Abstract
Objective:
Interictal epileptiform discharges (IEDs) were shown to be associated with cognitive impairment in persons with epilepsy. Previous studies indicated that IED rate, location, timing, and spatial relation to the seizure onset zone could predict an IEDs impact on memory encoding and retrieval in lateral temporal, mesial temporal, and parietal regions. In this study, we explore the influence that other IED properties (e.g., amplitude, duration, white matter classification) have on memory performance. We were specifically interested in investigating the influence that lateral temporal IEDs had on memory encoding.
Methods:
Two hundred sixty-one subjects with medication-refractory epilepsy undergoing intracranial electroencephalography monitoring performed multiple sessions of a delayed free-recall task (n = 671). Generalized linear mixed models were utilized to examine the relationship between IED properties and memory performance.
Results:
We found that increased IED rate, IEDs propagating in white matter, and IEDs localized to the left middle temporal region were associated with poorer memory performance. For lateral temporal IEDs, we observed a significant interaction between IED white matter categorization and amplitude, where IEDs with an increased amplitude and white matter propagation were associated with reduced memory performance. Additionally, changes in alpha power after an IED showed a significant positive correlation with memory performance.
Significance:
Our results suggest that IED properties may be useful for predicting the impact an IED has on memory encoding. We provide an essential step towards understanding pathological versus potentially beneficial interictal epileptiform activity.
Keywords: Epilepsy, Interictal Epileptiform Discharges, Cognition, Memory, Intracranial Monitoring
1. Introduction
Apart from seizures, persons with epilepsy are at an increased risk for many other comorbidities. Of which, cognitive issues often rank as one of their top concerns1. Studies from as early as the 1930s reported an association between cognitive impairment and subclinical interictal epileptiform discharges (IEDs)2. Numerous studies provide additional support for the association between cortical IEDs and memory impairment3–7. Lateral temporal and parietal IEDs were shown to impair performance on a free-recall task if they occurred during encoding and recall phases3,4. There is also evidence that IEDs in the left lateral temporal cortex impair memory by as much as 15% per IED; however, this effect was only present for IEDs outside of the seizure onset zone (SOZ) during memory encoding4.
While IEDs were harder to detect with scalp electroencephalography (EEG), intracranial EEG (iEEG) offers a highly sensitive method for detecting IEDs and is thought to reflect true neural activity. Thus, iEEG is commonly used to define epileptogenic zones prior to surgery for focal refractory epilepsy. An emerging issue is that not all IEDs are the same, and accordingly, not all IEDs are helpful for the localization of SOZs8–10. Past studies have examined the importance of IED rate, location, timing, and spatial relation to the SOZ. However, they have not systematically considered other IED properties that may be useful for uncovering differences in IED populations. This poses a testable hypothesis in need of investigation: are specific IED properties more deleterious to successful memory encoding?
In this study, we examined the impact that certain properties of IEDs have on performance on a free-recall memory task. We were particularly interested in evaluating the impact that lateral temporal IEDs had on memory encoding. Our hypothesis was that specific IED properties, such as amplitude and rate, would be associated with greater reductions in memory performance. We also hypothesized, based on previous findings that lower frequency bands were significant predictors of free-recall memory performance11–13, that IED-related powerband changes in the lower frequency bands (i.e., theta and alpha) would be significantly correlated with memory performance. These findings would shed light on why certain IEDs are more detrimental to memory and provide insight into the debate of whether interictal epileptiform activity is “good” or “bad”14. It would also further our understanding of why certain IEDs may be more useful for localizing SOZs and predicting cognitive outcomes after surgical resections.
2. Methods
2.1. Participants
Two hundred sixty-one subjects with medication-refractory epilepsy were included in this study. Subjects contributed 671 unique sessions and comprised 127 females and 134 males, with an average age of 37.65 [range 18–66, standard deviation (SD) = 11.5]. Subjects underwent a surgical procedure to implant intracranial surface (grids/strips) and depth electrodes to best localize epileptic regions (herein referred to collectively as iEEG electrodes). Data was collected as part of a prospective, multi-center feasibility study of direct brain recording stimulation for memory enhancement (ClinicalTrials.gov Identifier: NCT04286776) from the following eight hospitals: Columbia University Hospital (New York, NY), Dartmouth-Hitchcock Medical Center (Lebanon, NH); Emory University Hospital (Atlanta, GA); Hospital of the University of Pennsylvania (Philadelphia, PA); Mayo Clinic (Rochester, MN); National Institutes of Health (Bethesda, MD); Thomas Jefferson University Hospital (Philadelphia, PA); University of Texas Southwestern Medical Center (Dallas, TX). The research protocol was approved by the Institutional Review Board at each participating hospital, and written informed consent was obtained from each participant (Protocol #: MEMES-001). Only non-stimulation testing sessions, collected before any brain stimulation was performed, were included in this study. All testing sessions were conducted at least four hours after the most recent seizure activity and after a minimum of 24 hours post-implantation. Demographic and clinical characteristics are listed in Table 1.
Table 1.
Subject information.
| Characteristics | Subjects (n = 261) |
|---|---|
| Experiment Sessions | 671 |
| Gender | |
| Female | 127 (48.66%) |
| Age | |
| Mean (SD) | 37.65 (± 11.50) |
| Seizure Onset Age | |
| Mean (SD) | 18.07 (± 12.65) |
| Education (years) | |
| Mean (SD) | 13.60 (± 2.33) |
| Prior Resection | |
| Yes | 42 (16.09%) |
| Seizure Onset Zone | |
| Left Hemisphere | 65 (24.90%) |
| Right Hemisphere | 182 (69.73%) |
| Bilateral | 14 (5.36%) |
| Electrode Type | |
| Depth | 248 (95.02%) |
| Grids or Strips | 143 (54.34%) |
| Electrode Coverage | |
| Bilateral | 178 |
| Unilateral Left | 51 |
| Unilateral Right | 32 |
| IED Amplitude (μV) | |
| Mean (SD) | 234.71 (± 182.71) |
| IED Duration (ms) | |
| Mean (SD) | 46.49 (± 13.27) |
| IED Region | |
| Ipsilateral SOZ | 5348 (61.26%) |
| Contralateral SOZ | 3382 (38.74%) |
| IED Frequency (IEDs/trial) | |
| Mean (SD) | 3.43 (± 2.51) |
| IED Spread (electrodes/IED) | |
| Mean (SD) | 1.39 (± 2.40) |
2.2. Memory Task
Each subject participated in a delayed free-recall memory task consisting of an encoding phase, delay phase, and retrieval phase (Fig.1A). During each trial, subjects were asked to memorize 12 random nouns from a pool of English nouns (http://memory.psych.upenn.edu/WordPools) during a 30-second encoding phase. Subjects were subsequently asked to complete arithmetic problems presented as A+B+C=? where A, B, and C were set to random single-digit integers during a 20-second distractor phase. Subjects were finally given 30 seconds to recall as many words as possible in any order. Trials were repeated up to 25 times per testing session, and an average of 2.57 (SD 1.59) sessions was completed per subject. Data was used from sessions where the word lists were categorized (categorical free-recall) and completely independent (free-recall).
