Abstract
Objective:
There are limited studies on the association of HIV infection with systemic inflammation during pregnancy.
Design:
A cohort study (N=220) of pregnant women with HIV (N=70) (all on antiretroviral therapy) and without HIV (N=150) were enrolled from an antenatal clinic in Pune, India.
Methods:
The following systemic inflammatory markers were measured in plasma samples using immunoassays: soluble CD163 (sCD163), soluble CD14 (sCD14), intestinal fatty acid-binding protein (I-FABP), C-reactive protein (CRP), alpha 1-acid glycoprotein (AGP), interferon-β (IFNβ), interferon-γ (IFNγ), interleukin (IL)-1β, IL-6, IL-13, IL-17A and tumor necrosis factor α (TNFα). Generalized estimating equation (GEE) and linear regression models were used to assess the association of HIV status with each inflammatory marker during pregnancy and by trimester, respectively.
Results:
Pregnant women with HIV had higher levels of markers for gut barrier dysfunction (I-FABP), monocyte activation (sCD14) and markers of systemic inflammation (IL-6 and TNFα), but surprisingly lower levels of AGP, an acute phase protein, compared to pregnant women without HIV, with some trimester-specific differences.
Conclusions:
Our data show that women with HIV had higher levels of markers of gut barrier dysfunction, monocyte activation and systemic inflammation. These markers, some of which are associated with preterm birth, might help explain the increase in adverse birth outcomes in women with HIV and could suggest targets for potential interventions.
Keywords: Pregnancy, Inflammation, HIV, India, Monocyte
Introduction
Chronic immune activation plays a major role in HIV pathogenesis and adverse disease outcomes. Studies in the pre-antiretroviral therapy (ART) era show that adults with HIV have increased inflammation compared to adults without HIV This inflammatory profile in individuals with HIV is characterized by increased levels of markers of systemic inflammation, acute phase proteins and gut barrier dysfunction with microbial translocation, along with monocyte and T cell activation [1,2,3,4,5]. Recent data show that ART does not fully correct for this excess inflammation [4] and importantly, among adults with HIV, those with the highest levels of these inflammatory markers are at increased risk of morbidity and mortality [2,3,4].
While there are many studies that have examined the association of HIV infection with inflammation in non-pregnant adults, there are limited studies of pregnant women with HIV. Pregnancy is characterized by an immune profile that is distinct from non-pregnant women [6]; furthermore, studies show that there are changes to immunity and the inflammatory profile within pregnancy [7,8]. For example, the second trimester and early third trimester of pregnancy is characterized by a state of immune suppression with increased production of anti-inflammatory cytokines; in contrast, a pro-inflammatory cytokine milieu plays a major role in the induction of labor [4]. Thus, in the context of HIV, it is important to understand whether and how the inflammatory profile during pregnancy, and by trimester, might be different by HIV status.
A small study conducted in Spain in 2015 compared circulating levels of four markers for microbial translocation (lipopolysaccharide-binding protein (LBP) and EndoCAb), monocyte activation (soluble CD14 (sCD14)) and systemic inflammation (Interleukin (IL)-6) in pregnant women with and without HIV [9]. They found that women with HIV had higher levels of sCD14 and LBP in the first and third trimester of pregnancy. In studies conducted in the United States and Botswana, inflammatory markers C-reactive protein (CRP), IL-6 and D-dimer decreased from late pregnancy to postpartum [10,11]. Whether and how other immune markers related to gut barrier integrity, acute phase proteins, T-helper cytokines and additional markers of monocyte activation and systemic inflammation are different by HIV status among pregnant women and by trimester (including second trimester) is not clear. Understanding these differences is important, as various studies in populations without HIV have shown that high inflammation during mid-pregnancy is associated with preterm birth and other adverse pregnancy outcomes such as preeclampsia [12,13]; the immune profile of pregnant women with HIV could help us better understand the increased risk of preterm delivery [9, 14] and other adverse infant health outcomes [15,16,17].
To address these gaps in knowledge, we compared levels of various inflammatory markers, at the both the second and third trimesters, by HIV status in a cohort of pregnant women from Pune, India. The rationale for studying the immune markers in the second and third trimester was due to the existing literature linking high inflammation during this period with adverse birth outcomes [9,12,14]. Pregnant women with HIV infection in this study were all taking ART at enrollment study. We assessed various plasma markers relevant to HIV and pregnancy including those involved in systemic inflammation (IL-6, IL1-β and interferon-β (IFNβ)), acute phase response (C-reactive protein (CRP), alpha 1-acid glycoprotein (AGP)), intestinal barrier dysfunction (intestinal fatty acid-binding protein (I-FABP)), T-helper cytokines (interferon-γ (IFNγ), tumor necrosis factor α (TNFα), Interleukin (IL)-17a and IL-13) and monocyte activation (sCD14 and soluble CD163 (sCD163)) [1,4,6,9,14].
