Abstract
In 2017 alone, 783,000 children aged 12 to 17 years misused opioids with 14,000 using heroin. Opioid misuse and opioid use disorder (OUD) in adolescents and young adults are significant barriers to ending the human immunodeficiency virus (HIV) epidemic. To address these synergistic scourges requires dedicated practitioners and improved access to life-saving evidence based treatment. Adolescents and young adults make up over 1 in 5 new HIV diagnoses even though they are less likely to be tested or know they are infected. Adolescents and young adults living with HIV are less likely to be retained in care or achieve virological suppression. OUD further leads to increased rates of risky behaviors (like sex without condoms), deceased retention in HIV care, and decreased rates of viral suppression in this vulnerable population. Medications for opioid use disorder (MOUD) are recommended for adolescents and young adults with severe OUD and help retain youth in HIV treatment and decrease risk of death. However, due to stigma and lack of experience prescribing MOUD in adolescents, MOUD is often perceived as a last line option. MOUD remains difficult to access for adolescents with a shortage of providers and decreased options for treatment as compared to adults. Addiction treatment is infection prevention, and integrated addiction and HIV services are recommended to improve health outcomes. A multipronged approach including patient education, provider training, and policy changes to improve access to treatment and harm reduction are urgently needed confront the drug use epidemic in youth.
Keywords: HIV, Adolescent, Opioid-related disorders, social stigma, buprenorphine, methadone, naltrexone
Introduction
As the United States (U.S.) focuses in on ending the human immunodeficiency virus (HIV) epidemic, opioid use disorder (OUD) and, specifically, injection drug use (IDU) remain significant barriers, especially in poor and rural areas [1]. Further, untreated OUD increases HIV transmission through IDU, increased risk behaviors, decreased retention in HIV treatment programs, and decreased viral load suppression [2, 3]. Not only are opioids, like heroin and fentanyl, readily available in the U.S., but addiction treatment is often inaccessible due to a lack of providers or insurance coverage together with restrictive policies that impede harm reduction programs. This disparity is heightened in , rural areas like Appalachia, the Midwest, and the Southern U.S. where widespread opioid use combined with addiction treatment deserts [4] is devastating, leading to so-called deaths of despair (overdose, suicide) and driving a decline in life expectancy for the entire nation [5, 6]. Preliminary data from the Centers of Disease Control and Prevention (CDC) indicate an alarming increase in overdose deaths in the U.S. since 2020 with an estimated 93,000 deaths between December 2019 and December 2020, a 29.4% increase from the previous year [7].
Unlike the heroin epidemic of the 1960s and 1970s that affected adults, namely Vietnam veterans, today OUD has developed in multiple generations living under the same roof and does not spare youth. Hundreds of thousands of children in the U.S. are misusing opioids [8], and many are dying as a result [9]. Further, substance use related behaviors directly contribute to HIV risk behaviors in adolescents and young adults, a group that is already disproportionately affected by HIV. One in five Americans newly diagnosed with HIV is between 13 and 24 years old [10], with the Southern US especially hard hit (Figure 1). Due to intersecting stigma of race and gender minority status, black, Latino, and multiracial gay youth may be at higher risk of depression and substance use [11], which contribute to the higher burden of HIV in persons of color[12, 13]. Of new HIV diagnosis in 2019, approximately 6.8 % were among persons who inject drugs (PWID) and another 4% were among PWID and male to male sexual contact [14]. With the escalating syndemic of stimulant (e.g., cocaine, methamphetamines) and opioid use [15, 16]. identifying evidence-based ways to engage and retain substance using adolescents in care is imperative and urgent.
Figure 1.
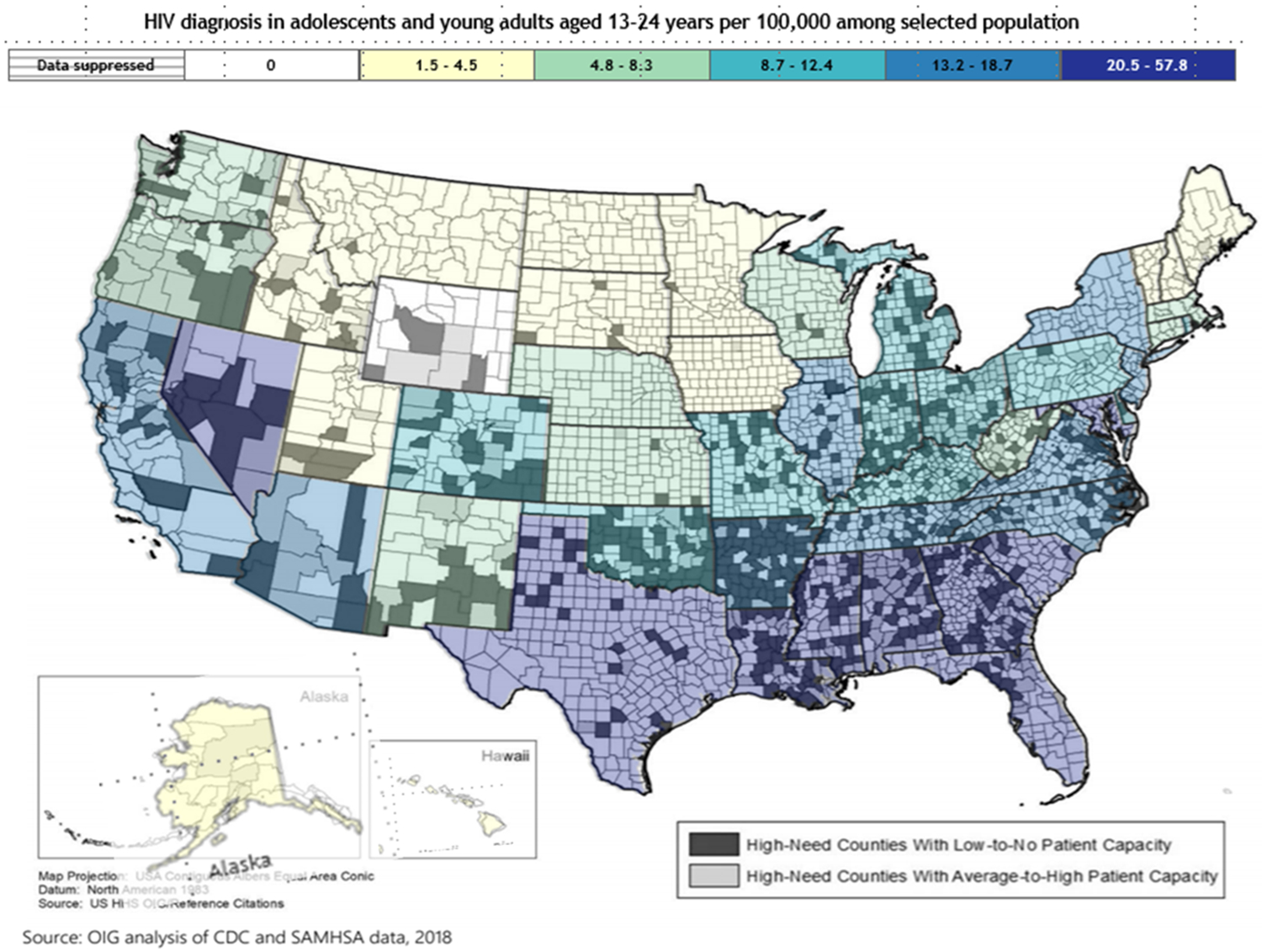
The overlap of HIV in adolescents and young adults aged 13-24 years and areas of high opioid misuse but low access to care.
