. Adjusted for potential confounders (infant sex and vaccination status; maternal age, education and type of housing), aIRR=2.8 (95% CI 1.5-5.4) for infectious-cause hospitalization among infants HEU vs. HUU (Model “A”). Increased maternal age and up-to-date vaccination status were associated with decreased rates of hospitalization.
Table 3:
Poisson regression models assessing associations with infectious-cause hospitalization in HIV uninfected infants between 29 days and 12 months of age
| All infants Unadjusted IRR (n=891) |
Model A: All infants Adjusted IRR (n=625) |
Model B: All infants Adjusted IRR (n=613) |
Model C: HEU infants Adjusted IRR (n=282) |
Model D: HEU infants Adjusted IRR (n=273) |
|
|---|---|---|---|---|---|
| HIV exposure | 1.76 (1.05-2.93) | 2.84 (1.49-5.39) | 1.95 (1.01-3.79) | ||
| Maternal education (secondary schooling completed) | 0.76 (0.44-1.32) | 0.70 (0.37-1.34) | 0.67 (0.35-1.26) | 0.80 (0.34-1.87) | 0.84 (0.37-1.92) |
| Maternal age at delivery (years) | 0.96 (0.92-1.00) | 0.94 (0.89-0.99) | 0.94 (0.89-0.99) | 0.92 (0.86-0.99) | 0.92 (0.86-0.99) |
| Reside in formal housing (vs informal dwelling) | 0.68 (0.41-1.14) | 0.78 (0.44-1.41) | 0.85 (0.48-1.52) | 0.55 (0.25-1.18) | 0.59 (0.28-1.23) |
| Male | 1.31 (0.79-2.17) | 1.44 (0.80-2.60) | 1.46 (0.83-2.59) | 1.40 (0.68-2.90) | 1.33 (0.66-2.65) |
| All vaccinations up-to-date | 0.52 (0.29-0.95) | 0.52 (0.29-0.95) | 0.61 (0.34-1.10) | 0.68 (0.32-1.42) | 0.88 (0.43-1.82) |
| Preterm birth (<37 weeks gestation) | 2.35 (1.24-4.45) | 2.20 (1.06-4.56) | 2.93 (1.28-6.73) | ||
| Very preterm birth (<32 weeks gestation) | 4.91 (1.35-17.81) | ||||
| Low birthweight (<2500g) | 2.88 (1.58-5.23) | ||||
| Very low birthweight (<1500g) | 5.05 (1.83-13.98) | ||||
| Previous neonatal admission | 2.58 (1.41-4.73) | ||||
| Previous infection-related neonatal admission | 3.80 (1.61-9.00) | ||||
| Breastfeeding duration (months) | 0.90 (0.84-0.96) | 0.90 (0.84-0.98) | 0.90 (0.81-1.00) | ||
| Breastfed >28 days | 0.70 (0.38-1.30) | ||||
| Breastfed >3 months | 0.67 (0.40-1.12) | ||||
| Breastfed >6 months | 0.39 (0.22-0.71) | ||||
| Maternal ART at conception | 1 | 1 | 1 | ||
| vs Maternal ART initiated during pregnancy | 1.20 (0.54-2.71) | 0.60 (0.23-1.57) | 0.58 (0.23-1.48) | ||
| vs Previous maternal ART history, resumed ART during pregnancy | 2.31 (1.04-5.13) | 1.19 (0.47-2.99) | 1.22 (0.51-2.89) | ||
| Maternal CD4 count <200 cells/μl near delivery | 2.26 (0.91-5.62) | 1.85 (0.66-5.18) | 1.57 (0.59-4.21) | ||
| Maternal VL ≥400 copies/ml before delivery | 1.39 (0.68-2.84) | 1.16 (0.51-2.63) | 1.03 (0.46-2.30) | ||
| Variance of random effect (95% CI) | 1.42 (0.65-3.11) | 1.07 (0.42-2.72) | 1.05 (0.33-3.29) | 0.55 (0.08-3.66) | |
Abbreviations: IRR - incidence rate ratio; g – grams; ART - antiretroviral treatment; VL - viral load; CI – confidence interval
Models A and C include possible confounders; Models B and D also include mediators (preterm birth and breastfeeding).
