Abstract
We assessed the impact of the Janus Kinase (JAK) 1 inhibitor itacitinib on xenogeneic graft-versus-host disease (xGVHD). XGVHD was induced by i.v. injection 20 × 106 human peripheral blood mononuclear cells (hPBMC) in NSG mice on day 0. Itacitinib (3 mg, ≈120 mg/kg) or methylcellulose was administered by force-feeding twice a day from day 3 to day 28. Mice were followed for xGVHD score and survival. In addition, human T-cell engraftment and as well as human T-cell subtypes were monitored in blood on days 14, 21, and 28 after transplantation. We observed that itacitinib-treated mice had significantly longer survival than control mice (median 45 versus 33 days; P < 0.001). Further, they also had lower absolute numbers of human CD4+ T cells on days 21 and 28 after transplantation as well as of human CD8+ T cells on days 14, 21, and 28 after transplantation. In addition, itacitinib-treated mice had higher frequencies of human regulatory T cells (Treg) on days 21 and 28 after transplantation. In summary, our data indicate that itacitinib decreases human T-cell engraftment, increases Treg frequencies and attenuates xGVHD in NSG mice transplanted with hPBMC.
Subject terms: Bone marrow transplantation, Preclinical research
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) still remains the best therapeutic option for many patients suffering from acute myeloid leukemia [1]. Its efficacy is largely due to graft-versus-leukemia effects mediated by donor T cells present in the graft [2]. Unfortunately, these donor T cells can also target healthy tissues of the recipient, causing graft-versus-host disease (GVHD) which has remained an important cause of treatment failure in allo-HCT recipients.
Janus kinases (JAKs) are intracellular tyrosine kinase proteins that mediate signal transduction of several cytokine receptors present at the surface of immune cells such as T cells, B cells, macrophages as well as dendritic cells [3]. Three JAKs are involved in the signaling of cytokines associated with GVHD pathogenesis [4]. Briefly, JAK1 is involved in the signal transduction of IL-2, IL-6, IL-7, IL-21 INFα, and IFNγ, JAK2 in the signal transduction of IFNγ, IL-6, IL-12, IL-23, and GM-CSF as well as EPO and TPO, while JAK3 is involved in the signal transduction of IL-2, IL-4, IL-7, and IL-21 [3].
Confirming data observed in mouse-to-mouse models of GVHD as well as early clinical observations, the JAK1/2 inhibitor ruxolutinib has recently been shown to induce higher response rates compared to other regimens in patients with steroid-refractory acute GVHD in a phase III trial [5]. However, a significant incidence of thrombocytopenia (and of anemia) was observed, probably due to JAK2 inhibition. These considerations support further studies assessing the efficacy of selective JAK1 inhibitors in GVHD.
Itacitinib is a specific JAK1 inhibitor currently assessed as treatment of acute and chronic GVHD in humans. Importantly, itacitinib has recently been shown to mitigate GVHD in a mouse-to-mouse model of GVHD [6]. Further, in that model, the drug reduced T-cell infiltration in GVHD target organs as well as serum levels of IL-1β, TNFα, and IFNγ [6]. While results of a phase I trial have been promising [7], itacitinib failed to improve the GVHD objective response rate at day 28 (primary endpoint of the GRAVITAS-301 study, 74% versus 66%, P = 0.08) when added or not to steroids as first line treatment of acute GVHD [8]. However, post-hoc analysis showed higher day 28 complete remission (CR) rates when stratified for acute GVHD risk status (OR = 1.66, P = 0.008) [8]. This stresses the need for further studies assessing the impact of itacitinib on human immune cells in experimental in vivo humanized GVHD models.
Here we assessed the impact of itacitinib on xenogeneic GVHD (xGVHD) induced by infusion of human peripheral blood mononuclear cells (hPBMC) in NOD.Cg-Prkdcscid Il2rgtm¹Wjˡ/SzJ (NSG) mice [9]. Besides using human cells to induce and potentially control xGVHD, this model has the advantage of taking into consideration genetic diversity (since immune cells from different donors can be used). Indeed, prior studies have observed that the severity of xGVHD in that model was partly donor-dependent with some donors inducing more severe xGVHD than others [10, 11]. In addition, we also assessed the impact of itacitinib on graft-versus-leukemia effects in a newly developed humanized mouse model combining xeno and allo-reactivity [12, 13].
Methods
Induction and assessment of xGVHD in NSG mice
NSG (The Jackson Laboratory, Bar Harbor, ME), aged 7–16 weeks, were infused with 2 × 107 hPBMCs to induce xGVHD on day 0. HPBMC were isolated by Ficoll-Paque density centrifugation (GE Healthcare, Freiburg, Germany) of buffy coats obtained in healthy blood donors (Belgian Red Cross). GVHD severity was assessed by a scoring system that incorporates 4 clinical parameters: weight loss, posture (hunching), mobility, and anemia as previously reported [10]. Each parameter received a score of 0 (minimum) to 2 (maximum). Mice were assessed for GVHD score thrice weekly and monitored daily during the experiments. Mice reaching a GVHD score of 6/8 were sacrificed in agreement with the request of our ethical committee. Final scores for dead animals reaching the ethical limit score were “6” for the remaining time points. Final weights for dead animals reaching the ethical limit score were kept in the data set for the remaining time points (last value carried forward). No blinding was done regarding treatment received but GVHD scorings were performed by a technician (SD) who is a charge of mice welfare and not interested in the study outcome. All animal experiments used in this study were reviewed and approved by the Institutional Animal Care and Use Ethics Committee of the University of Liège, Belgium (protocol # 2115). The “Guide for the Care and Use of Laboratory Animals,” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, was followed carefully.