Figure 1. Task Design and Analysis Methods.
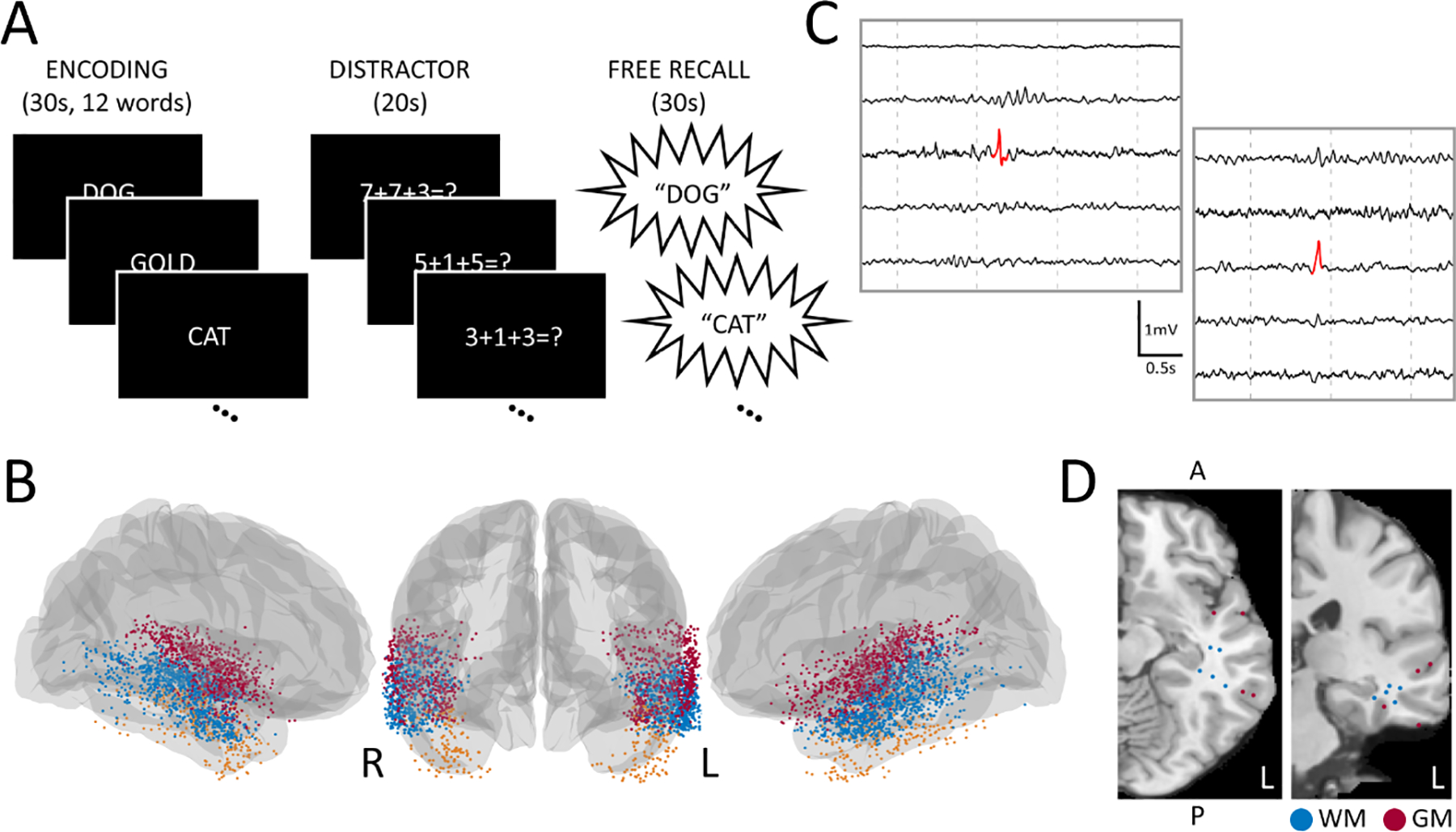
(A) Subjects performed a verbal free-recall memory task, where they were asked to encode 12 nouns, perform an arithmetic distraction, then verbally recall as many nouns as possible. (B) 261 subjects with intracranial electrodes in the lateral temporal region participated. Each point represents an electrode where at least one interictal epileptiform discharge was detected during the encoding phase of the memory task. Electrodes were classified based on if they were in the superior (red), middle (blue), or inferior (orange) temporal regions. (C) Sample interictal epileptiform discharges detected by our automated template-matching algorithm. (D) T1 MRI with a subject’s electrodes transformed onto an average brain with IED detections classified as either white matter or gray matter (i.e., IEDs with or without white matter propagation).
2.3. Anatomical Localization
Intracranial electrodes were localized with CT and MRI co-registration provided with the dataset. Pre-implant T1- and T2-weighted structural scans were aligned with post-implant CT scans for each subject using Advanced Normalization tools15. Freesurfer and the Desikan-Killiany atlas were used for cortical parcellation and subfield localization16. Two neuroradiologists reviewed output from this automated localization pipeline. Only electrodes localized to lateral temporal brain regions were included in this study. These electrodes were reclassified based on whether they were in the superior temporal region (ST), middle temporal region (MT), or inferior temporal region (IT). Fig.1B illustrates the spatial distribution of these electrodes on an average brain.
2.4. Intracranial EEG Data Processing
Intracranial EEG (iEEG) signals were recorded using one of the following systems at each testing site: Quantum LTM (Natus), Grass Telefactor, Nihon-Kohden, and custom Medtronic EEG systems. Signals were originally sampled at 500, 1,000, or 1,600 Hz and were bipolar referenced or referenced to a common contact placed intracranially, on the scalp, or on the mastoid process. Thus, iEEG signals were re-referenced to an averaged referential montage, linearly de-trended, and notch-filtered at 60 Hz and its odd harmonics. Signals were low-pass filtered with a Butterworth filter at 250 Hz, high-pass filtered with a Butterworth filter at 1 Hz, then downsampled to 500 Hz. Electrodes were excluded if the signal was greater than three standard deviations from the mean value across other recording channels.