Note: Adjusted IRRs (and 95% confidence intervals) were obtained from mixed-effects Poisson regression models (log link function; normally distributed random effect by infant; observation time as an offset). Second-born twins and infants with high-risk congenital anomalies were excluded from analyses.
The association of HIV exposure with infectious-cause hospitalization remained significant (aIRR=2.0; 95% CI 1.0-3.8) when breastfeeding duration and preterm birth were incorporated in a separate model (“B”) to evaluate their potential effects as mediators. Both were significant mediators of infectious-cause hospitalization: infant hospitalization rates were 10% lower for each month of breastfeeding and twice as high if infants were preterm. In stratified analysis conditioned on breastfeeding status at 3 months (refitting Model “A”), hospitalization rates were significantly higher in infants HEU vs. HUU who breastfed for <3 months (aIRR=3.5; 95% CI 1.1-11.0) and even in those who continued breastfeeding ≥3 months, rates were double (aIRR=2.4; 95% CI 0.9-6.2). Among infants HEU, we evaluated maternal HIV-related factors for associations with infectious-cause hospitalization. In adjusted models (“C” and “D”; with and without mediators), neither maternal CD4 count, VL nor timing of ART initiation were significantly associated with infant infectious-cause hospitalization (Table 3).
In all mixed-effects Poisson models, random effects at individual level showed significant variability, indicating that factors not included in the models could be having an effect. Random effects may represent either infant or maternal factors as their identities in the models were synonymous. Due to the large amount of missing vaccination data (30%) and the potential for this to create bias in regression models, we conducted sensitivity analyses excluding vaccination status as well as including vaccination status as unknown. Findings were reassuringly similar, as were those adjusted for time potentially out-of-province (see Supplemental Digital Content, Table 2 and 3). Because a considerable number of HIV-exposed infants (n=68; 15%) had low certainty of HIV infection status, (i.e. not tested for HIV at ≥6 weeks of age), we conducted additional sensitivity analyses excluding these infants. The association of HIV exposure with infectious-cause hospitalization remained similar after refitting Models “A” and “B” (aIRR=3.07 (95% CI 1.59-5.90 and aIRR=2.03 (95% CI 1.04-3.94) respectively) (see Supplemental Digital Content, Table 4).
Discussion
We found increased incidence rates of infectious-cause hospitalization in infants HEU vs. HUU (aIRR=2.8; 95% CI 1.5-5.4). Other studies report similar findings [8,15,18,32]. Although this did not result in mortality differences in our urban setting with good access to oxygen, respiratory support and intensive care, this difference could result in mortality differences in other contexts where access to hospital care is insufficient to mitigate the increased infectious disease morbidity in infants HEU. Considering the size of the global infant HEU population, increases in morbidity and hospitalization among infants HEU will add considerable burden to the paediatric healthcare services.
Increased incidence of preterm birth and shorter duration of breastfeeding among infants HEU contributed to increased hospitalization but did not account for all the risk. Causal mechanisms by which HIV exposure predispose infants to worse morbidity should continue to be investigated, although it is difficult to disentangle the effects of HIV/ART exposure and social disadvantages on infant outcomes. In this post-neonatal survivor cohort, we did not find significant associations between infant infectious-cause hospitalization and maternal CD4 count, VL or timing of ART initiation, although we previously found neonates HEU were 3 times more likely to be preterm if women initiated/resumed ART in the first trimester vs. prior to conception and 6 times more likely to be very preterm if women were viremic (VL>400 copies/ml) near delivery.[29] Although differences in housing and access to indoor toilets and running water were not significant, women with HIV had poorer living conditions and level of education. Addressing poverty and structural inequalities (education and housing) will likely improve child health outcomes.
Overall rates of timely infant vaccination were poor in both groups. More infants HEU were up-to-date with vaccinations; this may be fortuitous due to increased contact with healthcare services as mothers with HIV attend services regularly for HIV treatment, or perhaps mothers with HIV comply better with infant vaccination/clinic/study visits because of HIV transmission concerns. The protective association noted between vaccination status and infant hospitalization may be a proxy for connection to healthcare services rather than protection against vaccine-preventable infections per se.