The overlap of HIV in adolescents and young adults aged 13-24 years and areas of high opioid misuse but low access to MOUD.
This figure illustrates the geographic overlap of states with high rates of HIV diagnosis among adolescents and young adults aged 13-24 years in 2018[1] with counties with high need of opioid treatment based on high indicators of opioid misuse in that county[2, 3]. Counties were further delineated by whether the county had high or low capacity to provide MOUD treatment to people with OUD in 2018[2]. Patient capacity was determined by number of X-waivered providers present in a county and surrounding area.
The objective of this review is to increase awareness of the unique epidemiology and outcomes of OUD in adolescents with an emphasis on missed opportunities for HIV prevention and treatment.
Opioid Use Disorder in Adolescents
Epidemiology
Adolescent is defined as an individual between 10 and 18 years old [17, 18] and young adult as an individual between 19 and 25 years old. Non-medical opioid use is defined as opioid use without one’s own prescription or for non-medical purposes like experiencing feelings of euphoria. OUD is defined as a “problematic pattern of opioid use leading to clinically significant impairment or distress” and manifested by at least two of the symptoms and behaviors described in Table 1[19]. Significant impairment in daily life including health problems, disability, and failure to meet responsibilities at work, school, or home manifest in unique ways for adolescents [20] (Table 1). In 2017 alone, 783,000 children aged 12 to 17 years used opioids for non-medical purposes with 769,000 misusing prescription opioids and 14,000 adolescents using heroin [8]. The clinical characteristics of adolescent OUD includes non-medical use of opioids [21], male sex, Caucasian race, and co-occurring mental health and substance use disorders.
Table 1.
OUD Diagnostic Criteria and Manifestations in Adolescents
| DSM-5 Diagnostic Criteria for OUD | Examples of OUD Signs in Adolescents |
|---|---|
| 1. Opioids are taken in larger amounts, duration than intended |
|
| 2. Persistent desire/unsuccessful effort to cut down or control opioid use |
|
| 3. A great deal of time is spent obtaining, using or recovering from the effects of opioids |
|
| 4. Craving |
|
| 5. Recurrent use of opioid results in failure to fulfill major role obligations at work, school, or home. |
|
| 6. Continued use despite social/interpersonal substance - related problems. |
|
| 7. Important social, occupational, or recreational activities are given up or reduced because of substance use |
|
| 8. Recurrent use in hazardous situations |
|
| 9. Continued use despite knowledge of having a persistent or recurrent opioid-related physical or psychological problem that is likely caused or exacerbated by opioid use |
|
| 10. Tolerance | |
| 11. Withdrawal |
|
Non-medical opioid use is increasingly fatal among adolescents. Between 1999 and 2016, adolescent overdose deaths in 15- to 19-year-olds exploded with a 95% increase for prescription opioids, 405% increase for heroin, and over 2,900% increase in fatal overdoses for synthetic opioids such as fentanyl [9]. Between 2010 and 2016, there was a nearly 500% increase seen in lethal overdoses among adolescents involving combinations of illicit opioids such as heroin and fentanyl and prescription opioids.[22]
Initiation of Non-medical Opioid Use
Most young adults with OUD first use opioids as adolescents. Of adults aged 18 to 30 years requiring hospital admission for substance use disorder (SUD), 74% initiated illicit substance use at age 17 or younger [23]. A study in rural New England found that over half of persons who inject drugs (PWID) took their first opioids between ages 10 and 17 years with 13.5% of PWID first injecting between 10 and 13 years old and 90% initiating IDU by the age of 25 [24]. This is especially concerning as initiation of prescription non-medical opioid use prior to age 15 has since been linked to increased risk of transition to heroin [25]. While not all non-medical opioid use is consistent with OUD, any opioid use during adolescence is associated with increased risk of lifetime dependence [26-28] and even infrequent non-medical opioid use during high school has been prospectively associated with subsequent heroin use initiation[29].
Adolescents may first experience or observe non-medical opioid use in their home. The presence of a family member with SUD can normalize addiction. Children may witness non-medical opioid use or directly receive opioids from a family member [24, 30, 31]. In the US, an estimated 623,000 parents with OUD are living with children [32]. Further, a recent study found that 34% of parents and caregivers of adolescents reported having prescription opioids in the home, including active and leftover prescriptions. Many were unaware that opioids were commonly misused by adolescents (30%) and could lead to heroin addiction (39%)[33].
Physiology of Opioid Use Disorder in Adolescents and Young Adults
OUD is a chronic, relapsing, physiologic disease resulting in changes to the central nervous system (CNS), and poses a persistent threat to adolescent and young adult health. Adolescence is a time of critical hormonal, neurochemical, and behavioral changes and represents a period of unique vulnerability to addiction [34]. Adolescents in particular, experience increased impulsivity, novelty seeking, risk taking, and preference for immediate gratification increasing their risk for opioid initiation [35, 36]. On a neurochemical level, the early adolescent brain has an overproduction of dopamine receptors within reward centers compared to later adulthood [37]. This likely indicates increased sensitivity to the euphoric properties of opioids and is supported by animal models demonstrating increased dopamine levels in response to lower doses of oxycodone in adolescent mice as compared to adult mice [38].
OUD alters neural pathways, which perpetuate and solidify opioid addiction and are further reinforced by physiologic dependence and negative effects on mood and executive function [39-41]. Animal studies suggest that adolescents also may be more sensitive to the negative effects of OUD. Adolescent rats exposed to morphine had greater tolerance to opioid analgesia, greater pain sensitivity, and more pronounced symptoms of opioid withdrawal when later re-exposed to morphine as adults than rats who did not initiate opioid use until adulthood [42, 43]. As physiologic dependence and withdrawal symptoms negatively reinforce opioid addiction and exacerbate HIV risk behaviors, [24, 44] this suggests initiation of opioid use in adolescence increases the likelihood of developing OUD and a more difficulty achieving sustained abstinence from opioids.
OUD, HIV, and Comorbid Infections in Adolescents
Adolescents and young adults represent a significant and sustaining proportion of the HIV epidemic. In 2018, 21% of all new diagnoses in the U.S. (37,832) occurred in young people, aged 13 to 24 [10]. Young adults and adolescents are less likely to know they are infected or be tested for HIV [45]; only 9.3% of high school students ever receive HIV testing [46]. Even when diagnosed, adolescents and young adults are less likely to achieve virological suppression and be retained in care (relative to older adults) [47] allowing them to contribute to subsequent HIV transmission. Young people aged 18 to 24 who are receiving care for their HIV are more likely than older people with HIV to be living in households with low income levels, to have been recently homeless, recently incarcerated, or uninsured [48] further complicating their care. Any SUD, including OUD, increases the likelihood that youth will engage in IDU, transactional sex (TS), inconsistent condom use, and sex with persons of unknown HIV status- behaviors associated with HIV acquisition and transmission [49-52] and reduces the likelihood that HIV infected youth will be retained in HIV care or virologically suppressed [2, 53, 54]. In a national survey, 18.8% of adolescents admitted to using drugs or alcohol just before sex [46]. “Chemsex” is the recreational and non-medical use of illicit substances to facilitate and enhance sexual activity, especially among male to male sexual contact and has been associated with increased likelihood of HIV positive status [16], sexual activity with more partners and for longer duration [55]. In adolescent and young adult sexual minority males, chemsex has been associated with increased rates of condomless anal sex [56]. With the growing syndemic of stimulants combined with opioids, chemsex is a challenge to controlling the HIV epidemic. All these factors contribute to the unique challenges of preventing, identifying, and treating HIV infection in youth who use substances.