2.5. Automated IED Detection
An automated template-matching IED detector was used for the detection of all IEDs in this study. This detector was validated previously and performed comparably to clinicians and other published IED detectors3,17. The detection algorithm cross-correlated a 60-millisecond triangular template with preprocessed iEEG data, then computed the absolute value of the normalized cross-correlation from one-second sliding windows. Local peaks above a specified detection threshold were classified as IEDs (Fig.1C). Temporally overlapping IEDs were collapsed into a single marked event, while IEDs occurring within three seconds of another IED were excluded to account for bursts of high frequency activity. We elected to use automatic IED detection because (1) our highly sensitive IED detection algorithm provided an objective, quantifiable detection method, and (2) false positive IEDs were predicted to be distributed equally between subjects and experiment trials4.
The following parameters were collected for all IEDs detected during the encoding phase: amplitude, duration, region (ST, MT, IT), laterality (i.e., whether the IED was contralateral or ipsilateral to a subject’s SOZ), rate (total IEDs/ trial), hemisphere, and spread (i.e., if an IED occurred in a single channel or multiple channels). We also indicated if an IED propagated to white matter and the Euclidean distance an IED was from the hippocampal centroid (MNI coordinates [x,y,z] left: 226, 218.8, 217.2; right: 27.52, 218.2, 216.8)18. White matter classification of IEDs relied on an automated version of the MRIcron software in R (https://github.com/yunshiuan/label4MRI), which input MNI coordinates and output (1) a Broadmann Area (BA) brain region and (2) an output distance. Gray matter IEDs were classified as those with an output distance of 0, and white matter IEDs were classified as those with output distances greater than 0 (Fig.1D).
2.6. Spectral Power Analysis
The multitaper method was used to analyze spectral power in the pre- and post-IED interval (−2000 to −1000 ms relative to IED onset, and +1000 to +2000 ms after IED offset). This method convolved orthogonal Slepian sequences with the preprocessed iEEG, providing periodograms that were used to compute a final spectrum. The final spectrum was then utilized to calculate the average spectral power within the following frequency bands: delta (2–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (12–30 Hz), gamma (25–40 Hz), and high-frequency activity (HFA: 40–100 Hz)19. We used a 1 s gap to measure relevant power changes surrounding an IED, while minimizing IED perturbations from post-IED slow waves; this was motivated by our previous work, which revealed that the optimal powerband change was in the +1 to +2 second post-IED window20.
2.7. Statistical Analysis
We evaluated if specific properties of lateral temporal IEDs were more deleterious to memory encoding. Memory performance was assessed with a normalized metric, which was obtained by subtracting the total number of recalled words per trial from the average number of recalled words in that session, then dividing by the standard deviation of recalled words in that session for each subject. Generalized linear mixed models (GLMM) were used for significance testing to estimate within-subject correlation, adjust for data heterogeneity between subjects (i.e., different number of completed trials and electrodes per subject), test the effect of potential confounders, and account for differential performance due to experiment type (i.e., categorical free-recall versus free-recall).
We first used a GLMM to test the effect of subject-level variables (e.g., age, sex, and history of prior surgical resections) against memory performance and IED occurrence across all subjects. We next examined if any of the measured IED features were associated with worse overall memory performance. This model had fixed effects for IED amplitude, duration, distance to the hippocampal centroid, rate, laterality concerning the SOZ, hemisphere, spread, region, and white matter categorization. The dependent variable was the normalized memory performance metric. We incorporated random slopes and intercepts for both the subject and the experiment type. These models also included interactions for all IED parameters; thus, all parameters were refactored as either binary or continuous. Continuous parameters were standardized for ease of interpretation and to minimize the influence of multicollinearity from higher-order terms. Analogous models were used for pairwise comparisons between lateral temporal regions. Tests for multicollinearity showed no significant collinearity between these predictor variables [VIF range 1.03–1.94].
We subsequently tested whether IED-induced changes in spectral power were correlated with memory performance using similar hierarchical models. Independent models were used for each lateral temporal region (ST, MT, IT) and frequency band. Power changes were calculated by subtracting the post-IED average power from the pre-IED average power, then z-scoring within subject and frequency band. The dependent variable was normalized memory performance. Independent variables included normalized power change and IED features that significantly influenced memory in our prior analysis (e.g., IED rate, IED white matter category, IED hemisphere). Random slopes and intercepts were included for both subject and experiment type.
A post hoc analysis was performed to compare the pre- and post-IED alpha power in the middle temporal region relative to other lateral temporal regions (ST/IT regions combined). Independent tests were used for all IEDs corresponding to either increased alpha activity or decreased alpha activity to determine if changes in alpha power were due to (1) post-IED power alterations, (2) pre-IED power alterations, or (3) a combination of pre- and post-IED alterations. Similar hierarchical models were fit with pre-IED and post-IED alpha power as outcome variables, region as the independent variable, and random effects for subject and experiment. We performed a nonparametric permutation test to validate the correlation between alpha power changes in the middle temporal region and memory performance. Alpha power values were permuted 10,000 times, storing model coefficients and p-values for each iteration.
Another post hoc analysis used similar hierarchical models with change in alpha power as the outcome and IED features as independent variables to evaluate if any of the IED features were correlated with the degree of change in peri-IED alpha in the middle temporal region. Finally, to understand the role of alpha in the direct context of this free recall memory task, we examined alpha power for trails without any IEDs using a binomial GLMM. The outcome variable was memory performance, discretized based on increased or decreased performance relative to the within-subject mean. Alpha power was used as the independent variable, with random effects for subject and experiment.
The trial number was included in all models assessing memory and IEDs, as it was significantly associated with memory performance and IED occurrence. Sensitivity tests were performed with simpler models to confirm the overall results reported by our full models. Wald chi-square tests were used to obtain the significance of fixed effects. Here, a ß value for memory performance above zero indicates a positive correlation with the predictor, and a value below zero indicates a negative correlation with the predictor; larger ß values correspond to increased predictor effects. The false discovery rate was controlled at 0.05 with the Benjamini-Hochberg (BH) procedure for all models21; thus, all reported values were adjusted, unless otherwise stated. Distributional assumptions were verified for all models in this study. Code for analyses in this study was written in R version 3.6.2 and Python version 3.6.7.
2.8. Data Availability
All data is available through the Defense Advanced Research Projects Agency (DARPA) Restoring Active Memory (RAM) Public Data Release (http://memory.psych.upenn.edu/RAM). Code used for our analyses is available upon reasonable request.
3. Results
Subjects had an average recall rate of 25.81% (standard deviation [SD] 10.65%) for the free-recall task and 31.48% (SD 15.17%) for the categorical free-recall task. Of the subject-level variables examined, a higher trial number was significantly associated with a decrease in memory performance (adjusted p < 0.001) and a slight decrease in IED rates (adjusted p = 0.031) (Fig.S1). There was no significant difference in memory performance or IED rates by age, age of seizure onset, history of prior resection, sex, and SOZ hemisphere (Fig.S1). Raw IED amplitudes and durations are illustrated in Fig.S2.