Itacitinib administration
Itacitnib (kindly provided by Incyte Biosciences, Morges, Switzerland) was given orally by force-feeding at the dose of 3 mg (≈120 mg/kg) in 200 μL of a methylcellulose solution twice a day from day 3 to day 28 after transplantation. Control mice were given 200 μL of methylcellulose solution only.
Flow cytometry
Peripheral blood (PB) was drawn on days 14, 21, and 28 during survival experiments. PB was depleted of erythrocytes using RBC lysis buffer (eBioscience, San Diego, CA) according to the manufacturer’s instructions. The following antibodies specific for human antigens were used: anti-mouse CD45-PECY5 (30-F11, Invitrogen), anti-human CD8-PECy7 (HIT8a, Biolegend), anti-human CD62L-APCCY7 (DREG56, Invitrogen), anti-human CD4-eFluor450 (RPA-T4, invitrogen), anti-human CD45-BV510 (HI30, BD), anti-human HLA-DR-BV605 (L243, Biolegend), anti-human CD27-BV650 (L128, BD), anti-human CD45RA-BV786 (HI100, Biolegend), anti-human CD25-BUV395 (2A3, BD), anti-human CD4-BV786 (SK3, BD), anti-human CD8-FITC (HIT8a, eBioscience), anti-human CD45RA-AF700 (HI100, BD), anti-human CD27-PE (BD), biotin anti-human CD194 (BD), streptavidin-APC (Biolegend), anti-human Granzyme B-FITC (BD), anti-human BCl2-PE (BD), anti-human Ki67-PerCP-Cy5.5 (B56, BD), anti-human FOXP3-PECF594 (259D/C7, BD), anti-human IL10-BV421 (JES3-9D7, Sony), anti-human IFNg-PECY7 (4SB3, Invitrogen), anti-human TNFa-APCCY7 (MAb11, Sony), anti-human IL17-APC (eBio64DEC17, eBioscience), anti-human IL2-BV650 (5344.111, BD). Cells (1.5–2 × 106 cells/sample), resuspended in 50 µl of PBS + 3% FBS, were incubated with surface antibodies for 20 min at 4 °C in the dark and washed twice with PBS + 3% FBS. Intracellular staining for Granzyme B, BCl2, FOXP3, Ki67, and cytokines was performed by using the FOXP3 Staining Buffer Set (eBioscience). For intracellular IL-2 staining, samples were stimulated for 4 h in RPMI supplemented with 10% FBS, in presence of PMA/ionomycin, brefeldin A and monensin (Cell Stimulation Cocktail + Protein Transport Inhibitors, eBioscience). Total cell counts of human CD45+ cells in blood were calculated based on the absolute number of white blood cells (counted by using a Sysmex XS-800i cell counter) and on human cell chimerism (frequency of human CD45+ cells among the total white blood cell population (%humanCD45+ + %mouseCD45+)). Data were acquired on a FACS FORTESSA flow cytometer (Becton Dickinson) and analyzed with the Flowjo software 10.0 (Tree Star Inc., Ashland, OR). In gating strategies, regulatory T cells (Treg) were defined as CD4+CD25+FOXP3+ while remaining CD4+ T cells were termed conventional T cells (Tconv). Naive T cells were defined as CD45RA+CD27+, effector T cells (TE) as CD45RA−CD27−, effector memory T cells (TEM) as CD45RA−CD27+CD62L−, and central memory T cells (TCM) as CD45RA−CD27+CD62L+.
Graft-versus-leukemia effects and bioluminescence imaging
For graft-versus-leukemia effect assessment, NSG-HLA-A2/HHD mice (The Jackson Laboratory, Bar Harbor, ME) were co-injected with 3 millions luciferase-expressing THP-1 cells with or without 20 millions hPBMC from a non-HLA-A0201 donor as previously reported [13]. Itacitinib was given as for the GVHD experiments. Bioluminescence analyses were made as previously reported [13]. In brief, mice were injected s.c. with 3 mg of D-luciferin (Promega, Madison, WI, USA) in PBS and imaged 12 min later. Tumor growth was evaluated by measuring the bioluminescence of THP-1 cells transfected to express the luciferase reporter gene by using the bioluminescent IVIS imaging system (Xenogen-Caliper, Hopkinton, MA). Mice were anesthetized using isoflurane (2.5% vaporized in O2). For analysis, total photon flux (photons per second) was measured from a fixed region of interest over the entire abdomen using Living Image 4.5.2.
Serum cytokine levels
IL-1β, IL-2, IL-10, IL-12 p70, IFNγ, and TNFα serum concentrations were quantified using Luminex Performance Human High Sensitivity Cytokine Magnetic Panel A assay (6-Plex, FCSTM09-06, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Analysis of each sample was performed in duplicate. Data were collected and analyzed using a Luminex MAGPIX analyzer (APX1042, ThermoFisher Scientific). Data were collected and analyzed using a Luminex MAGPIX analyzer (APX1042, ThermoFisher Scientific).