In May 2018, HIV pre-exposure prophylaxis (PrEP) was approved for adolescents weighing at least 35 kilograms (kg) and recommended for any youth at risk of HIV infection due to substance use or other risks[57]. Yet, uptake among adolescents and young adults in the US remains low [58-60]. Misinformation and mystery continue to surround HIV care and prevention in young adults. A 2017 study from the Kaiser Family Foundation, found that only 27% of adults aged 18-30 had heard anything about PrEP and of those, only 18% believed it was “very effective [61].” Over half of participants did not believe people who wanted PrEP could actually access it. This lack of awareness about PrEP and HIV prevention strategies places young adults and adolescents at increased risk for HIV acquisition.
Furthermore, young adults and adolescents contemplating PrEP cite possible disclosure of sexual status and orientation to their parents as a significant barrier to PrEP initiation and adherence [62]. However, a recent survey out of the deep South found that primary support persons of adolescents and young adults seen at an academic pediatric hospital overwhelming (98%) %) indicated that they would support an adolescent or young adult taking PrEP [63]. These support persons were will to provide transportation to appointments, assistance with refilling prescriptions, medication reminders, and encouragement suggesting they are underutilized allies in prevention [63]. Other barriers to PrEP use in adolescents and young adults included perceived inconvenience of trimonthly follow-up and cost without parental insurance [62]. Mobile phone applications, or apps, hold promise for increasing access and adherence to PrEP [64, 65]. The UNC/Emory Center for Innovative Technology (iTech) evaluated PrEP related apps in youth and found high acceptability among youth participants [65]. This continues to be an area of ongoing study.
Additional infections, such as Hepatitis C and sexually transmitted infections (STI), are also associated with OUD and increasingly infecting youth and young adults. Sexual risk behaviors increase with misuse of prescription medications [66], putting adolescents at risk for bacterial STIs and hepatitis C virus (HCV). In addition to sexual transmission, injection of opioids continues to be a significant risk factor for HCV among adolescents and young adults: in a study of street-involved Canadian youth, HCV infection was significantly associated with injection drug use along with sex work and non-fatal overdose [67].
OUD Treatment in Adolescents and Young Adults
Medications for opioid use disorder (MOUD) are the cornerstone of care due to their effectiveness in improving addiction outcomes. Physiologic dependence and symptoms of withdrawal negatively reinforce opioid addiction and exacerbate HIV risk behaviors, [24, 44]. MOUD, such as buprenorphine, have been shown to increase rates of opioid use cessation, decrease injection drug events and [3, 68-70] supporting sustained abstinence from non-medical opioid use. In adults, MOUD use is cost effective [71, 72]. Most importantly, MOUD significantly increases survival [73-75]. Treatment with MOUD is beneficial towards ending the HIV epidemic as well. MOUD is associated with a 40-60% decrease in injection events among PWID and decreased needle sharing among people with HIV (PWH) [76] decreasing risk of transmission of blood borne pathogens such a HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) [3, 77]. IVDU continues to drive epidemics of HCV and HIV, and over 200 U.S. counties have been identified as high risk for outbreaks among PWID. [78] Even in people who do not inject drugs, receipt of MOUD decreases HIV risk [79-81] and increases retention in HIV care [82, 83].
The American Academy of Pediatrics (AAP) and the Society for Adolescent Health and Medicine (SAHM) recommend using MOUD to treat adolescents with severe OUD [84, 85]. However, data and policy to support OUD treatment of adolescents are woefully lacking [4, 86] and access is often limited [4, 8, 87]. There are three Food and Drug Administration (FDA) approved medications currently licensed for the treatment of opioid use disorder in adults: buprenorphine, methadone, and naltrexone (Table 2). However, only buprenorphine is approved for the treatment of substance use disorder in adolescents (approved for ages 16 years and above) [88].
Table 2.
FDA Approved Medications for OUD, Adolescent Criteria, and HIV Outcomes
| Medication | Mechanism | Benefits | Adverse Effects | Use in Adolescents | Effect on HIV Outcomes |
|---|---|---|---|---|---|
| Buprenorphine | Partial μ-receptor agonist often co-formulated with naloxone | Decreased risk of overdose and diversion (when combined with naloxone) |
|
Recommended by the AAP for all patients ≥16 y/o with severe OUD | |
| Methadone | Full μ-receptor agonist | Will not precipitate withdraw |
|
Use in patients <18 is restricted | |
| Extended-release naltrexone | Full μ-receptor antagonist | Has decreased overdoses compared to oral naltrexone [6] |
|
FDA approved for 18 years and older. Can be used in patients younger than 18 in certain circumstances |
Although few models for pediatric OUD care have been rigorously studied, available data supports an interdisciplinary approach, such as the Vermont Hub-and-Spoke model, the Project ECHO model, and the Massachusetts nurse care manager model [89, 90]. Each of these models offers integrated care that addresses both psychosocial and pharmacologic treatment, including buprenorphine. Project ECHO has the added advantage of facilitating tele-mentoring of prescribers new to the field of OUD care. Using virtual training and hands on mentoring, Project ECHO model can help grow the workforce of adolescent addiction treatment providers.
Barriers to Addiction Treatment in Adolescents and Young Adults
Diagnosis
The U.S. Preventative Services Task Force (USPSTF) and the AAP recommend routine screening for SUD in adolescents and young adults [91-93] but the diagnosis of OUD in adolescents remains especially challenging. Adolescents often do not disclose substance use due to concerns of parental discovery or lack of insight into their substance use disorder [94]. Even after admission to the hospital for non-fatal opioid overdose, less than 10% of adolescents receive a diagnosis of OUD [95] with female adolescents significantly less likely to receive a diagnosis of OUD than male counterparts[95]. Substance use among peers and family members, childhood trauma, and poverty are all strongly associated with opioid use in adolescents [24, 27, 28, 96-98] and should prompt more in-depth screening for non-medical opioid use. Because substance use is more common among youth in juvenile justice systems, all youth in these settings should receive OUD screening and referral to services when indicated [99].
These barriers to diagnosis may be amplified in lesbian, gay, bisexual, transgender, or queer (LGBTQ) populations who may experience intersecting stigma around multiple domains: gender/sexual minority status, substance use/criminalization, and often race/ethnicity [11]. Stigma erodes trust in the healthcare system and precludes people from disclosing stigmatizing behaviors. Further, food insecurity is associated with increased risk of nonmedical prescription opioid use as well as increased rates of TS, STI, and HIV among gay or bisexual males and all females [100]. Yet, complex relationships between the stigma of poverty, HIV, and minority status all make it difficult for patients to disclose illicit opioid use. The rising epidemic of adolescent OUD necessitates a high index of suspicion and underscores the need for routine screening in all adolescents. More in-depth screening should be offered to adolescents identifying as LGBTQ and those experiencing trauma, poverty, and substance use in the home [92].