Methods
Setting
For this analysis on the relationship of HIV and inflammation during pregnancy, we used data from a prospective cohort study, ‘Impact of Immune Changes of HIV and Stages of Pregnancy on Tuberculosis (PRACHITi)’. In this cohort study, we recruited pregnant women who were attending the BJ Medical Government College (BJGMC)/Sassoon General Hospital for antenatal care in Pune, India between June 2016 and June 2019.
Population
As the primary objective of the parent study was to assess changes in immune responses by HIV and latent tuberculosis infection (LTBI) status, four groups of pregnant women were enrolled based on their LTBI and HIV status: 1) LTBI+HIV+ 2) LTBI+HIV− 3) LTBI−HIV+ 4) LTBI−HIV−. We used past medical records or a rapid test to determine HIV status. If the test was positive, it was further confirmed by enzyme-linked immunosorbent assay (ELISA). For participant on antiretroviral therapy (ART), a repeat diagnosis was not performed. An Interferon Gamma Release Assay (IGRA Quantiferon TB-Gold) cutoff of TB antigen minus nil ≥0.35 IU/mL was used to determine LTBI status for both women with and without HIV.
Ethics Statement is detailed in online supplement
Study Design and Eligibility Criteria
Adult pregnant women between the ages of 18 to 40, with a gestational age (determined by early second trimester ultrasound) between 13 and 34 weeks, and receiving antenatal care at BJGMC were eligible for enrollment into the PRACHITi cohort. Pregnant women with active TB disease, severe anemia (Hb < 75 g/l during the 30 days prior to enrollment) [18], taking antibiotics or immunosuppressive medications for greater than 14 days at enrollment, or with a history of autoimmune or immunosuppressive disease were excluded from the study. A sample of 234 pregnant women were enrolled into the PRACHITi study through convenience sampling. All women from PRACHITi with available inflammation data were included in this analysis.
Data Collection
Sociodemographic information was collected from pregnant women in PRACHITi at enrollment during second (13–27 gestational weeks) or third (week 28 onwards) trimester. Clinical data were collected at enrollment (second or third trimester), at the third trimester visit (if enrolled in second trimester), at delivery, 6 weeks, 3 months, 6 months and 12 months post-partum. We also measured mid-upper arm circumference (MUAC) at all visits, to define undernutrition in pregnancy as MUAC less than 23 cm and overweight as MUAC greater than 30.5 cm [19]. Assessments of anemia and gestational diabetes are detailed in online supplement.
At each visit, blood samples were collected in heparin tubes, and plasma samples were extracted and stored in −80°C until further use. To measure levels of sCD163, sCD14, I-FABP, CRP, AGP, and IFNβ in plasma samples collected from the second and third trimester, we conducted single-plex immunoassays according to the manufacturer’s directions (R&D Systems, Minneapolis, MN). Levels of IFNγ, IL-1β, IL-6, IL-13, IL-17A and TNFα in plasma were measured using a multiplex immunoassay following manufacturer guidelines (Luminex R&D Systems). Among these markers, sCD163, sCD14, I-FABP, CRP, AGP, IL-1β, IL-6, IL-17A and TNFα have been linked with both HIV and preterm birth, while IFNγ and IFNβ were selected because of their association with HIV.
Statistical Analysis
We included both HIV+ (LTBI+ and LTBI−) and HIV− (LTBI+ and LTBI−) women to study the relationship between HIV status and inflammatory markers during pregnancy. Descriptive statistics are detailed in online supplement.
To understand whether the specific inflammatory markers measured in our study were different by trimester in the overall population, we examined the intra-individual variation between the second and third trimester of these markers using intraclass correlation coefficient (ICC). Type A ICCs based on an absolute agreement were calculated using a two-way mixed effects model assuming people effects are random and measure effects are fixed.
The inflammatory markers were transformed to a log2 scale to reduce the impact of higher leverage values. To determine the relationship of HIV status with inflammation during the second and third trimester of pregnancy, we conducted univariable and multivariable analysis using generalized linear model with identity link function. As the majority of women in this study (N = 175) contributed more than one observation (i.e. in both second and third trimester), generalized estimating equation (GEE) method was employed to account for within subject correlation due to repeated measures from the same subject. In the univariable variable, HIV status is the binary exposure variable and each log2-transformed inflammatory marker is a continuous outcome variable. We considered two multivariable models with HIV status as the key covariate of interest. Multivariable model 1 was adjusted for age, mid-upper arm circumference (MUAC), and education while multivariable model 2 was further adjusted for LTBI status, smoking, gestational diabetes, and anemia. Any unadjusted p-values less than or equal to 0.05 was considered as statistically significant (further details in online supplement). In addition to the GEE analysis (using both second and third trimester data), we also used univariable and multivariable linear regression to assess the relationship of HIV with inflammation, separately for the second and third trimester. All analyses were conducted in SAS (University Edition, NC, USA) or SPSS Version 27.0.