Statistical analyses
The Mann–Whitney test was used to compare flow-cytometry data between 2 different groups. Comparisons between GVHD score curves were made using the 2-way ANOVA test. Survival curves were modeled using the Kaplan-Meier methods. Comparisons between groups were made with the log-rank test. Prior studies have shown that in the NSG mouse model of GVHD cohorts of 8–10 animal per groups are optimal to detect a significant impact of the treatment [13]. Since they can be a donor variability in the NSG xGVHD model, we elected to perform three independent experiments with three different PBMC donors. Mice were allocated to treatment group before the start of the experiment (transplantation) but no randomization was performed. Thus, a multivariate Cox model was also performed to account for possible confounding factors (mouse weight, mouse age, mouse sex, PBMC donor). Parameters included in the Cox model included itacitinib or not, hPBMC donor (one donor by cohort), mouse weight, age, and sex. Data from all animals were taken into consideration in survival and GVHD score analyses. Data from all animals alive at that time point were taken into consideration for flow cytometry analyses. P values < 0.05 were considered as statistically significant and all P values were 2-sided. Statistical analyses were carried out with Graphpad Prism 8.0 (Graphpad Software, San Diego, CA, USA) and with SAS.
Results
Itacitinib mitigates xenogeneic GVHD
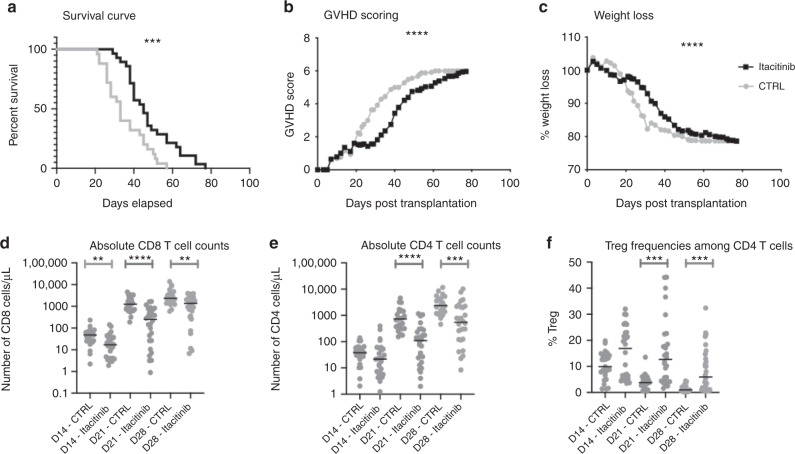
The impact of itacitinib on xGVHD was assessed in 3 independent experiments using hPBMC from 3 different donors. Combining data from the 3 experiments, itacitinib-treated mice had significantly longer survival than control mice (median 45 versus 33 days; P < 0.001) (Fig. 1). This was confirmed in a multivariate Cox model adjusted for PBMC donor, mouse weight, age and sex (HR = 0.33, 95% CI 0.18-0.61, P = 0.0004) (supplemental table 1). Similarly, GVHD scores and weight losses were lower in itacitinib-treated mice than in controls. Looking at the 3 cohorts separately, in the first experiment, itacitinib did not attenuate xGVHD as shown by comparable GVHD score, weight losses and survival in the 2 groups of mice (Supplementary Fig. 1). In contrast, in the second and third cohorts, itacitinib attenuated xGVHD as illustrated by lower xGVHD scores, slower weight losses and improved survival in itacitinib-treated mice (Supplementary Fig. 1).
Fig. 1. Impact of itacitinib on xGVHD.
Survival (a), GVHD scoring (b) and weight loss (c) in the three cohorts (with three different PBMC donors) combined. Gray line with circles shows control mice (n = 25) while black line with squares shows itacitinib mice (n = 28). Absolute CD8 T-cell counts (d), absolute CD4 T-cell counts (e) and Treg frequencies among CD4 T-cells (f) in the three cohorts combined. NSG mice were injected with 2 × 107 human PBMCs. Mice were then treated twice daily with Itacitinib (≈120 mg/kg) or a methylcellulose solution from day 3 to day 28 after transplantation. a–c Survival, GVHD scoring and weight loss curves of three groups of mice transplanted with three different healthy donors (n = 15 for donor 1, n = 18 for donor 2 and n = 20 for donor 3, including 25 in the control and 28 in the Itacitinib group). d Comparison of absolute CD8+ T-cell counts for each mouse treated or not with Itacitinib. e Comparison of absolute CD4+ T-cell counts for mice treated or not with Itacitinib. f Comparison of Treg frequencies among CD4+ T-cells for mice treated or not with Itacitinib. Data show median values (*P < 0.05, **P < 0.005, ***P < 0.0005).
Itacitinib decreases human T-cell engraftment
Absolute human CD45 counts were significantly lower in itacitinib-treated mice than in controls at days 14, 21, and 28 after transplantation (Supplemental Fig. 2). Accordingly, absolute numbers of human CD8+ T cells in the peripheral blood of transplanted NSG mice were significantly lower in itacitinib-treated than in control mice on days 14, 21, and 28 after transplantation (Fig. 1d). Similarly, itacitinib-treated mice had lower absolute numbers of CD4+ T cells on days 21 and 28 after transplantation (Fig. 1e). CD4/CD8 ratios were mostly not affected by itacitinib (data not shown). Over time, the proportion of naive T cells decreased in both groups in favor of an effector memory phenotype. This was observed both in CD4+ conventional T cells and in CD8+ T cells (Supplementary Fig. 3).