3.1. IED Features and Memory Performance
This study’s primary focus was to characterize if features of IEDs were significantly correlated with memory performance for IEDs that occurred in the lateral temporal region during encoding. A higher IED rate was significantly correlated with worse memory performance (adjusted p < 0.001; Fig.2A). Regardless of the SOZ hemisphere, IEDs in the left lateral temporal lobe were associated with lower recall relative to right lateralized IEDs (adjusted p = 0.024; Fig.2A). Among lateral temporal brain regions, IEDs occurring in the middle temporal region were more detrimental to memory performance (adjusted p = 0.010; Fig.2A). IEDs propagating in white matter were more deleterious than purely gray matter IEDs (adjusted p = 0.047; Fig.2A).
Figure 2. Features of Interictal Epileptiform Discharges associated with Memory.
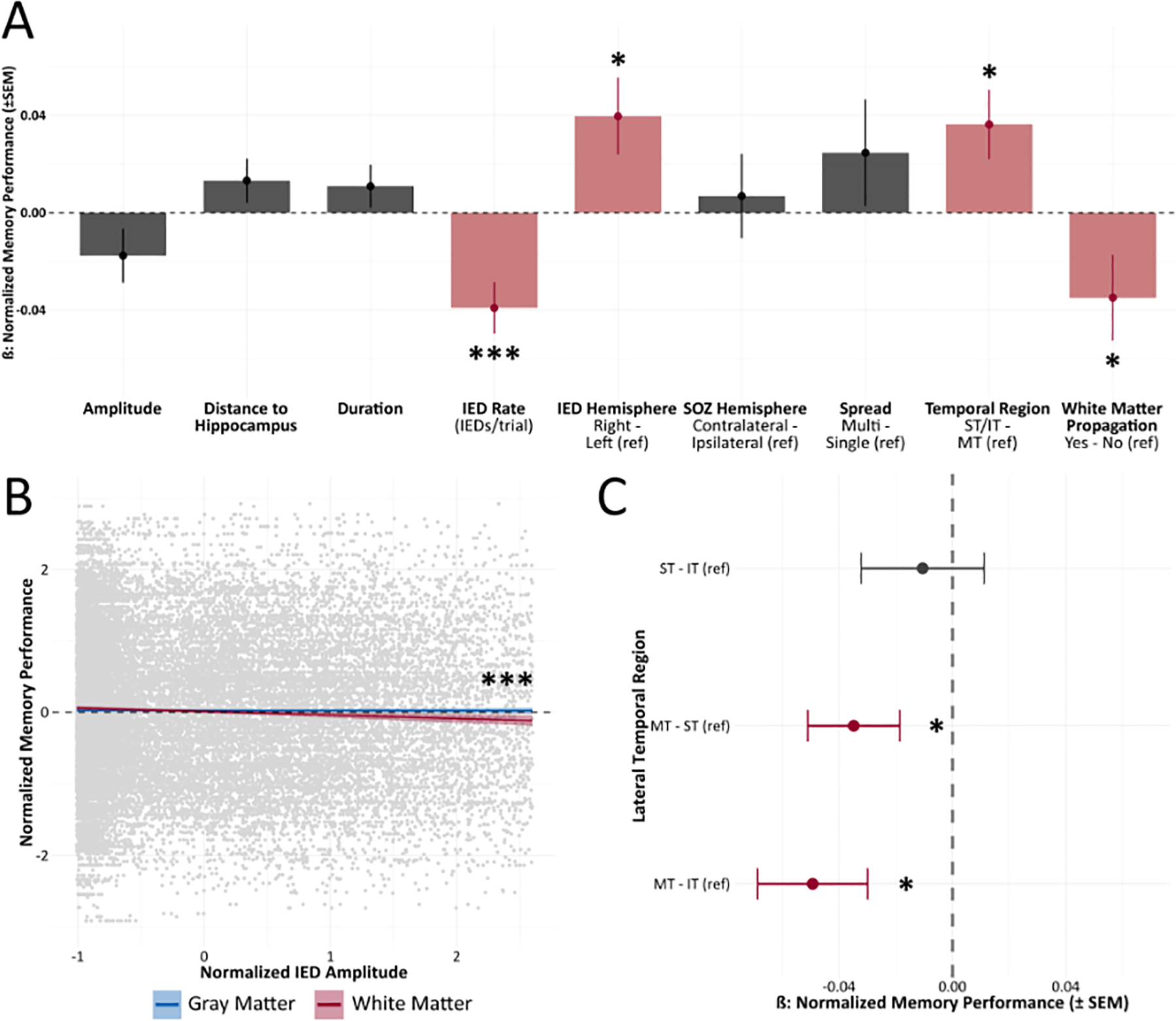
(A) IED rate (IEDs/trial), hemisphere, lateral temporal localization, and white matter classification were significantly associated with memory performance. Increased IED rate localized to the left middle temporal region with white matter propagation predicted worse memory performance. (B) IED white matter categorization and amplitude showed a significant interaction; an increased amplitude of IEDs with white matter propagation was associated with lower memory performance. (C) Pairwise comparisons of all lateral temporal brain regions revealed IEDs in the middle temporal region (MT) predicted reduced memory performance. * p < 0.05, ** p < 0.01, *** p < 0.001
There was a significant interaction between IED amplitude and the white/gray matter classification (adjusted p = 0.011; Fig.2B). That is, elevated IED amplitude had a greater impact on memory performance for IEDs that propagated to white matter relative to gray matter IEDs. A pairwise comparison between all lateral temporal regions demonstrated that IEDs in the middle temporal region predicted worse overall memory performance relative to both the inferior temporal region (adjusted p = 0.011; Fig.2C) and the superior temporal region (adjusted p = 0.032; Fig.2C). In back-transforming our model estimates for ease of interpretation, we show that IEDs in the middle temporal region with white matter propagation reduced the average recall by approximately 8% per IED. Further, holding the features above constant, we reveal an additional 3% reduction in the average recall for every 183 μV increase in IED amplitude.