Results
Study Population Characteristics
From our cohort study, we had inflammation data available from 220 pregnant women. Overall study population characteristics are presented in Table 1 and described in online supplement. In this study, 70 women were HIV+ and 150 women were HIV−. Study population characteristics did not differ by HIV status except that HIV-positive women had a higher proportion of third trimester anemia (p-value = 0.037), income below India’s poverty line (p-value = 0.047), and a lower proportion of LTBI (p-value < 0.001). There were a higher proportion of women with HIV with an education level of secondary or less (p-value = 0.058), and gestational diabetes (p-value = 0.136), but these differences were not statistically significant (Table 1). Among women with HIV, the median CD4 count was 423 cells/mm3 and the median viral load was 40 copies/ml; 71% were on TDF/3TC/EFV ART regimen, 11% were on AZT/3TC/NVP ART regimen, and 18% were taking other forms of ART.
Table 1:
Characteristics of the study population (N = 220)
| Overall (N=220) |
HIV+ (N=70) |
HIV− (N=150) |
P-value | |
|---|---|---|---|---|
| Age median (IQR) | 23 (21–27) | 25 (22–30) | 23 (20–26) | 0.002 |
| Monthly Income | ||||
| ≤ Rs. 10,255 | 75 (34) | 31 (44) | 44 (30) | 0.06 |
| > Rs. 10,255 | 143 (66) | 39 (56) | 104 (70) | |
| Education | ||||
| None to primary | 54 (25) | 24 (34) | 30 (20) | |
| Middle school to high school | 139 (63) | 40 (57) | 99 (66) | 0.06 |
| Post-high school | 27 (12) | 6 (9) | 21 (14) | |
| Smoking status | ||||
| Yes | 26 (12) | 6 (9) | 20 (13) | 0.38 |
| No | 194 (88) | 64 (91) | 130 (87) | |
| Mid-upper arm circumference | ||||
| < 23 cm | 62 (28) | 19 (27) | 43 (29) | |
| 23 – 30.5 cm | 143 (65) | 46 (67) | 97 (65) | 0.95 |
| >30.5 cm | 14 (6) | 4 (6) | 10 (7) | |
| Anemia | ||||
| Yes | 88 (41) | 35 (51) | 53 (36) | 0.02 |
| No | 129 (59) | 33 (48) | 96 (64) | |
| Gestational Diabetes status | ||||
| Yes | 21 (10) | 10 (15) | 11 (7) | 0.08 |
| No | 195 (90) | 58 (84) | 137 (93) | |
| LTBI | ||||
| Yes | 155 (70) | 31 (44) | 124 (83) | <0.001 |
| No | 65 (30) | 39 (56) | 26 (17) |
Data are presented as number (%) of subjects unless otherwise stated. P-values were calculated using Fisher’s exact test for the monthly income, anemia, and gestational diabetes variables, Chi-squared test for the smoking status, LTBI, mid-upper arm circumference, and education variables, and Wilcoxon rank-sum for continuous variables, age, to determine the difference between HIV+ and HIV− pregnant women.
Anemia was characterized as having a hemoglobin level less than 110 g/L.
Gestational diabetes was characterized as either having a fasting plasma glucose between 92–125 mg/dl or a 2-hour plasma glucose following glucose load between 153–199 mg/dl.
Association of HIV Status with Inflammation During Pregnancy Using Both Second and Third Trimester Data
Findings from univariable GEE analysis suggested that women with HIV had significantly higher levels of I-FABP (mean log2 difference: 0.43, 95% confidence interval (CI): 0.08 to 0.78, p-value = 0.014) and sCD14 (mean log2 difference: 0.47, 95% CI: 0.25 to 0.69; p-value < 0.001), and lower levels of AGP (mean log2 difference: −0.67, 95% CI: −1.20 to −0.15; p-value=0.014) compared to women without HIV (Figure 1).
Figure 1: Association of HIV status with inflammation (N = 487 samples from 220 women).