Itacitinib increases Treg frequencies
Given that prior studies demonstrated that Treg play an important role for xGVHD prevention in the NSG xGVHD model [10], we assessed the impact of itacitinib on Treg frequencies. We observed significantly higher Treg frequencies (among CD4+ T cells) in itacitinib-treated than in control mice on days 21 and 28 after transplantation (and a similar trend for day 14) (Fig. 1f and Supplementary Fig. 4). However, absolute Treg numbers were significantly lower in itacitinib-treated mice than in controls.
Given that Treg depend on IL-2 for their homeostasis [14], we assessed the production of IL-2 by CD4+ T cells from mice from the cohort 3 on day 28 after PBMC infusion (Supplementary Fig. 5A). After in vitro stimulation by PMA/ionomycin we observed a higher proportion of CD4+ T cells from itacitinib-treated mice (median of 13.7%) than from control mice (median of 5.8%, P = 0.0001) producing IL-2 in intracellular staining. Accordingly, Treg from itacitinib-treated mice had higher expression of CD25 (MFI of 2283 versus 1777, P = 0.0330) and FoxP3 (MFI of 534 versus 396, P = 0.0005) than those from control mice, suggesting higher IL-2 signaling (Supplementary Fig. 5B–C). Further, assessment of serum cytokine levels on day 28 in 5 itacitinib-treated and 5 control mice showed a suggestion for lower INFγ (609 versus 1923 pg/mL, P = 0.09) and TNFα (37 versus 78 pg/mL, P = 0.09) in itacitinib-treated mice, IL-2 serum concentrations (3.2 versus 2.2 pg/mL, P = 0.31) were comparable despite of lower absolute T cell numbers in itacitinib-treated mice (Supplementary Fig. 5D–F).
Impact of itacitinib on peripheral blood T-cell subsets
We further assessed the impact of itacitinib on peripheral blood T-cell subsets on days 21 and 28 after transplantation (in mice from cohort 3) using t-SNE analyses in human CD4+ and CD8+ T-cell subpopulations. On day 21, we observed higher frequencies of CD4+CD45RA+BCL2+ cells (P = 0.0001), as well as Ki67+ Treg (P = 0.0001) and Ki67− Treg (P = 0.002), in itacitinib-treated mice (Supplementary Fig. 6). Regarding CD8+ T-cell subsets, the main changes included a higher proportion of naive CD8+ T cells (CD45RA+CD27+CD62L+; P = 0.002), a higher proportion of CD8+CD45RA+KI67+GranzB+ cells (P = 0.002) and of CD8+KI67+CD27+GranzB− cells (P = 0.0001) (Supplementary Fig. 7).
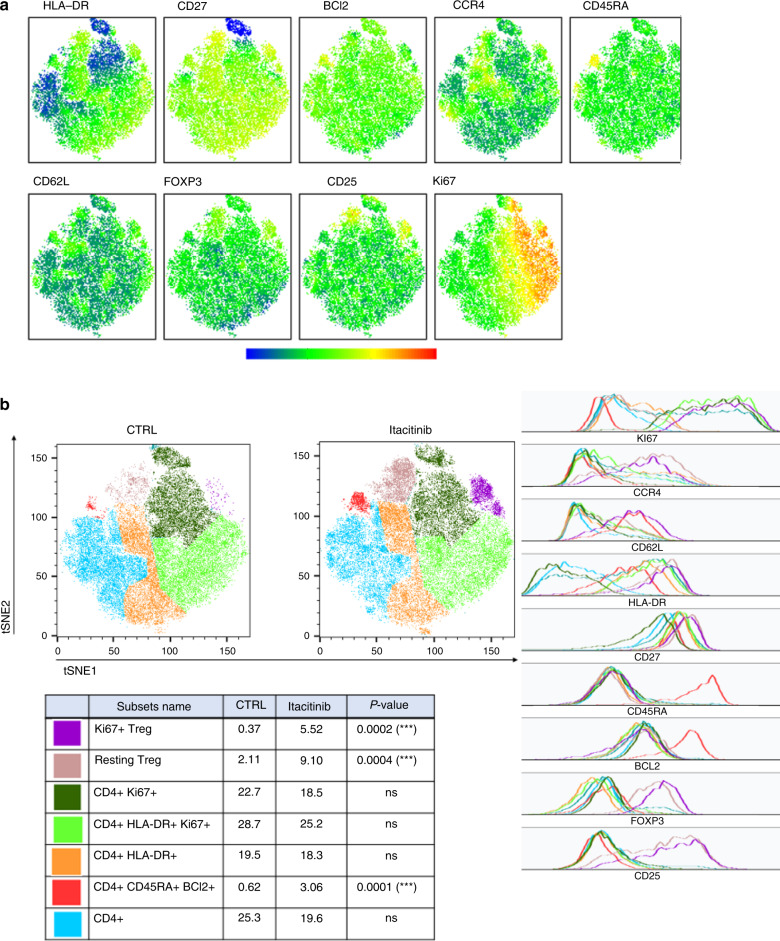