3.2. Spectral Power Changes following an IED Influence Memory Encoding
After determining that IEDs had location-dependent influences on memory encoding, we sought to predict if an IED would be detrimental to memory performance based on its impact on local network oscillations. There were nonsignificant correlations between power changes and memory performance for all frequency bands except for the alpha band (adjusted p = 0.029; Fig.3A). A permutation test validated these alpha power findings for IEDs in the middle temporal region (permuted p = 0.026; Fig.3B). Repeating this analysis for IEDs in the superior temporal region and inferior temporal region revealed null findings for all frequency bands (Fig.S3). Thus, we used the ST/IT regions as a reference to distinguish when alpha power changes were occurring relative to the IED (i.e., pre-IED, post-IED, or both). For observed IED-related increases in alpha activity, our analysis showed a significant increase in alpha power after an IED (adjusted p = 0.0073; Fig.3C), but not prior to an IED (adjusted p = 0.55; Fig.3C). For observed IED-related decreases in alpha activity, we showed a significant decrease in alpha power after an IED (adjusted p = 0.0068; Fig.3C), but not prior to an IED (adjusted p = 0.11; Fig.3C). A back-transformation of peri-IED power changes in the middle temporal region showed a 4.5% reduction in the average recall for every 6 dB decrease in post-IED alpha power.
Figure 3. IED Power Spectral Perturbations and Memory Performance.
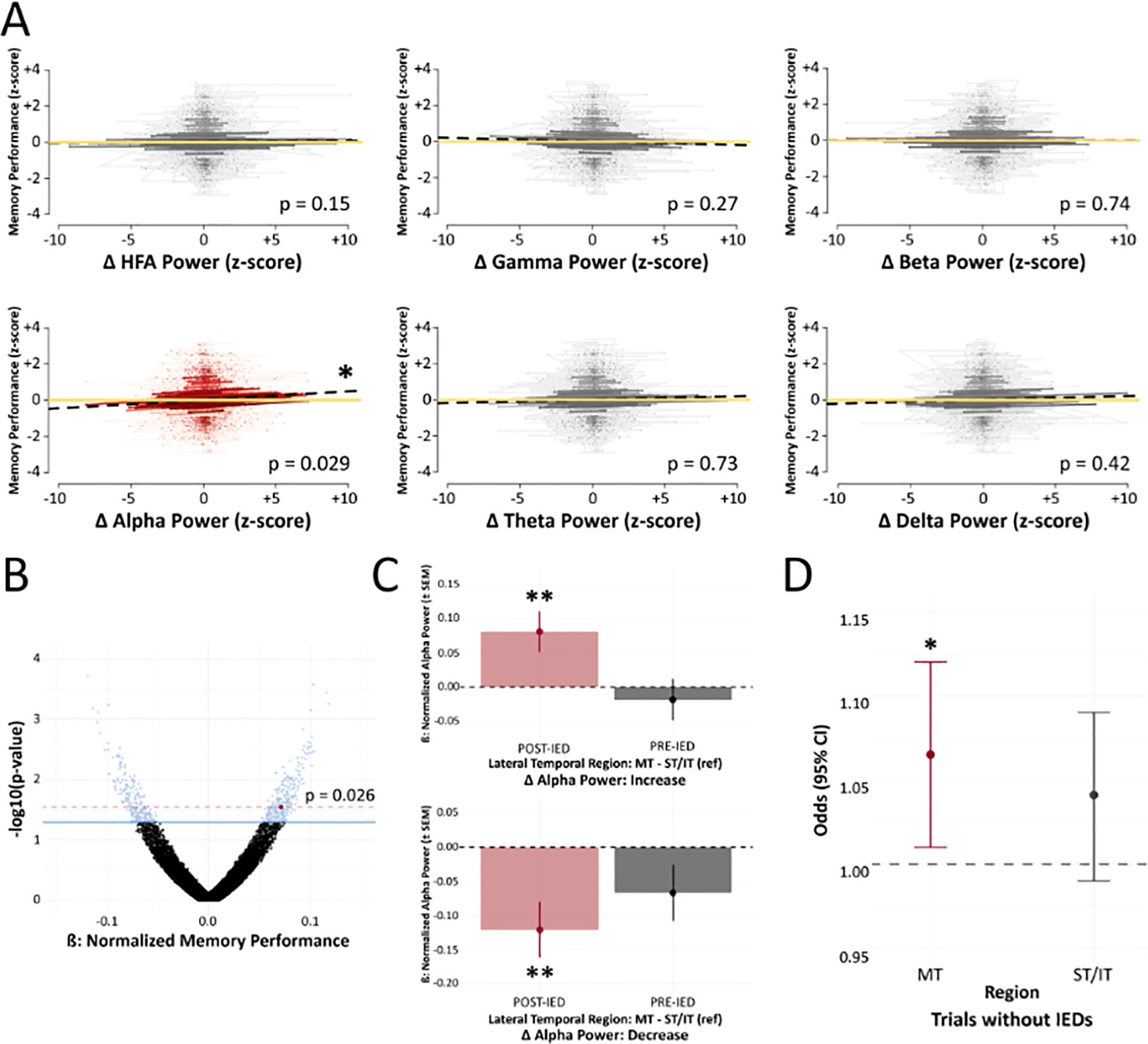
(A) Changes in power were calculated by comparing pre-IED and post-IED (−2000 to −1000 ms relative to IED onset, and +1000 to +2000 ms after IED offset) windows. Power changes were evaluated in each frequency band for electrodes in the middle temporal region during the encoding phase. Alpha power showed a significant positive correlation with memory performance. Dashed black lines represent correlations, while yellow lines were plotted as a reference (z-score = 0). (B) A nonparametric permutation procedure validated alpha power findings. The red point denotes our observed p-value; blue points denote permuted p-values < 0.05. (C) To determine if alpha power changes were due to shifts before or after IEDs, we compared alpha power in the middle temporal region to alpha power in other lateral temporal regions (superior, inferior) for IEDs with overall increases and decreases in alpha activity. In the middle temporal region, increases in post-IED alpha were driving IED-related increases in alpha activity, while decreases in post-IED alpha were driving IED-related decreases in alpha activity. IEDs showed nonsignificant effects on pre-IED alpha activity. (D) There was an approximate 6.5% increased odds of recall for every 12.21 dB increase in alpha power during encoding periods without any IEDs (p = 0.036). Red indicates significant changes. * p < 0.05, ** p < 0.01, *** p < 0.001
While investigating if peri-IED alpha in the middle temporal region was correlated with any of the IED features, we found that peri-IED alpha changes were negatively correlated with IED amplitude (adjusted p < 0.001). None of the other IED features were significantly associated with peri-IED alpha. Our final post hoc analysis evaluated alpha trends in trials without any IEDs during encoding. This revealed a 6.5% increased odds of recall for every 12.21 dB increase in alpha power in the middle temporal region (adjusted p = 0.036).