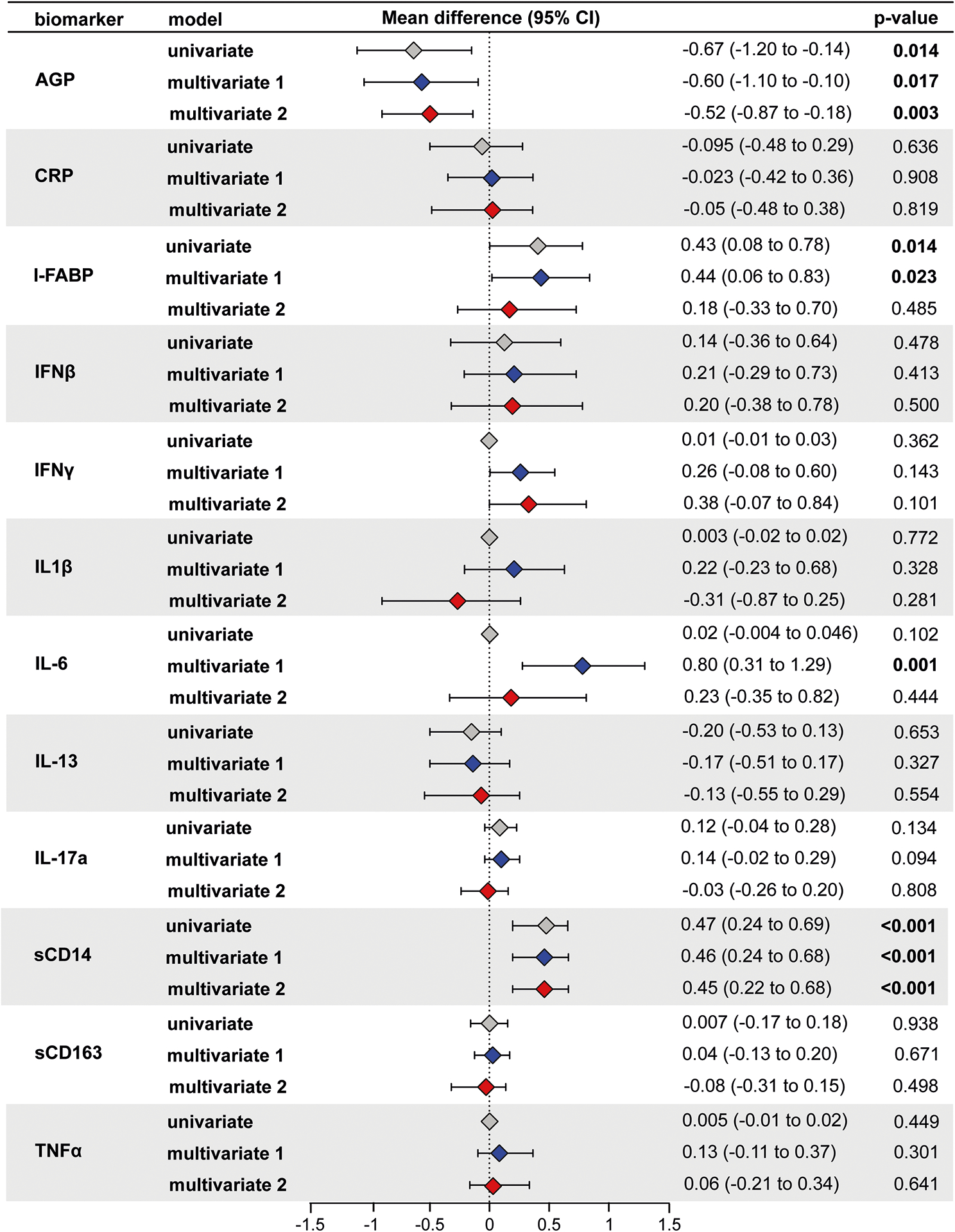
Using generalized linear model with identity link and GEE to account for the within subject correlation due to repeated measures, the mean difference in log2 concentrations of each inflammation marker and 95% confidence intervals (95% CI) among HIV+ individuals compared to HIV− individuals (HIV+ minus HIV−) is shown in the forest plot. Multivariate model 1 was adjusted for age, mid-upper arm circumference, and education. Multivariable model 2 was further adjusted for anemia, smoking, LTBI, and GDM. A p-value less than or equal to 0.05 was considered statistically significant.. Color legends: Gray for univariable, blue for multivariable model 1 and red for multivariable model 2.
After adjusting for age, MUAC, and education (i.e., multivariable model 1), levels of I-FABP (mean log2 difference: 0.44, 95% CI: 0.06 to 0.83, p-value = 0.023) and sCD14 (mean log2 difference: 0.46, 95% CI: 0.24 to 0.68, p-value < 0.001) remained significantly higher, and levels of AGP (mean log2 difference: −0.60, 95% CI: −1.10 to −0.11, p-value = 0.017) were significantly lower in women with HIV compared to women without HIV. In addition, levels of IL-6 (mean log2 difference: 0.80, 95% CI: 0.31 to 1.29, p-value = 0.001) were significantly higher in women with HIV compared to women without HIV (Figure 1). Of note, sCD14 and IL-6 results were robust to adjustment for Bonferroni multiple comparison adjustments (p-value < 0.004).
In the multivariable analysis further adjusting for LTBI, anemia, smoking, and gestational diabetes (i.e., multivariable model 2), we found sCD14 (mean log2 difference: 0.45, 95% CI: 0.22 to 0.69, p-value < 0.001) was significantly higher and AGP (mean log2 difference: −0.53, 95% CI: −0.88 to −0.18, p-value = 0.003) was significantly lower, after accounting for Bonferroni multiple comparison adjustments. Interestingly, in contrast to multivariable model 1, I-FABP (mean log2 difference: 0.18, 95% CI: −0.33 to −0.70, p-value = 0.485) and IL-6 (mean log2 difference: 0.23, 95% CI: −0.35 to 0.82, p-value = 0.444) levels were not significantly different in this model (Figure 1). Adjustment of LTBI drove these differences between the two multivariable models. Further adjusting for parity did not change our results (data not shown).