Although contributing to risk, neither up-to-date vaccination nor longer breastfeeding fully mitigated the effects of HIV exposure on hospitalization risk. Nevertheless, breastfeeding is crucial for child health, development and survival. In countries with high HIV prevalence and settings in which diarrhoea, pneumonia and undernutrition are common causes of infant mortality, WHO guidelines strongly recommend that in the context of universal maternal ART, mothers with HIV should breastfeed for at least 12 months [37]. In this cohort, only 40% of women overall breastfed for ≥6 months; significantly fewer women with HIV than without (28% vs. 52%; p<0.001). There is a need to actively implement services/activities in healthcare facilities, workplaces, communities and homes to protect, promote and support breastfeeding, especially among women with HIV (provided they are virally suppressed). Although exclusive breastfeeding is recommended, women with HIV who do not or cannot exclusively breastfeed should be reassured that ART reduces the risk of postnatal HIV transmission and practicing mixed feeding is not a reason to stop breastfeeding [37]. Women who do not plan to breastfeed for 12 months should be reassured that shorter durations of breastfeeding are better than never initiating breastfeeding at all.
Birth PCR testing was well-implemented in this cohort (96%), but it is concerning that only 77% of HIV-exposed infants received testing at 10 weeks, and that at age 12 months, 15% of HIV-exposed infants had no record of further PCR testing in the province since birth. This may be due to healthcare workers not implementing guidelines adequately or mothers not presenting their infants for follow-up care. Furthermore, population mobility (particularly to other provinces) and fragmentation of healthcare systems could make it difficult for healthcare workers to identify whether infants were HIV-exposed and require testing.
Unique strengths of the study are the ability to examine contemporaneous cohorts of infants HEU and HUU during the universal ART era, with standardized outcome definitions applied systematically by a neutral observer and not relying on the treating clinicians’ diagnoses. The 2 groups of women were comparable; all resided in the same area and in similar socio-economic conditions. However, despite adjustment for possible confounders, the potential for unmeasured differences and residual confounding exists. More infants HEU vs. HUU were hospitalized with severe or very severe infections, suggesting that the increased incidence of infectious-cause hospitalization among infants HEU is unlikely due to “over-hospitalization bias”, whereby infants HEU without severe illness may be hospitalized as a “precautionary” measure. We were able to track hospitalizations electronically within the province, therefore loss to follow-up was minimal. However, a potential weakness of the study is that we may have missed hospitalization or deaths in other provinces of South Africa, as well as out-of-hospital deaths. Several infants (10%) were known to have spent time in other provinces and we did not have hospitalization data for these periods. Details of the deaths were not documented; they occurred out of hospital and were self-reported by the mothers. Specific infectious-cause aetiologic agents could not be evaluated. There was the potential for misclassification of infant HIV infection and exposure status, as 15% of (known) HIV-exposed infants did not have 10 week (i.e. 6-14 week) or later PCR testing, nor was reliable data available on postnatal HIV testing among women without HIV. However, findings were robust in sensitivity analyses when infants with low certainty of HIV infection status were excluded.
Conclusion
We observed an increased rate of infectious-cause hospitalization among infants HEU vs. HUU. Higher incidence of preterm birth and lower breastfeeding rates among infants HEU contributed to the risk. Interventions to support breastfeeding could partially mitigate this risk. Considering the substantial size of the infant HEU population, the increased infectious disease burden in these infants will place increased strain on pediatric health services in sub-Saharan Africa.
Supplementary Material
Acknowledgements
The mothers, infants and Western Cape service providers are acknowledged. We thank the Western Cape Provincial Health Data Centre for collating and sharing data.
Funding
The project was supported by Grant Number R01HD080465 from NIH (NICHD); PI: A Boulle.
Footnotes
Conflicts of Interest: Kim Anderson and Mary-Ann Davies have received funding from Viiv Healthcare which is not related to the current project.