4. Discussion
Whereas several studies indicate that IED rate, IED laterality, and IED time-dependence significantly impact memory networks3,4,22, they did not account for IED features, such as peri-IED rate compositions and white matter propagation, which may be related to memory. Here, we showed that certain properties of lateral temporal IEDs were more deleterious to successful memory encoding. We demonstrated that increased IEDs in the left middle temporal region with white matter propagation were most harmful to memory encoding and resulted in significant reductions in recall. IED amplitude showed a significant interaction with white matter categorization, whereby IEDs with higher amplitudes and white matter propagation were associated with lower memory performance. Finally, we observed that IED-related modulations in alpha power were correlated with memory performance. These results uncover IED properties that may be useful for differentiating “bad spikes” from “good spikes” as they relate to memory encoding14.
Similar to past reports, we observed greater memory impairment for an increased rate of left lateralized IEDs3,4. The magnitude of our observed effect was comparable to Ung et al.’s (2017) past report of a 6.6% reduced odds of recall for each IED in the left middle temporal gyrus (BA 21)4. These findings were likely linked with the verbal and auditory word processing functions of the left temporal lobe, which are necessary to perform the verbal word memory task5. Wagner et al.’s (1998) fMRI study also demonstrated that activity in the left prefrontal and temporal regions was jointly required for memory formation, especially for recalling verbalizable events23.
We observed that left lateralized IED effects were irrespective of the subjects’ SOZ, as IED-SOZ laterality did not significantly impact encoding4,22. This contrasts past studies, which include: Kleen et al.’s (2013) report that hippocampal IEDs bilateral and contralateral to the seizure focus decreased memory performance22, and Ung et al.’s (2017) report that IEDs outside of the SOZ had a greater impact on recall than IEDs within the SOZ4. These discrepancies could be explained by our increased sample size and control for IED-related properties (e.g., amplitude, duration, spread), which may have confounded past observations. Heterogeneity surrounding the importance of the SOZ for predicting IED effects on memory is further demonstrated by Horak et al.’s (2016) finding that IEDs in the SOZ had a greater impact on memory3.
During our regional analysis of IEDs, we discovered that IEDs in the middle temporal region affected recall greater than IEDs in the inferior and superior temporal regions. Previous intracranial EEG studies showed that the inferior temporal lobe was involved in memory encoding3 and that hippocampal IEDs reduced memory performance during memory retrieval but not encoding22,24. These findings support the theory that different memory processes (i.e., encoding versus retrieval) may depend on different brain regions; medial structures, such as the hippocampus, may be more involved with memory retrieval, while lateral temporal structures may be essential for memory encoding.
Past studies of human episodic memory offer additional support for this theory. Mainly, there is a flow of information from the neocortex to the hippocampus during memory encoding and a reverse in this flow during memory retrieval25,26. The successful recall of an event was also shown to depend on the reinstatement of an oscillatory state27,28. In a study with 69 intracranial EEG subjects, Manning et al. (2011) showed that the pattern of brain activity during successful recall was similar to patterns observed during memory encoding for that item and neighboring list items during a free-recall memory task27. Another intracranial EEG study of 11 subjects further demonstrated that this reinstatement process was dependent on both time and space and could be predicted by the magnitude of phase synchronization between the hippocampus and lateral temporal cortex29. Whether this oscillatory reinstatement spans across broad brain regions or is focal at specific sites is still up for debate30,31; nevertheless, IEDs are likely to disrupt oscillatory patterns necessary for proper memory encoding and retrieval.
Our work shows that IEDs with white matter propagation in the lateral temporal region were more detrimental to memory encoding. These results are unsurprising, as white matter tracts have a known association with the propagation of electrical stimulation in the brain32,33. If viewing IEDs as internally generated equivalents to exogenous electrical stimulation, our results agree with Solomon et al.’s (2018) finding that stimulation induces spectral power changes in the theta range across a broad network of brain regions if stimulation occurred in or near white matter34. In a subsequent study, Solomon et al. (2019) also showed that increased theta synchrony in medial temporal regions indicated periods of successful memory encoding11. Thus, our next step was to evaluate the association between spectral power changes between pre- and post-IED windows and memory.
Of all the power bands, only changes in alpha activity were significantly correlated with memory performance. We witnessed a positive relationship between alpha power changes and memory performance in middle temporal regions, through which IED-related increases in alpha predicted better memory performance and IED-related decreases in alpha predicted worse memory performance. We showed that power changes following an IED were driving these alpha alterations. Additionally, IEDs with higher amplitudes were correlated with more negative peri-IED alpha changes (i.e., worse memory performance). Two prominent theories may explain the functional significance of alpha activity for cognition: (1) alpha activity is thought to be involved with cortical idling, with increases occurring during rest states35, and (2) alpha activity is thought to contribute to memory retention, where task-related increases in alpha were seen with increasing task load36,37.
Recent research provides increasing support for the latter theory owing to consistent observations of task-related increases in alpha activity. Moreover, these reports exposed two suspected roles for increased alpha during memory retention. The first role deals with the inhibition timing hypothesis, where alpha is thought to filter out task-irrelevant information, preventing its interference with memory12,38,39. Second, alpha activity is thought to be directly responsible for maintaining relevant memory contents and controlling the timing within task-relevant networks40–43. Both roles support our findings that increased post-IED alpha power is conducive to enhanced memory performance – and that middle temporal alpha was positively correlated with memory performance, even in the absence of IEDs. An alternative explanation for these findings is that “good” IEDs did not interfere with a subject’s ability to engage cognitively (i.e., did not perturb underlying alpha oscillations). An epiphenomenon could also explain these findings whereby trials that a subject engaged cognitively resulted in enhanced alpha oscillations, coincidentally timed right after an IED.
Analogous to other neural oscillations, the task-related role of alpha is much more complex than the above simplifications. For instance, Jensen et al. (2002) observed increased posterior-central alpha with lateralized asymmetries during Sternberg retention, which was present during the last 2.5 s of the retention period36. Palva et al. (2011) also showed that frontoparietal high-alpha was suppressed at higher memory loads, while high-alpha and beta bands were positively correlated with memory load from 400 ms to the end of the visual working memory retention period37. Both studies suggest that the location and timing of alpha and other frequencies are complex. Adding to this complexity, alpha changes witnessed in these studies are likely different from alpha changes associated with eyes-opened and eyes-closed states (i.e., eyes-opened alpha desynchronization)44. Future work should examine how location and timing may affect IED-related changes in alpha to further uncover the relationship between IED-related shifts in alpha and memory.