Treatment Access
For those who are diagnosed with OUD, there are myriad additional barriers to addiction treatment. Pediatric patients are significantly less likely to receive a referral and enroll in MOUD programs [101] with female, Black, and Hispanic adolescents even less likely to be referred [86]. Timely adolescent enrollment in MOUD treatment (within 3 months of diagnosis) results in significantly longer retention in OUD treatment and care [101], yet due to stigma, treatment with MOUD is often perceived as a last line option by practitioners, parents, and patients causing delays in referral and engagement in care [87, 102, 103]. A 2017 study by the Johns Hopkins School of Public Health found that only 2.4% of adolescents in treatment for heroin abuse received treatment with MOUD as compared to 26% of adults similarly enrolled for heroin treatment [104]. Adolescents who misuse oral opioids are even less likely to be referred for MOUD [27]. Even when adolescents and young adults present with life-threatening manifestations of OUD, rates of treatment are still astonishingly low [105]. Alinsky et al., evaluated over 3,700 adolescents and young adults presenting with non-fatal opioid overdose, and found that most were discharged without any addiction treatment. Around 30% of adolescents and young adults received behavioral health recommendations alone and only 1.9% received evidence-based pharmacotherapy within 30 days of their admission for opioid overdose [106]. Many youths with OUD lack insight and may not perceive any need for intervention [94]. Co-morbid mental health illness [107, 108] is common, especially among adolescents who use heroin, making retention and treatment in this cohort even more challenging. Adolescents may refuse OUD treatment due to stigma relating to the diagnosis of OUD [94, 103]. If adolescents do enroll in treatment for OUD, they cannot use their parents’ insurance without insurance companies disclosing the expense to their parents along with their diagnosis of OUD. The inability to assure confidential and affordable care can discourage enrollment in OUD treatment programs among adolescents [94]. However, parents can be an ally in the treatment of OUD and often serve as the impetus for adolescents to get the help they need [92, 107]. Adolescents who have disclosed non-medical opioid use to their parents are more likely to engage in treatment for their OUD [85, 94]. Whenever possible, parents should be involved in their child’s care [85]. For juvenile justice-involved youth, this touch point provides an opportunity to integrate addiction services and support, such as case management, but is often under-utilized for the treatment of OUD [109]. Research is ongoing to identify optimal ways to implement evidence-based substance use services in juvenile justice settings.
Even if referred to addiction treatment, adolescents and young adults may be unable to access treatment in a timely manner due to a paucity of programs and practitioners treating adolescents with OUD. Because OUD often develops in adolescence and has implications for long term clinical outcomes, it is like other chronic diseases (e.g., diabetes) that have been championed by the adolescent and combined internal medicine/pediatrics field. In recent years, the field of addiction has been bolstered by a cadre of infectious diseases clinicians and researchers focused on HIV prevention and treatment in substance using adults. Yet, pediatric champions that provide treatment at the nexus of OUD and infectious diseases are lacking. Only around 1% of X-waivered physicians identify as pediatricians [110]. Access is even more limited in rural areas, especially in the Southern U.S. and Appalachia, due to geographic isolation from OUD treatment centers and MOUD providers[4] (Figure 1). Patients with OUD are less likely to be insured, are often economically disadvantaged, and may struggle with transportation or taking time off work [111-113]. To improve access to MOUD, the U.S. Department of Health and Human Services and the Official of National Drug Control Policy are working with interagency partners to relax barriers to MOUD, including the “X” waiver, with the goal to reduce hurdles to effective OUD treatment [114]. We continue to need pediatric and adolescent providers educated in MOUD and trained in the treatment of substance use disorders including initiating and managing MOUD.
Access to Evidence-based Pharmacotherapy
Buprenorphine, a long-acting partial μ-receptor agonist, is an FDA approved OUD treatment for those aged 16 years and older [115]. Buprenorphine increases retention of adolescents and young adults in OUD treatment programs (with longer treatment courses associated with higher rates of retention) and. decreases illicit substance use and injection drug events [116-118] in adolescents. Compared to clonidine alone, buprenorphine treatment in adolescents was associated with a 33% higher relative rate of retention in OUD care and an increased number of opioid negative urine drug screens [116]. Furthermore, another recent study evaluating adolescents with OUD from ages 10 to 19 years old, found enrollment in an outpatient buprenorphine treatment program was associated with a 90% decrease in odds of requiring an acute emergency department visit, urgent care visit, or inpatient hospitalization for an opioid-related event [119]. Interestingly, outpatient primary care visits increased for non-opioid-related complaints suggesting greater engagement in the healthcare system among patients enrolled in OUD treatment with buprenorphine [119]. Further barriers include expense, with buprenorphine costing upwards of 5,000 dollars a year for uninsured patients [120]. Even with medical insurance, insurance plans often require lengthy prior authorizations which have been linked to decreased retention in care, increased rates of return to non-medical opioid use, and delays in treatment [121, 122]. Lastly, even if an adolescent or young adult obtains a prescription for buprenorphine or naloxone, many pharmacies in the U.S. do not routinely carry these medications and may even refuse to order them, further limiting access to MOUD and increasing stigma [123].
Methadone is a full long-acting μ-receptor agonist that does not typically produce euphoria in people with OUD due to the slow prolonged μ-receptor binding [115]. While also effective for the treatment of OUD, methadone is not approved for the treatment of OUD in pediatric patients and is rarely used to treat OUD in people less than 18 years old in the U.S. [124]. Like buprenorphine, there are restrictions on the types of clinics and providers who may prescribe methadone in the U.S., creating additional barriers for young adults who would otherwise qualify. However, methadone is approved for MOUD treatment in adolescents in Europe and enrollment in treatment programs using methadone demonstrated decreased heroin use among youth with severe OUD [70]. In adults, enrollment in methadone treatment programs is associated with decreased risk of HIV acquisition among PWID and decreased HIV and HCV related risk behaviors [79, 80]. Enrollment in methadone treatment programs have also been associated with increased uptake in HIV testing, increased initiation and adherence to HIV antiviral therapy, and increased HIV virologic suppression [83].
Naltrexone is an opioid antagonist and can be prescribed by any physician without the requirement of special licensing, but it is not FDA approved for adolescents under the age of 18. However, it can be obtained for youth in certain circumstances of severe OUD. Since it is an opioid antagonist, there is no abuse potential and is thus preferred by some families and patients. Naltrexone has the benefit of an oral formulation and a long-acting once monthly injection, but the oral form has not been shown to be superior to placebo or no medication at all in the treatment of OUD [125] and has high rates of discontinuation [125-127]. Other countries have shown some success with extended release implantable and injectable naltrexone in decreasing overdoses as compared to oral naltrexone in adolescents and young adults with severe OUD [128, 129]. In the U.S., a small case report of adolescents and young adults treated with long-acting injectable naltrexone demonstrated decreased opioid use during a 4 month follow up period [129]. A randomized controlled trial of 159 adults comparing buprenorphine and extended injection naltrexone showed naltrexone was non-inferior to buprenorphine in retention in OUD care and number of opioid negative urine drug tests [130]. As naltrexone is an opioid antagonist, patients must undergo detoxification and withdrawal before initiating and can lead to higher rates of early discontinuation [131]. An important limitation to extended-release naltrexone injection is that patients develop decreased opioid tolerance and risk potentially fatal overdose if they do return to non-medical opioid use after discontinuing naltrexone or try to overcome the naltrexone opioid blockade [132].