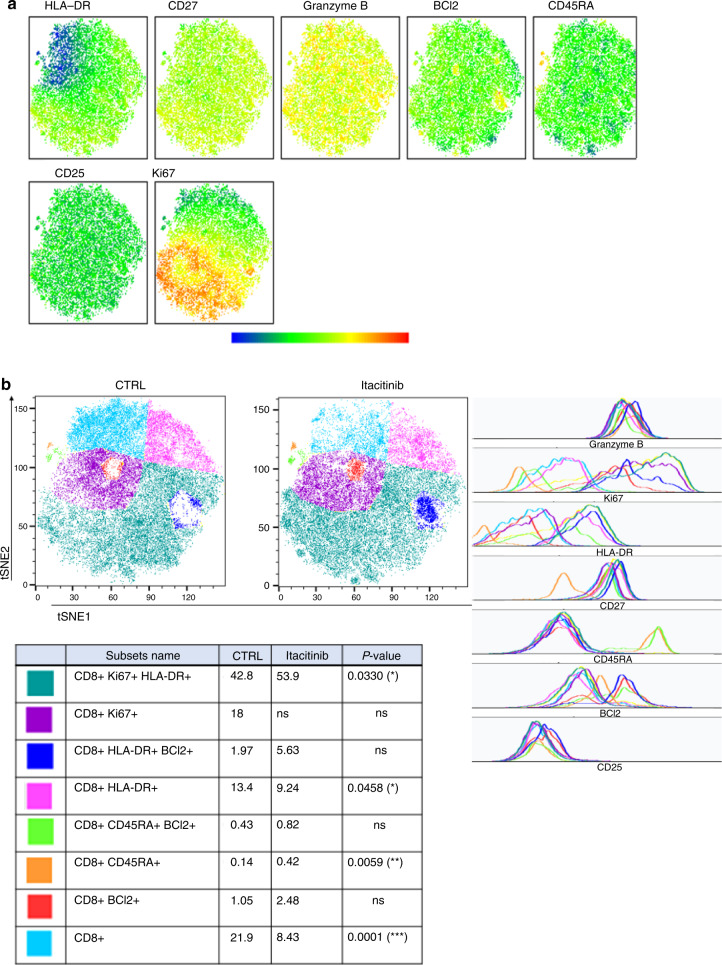
On day 28, regarding CD4+ T-cell subsets, we observed higher frequencies of CD4+CD45RA+BCL2+ cells (P = 0.0001), as well as Ki67+ Treg (P = 0.0002) and Ki67− Treg (P = 0.0004) in itacitinib-treated mice (Fig. 2). Higher frequencies of Ki67+ Treg (P = 0.0002) and Ki67- Treg (P = 0.008) were also observed in mice from the second cohort of itacitinib-treated mice (Supplementary Fig. 8). Regarding CD8+ T-cell subsets, the main changes included a higher proportion of CD8+KI67+HLA-DR+ cells (P = 0.03) (Fig. 3). This was also observed in mice from the second cohort (Supplementary Fig. 9).
Fig. 2. T-SNE of CD4+ T cells in peripheral blood from mice in cohort 3 on day 28 after transplantation.
T-SNE were created on 20,135 CD4+ T cells and included HLA-DR, CD27, BCl2, CCR4, CD45RA, CD62L, FOXP3, CD25, and Ki67 markers. a Visualization of marker expression (MFI) among CD4+ T cells. b Seven populations were determined based on different marker expressions (geometric mean), i.e., Treg Ki67+ (purple), resting Treg (beige), CD4+ Ki67+ (dark green), CD4+HLA-DR+Ki67+ (light green), CD4+HLA-DR+ (orange), CD4+CD45RA+BCl2+ (red), and other CD4+ (blue) in the control and the itacitinib groups. Ki67+ Treg (purple, P = 0.0002), resting Treg (beige, P = 0.0004) and CD4+CD45RA+BCl2+ T cells (red, P = 0.0001) were increased in the treated group at day 28 in the cohort 3. Histograms show marker expression in subpopulations.
Fig. 3. T-SNE of CD8+ T cells in peripheral blood from mice in cohort 3 on day 28 after transplantation.
T-SNE were created on 21,000 CD8+ T cells and included HLA-DR, CD27, Granzyme B, BCl2, CD45RA, CD25 and Ki67 markers. a Visualization of marker expression (MFI) among CD8 T cells. b Eight populations were determined based on different marker expressions (geometric mean), i.e. CD8+Ki67+HLA-DR+ (dark green), CD8+Ki67+ (purple), CD8+HLA-DR+BCl2+ (dark blue), CD8+HLA-DR+ (pink), CD8+CD45RA+BCl2+ (light green), CD8+CD45RA+ (orange), CD8+BCl2+ (red), and other CD8+ (light blue) in the control and the treated groups at day 28 in cohort 3. CD8+Ki67+HLA-DR+ (dark green, P = 0.033) and CD8+CD45RA+ (orange, P = 0.0059) were increased in the treated group at day 28 in the cohort 3. CD8+HLA-DR+ (pink, P = 0.0458) and other CD8+ T cells (light blue, P = 0.0001) were decreased in the treated group at day 28 in cohort 3. Histograms show marker expression of subpopulations.