References
- 1.Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000-18: a modelling study. Lancet Glob Heal 2020; 8(1):e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, et al. The 2017 National Antenatal Sentinel HIV Survey Key Findings, South Africa, National Department of Health [NICD website]. July 2019. https://www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report_24July19.pdf. [Accessed 12 August 2020]. [Google Scholar]
- 3.Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. South African Med J 2014; 104(3):235. [DOI] [PubMed] [Google Scholar]
- 4.Evans C, Humphrey JH, Ntozini R, Prendergast AJ. HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Front Immuno. 2016; 7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012; 206(11):1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malaba TR, Phillips T, Le Roux S, Brittain K, Zerbe A, Petro G, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017; 46(5):1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedi COO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016; 3(1):e33–48. [DOI] [PubMed] [Google Scholar]
- 8.Labuda SM, Huo Y, Kacanek D, Patel K, Huybrechts K, Jao J, et al. Rates of Hospitalization and Infection-Related Hospitalization Among Human Immunodeficiency Virus (HIV)-Exposed Uninfected Children Compared to HIV-Unexposed Uninfected Children in the United States, 2007-2016. Clin Infect Dis 2020; 71(2):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dara JS, Hanna DB, Anastos K, Wright R, Herold BC. Low birth weight in human immunodeficiency virus–exposed uninfected infants in Bronx, New York. J Pediatric Infect Dis Soc 2018; 7(2):E24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santosa WB, Staines-Urias E, Tshivuila-Matala COO, Norris SA, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS 2019; 33(10):1623–33. [DOI] [PubMed] [Google Scholar]
- 11.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 2016; 21(2):68–73. [DOI] [PubMed] [Google Scholar]
- 12.Coathup V, Boyle E, Carson C, Johnson S, Kurinzcuk JJ, Macfarlane A, et al. Gestational age and hospital admissions during childhood: population based, record linkage study in England (TIGAR study). BMJ 2020; 371:m4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan AT, Bonawitz R, Gill CJ, Thea DM, Kleinman M, Long L, et al. A Meta-analysis Assessing Diarrhea and Pneumonia in HIV-Exposed Uninfected Compared with HIV-Unexposed Uninfected Infants and Children. J Acquir Immune Defic Syndr 2019; 82:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-Exposed Uninfected Infants are at Increased Risk for Severe Infections in the First Year of Life. J Trop Pediatr 2012; 58(6):505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetghebuer T, Smolen KK, Adler C, Das J, McBride T, Smits G, et al. Initiation of Antiretroviral Therapy Before Pregnancy Reduces the Risk of Infection-related Hospitalization in Human Immunodeficiency Virus–exposed Uninfected Infants Born in a High-income Country. Clin Infect Dis 2019; 68(7):1193–203. [DOI] [PubMed] [Google Scholar]
- 16.Rupérez M, González R, Maculuve S, Quintó L, López-Varela E, Augusto O, et al. Maternal HIV infection is an important health determinant in non-HIV-infected infants. AIDS 2017; 31(11):1545–53. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does Severity of HIV Disease in HIV-Infected Mothers Affect Mortality and Morbidity among Their Uninfected Infants? Clin Infect Dis 2005; 41(11):1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.le Roux SM, Abrams EJ, Donald KA, Brittain K, Phillips TK, Zerbe A, et al. Infectious morbidity of breastfed, HIV-exposed uninfected infants under conditions of universal antiretroviral therapy in South Africa: a prospective cohort study. Lancet Child Adolesc Heal 2020; 4:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Mollendorf C, von Gottberg A, Tempia S, Meiring S, de Gouveia L, Quan V, et al. Increased Risk for and Mortality From Invasive Pneumococcal Disease in HIV-Exposed but Uninfected Infants Aged <1 Year in South Africa, 2009–2013. Clin Infect Dis 2015; 60(9):1346–56. [DOI] [PubMed] [Google Scholar]