Access to Harm Reduction Services
Finally, harm reduction interventions, namely syringe service programs (SSPs), are an evidence-based intervention that reduce HIV and HCV transmission and enable a range of support services [133-136]. By providing co-located services from sterile syringes and naloxone to HIV PrEP and HCV treatment, SSPs can facilitate a range of ancillary care. These programs also allow vulnerable patients to access counseling, education, and linkage to routine health services [134, 136]. Yet, few SSPs cater to youth and young adults- serving as another barrier to prevention and treatment for both addiction and infectious diseases [137]. Fear of arrest and stigma also remain important deterrents to youth utilization of SSPs, especially in the rural U.S. [137, 138].
Even less controversial forms of harm reduction are often underutilized for adolescents and young adults with OUD. Naloxone is the standard of care to safely and rapidly reverse most opioid overdoses [139]. However, while there are multiple studies evaluating the positive effects of expanded community access to naloxone in adults, there are few programs which specifically target youth [140].
Lack of access to harm reduction services means youth with IDU are less likely to be aware of safe injecting practices or overdose prevention strategies increasing their risks of dangerous outcomes [140, 141]. The lack of youth-facing programs with a multi-faceted approach to HIV prevention (e.g., PrEP, MOUD, and harm reduction) is an obstacle to progress in ending the HIV epidemic [136].
Conclusion
The lack of initiatives and experts centered on OUD treatment in adolescents is a missed opportunity to address HIV risk and transmission in young Americans and exposes our vulnerability to emerging drug epidemics, including methamphetamines. Inadequate OUD diagnosis and referral underscore a lack of awareness on behalf of both the young patient and provider that addiction is treatable and addiction treatment is infection prevention. Rural, poor, and LGBTQ adolescents are particularly vulnerable to both OUD and HIV with decreased access to MOUD, harm reduction (e.g., PrEP), and addiction treatment services. Lastly, stigma surrounding MOUD continues to be a significant barrier in the treatment of adolescents and may be alleviated with more awareness, data, and policies that support adolescent addiction as a treatable chronic disease. To end the HIV epidemic, we must address the drug use epidemic effecting our youth by increasing research into OUD treatment in adolescents, access to harm reduction and addiction services, and education among patients, parents, and providers.
Conflicts of Interest and Source of Funding:
None of the authors have any conflicts of interest. EE receives research support to UAB from NIH (R01 MH124633-01), SAMHSA (1H79SP082270-01).
References
- 1.Lerner AM, Fauci AS. Opioid Injection in Rural Areas of the United States: A Potential Obstacle to Ending the HIV Epidemic. JAMA 2019. [DOI] [PubMed] [Google Scholar]
- 2.Hartzler B, Dombrowski JC, Williams JR, Crane HM, Eron JJ, Geng EH, et al. Influence of Substance Use Disorders on 2-Year HIV Care Retention in the United States. AIDS and behavior 2018; 22(3):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzger DS, Woody GE, O'Brien CP. Drug treatment as HIV prevention: a research update. Journal of acquired immune deficiency syndromes (1999) 2010; 55 Suppl 1(Suppl 1):S32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm CA. Geographic Disparities Affect Access to Buprenorphine Services for Opioid Use Disorder. In; 2020. [Google Scholar]
- 5.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morbidity and mortality weekly report 2018; 67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson N, Kariisa M, Seth P, Smith Ht, Davis NL. Drug and Opioid-Involved Overdose Deaths - United States, 2017-2018. MMWR Morbidity and mortality weekly report 2020; 69(11):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital Statistics Rapid Release Provisional Drug Overdose Counts. In; 2021. [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. In. Rockville, MD: 2018. [Google Scholar]
- 9.Gaither JR, Shabanova V, Leventhal JM. US National Trends in Pediatric Deaths From Prescription and Illicit Opioids, 1999-2016. JAMA network open 2018; 1(8):e186558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. HIV Surveillance Report, 2018. In. Atlanta, GA; 2020. [Google Scholar]
- 11.English D, Rendina HJ, Parsons JT. The Effects of Intersecting Stigma: A Longitudinal Examination of Minority Stress, Mental Health, and Substance Use among Black, Latino, and Multiracial Gay and Bisexual Men. Psychology of violence 2018; 8(6):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social science & medicine (1982) 2003; 57(1):13–24. [DOI] [PubMed] [Google Scholar]
- 13.Logie CH, Wang Y, Lacombe-Duncan A, Wagner AC, Kaida A, Conway T, et al. HIV-related stigma, racial discrimination, and gender discrimination: Pathways to physical and mental health-related quality of life among a national cohort of women living with HIV. Preventive medicine 2018; 107:36–44. [DOI] [PubMed] [Google Scholar]
- 14.Prevention CfDCa. HIV Surveillance Report, 2019. In; 2021. [Google Scholar]
- 15.Cano M, Huang Y. Overdose deaths involving psychostimulants with abuse potential, excluding cocaine: State-level differences and the role of opioids. Drug and alcohol dependence 2021; 218:108384. [DOI] [PubMed] [Google Scholar]
- 16.Nerlander LMC, Hoots BE, Bradley H, Broz D, Thorson A, Paz-Bailey G. HIV infection among MSM who inject methamphetamine in 8 US cities. Drug and alcohol dependence 2018; 190:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. The Lancet Child & adolescent health 2018; 2(3):223–228. [DOI] [PubMed] [Google Scholar]
- 18.Moreno M, Thompson L. What Is Adolescent and Young Adult Medicine? JAMA pediatrics 2020; 174(5):512–512. [DOI] [PubMed] [Google Scholar]
- 19.Substance-Related and Addictive Disorders. In: Diagnostic and Statistical Manual of Mental Disorders; 2013. [Google Scholar]
- 20.Substance Abuse and Mental Health Services Administration. Prevention and Treatment of HIV Among People Living with Substance Use and/or Mental Disorders. In. Rockville, MD: National Mental Health and Substance Use Policy Laboratory, . ; 2020. [Google Scholar]
- 21.McCabe SE, Veliz PT, Boyd CJ, Schepis TS, McCabe VV, Schulenberg JE. A prospective study of nonmedical use of prescription opioids during adolescence and subsequent substance use disorder symptoms in early midlife. Drug and alcohol dependence 2019; 194:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohm MK, Clayton HB. Nonmedical Use of Prescription Opioids, Heroin Use, Injection Drug Use, and Overdose Mortality in U.S. Adolescents. Journal of studies on alcohol and drugs 2020; 81(4):484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strashny A Age of Substance Use Initiation Among Treatment Admissions Aged 18 to 30. In: The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013. pp. 1–9. [PubMed] [Google Scholar]
- 24.Nolte K, Drew AL, Friedmann PD, Romo E, Kinney LM, Stopka TJ. Opioid initiation and injection transition in rural northern New England: A mixed-methods approach. Drug and alcohol dependence 2020; 217:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson RG, Nahhas RW, Martins SS, Daniulaityte R. Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: A natural history study. Drug and alcohol dependence 2016; 160:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miech R, Johnston L, O'Malley PM, Keyes KM, Heard K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015; 136(5):e1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarino H, Mateu-Gelabert P, Teubl J, Goodbody E. Young adults' opioid use trajectories: From nonmedical prescription opioid use to heroin, drug injection, drug treatment and overdose. Addictive behaviors 2018; 86:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe SE, Schulenberg J, McCabe VV, Veliz PT. Medical Use and Misuse of Prescription Opioids in US 12th-Grade Youth: School-Level Correlates. Pediatrics 2020; 146(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley-Quon LI, Cho J, Strong DR, Miech RA, Barrington-Trimis JL, Kechter A, et al. Association of Nonmedical Prescription Opioid Use With Subsequent Heroin Use Initiation in Adolescents. JAMA pediatrics 2019; 173(9):e191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parolin M, Simonelli A, Mapelli D, Sacco M, Cristofalo P. Parental Substance Abuse As an Early Traumatic Event. Preliminary Findings on Neuropsychological and Personality Functioning in Young Drug Addicts Exposed to Drugs Early. Frontiers in psychology 2016; 7:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCabe SE, Veliz P, Wilens TE, West BT, Schepis TS, Ford JA, et al. Sources of Nonmedical Prescription Drug Misuse Among US High School Seniors: Differences in Motives and Substance Use Behaviors. Journal of the American Academy of Child and Adolescent Psychiatry 2019; 58(7):681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemans-Cope L, Lynch V, Epstein M, Kenney GM. Opioid and Substance Use Disorder and Receipt of Treatment Among Parents Living With Children in the United States, 2015-2017. Annals of family medicine 2019; 17(3):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbutt JM, Kulka K, Dodd S, Sterkel R, Plax K. Opioids in Adolescents' Homes: Prevalence, Caregiver Attitudes, and Risk Reduction Opportunities. Academic pediatrics 2019; 19(1):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American journal of psychiatry 2003; 160(6):1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, et al. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental cognitive neuroscience 2018; 32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug and alcohol dependence 2014; 145:1–33. [DOI] [PubMed] [Google Scholar]
- 37.Teicher MH, Andersen SL, Hostetter JC Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain research Developmental brain research 1995; 89(2):167–172. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2009; 34(4):912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine 2016; 374(4):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 2016; 3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience 2011; 12(11):652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmanzadeh H, Azizi H, Semnanian S. Adolescent chronic escalating morphine administration induces long lasting changes in tolerance and dependence to morphine in rats. Physiology & behavior 2017; 174:191–196. [DOI] [PubMed] [Google Scholar]
- 43.Ghasemi E, Pachenari N, Semnanian S, Azizi H. Adolescent morphine exposure increases nociceptive behaviors in rat model of formalin test. Developmental psychobiology 2019; 61(2):254–260. [DOI] [PubMed] [Google Scholar]
- 44.Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues in clinical neuroscience 2017; 19(3):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vital signs: HIV infection, testing, and risk behaviors among youths - United States. MMWR Morbidity and mortality weekly report 2012; 61(47):971–976. [PubMed] [Google Scholar]
- 46.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Queen B, et al. Youth Risk Behavior Surveillance - United States, 2017. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 2018; 67(8):1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS patient care and STDs 2014; 28(3):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. HIV and Youth. In: HIV: Centers for Disease Control and Prevention, . 2020. [Google Scholar]
- 49.Berg RC, Weatherburn P, Marcus U, Schmidt AJ. Links between transactional sex and HIV/STI-risk and substance use among a large sample of European men who have sex with men. BMC infectious diseases 2019; 19(1):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koblin B, Chesney M, Coates T. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet (London, England) 2004; 364(9428):41–50. [DOI] [PubMed] [Google Scholar]
- 51.Patrick ME, O'Malley PM, Johnston LD, Terry-McElrath YM, Schulenberg JE. HIV/AIDS risk behaviors and substance use by young adults in the United States. Prevention science : the official journal of the Society for Prevention Research 2012; 13(5):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatia D, Mikulich-Gilbertson SK, Sakai JT. Prescription Opioid Misuse and Risky Adolescent Behavior. Pediatrics 2020; 145(2). [DOI] [PubMed] [Google Scholar]
- 53.Thompson RG Jr., Auslander WF. Substance use and mental health problems as predictors of HIV sexual risk behaviors among adolescents in foster care. Health & social work 2011; 36(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flores J, Liang Y, Ketchum NS, Turner BJ, Bullock D, Villarreal R, et al. Prescription Opioid Use is Associated with Virologic Failure in People Living with HIV. AIDS and behavior 2018; 22(4):1323–1328. [DOI] [PubMed] [Google Scholar]
- 55.Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings ('chemsex') and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sexually transmitted infections 2015; 91(8):564–568. [DOI] [PubMed] [Google Scholar]
- 56.Cain D, Samrock S, Jones SS, Jimenez RH, Dilones R, Tanney M, et al. Marijuana and illicit drugs: Correlates of condomless anal sex among adolescent and emerging adult sexual minority men. Addictive behaviors 2021; 122:107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanner MR, Miele P, Carter W, Valentine SS, Dunville R, Kapogiannis BG, et al. Preexposure Prophylaxis for Prevention of HIV Acquisition Among Adolescents: Clinical Considerations, 2020. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports 2020; 69(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosek S, Henry-Reid L. PrEP and Adolescents: The Role of Providers in Ending the AIDS Epidemic. Pediatrics 2020; 145(1). [DOI] [PubMed] [Google Scholar]
- 59.Yusuf H, Fields E, Arrington-Sanders R, Griffith D, Agwu AL. HIV Preexposure Prophylaxis Among Adolescents in the US: A Review. JAMA pediatrics 2020. [DOI] [PubMed] [Google Scholar]
- 60.Siegler AJ, Mouhanna F, Giler RM, Weiss K, Pembleton E, Guest J, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Annals of epidemiology 2018; 28(12):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaiser Family Foundation. National Survey of Young Adults on HIV/AIDS. In: HIV. Menlo Park, CA; 2017. [Google Scholar]
- 62.Owens C, Moran K, Mongrella M, Moskowitz DA, Mustanski B, Macapagal K. "It's Very Inconvenient for Me": A Mixed-Method Study Assessing Barriers and Facilitators of Adolescent Sexual Minority Males Attending PrEP Follow-Up Appointments. AIDS and behavior 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill SV, Johnson J, Rahman F, Dauria EF, Mugavero M, Matthews LT, et al. Exploring adults as support persons for improved pre-exposure prophylaxis for HIV use among select adolescents and young adults in the Deep South. PloS one 2021; 16(3):e0248858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan PS, Hightow-Weidman L. Mobile apps for HIV prevention: how do they contribute to our epidemic response for adolescents and young adults? mHealth 2021; 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giovenco D, Muessig KE, Horvitz C, Biello KB, Liu AY, Horvath KJ, et al. Adapting technology-based HIV prevention and care interventions for youth: lessons learned across five U.S. Adolescent Trials Network studies. mHealth 2021; 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonar EE, Cunningham RM, Chermack ST, Blow FC, Barry KL, Booth BM, et al. Prescription drug misuse and sexual risk behaviors among adolescents and emerging adults. Journal of studies on alcohol and drugs 2014; 75(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerr T, Marshall BD, Miller C, Shannon K, Zhang R, Montaner JS, et al. Injection drug use among street-involved youth in a Canadian setting. BMC public health 2009; 9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Wang H, Li W, Zhu J, Gold MS, Zhang D, et al. Reduced responses to heroin-cue-induced craving in the dorsal striatum: effects of long-term methadone maintenance treatment. Neuroscience letters 2014; 581:120–124. [DOI] [PubMed] [Google Scholar]
- 69.Fanucchi L, Springer SA, Korthuis PT. Medications for Treatment of Opioid Use Disorder among Persons Living with HIV. Current HIV/AIDS reports 2019; 16(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smyth BP, Elmusharaf K, Cullen W. Opioid substitution treatment and heroin dependent adolescents: reductions in heroin use and treatment retention over twelve months. BMC pediatrics 2018; 18(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health technology assessment (Winchester, England) 2007; 11(9):1–171, iii-iv. [DOI] [PubMed] [Google Scholar]