Impact of itacitinib on graft-versus-leukemia effects
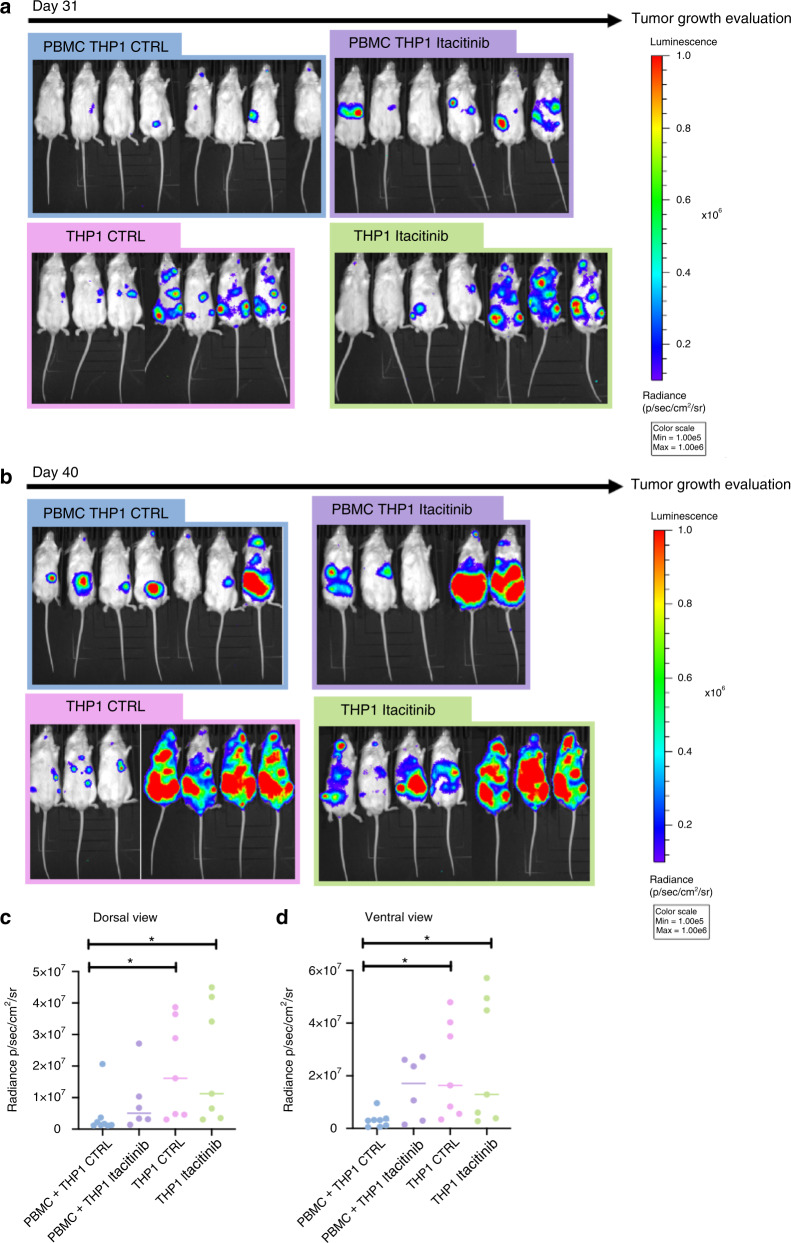
We finally assessed the impact of itacitinib on graft-versus-leukemia effects. We observed that itacitinib failed to improve survival in comparison to control mice. On day 31 after PBMC infusion, mice given hPBMC + THP-1 cells had significantly lower tumor burden than mice not given hPBMC (and given or not itacitinib) (Fig. 4). This was not observed in mouse given hPBMC + THP-1 cells + itacitinib suggesting that the drug might somewhat decrease graft-versus-leukemia effects.
Fig. 4. Impact of itacitinib on GvL effects.
NSG-A2 mice were infused intravenously with 2 × 107 human PBMCs and 3 × 106 THP-1-luc cells. Mice were then treated twice daily with Itacitinib (≈120 mg/kg) or a methylcellulose solution from day 3 to day 28 after transplantation. a Bioluminescence was monitored. Images acquired at day 31 are shown with the Y axis indicating radiance (photon/sec/cm2/sr) measured from the dorsal view and the ventral view with a region of interest drawn over the entire body of each mouse and compared in subfigures (c, d). b Images acquired at day 40. c Comparison of bioluminescence at day 31 in dorsal view. D Comparison of bioluminescence at day 31 in ventral view. Data show median values (*P < 0.05, **P < 0.005, ***P < 0.0005).
Discussion
The pathogenesis of xGVHD induced by hPBMC infusion in NSG mice has been partly elucidated. Studies have demonstrated that the disease is caused by a fraction of human T cells [12], reacting against mouse MHC [9, 15–17]. The second signal (also required for xGVHD) is provided by interaction between mouse B7.1 and B7.2 and CD28 expressed on human T cells [18]. The third signal for CD8+ T-cell activation is provided by cytokines secreted by activated human CD4+ T cells [15] (i.e. mainly IL-2, INFγ, and TNFα but also IL-17 [19–22]). While it has been recently established that JAK-1/2 inhibitors are effective as treatment for steroid-refractory GVHD [23], it remains to be established whether similar results can be achieved with specific JAK1 inhibitors (which will likely induce less cytopenia since they do not target EPO and TPO signaling transduction). Here, we used the NSG humanized mouse model to assess the impact of itacitinib on xGVHD. We believed that this is highly relevant given the important differences between mouse and human T cell biology (particularly for Treg). Several observations were made.