- 20.Brennan AT, Bonawitz R, Gill CJ, Thea DM, Kleinman M, Useem J, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016; 30(15):2351–60. [DOI] [PubMed] [Google Scholar]
- 21.Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Front Immunol 2016; 7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ásbjörnsdóttir KH, Slyker JA, Maleche-Obimbo E, Wamalwa D, Otieno P, Gichuhi CM, et al. Breastfeeding Is Associated with Decreased Risk of Hospitalization among HIV-Exposed, Uninfected Kenyan Infants. J Hum Lact 2016; 32(3):NP61–NP66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bork KA, Cournil A, Read JS, Newell M-L, Cames C, Meda N, et al. Morbidity in relation to feeding mode in African HIV-exposed, uninfected infants during the first 6 mo of life: the Kesho Bora study. Am J Clin Nutr 2014; 100(6):1559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taron-Brocard C, Le Chenadec J, Faye A, Dollfus C, Goetghebuer T, Gajdos V, et al. Increased Risk of Serious Bacterial Infections Due to Maternal Immunosuppression in HIV-Exposed Uninfected Infants in a European Country. Clin Infect Dis 2014; 59(9):1332–45. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg A, Mussi-Pinhata MM, Yu Q, Cohen RA, Almeida VC, Amaral FR, et al. Factors Associated with Lower Respiratory Tract Infections in HIV-Exposed Uninfected Infants. AIDS Res Hum Retroviruses 2018; 34(6):527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeganeh N, Watts DH, Xu J, Kerin T, Joao EC, Pilotto JH, et al. Infectious Morbidity, Mortality and Nutrition in HIV-Exposed, Uninfected, Formula Fed Infants. Pediatr Infect Dis J 2018; 37: 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mofenson LM. New challenges in the elimination of pediatric HIV infection: The expanding population of HIV-exposed but uninfected children [Editorial Commentary]. Clin Infect Dis 2015; 60:1357–1360. [DOI] [PubMed] [Google Scholar]
- 28.Goga A, Slogrove A, Wedderburn CJ, Feucht U, Wessels J, Ramokolo V, et al. The impact of health programmes to prevent vertical transmission of HIV. Advances, emerging health challenges and research priorities for children exposed to or living with HIV: Perspectives from South Africa. S Afr Med J 2019; 109(11b):77–82. [DOI] [PubMed] [Google Scholar]
- 29.Anderson K, Kalk E, Madlala HP, Nyemba DC, Jacob N, Slogrove A, et al. Preterm Birth and Severe Morbidity in Hospitalized Neonates who are HIV Exposed and Uninfected compared to HIV Unexposed. AIDS 2021; 35(6):921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulle A, Heekes A, Tiffin N, Smith M, Mutemaringa T, Zinyakatira N, et al. Data Centre Profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Popul Data Sci 2019; 4(2):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slogrove AL, Esser MM, Cotton MF, Speert DP, Kollmann TR, Singer J, et al. A Prospective cohort study of common childhood infections in South African HIV-exposed uninfected and HIV-unexposed infants. Pediatr Infect Dis J 2017; 36(2):e38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Metropolitan Atlanta Congenital Defects Program: MACDP 6-Digit Code Defect List [Internet]. [cited 2020 Jul 17]. Available from: https://www.cdc.gov/ncbddd/birthdefects/macdp.html
- 34.South African National Department of Health. National Consolidated Guidelines for the Prevention of Mother-To-Child Transmission of HIV (PMTCT) and the Managment of HIV in Children, Adolescents and Adults [Internet]. 2015. [cited 2021 Jan 18]. p. 1–128. Available from: https://sahivsoc.org/Files/ARTGuidelines15052015.pdf [Google Scholar]
- 35.Western Cape Government. Expanded Programme of Immunisation - Revised Childhood Immunisation Schedule [Internet]. 2015. [cited 2020 Dec 14]. Available from: https://www.westerncape.gov.za/assets/departments/health/2016_schedule.pdf
- 36.WHO Anthro Survey Analyser and other tools [Internet]. [cited 2020 Dec 14]. Available from: https://www.who.int/toolkits/child-growth-standards/software
- 37.WHO ∣ Updates on HIV and infant feeding [Internet]. [cited 2021 Aug 1]. Available from: https://apps.who.int/iris/bitstream/handle/10665/246260/9789241549707-eng.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