The literature also suggests that theta oscillations are important for successful memory encoding and retrieval45,46. Theta is thought to drive hippocampus-dependent memory processing and inter-regional brain communication11,13,47. Zhang et al. (2018) provide a connection between alpha oscillations, theta oscillations, and memory in showing that theta and alpha oscillations are traveling waves in the human neocortex, which serve to organize neural processes across space and time48. These traveling waves are thought to temporally and spatially segment neural representations; the neocortex (e.g., lateral temporal structures) was shown to prefer alpha oscillations48–50, while mesial structures (e.g., hippocampus) preferred theta oscillations51,52. Despite these findings, the question of causality remains. Future work is encouraged to determine if certain IEDs are beneficial to memory via increased alpha activity or if increased alpha activity coinciding with better internal memory states suppresses IEDs.
Several limitations should be noted. First, we acknowledge that automated IED detection could introduce bias, especially since our detector is not perfectly concordant with human review17. However, our automated algorithm allowed for the objective quantification of IEDs in a large iEEG dataset while limiting bias attributable to differences in inter-rater reliability. The implications of these findings were further limited by missing surgical outcome data, which reduced confidence in labeled SOZs. Electrode segmentation was also based on MNI coordinates due to the sheer volume of subjects and detected IEDs. We acknowledge the presence of selection bias because we did not select subjects to specifically examine the relationship between IEDs and memory. Furthermore, we could not correlate memory performance with the subject’s lateralized memory dominance.
Additional sources of bias result from the varying placement of electrodes, different number of experiment sessions completed per subject, and other uncontrollable factors commonly associated with intracranial EEG research. Bias may also result from our less conservative control for multiple comparisons (i.e., Benjamini-Hochberg versus Bonferroni correction). Although our statistical models accounted for many of these limitations, there remains a need for controlled experiments specifically designed to examine the influence that different IED properties have on cognition. We also utilized canonical frequency bands classically defined for scalp EEG; thus, future work should evaluate frequencies with finer granularity (e.g., using smaller logarithmically spaced frequency bins) to better identify peri-IED frequencies that may be crucial for memory encoding.
5. Conclusion
In this study, we showed that IEDs impair memory performance when they occur in the left middle temporal region during memory encoding. Higher amplitude IEDs with white matter propagation had adverse effects on memory, while increased post-IED alpha power predicted better memory performance. These results suggest that certain properties of IEDs may be useful for differentiating pathological from potentially beneficial IEDs concerning their influence on cognition. Our findings support the future examination of IED properties, as targeting IED populations detrimental to cognition may improve surgical outcomes and the quality of life for persons with epilepsy.
Supplementary Material
Figure S1. Assessment of Baseline Factors.
The trial number was the only baseline variable significantly associated with memory performance and IED occurrence. Increased trials were associated with lower memory performance and slight decreases in IED rates. * p < 0.05, ** p < 0.01, *** p < 0.001
Figure S2. IED Amplitude and Duration Distributions.
Raw density plots of IED amplitudes and durations aggregated across subjects. Dashed lines indicate amplitude and duration means.
Figure S3. Evaluation of Power Changes in Other Lateral Temporal Regions.
Power changes were calculated by comparing pre-IED (−2000 to −1000 ms relative to IED onset) and post-IED (+1000 to +2000 ms after IED offset) windows. Power changes were assessed in each frequency band for electrodes in the superior and inferior temporal region during the encoding phase. All powerbands showed nonsignificant associations with memory performance.
Key Points.
Interictal epileptiform discharges (IEDs) in the left middle temporal region impair memory encoding
Higher amplitude IEDs with white matter propagation predicted poorer memory performance
Deleterious IEDs reduced the average memory performance by approximately 8% per IED
Increased post-IED alpha power in the middle temporal region was positively correlated with memory performance
Acknowledgements
We thank the patients who participated in this study, the clinical staff, and the research teams that made this study possible. We also thank the Penn Computational Memory Lab for providing data and supporting information used in this study. This work was supported by the National Institutes of Health [Grant Number 05-T32LM012204-03], the Burroughs Wellcome Fund [Grant Number 1014106], the National Science Foundation [Award Number 1632738], and the Diamond Foundation [Research Development Award].
Disclosures
B.C.J. received research support from the following: CDC, DARPA, Diamond Foundation, Eisai, Harvard Pilgrim, Neuropace, NIH, NSF, and the Louis and Ruth Frank Professorship of Neurosciences. She serves as an Associate Editor of Neurology. None of the other authors has any conflict of interest to disclose.
Footnotes
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015; 17(2):101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab RS. A method of measuring consciousness in petit mal epilepsy. J Nerv Ment Dis. 1939; 89:690–1. [Google Scholar]
- 3.Horak PC, Meisenhelter S, Song Y, Testorf ME, Kahana MJ, Viles WD, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. 2017; 58(3):373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. 2017; 140(8):2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarts JHP, Binnie CD, Smit AM, Wilkins AJ. SELECTIVE COGNITIVE IMPAIRMENT DURING FOCAL AND GENERALIZED EPILEPTIFORM EEG ACTIVITY. Brain. 1984; 107(1):293–308. [DOI] [PubMed] [Google Scholar]
- 6.Binnie CD, Kasteleijn-Nolst Trenité DGA, Smit AM, Wilkins AJ. Interactions of epileptiform EEG discharges and cognition. Epilepsy Res. 1987; 1(4):239–45. [DOI] [PubMed] [Google Scholar]
- 7.Shewmon DA, Erwin RJ. Focal spike-induced cerebral dysfunction is related to the after-coming slow wave. Ann Neurol. 1988; 23(2):131–7. [DOI] [PubMed] [Google Scholar]
- 8.Goncharova II, Spencer SS, Duckrow RB, Hirsch LJ, Spencer DD, Zaveri HP. Intracranially recorded interictal spikes: Relation to seizure onset area and effect of medication and time of day. Clin Neurophysiol. 2013; 124(11):2119–28. [DOI] [PubMed] [Google Scholar]
- 9.Marsh ED, Peltzer B, Brown III MW, Wusthoff C, Storm PB Jr, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2010; 51(4):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncharova II, Alkawadri R, Gaspard N, Duckrow RB, Spencer DD, Hirsch LJ, et al. The relationship between seizures, interictal spikes and antiepileptic drugs. Clin Neurophysiol. 2016; 127(9):3180–6. [DOI] [PubMed] [Google Scholar]