The first and most important observation was that itacitinib attenuated xGVHD. This observation is in line with recent observations made in a mouse-to-mouse model of GVHD [6]. The ability of JAK1 inhibitor to attenuate xGVHD should be seen in line with prior observations showing that a selective JAK-2 inhibitor failed to prevent xGVHD when given alone (although it increases the anti-xGVHD potential of an aurora kinase A inhibitor) in the NSG mouse model [24]. However, our data indicate that the benefit of itacitinib might be donor-dependent, since it was not observed with 1 of the 3 donors that provided hPBMC for xGVHD induction. Prior observations have demonstrated that the severity of xGVHD in the NSG model is donor-dependent, possibly because the immune reaction is launched by human T cells recognizing mouse MHC by antigen mimicking (which might differ from one donor to another) [12].
A second observation of our study was that itacitinib decreased the number of CD4+ and CD8+ T cells after hPBMC infusion. This is in concordance to what has been recently observed in a mouse-to-mouse model of GVHD [6].
A third observation of the study was that itacitinib increased Treg frequency. This is in line with what has been observed with the JAK-1-2 inhibitor ruxolutinib in mouse-to-mouse models of GVHD [23]. This observation might be of importance given prior studies showing the important role of Treg for xGVHD prevention in the NSG mouse model [10, 25, 26]. However, it should be noted that absolute Treg numbers were actually lower in itacitinib-treated than in control mice, stressing that itacitinib did not per se promote Treg but rather impacted Tconv more than Treg. Since Treg depend on IL-2 for their homeostasis [14, 27] as well as for their ability to prevent GVHD in mouse-to-mouse models of GVHD [28, 29], we compared IL-2 production by CD4+ T cells from itacitinib-treated versus control mice after in vitro stimulation by PMA/ionomycin. Indeed, human T cells are the only source of IL-2 in this humanized mouse model of GVHD and low-dose IL-2 administration in NSG mice infused by human PBMC results in higher Treg proportion [12, 22, 19]. We observed a higher production of IL-2 by CD4+ T cells from itacitinib-treated than from control mice. Further, we observed higher CD25 and FoxP3 expression by Treg from itacitinib than from control mice suggesting that IL-2 signal transduction by JAK3 is predominant for Treg homeostasis in the humanized mouse model.
Finally our data suggest that itacitinib might decrease graft-versus-leukemia effects. This was observed in a humanized mouse model of GvL effects in which one strong antigen (the HLA-A0201) is shared between the GVHD and GvL effects despite the GvL reaction is allogeneic and not xenogeneic [12]. This observation might not be surprising since several clinical studies have observed a strong link between occurrence of GVHD and graft-versus-leukemia effects [30–35]. This observation is however in contrast to what has been observed with other molecules such as azacytidine (which was able to reduce GVHD without affecting graft-versus-leukemia effects in this model [13]), possibly because of the direct impact of azacytidine on THP-1 cells.
In summary, our data show that itacitinib mitigated xGVHD induced by infusion of hPBMC, possibly through decreasing absolute human T-cell numbers and perhaps increasing Treg frequencies.
Supplementary information
Acknowledgements
We are grateful to Sandra Ormenese, Raafat Stephan, and Céline Vanwinge from the Imaging and Flow Cytometry Platform of the GIGA for help with flow cytometry analyses. We are also grateful to INCYTE Biosciences who kindly gave us itacitinib.
Author contributions
FB designed the study; JC, CR, and SD performed the experiments; LC, BV, and CD helped in the experiments; JC, LS and CR analyzed the data; JC, CR, GE, and FB interpreted the data; SS, JCA and YB helped in data interpretation; FB and JC wrote the article; all authors reviewed and edited the manuscript.
Funding
This study was supported by funds from: the National Fund for Scientific Research (FNRS) (grant numbers T.0069.15 and T.0016.20), The Belgian Fondation contre le cancer (grant # FBC # FAF-C/2016/889), and the Leon Fredericq fund and Anti-Cancer Center at the University of Liège. GE is a Télévie Research Assistant, and FB is a senior research associate of the National Fund for Scientific Research (FNRS) Belgium.
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files. Raw files used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
Frédéric Baron has received travel grants from Celgene, Abbvie, Novartis, INCYTE Biosciences, and Sanofi as well as honoraria from Merck and Abbvie. The remaining authors declare that they have no relevant conflict of interest in regard to this study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Justine Courtois, Caroline Ritacco
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-021-01363-1.