- 72.Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and utilization outcomes of opioid-dependence treatments. The American journal of managed care 2011; 17 Suppl 8:S235–248. [PubMed] [Google Scholar]
- 73.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ (Clinical research ed) 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction (Abingdon, England) 2017; 112(8):1408–1418. [DOI] [PubMed] [Google Scholar]
- 75.Pearce LA, Min JE, Piske M, Zhou H, Homayra F, Slaunwhite A, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ (Clinical research ed) 2020; 368:m772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edelman EJ, Chantarat T, Caffrey S, Chaudhry A, O'Connor PG, Weiss L, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug and alcohol dependence 2014; 139:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O'Connor PG, et al. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of substance abuse treatment 2008; 35(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Handel MM, Rose CE, Hallisey EJ, Kolling JL, Zibbell JE, Lewis B, et al. County-Level Vulnerability Assessment for Rapid Dissemination of HIV or HCV Infections Among Persons Who Inject Drugs, United States. Journal of acquired immune deficiency syndromes (1999) 2016; 73(3):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartel DM, Schoenbaum EE. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public health reports (Washington, DC : 1974) 1998; 113 Suppl 1(Suppl 1):107–115. [PMC free article] [PubMed] [Google Scholar]
- 80.Wong KH, Lee SS, Lim WL, Low HK. Adherence to methadone is associated with a lower level of HIV-related risk behaviors in drug users. Journal of substance abuse treatment 2003; 24(3):233–239. [DOI] [PubMed] [Google Scholar]
- 81.MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ (Clinical research ed) 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. Journal of urban health : bulletin of the New York Academy of Medicine 2010; 87(4):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohd Salleh NA, Voon P, Karamouzian M, Milloy MJ, Richardson L. Methadone maintenance therapy service components linked to improvements in HIV care cascade outcomes: A systematic review of trials and observational studies. Drug and alcohol dependence 2021; 218:108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medication-Assisted Treatment of Adolescents With Opioid Use Disorders. Pediatrics 2016; 138(3). [DOI] [PubMed] [Google Scholar]
- 85.Medication for Adolescents and Young Adults With Opioid Use Disorder. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2021; 68(3):632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001-2014. JAMA pediatrics 2017; 171(8):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alinsky RH, Zima BT, Rodean J, Matson PA, Larochelle MR, Adger H Jr, et al. Receipt of Addiction Treatment After Opioid Overdose Among Medicaid-Enrolled Adolescents and Young Adults. JAMA pediatrics 2020; 174(3):e195183–e195183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Squeglia LM, Fadus MC, McClure EA, Tomko RL, Gray KM. Pharmacological Treatment of Youth Substance Use Disorders. Journal of child and adolescent psychopharmacology 2019; 29(7):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson CA, Wilson JD. Management of Opioid Misuse and Opioid Use Disorders Among Youth. Pediatrics 2020; 145(Suppl 2):S153–s164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, et al. Primary Care-Based Models for the Treatment of Opioid Use Disorder: A Scoping Review. Annals of internal medicine 2017; 166(4):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for Unhealthy Drug Use: US Preventive Services Task Force Recommendation Statement. Jama 2020; 323(22):2301–2309. [DOI] [PubMed] [Google Scholar]
- 92.Levy SJ, Williams JF. Substance Use Screening, Brief Intervention, and Referral to Treatment. Pediatrics 2016; 138(1). [DOI] [PubMed] [Google Scholar]
- 93.Substance Use Screening, Brief Intervention, and Referral to Treatment. Pediatrics 2016; 138(1). [DOI] [PubMed] [Google Scholar]
- 94.Wu LT, Blazer DG, Li TK, Woody GE. Treatment use and barriers among adolescents with prescription opioid use disorders. Addictive behaviors 2011; 36(12):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bagley SM, Gai MJ, Earlywine JJ, Schoenberger SF, Hadland SE, Barocas JA. Incidence and Characteristics of Nonfatal Opioid Overdose Among Youths Aged 11 to 24 Years by Sex. JAMA network open 2020; 3(12):e2030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public health 2017; 2(8):e356–e366. [DOI] [PubMed] [Google Scholar]
- 97.Taplin C, Saddichha S, Li K, Krausz MR. Family history of alcohol and drug abuse, childhood trauma, and age of first drug injection. Substance use & misuse 2014; 49(10):1311–1316. [DOI] [PubMed] [Google Scholar]
- 98.Winstanley EL, Stover AN. The Impact of the Opioid Epidemic on Children and Adolescents. Clin Ther 2019; 41(9):1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belenko S, Knight D, Wasserman GA, Dennis ML, Wiley T, Taxman FS, et al. The Juvenile Justice Behavioral Health Services Cascade: A new framework for measuring unmet substance use treatment services needs among adolescent offenders. Journal of substance abuse treatment 2017; 74:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagata JM, Palar K, Gooding HC, Garber AK, Tabler JL, Whittle HJ, et al. Food Insecurity, Sexual Risk, and Substance Use in Young Adults. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hadland SE, Bagley SM, Rodean J, Silverstein M, Levy S, Larochelle MR, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. JAMA pediatrics 2018; 172(11):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bagley SM, Hadland SE, Carney BL, Saitz R. Addressing Stigma in Medication Treatment of Adolescents With Opioid Use Disorder. Journal of addiction medicine 2017; 11(6):415–416. [DOI] [PubMed] [Google Scholar]
- 103.Hadland SE, Park TW, Bagley SM. Stigma associated with medication treatment for young adults with opioid use disorder: a case series. Addiction science & clinical practice 2018; 13(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feder KA, Krawczyk N, Saloner B. Medication-Assisted Treatment for Adolescents in Specialty Treatment for Opioid Use Disorder. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2017; 60(6):747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chatterjee A, Larochelle MR, Xuan Z, Wang N, Bernson D, Silverstein M, et al. Non-fatal opioid-related overdoses among adolescents in Massachusetts 2012-2014. Drug and alcohol dependence 2019; 194:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alinsky RH, Zima BT, Rodean J, Matson PA, Larochelle MR, Adger H Jr. et al. Receipt of Addiction Treatment After Opioid Overdose Among Medicaid-Enrolled Adolescents and Young Adults. JAMA pediatrics 2020; 174(3):e195183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore SK, Guarino H, Marsch LA. "This is not who I want to be:" experiences of opioid-dependent youth before, and during, combined buprenorphine and behavioral treatment. Substance use & misuse 2014; 49(3):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. In. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2020. [Google Scholar]
- 109.Becan JE, Fisher JH, Johnson ID, Bartkowski JP, Seaver R, Gardner SK, et al. Improving Substance Use Services for Juvenile Justice-Involved Youth: Complexity of Process Improvement Plans in a Large Scale Multi-site Study. Administration and policy in mental health 2020; 47(4):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of family medicine 2015; 13(1):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu LT, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug and alcohol dependence 2016; 169:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barocas JA, Wang J, Marshall BDL, LaRochelle MR, Bettano A, Bernson D, et al. Sociodemographic factors and social determinants associated with toxicology confirmed polysubstance opioid-related deaths. Drug and alcohol dependence 2019; 200:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pear VA, Ponicki WR, Gaidus A, Keyes KM, Martins SS, Fink DS, et al. Urban-rural variation in the socioeconomic determinants of opioid overdose. Drug and alcohol dependence 2019; 195:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Announcement of Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. In; 2021.