- 11.Solomon EA, Stein JM, Das S, Gorniak R, Sperling MR, Worrell G, et al. Dynamic Theta Networks in the Human Medial Temporal Lobe Support Episodic Memory. Curr Biol. 2019; 29(7):1100–1111.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, et al. Medial Temporal Theta/Alpha Power Enhancement Precedes Successful Memory Encoding: Evidence Based on Intracranial EEG. J Neurosci. 2011; 31(14):5392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci. 2009; 106(13):5365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staley KJ, White A, Dudek FE. Interictal spikes: Harbingers or causes of epilepsy? Neurosci Lett. 2011; 497(3):247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008; 12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006; 31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 17.Horak PC, Meisenhelter S, Testorf ME, Connolly AC, Davis KA, Jobst BC. Implementation and evaluation of an interictal spike detector. Proc SPIE. 2015; :96000N1-11. [Google Scholar]
- 18.Robinson JL, Barron DS, Kirby LAJ, Bottenhorn KL, Hill AC, Murphy JE, et al. Neurofunctional topography of the human hippocampus. Hum Brain Mapp. 2015; 36(12):5018–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prerau MJ, Brown RE, Bianchi MT, Ellenbogen JM, Purdon PL. Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis. Physiology. 2017; 32(1):60–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisenhelter S, Quon RJ, Steimel SA, Testorf ME, Camp EJ, Moein P, et al. Interictal Epileptiform Discharges are Task Dependent and are Associated with Lasting Electrocorticographic Changes. Cereb Cortex Commun. 2021; 2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995; 57(1):289–300. [Google Scholar]
- 22.Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013; 81(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner AD. Building Memories: Remembering and Forgetting of Verbal Experiences as Predicted by Brain Activity. Science. 1998; 281(5380):1188–91. [DOI] [PubMed] [Google Scholar]
- 24.Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010; 67(2):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staresina BP, Cooper E, Henson RN. Reversible Information Flow across the Medial Temporal Lobe: The Hippocampus Links Cortical Modules during Memory Retrieval. J Neurosci. 2013; 33(35):14184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linde-Domingo J, Treder MS, Kerrén C, Wimber M. Evidence that neural information flow is reversed between object perception and object reconstruction from memory. Nat Commun. 2019; 10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning JR, Polyn SM, Baltuch GH, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc Natl Acad Sci. 2011; 108(31):12893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staresina BP, Michelmann S, Bonnefond M, Jensen O, Axmacher N, Fell J. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. eLife. 2016; 5:e17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacheco Estefan D, Sánchez-Fibla M, Duff A, Principe A, Rocamora R, Zhang H, et al. Coordinated representational reinstatement in the human hippocampus and lateral temporal cortex during episodic memory retrieval. Nat Commun. 2019; 10(1):2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaffe RB, Kerr MSD, Damera S, Sarma SV, Inati SK, Zaghloul KA. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc Natl Acad Sci. 2014; 111(52):18727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang AI, Wittig JH, Inati SK, Zaghloul KA. Human Cortical Neurons in the Anterior Temporal Lobe Reinstate Spiking Activity during Verbal Memory Retrieval. Curr Biol. 2017; 27(11):1700–1705.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawasli AH, Kim D, Ledbetter NM, Dahiya S, Barbour DL, Leuthardt EC. Influence of White and Gray Matter Connections on Endogenous Human Cortical Oscillations. Front Hum Neurosci. 2016; 10(330). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004; 127(10):2316–30. [DOI] [PubMed] [Google Scholar]
- 34.Solomon EA, Kragel JE, Gross R, Lega B, Sperling MR, Worrell G, et al. Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat Commun. 2018; 9(1):4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfurtscheller G, Stancák A, Neuper Ch. Event-related synchronization (ERS) in the alpha band — an electrophysiological correlate of cortical idling: A review. Int J Psychophysiol. 1996; 24(1–2):39–46. [DOI] [PubMed] [Google Scholar]
- 36.Jensen O. Oscillations in the Alpha Band (9–12 Hz) Increase with Memory Load during Retention in a Short-term Memory Task. Cereb Cortex. 2002; 12(8):877–82. [DOI] [PubMed] [Google Scholar]
- 37.Palva S, Kulashekhar S, Hamalainen M, Palva JM. Localization of Cortical Phase and Amplitude Dynamics during Visual Working Memory Encoding and Retention. J Neurosci. 31(13):5013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poch C, Valdivia M, Capilla A, Hinojosa JA, Campo P. Suppression of no-longer relevant information in Working Memory: An alpha-power related mechanism? Biol Psychol. 2018; 135:112–6. [DOI] [PubMed] [Google Scholar]
- 39.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res Rev. 2007; 53(1):63–88. [DOI] [PubMed] [Google Scholar]
- 40.Manza P, Hau CLV, Leung H-C. Alpha Power Gates Relevant Information during Working Memory Updating. J Neurosci. 2014; 34(17):5998–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn Sci. 2014; 18(1):16–25. [DOI] [PubMed] [Google Scholar]
- 42.Vogelsang DA, Gruber M, Bergström ZM, Ranganath C, Simons JS. Alpha Oscillations during Incidental Encoding Predict Subsequent Memory for New “Foil” Information. J Cogn Neurosci. 2018; 30(5):667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012; 16(12):606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gómez-Ramírez J, Freedman S, Mateos D, Pérez Velázquez JL, Valiante TA. Exploring the alpha desynchronization hypothesis in resting state networks with intracranial electroencephalography and wiring cost estimates. Sci Rep. 2017; 7(1):15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012; 22(4):748–61. [DOI] [PubMed] [Google Scholar]
- 46.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013; 16(2):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005; 15(7):827–40. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Watrous AJ, Patel A, Jacobs J. Theta and Alpha Oscillations Are Traveling Waves in the Human Neocortex. Neuron. 2018; 98(6):1269–1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanRullen R, Koch C. Is perception discrete or continuous? Trends Cogn Sci. 2003; 7(5):207–13. [DOI] [PubMed] [Google Scholar]
- 50.Samaha J, Postle BR. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr Biol. 2015; 25(22):2985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta AS. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012; 15(7):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon EA, Lega BC, Sperling MR, Kahana MJ. Hippocampal theta codes for distances in semantic and temporal spaces. Proc Natl Acad Sci. 2019; 116(48):24343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Assessment of Baseline Factors.
The trial number was the only baseline variable significantly associated with memory performance and IED occurrence. Increased trials were associated with lower memory performance and slight decreases in IED rates. * p < 0.05, ** p < 0.01, *** p < 0.001
Figure S2. IED Amplitude and Duration Distributions.
Raw density plots of IED amplitudes and durations aggregated across subjects. Dashed lines indicate amplitude and duration means.
Figure S3. Evaluation of Power Changes in Other Lateral Temporal Regions.
Power changes were calculated by comparing pre-IED (−2000 to −1000 ms relative to IED onset) and post-IED (+1000 to +2000 ms after IED offset) windows. Power changes were assessed in each frequency band for electrodes in the superior and inferior temporal region during the encoding phase. All powerbands showed nonsignificant associations with memory performance.
Data Availability Statement
All data is available through the Defense Advanced Research Projects Agency (DARPA) Restoring Active Memory (RAM) Public Data Release (http://memory.psych.upenn.edu/RAM). Code used for our analyses is available upon reasonable request.