References
- 1.Baron F, Efficace F, Cannella L, Muus P, Trisolini S, Halkes CJM, et al. Impact of the type of anthracycline and of stem cell transplantation in younger patients with acute myeloid leukemia: Long-term follow up of a phase III study. Am J Hematol. 2020;95:749–58. doi: 10.1002/ajh.25795. [DOI] [PubMed] [Google Scholar]
- 2.Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 3.Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder MA, Choi J, Staser K, DiPersio JF. The role of janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transpl. 2018;24:1125–34. doi: 10.1016/j.bbmt.2017.12.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl J Med. 2020;382:1800–10. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 6.Covington M, He X, Scuron M, Li J, Collins R, Juvekar A, et al. Preclinical characterization of itacitinib (INCB039110), a novel selective inhibitor of JAK1, for the treatment of inflammatory diseases. Eur J Pharm. 2020;885:173505. doi: 10.1016/j.ejphar.2020.173505. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder MA, Khoury HJ, Jagasia M, Ali H, Schiller GJ, Staser K, et al. A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv. 2020;4:1656–69. doi: 10.1182/bloodadvances.2019001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiser R, Socié G, Schroeder MA, Abhyankar S, Pinho Vaz C, Kwon M et al. GRAVITAS-301: A Randomized, double-blind phase 3 study of itacitinib or placebo in combination with corticosteroids for initial treatment of patients with acute graft-versus-host disease. EHA Library 2020;: S256. [DOI] [PubMed]
- 9.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–18. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon M, Lechanteur C, Lucas S, Somja J, Seidel L, Belle L, et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014;54:353–63. doi: 10.1111/trf.12666. [DOI] [PubMed] [Google Scholar]
- 11.Gregoire C, Ritacco C, Hannon M, Seidel L, Delens L, Belle L, et al. Comparison of mesenchymal stromal cells from different origins for the treatment of graft-vs.-host-disease in a humanized mouse model. Front Immunol. 2019;10:619. doi: 10.3389/fimmu.2019.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehx G, Somja J, Warnatz H-J, Ritacco C, Hannon M, Delens L, et al. Xenogeneic graft-versus-host disease in humanized NSG and NSG-HLA-A2/HHD mice. Front Immunol. 2018;9:1943. doi: 10.3389/fimmu.2018.01943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehx G, Fransolet G, de Leval L, D’Hondt S, Lucas S, Hannon M, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2017;6:e1314425. doi: 10.1080/2162402X.2017.1314425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–65. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki Y, Sato K, Hayakawa H, Takayama N, Nakano H, Ito R, et al. Comprehensive analysis of the activation and proliferation kinetics and effector functions of human lymphocytes, and antigen presentation capacity of antigen-presenting cells in xenogeneic graft-versus-host disease. Biol Blood Marrow Transpl. 2018;24:1563–74. doi: 10.1016/j.bbmt.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Brehm MA, Kenney LL, Wiles MV, Low BE, Tisch RM, Burzenski L, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019;33:3137–51. doi: 10.1096/fj.201800636R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coman T, Rossignol J, D’Aveni M, Fabiani B, Dussiot M, Rignault R, et al. Human CD4- invariant NKT lymphocytes regulate graft versus host disease. Oncoimmunology. 2018;7:e1470735. doi: 10.1080/2162402X.2018.1470735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Søndergaard H, Kvist PH, Haase C. Human T cells depend on functional calcineurin, tumour necrosis factor-α and CD80/CD86 for expansion and activation in mice. Clin Exp Immunol. 2013;172:300–10. doi: 10.1111/cei.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérol L, Martin GH, Maury S, Cohen JL, Piaggio E. Potential limitations of IL-2 administration for the treatment of experimental acute graft-versus-host disease. Immunol Lett. 2014;162:173–84. doi: 10.1016/j.imlet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Abraham S, Guo H, Choi J-G, Ye C, Thomas MB, Ortega N, et al. Combination of IL-10 and IL-2 induces oligoclonal human CD4 T cell expansion during xenogeneic and allogeneic GVHD in humanized mice. Heliyon. 2017;3:e00276. doi: 10.1016/j.heliyon.2017.e00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delens L, Ehx G, Somja J, Vrancken L, Belle L, Seidel L, et al. In Vitro Th17-Polarized Human CD4(+) T Cells Exacerbate Xenogeneic Graft-versus-Host Disease. Biol Blood Marrow Transpl. 2019;25:204–15. doi: 10.1016/j.bbmt.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Ehx G, Ritacco C, Hannon M, Dubois S, Delens L, Willems E et al. Comprehensive analysis of the immunomodulatory effects of rapamycin on human T cells in graft-versus-host disease prophylaxis. Am J Transplant. 2021. 10.1111/ajt.16505. [DOI] [PubMed]
- 23.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 24.Betts BC, Veerapathran A, Pidala J, Yang H, Horna P, Walton K et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med 2017; 9. 10.1126/scitranslmed.aai8269. [DOI] [PMC free article] [PubMed]
- 25.Wang H, Song H, Pham AV, Cooper LJ, Schulze JJ, Olek S, et al. Human LAP(+)GARP(+)FOXP3(+) regulatory T cells attenuate xenogeneic graft versus host disease. Theranostics. 2019;9:2315–24. doi: 10.7150/thno.30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuende J, Liénart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, et al. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med. 2015;7:284ra56. doi: 10.1126/scitranslmed.aaa1983. [DOI] [PubMed] [Google Scholar]
- 27.Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–65. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 28.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–9. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7:458–62. doi: 10.4161/cc.7.4.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8. doi: 10.1038/leu.2012.135. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. doi: 10.1182/blood.V75.3.555.555. [DOI] [PubMed] [Google Scholar]
- 32.Ringdén O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–8. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron F, Labopin M, Savani BN, Beohou E, Niederwieser D, Eder M, et al. Graft-versus-host disease and graft-versus-leukaemia effects in secondary acute myeloid leukaemia: a retrospective, multicentre registry analysis from the Acute Leukaemia Working Party of the EBMT. Br J Haematol. 2020;188:428–37. doi: 10.1111/bjh.16185. [DOI] [PubMed] [Google Scholar]
- 34.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transpl. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boucault L, Lopez Robles M-D, Thiolat A, Bézie S, Schmueck-Henneresse M, Braudeau C, et al. Transient antibody targeting of CD45RC inhibits the development of graft-versus-host disease. Blood Adv. 2020;4:2501–15. doi: 10.1182/bloodadvances.2020001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. Raw files used and/or analyzed during the current study are available from the corresponding author on reasonable request.