Reliability of Inflammatory Markers over Trimester
While the above GEE analysis assessed the relationship of HIV with inflammation during both the second and third trimester, there are known differences in immunity by trimester of pregnancy [3]. To understand whether the specific inflammatory markers measured in our study were different by trimester in the overall population, we examined the intraclass correlation coefficient of these markers between the second and third trimester. The ICC varied between the markers with a range of 0.19–0.85 (Table 2). Some markers with relatively low ICC included CRP (0.19), I-FABP (0.23), and IFNγ (0.29), indicating high intra-individual variation over trimester (Table 2). Some markers with relatively high ICC included sCD14 (0.85), IL1β (0.83) and IL-6 (0.74), indicating relative stability in levels over trimester (Table 2). While the high ICC for several markers provided evidence to support the validity of analysis for combined sample, given the low reliability of some other inflammation markers, we also next assessed the relationship of HIV with inflammation separately for second and third trimester samples and formally compared their strength of association by trimester.
Table 2:
Intraclass Correlation Coefficient (ICC) Overall
| N 2nd and 3rd |
ICC 2nd and 3rd |
|
|---|---|---|
| Log2 IFN-g | 167 | 0.29 |
| Log 2 IFN-b | 157 | 0.39 |
| Log2 IL-6 | 167 | 0.74 |
| Log2 IL-17 | 167 | 0.55 |
| Log2 Il-13 | 167 | 0.63 |
| Log2 IL-1b | 167 | 0.83 |
| Log2 CRP | 158 | 0.19 |
| Log2 AGP | 150 | 0.65 |
| Log2 TNF-a | 167 | 0.63 |
| Log2 I-FABP | 165 | 0.23 |
| Log2 sCD14 | 161 | 0.85 |
| Log2 sCD163 | 161 | 0.62 |
Type A ICCs based on an absolute agreement definition were calculated using a two-way mixed effects model where people effects are random and measured effects are fixed.
Association of HIV Status with Inflammation by Trimester
In the second trimester, levels of sCD14 (p < 0.001), TNFα (p = 0.011), IL-6 (p = 0.002), and IL-17a (p = 0.007) were significantly higher, using Wilcoxon-rank sum tests, in women with HIV compared to women without HIV (Supplementary Figure 1). Conversely, levels of sCD163 (p=0.028) were significantly higher in women without HIV compared to women with HIV. In contrast, in the third trimester, only levels of sCD14 (p<0.0001) and IL-6 (p=0.026) were significantly higher in women with HIV compared to women without HIV (Supplementary Figure 2).
Next, we assessed the relationship of inflammation with HIV status using univariable and multivariable linear regression models separately for second and third trimester. The second trimester results were similar to the GEE analysis for sCD14 (significant in all models), IL-6 (significant for univariable and multivariable 1 but not multivariable 2) and AGP (significant in all models) (Figure 2). In contrast to the GEE analysis, second trimester TNFα was also significantly higher in univariable and multivariable model 1, but not multivariable model 2.
Figure 2: Association of HIV status with second trimester inflammation (N=187).
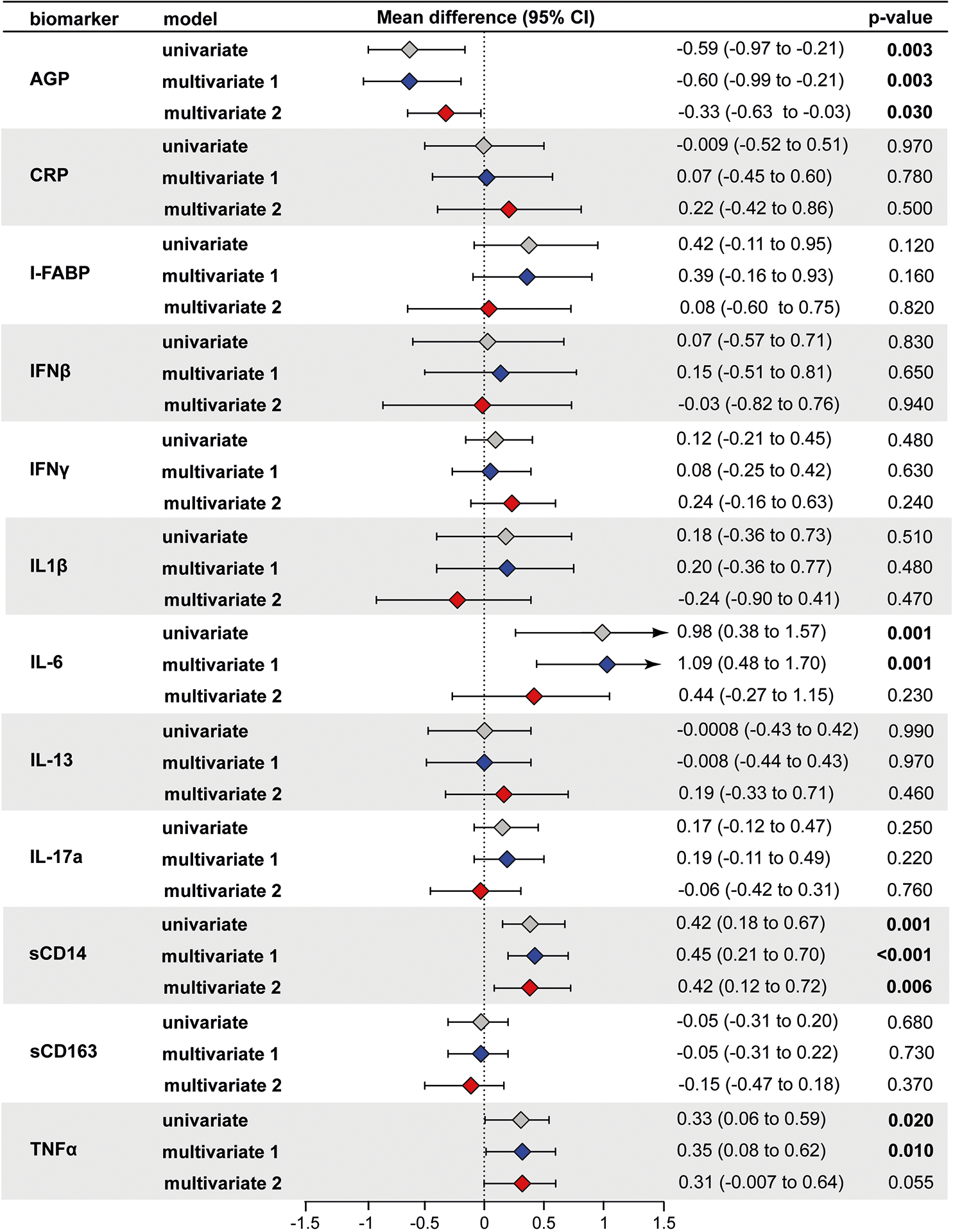