- 115.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA psychiatry 2019; 76(2):208–216. [DOI] [PubMed] [Google Scholar]
- 116.Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Archives of general psychiatry 2005; 62(10):1157–1164. [DOI] [PubMed] [Google Scholar]
- 117.Marsch LA, Moore SK, Borodovsky JT, Solhkhah R, Badger GJ, Semino S, et al. A randomized controlled trial of buprenorphine taper duration among opioid-dependent adolescents and young adults. Addiction (Abingdon, England) 2016; 111(8):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA 2008; 300(17):2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walker KS, Bonny AE, McKnight ER, Nahata MC. Impact of Office-based Opioid Treatment on Emergency Visits and Hospitalization in Adolescents with Opioid Use Disorder. The Journal of pediatrics 2020; 219:236–242. [DOI] [PubMed] [Google Scholar]
- 120.National Institute on Drug Abuse. Medications to treat opioid use disorder. In; 2018. [Google Scholar]
- 121.Clark RE, Baxter JD, Barton BA, Aweh G, O'Connell E, Fisher WH. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence. Health services research 2014; 49(6):1964–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Accurso AJ, Rastegar DA. The Effect of a Payer-Mandated Decrease in Buprenorphine Dose on Aberrant Drug Tests and Treatment Retention Among Patients with Opioid Dependence. Journal of substance abuse treatment 2016; 61:74–79. [DOI] [PubMed] [Google Scholar]
- 123.Hill LG, Loera LJ, Evoy KE, Renfro ML, Torrez SB, Zagorski CM, et al. Availability of buprenorphine/naloxone films and naloxone nasal spray in community pharmacies in Texas, USA. Addiction (Abingdon, England) 2020. [DOI] [PubMed] [Google Scholar]
- 124.Certification of Opioid Treatment Programs, 42 Code of Federal Regulations (CFR) 8. In. Washington (DC): U.S. Government Publishing Office; 2017. [Google Scholar]
- 125.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011; (2):Cd001333. [DOI] [PubMed] [Google Scholar]
- 126.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of substance abuse treatment 2018; 85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sullivan MA, Bisaga A, Pavlicova M, Carpenter KM, Choi CJ, Mishlen K, et al. A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder. The American journal of psychiatry 2019; 176(2):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hulse GK, Tait RJ. A pilot study to assess the impact of naltrexone implant on accidental opiate overdose in 'high-risk' adolescent heroin users. Addiction biology 2003; 8(3):337–342. [DOI] [PubMed] [Google Scholar]
- 129.Fishman MJ, Winstanley EL, Curran E, Garrett S, Subramaniam G. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction (Abingdon, England) 2010; 105(9):1669–1676. [DOI] [PubMed] [Google Scholar]
- 130.Tanum L, Solli KK, Latif ZE, Benth J, Opheim A, Sharma-Haase K, et al. Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA psychiatry 2017; 74(12):1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet (London, England) 2018; 391(10118):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haffer A Warning Letter Alkermes, Inc. In. Silver Spring, MD; 2019. [Google Scholar]
- 133.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017; 9(9):Cd012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perlman DC, Jordan AE. The Syndemic of Opioid Misuse, Overdose, HCV, and HIV: Structural-Level Causes and Interventions. Current HIV/AIDS reports 2018; 15(2):96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ijioma SC, Pontinha VM, Holdford DA, Carroll NV. Cost-effectiveness of syringe service programs, medications for opioid use disorder, and combination programs in hepatitis C harm reduction among opioid injection drug users: a public payer perspective using a decision tree. Journal of managed care & specialty pharmacy 2021; 27(2):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kimmel SD, Gaeta JM, Hadland SE, Hallett E, Marshall BDL. Principles of Harm Reduction for Young People Who Use Drugs. Pediatrics 2021; 147(Suppl 2):S240–s248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krug A, Hildebrand M, Sun N. "We don't need services. We have no problems": exploring the experiences of young people who inject drugs in accessing harm reduction services. Journal of the International AIDS Society 2015; 18(2 Suppl 1):19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davis SM, Kristjansson AL, Davidov D, Zullig K, Baus A, Fisher M. Barriers to using new needles encountered by rural Appalachian people who inject drugs: implications for needle exchange. Harm reduction journal 2019; 16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amur S, Xu Y, Hertz S, Clingman C. FDA Experience With Regulation of Naloxone Products to Address the Opioid Crisis and Opportunities for Leveraging Scientific Engagement. Clinical pharmacology and therapeutics 2021; 109(3):569–572. [DOI] [PubMed] [Google Scholar]
- 140.Marshall BD, Green TC, Yedinak JL, Hadland SE. Harm reduction for young people who use prescription opioids extra-medically: Obstacles and opportunities. The International journal on drug policy 2016; 31:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frank D, Mateu-Gelabert P, Guarino H, Bennett A, Wendel T, Jessell L, et al. High risk and little knowledge: overdose experiences and knowledge among young adult nonmedical prescription opioid users. The International journal on drug policy 2015; 26(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.AtlasPlus. HIV diagnoses ∣ 2018 ∣ 13-24 ∣ All races/ethnicities ∣ Both sexes ∣ All transmission categories ∣ US Map-State Level. In. Edited by Centers for Disease Control and Prevention. 1600 Clifton Road Atlanta, GA 30329-4027 USA; 2021. [Google Scholar]
- 2.Grimm CA. Geographic Disparities Affect Access to Buprenorphine Services for Opioid Use Disorder. In. Edited by United States' Department of Health and Human Services; 2020. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. In. Edited by Center for Behavioral Health Statistics and Quality Substance Abuse and Mental Health Services Administration. Rockville, MD 2018. [Google Scholar]
References
- 1.Springer SA, Chen S, and Altice FL, Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health, 2010. 87(4): p. 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth BP, Elmusharaf K, and Cullen W, Opioid substitution treatment and heroin dependent adolescents: reductions in heroin use and treatment retention over twelve months. BMC Pediatr, 2018. 18(1): p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan MA, et al. A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder. Am J Psychiatry, 2019. 176(2): p. 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelman EJ, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug Alcohol Depend, 2014. 139: p. 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohd Salleh NA, et al. Methadone maintenance therapy service components linked to improvements in HIV care cascade outcomes: A systematic review of trials and observational studies. Drug Alcohol Depend, 2021. 218: p. 108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulse GK and Tait RJ, A pilot study to assess the impact of naltrexone implant on accidental opiate overdose in 'high-risk' adolescent heroin users. Addict Biol, 2003. 8(3): p. 337–42. [DOI] [PubMed] [Google Scholar]
- 7.Haffer A, Warning Letter Alkermes, Inc., O.o.P.D.P.U.S.F.a.D. Administration, Editor. 2019: Silver Spring, Md. [Google Scholar]
- 8.Tanum L, et al. , Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry, 2017. 74(12): p. 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JD, et al. , Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet, 2018. 391(10118): p. 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]