Using linear regression, the mean difference in log2 concentrations of each inflammation marker and 95% confidence intervals (95% CI) among HIV+ individuals compared to HIV− individuals (HIV+ minus HIV−) is shown in the forest plot. Multivariate model 1 was adjusted for age, mid-upper arm circumference, and education. Multivariable model 2 was further adjusted for anemia, smoking, LTBI, and GDM. A p-value less than or equal to 0.05 was considered statistically significant. Color legends: Gray for univariable, blue for multivariable model 1 and red for multivariable model 2.
The third trimester results were also similar to the GEE analysis for sCD14 (significant in all models), IL-6 and I-FABP (significant in univariable and multivariable model 1 but not multivariable model 2 with adjustment for LTBI) (Figure 3). Comparing the results from second and third trimester results suggest that the association of HIV with I-FABP, AGP and TNFα are different by trimester while HIV had a similar association with the other markers over the second and third trimester.
Figure 3: Association of HIV status with third trimester inflammation (N=220).

Using linear regression, the mean difference in log2 concentrations of each inflammation marker and 95% confidence intervals (95% CI) among HIV+ individuals compared to HIV− individuals (HIV+ minus HIV−) is shown in the forest plot. Multivariate model 1 was adjusted for age, mid-upper arm circumference, and education. Multivariable model 2 was further adjusted for anemia, smoking, LTBI, and GDM. A p-value less than or equal to 0.05 was considered statistically significant. Color legends: Gray for univariable, blue for multivariable model 1 and red for multivariable model 2.
Discussion
In our study comparing pregnant women with HIV taking ART to pregnant women without HIV, we studied the association of HIV infection status and inflammation during pregnancy. Overall, we saw that HIV status was associated with higher levels of markers of gut integrity, monocyte activation and systemic inflammation, and lower levels of AGP, an acute phase protein. While there were trimester-specific differences in certain markers, overall, the changes were similar by HIV status. Increases in these markers, some of which are associated with preterm birth, might help to explain the higher risk of adverse birth outcomes in HIV-infected populations, and could suggest targets for potential interventions.
In this study of pregnant women from India, we examined the association of HIV with inflammation over the course of second and third trimester of pregnancy. While the primary analysis focused on GEE analysis that included samples from both the second and third trimesters, we also decided to conduct separate analysis for each trimester after observing some markers with high intra-individual variation (low ICC) over trimesters. While variability in levels of inflammatory markers within trimesters of pregnancy has been noted by other studies, data on ICCs over trimester are lacking. Our data suggests that there is high reliability between certain markers, such as sCD14 and IL1β, while other markers such as CRP and I-FABP have low reliability between the second and third trimester. Differences in levels of some of these markers by trimester are partly explained by the immune clock of human pregnancy, where the levels and function of various components of immunity changes with stage of pregnancy [20].
In the present study, sCD14 remained significantly higher among women with HIV compared to women without HIV in all univariable and multivariable GEE and trimester-specific regression analysis. These results are similar to what has been observed in non-pregnant adults with HIV [4]; in addition, we confirm and expand upon findings from a previous study of pregnant women with and without HIV by showing that, the levels are higher in both the second and third trimester [9]. sCD14 is a marker of monocyte activation, and circulating levels have been shown to increase in response to HIV-associated gut barrier dysfunction and microbial translocation [4], even after exposure to ART. These findings have significance among pregnant women as we [14] and others [9] have shown that higher levels of sCD14 during pregnancy was associated with preterm birth. We also discuss whether potential efavirenz use might explain these results in online supplement.
Related to gut barrier dysfunction, we also observed that I-FABP, a marker increased during intestinal damage [4], was higher in pregnant women with HIV compared to those without HIV from the in univariable and multivariable 1 model in the GEE analysis. This was also observed in the trimester-specific results although only the third trimester results were statistically significant. As described above, various studies in non-pregnant adults have also observed higher levels of I-FABP in individuals with HIV [4], although to our knowledge, potential differences by HIV status during pregnancy has not been previously explored. Our previous study [14] and the study from Spain described earlier [9] showed that higher levels of sCD14 and I-FABP levels during pregnancy was associated with preterm birth; given these previous findings, our results from this study suggest that these markers could potentially help explain the increased preterm delivery by mothers with HIV.
Interestingly, when we adjusted for LTBI status (multivariable model 2), the I-FABP results were no longer significant. The rationale to adjust for LTBI is presented in the online supplement. This result was surprising and warrants further investigation, especially as we have also shown that LTBI status affects various inflammatory markers including I-FABP and IL-6 levels during pregnancy [7]. Similar to the I-FABP results, HIV status was associated with increased IL-6 levels in the GEE and trimester-specific analysis only in the univariable and multivariable model 1, but not multivariable model 2. Once again, LTBI adjustment accounted for the change in significance. Given that multiple studies in non-pregnant adults, that do not adjust for LTBI status, have shown a positive association of HIV with IL-6 and I-FABP [1,4,21], our results suggest that future studies should address whether the observed associations might be confounded by LTBI status, or whether this observation reflects chance findings due to our study design, or alternatively if it reflects the real impact of LTBI only during pregnancy. A limitation in the interpretation of this data is the potential challenges in determining LTBI status among people with HIV; for example, LTBI+ could potentially be misclassified, due to anergy, as LTBI− in women with HIV, and this could result in biased inferences.
Levels of AGP were significantly lower in women with HIV compared to women without HIV in the second trimester as well as during pregnancy overall. Studies have examined the level of AGP in plasma, and have found that levels are actually elevated in many infections, including HIV infection [22,23]. However, studies examining the levels of AGP in pregnancy show varied results with levels decreasing during pregnancy or levels remaining unchanged [7,24]. The potential reasons for lower levels of AGP in HIV is not clear. A study of healthy pregnant women showed that AGP actually developed anti-inflammatory properties over the course of pregnancy, due to structural changes characterized by increases in degree of glycan branching and decreases in the degree of α3-fucosylation [25]. These changes were partly caused by increases in estrogen levels during pregnancy [25]. Thus, in the context of a more pro-inflammatory profile of HIV or reduced estrogen with EFV-based regimens [26] (i.e. the majority of women in this study), there might be reduced levels of AGP (with anti-inflammatory properties).
The strengths of our study include assessment of various inflammatory markers, informing on difference aspects of immunity, for pregnant women with and without HIV. Furthermore, this study examines the relationship of HIV with these inflammatory markers during pregnancy overall and by specific trimesters. We also acknowledge limitations to our study. First, the sample size of the study, while being among the largest to address this question, was limited. Second, we did not have data from the first trimester of pregnancy, the postpartum period or from non-pregnant controls; regardless, our study provides an important comparison by HIV status in the second and third trimester. A further limitation of our study is that we did not adjust for potential unmeasured (e.g., CMV infections status) or unknown confounders in the analyses. Additional limitations are detailed in online supplement.
In conclusion, our study found that HIV status during pregnancy was associated with higher levels of markers of gut integrity, monocyte activation and systemic inflammation, and lower levels of AGP, an acute phase protein. As some of these markers are associated with adverse birth outcomes, our results could help explain the increased risk of preterm delivery among women with HIV and suggest targets for potential interventions (e.g., dietary fiber or probiotics to improve gut integrity and reduce monocyte activation).
Supplementary Material
Acknowledgments:
The authors thank the study participants for their time and contributions as well as the study staff who meticulously collected detailed data.
Funding:
Research reported in this publication was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R00HD089753 to RS and R01HD081929 to AG. JSM received support from NIAID (K23AI129854). Additional support for this work was the NIH-funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks (UM1AI069465 to AG). BBA is a senior investigator from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. MAP received a research fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; finance code 001). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors also acknowledge support from Persistent Systems in kind.
Footnotes
Competing interests: None declared
References
- 1.Mudd JC, Brenchley JM. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. Journal of Infectious Diseases, 2016. October 1; 214(Suppl 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shivakoti R, Gupte N, Tripathy S, Poongulali S, Kanyama C, Berendes S, et al. Inflammation and micronutrient biomarkers predict clinical HIV treatment failure and incident active TB in HIV-infected adults: a case-control study. BMC Med. 2018. September 24;16(161). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. PLoS Medicine. 2008. October 21;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis;214 Suppl 2(Suppl 2):S44–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley J, Price D, Schacker T, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006. November 16;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 6.Mor G, Cardenas I. The Immune System in Pregnancy: A Unique Complexity. Am J Reprod Immunol. 2011. January 24;63(6):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson A, Palm M, Hansson L‐ O, Basu S, Axelsson O. Reference values for α1‐acid glycoprotein, α1‐antitrypsin, albumin, haptoglobin, C‐reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet Gynecol Scand. 2008;87(10):1084–1088. [DOI] [PubMed] [Google Scholar]
- 8.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2015. December 1;70(2):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López M, Figueras F, Coll O, Goncé A, Hernández S, Loncá M, et al. Inflammatory Markers Related to Microbial Translocation Among HIV-Infected Pregnant Women: A Risk Factor of Preterm Delivery. Journal of Infectious Diseases. 2016. February 1;213(3):343–350. [DOI] [PubMed] [Google Scholar]
- 10.Russell ES, Mohammed T, Smeaton L, Jorowe B, MacLeod IJ, Hoffman RM, et al. Immune activation markers in peripartum women in Botswana: association with feeding strategy and maternal morbidity. PLoS One. 2014. March 21;9(3):e89928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman RM, Leister E, Kacanek D, Shapiro DE, Read JS, Bryson Y, et al. Biomarkers from late pregnancy to 6 weeks postpartum in HIV-infected women who continue versus discontinue antiretroviral therapy after delivery. J Acquir Immune Defic Syndr. 2013. August 15;63(5):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2008. June 28;65:194–202. [DOI] [PubMed] [Google Scholar]
- 13.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016. March;130(6):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivakoti R, Gupte N, Kumar NP, Kulkarni V, Balasubramanian U, Bhosale R, et al. Intestinal Barrier Dysfunction and Microbial Translocation in Human Immunodeficiency Virus-Infected Pregnant Women Are Associated With Preterm Birth. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2018. August 31;67(7):1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Raya B, Kollmann TR, Marchant A, MacGillivray DM. The Immune System of HIV-Exposed Uninfected Infants. Front Immunol. 2016. September 28;7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Front Immunol. 2016. May 6;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevenoaks T, Wedderburn CJ, Donald KA, Barnett W, Zar HJ, Stein DJ, et al. Association of maternal and infant inflammation with neurodevelopment in HIV-exposed uninfected children in a South African birth cohort. Brain Behav Immun. 2021. January; 91:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Annals of the New York Academy of Sciences. 2019. August;1450(1):47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakier A, Petro G, Fawcus S. Mid-upper arm circumference: A surrogate for body mass index in pregnant women. South African Medical Journal. 2017. June;107(7):606. [DOI] [PubMed] [Google Scholar]
- 20.Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, Van Gassen S, et al. An immune clock of human pregnancy. Science Immunology. 2017. September 17;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Larrañaga GF, Petroni A, Deluchi G., Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis. 2002. December 31;14(1):15–18. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Schooley RT, Gerber JG. The Effect of Increasing α1-Acid Glycoprotein Concentration on the Antiviral Efficacy of Human Immunodeficiency Virus Protease Inhibitors. J Infect Dis. 1999. December;180(6):1833–1837. [DOI] [PubMed] [Google Scholar]
- 23.Jones K, Hoggard PG, Khoo S, Maher B, Back DJ. Effect of α1‐acid glycoprotein on the intracellular accumulation of the HIV protease inhibitors saquinavir, ritonavir and indinavir in vitro. Br J Clin Pharmacol. 2001. January;51(1):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu CYT, Singla VP, Wang H, Sweet B, Lai LTY. Plasma α1-acid glycoprotein levels in pregnancy. Clinica Chimica Acta. 1980. September 2;112(2):235–240. [DOI] [PubMed] [Google Scholar]
- 25.Havenaar EC, Axford JS, Brinkman-van der Linden EC, Alavi A, Van Ommen EC, van het Hof B, et al. Severe rheumatoid arthritis prohibits the pregnancy-induced decrease in alpha3-fucosylation of alpha1-acid glycoprotein. Glycoconj J 1998; 15(7):723–729. [DOI] [PubMed] [Google Scholar]
- 26.Balogun KA, Guzman Lenis MS, Papp E, Loutfy M, Yudin MH, MacGillivray J, et al. Elevated Levels of Estradiol in Human Immunodeficiency Virus-Infected Pregnant Women on Protease Inhibitor-Based Regimens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018; 66(3):420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


