Abstract
Objective:
This study was undertaken to characterize trajectories of antiseizure medication (ASM) adherence in adults with newly treated epilepsy and to determine predictors of trajectories.
Methods:
This was a retrospective cohort study using Medicare. We included beneficiaries with newly treated epilepsy (one or more ASM and none in the preceding 2 years, plus International Classification of Diseases codes) in 2010–2013. We calculated the proportion of days covered (proportion of total days with any ASM pill supply) for 8 quarters or until death. Group-based trajectory models characterized and determined predictors of trajectories.
Results:
We included 24 923 beneficiaries. Models identified four groups: early adherent (60%), early nonadherent (18%), late adherent (11%), and late nonadherent (11%). Numerous predictors were associated with being in the early nonadherent versus early adherent group: non-White race (e.g., Black, odds ratio [OR] = 1.7, 95% confidence interval [CI] = 1.5–1.8), region (e.g., South vs. Northeast: OR = 1.2, 95% CI = 1.1–1.4), and once daily initial medication (OR = 1.1, 95% CI = 1.0–1.3). Predictors associated with decreased odds of being in the early nonadherent group included older age (OR = .9 per decade, 95% CI = .9–.9), female sex (OR = .9, 95% CI = .8–1.0), full Medicaid eligibility (OR = .6, 95% CI = .4–.8), neurologist visit (OR = .6, 95% CI = .6–.7), and initial older generation ASM (OR = .6, 95% CI = .6–.7).
Significance:
We identified four ASM adherence trajectories in individuals with newly treated epilepsy. Whereas risk factors for early nonadherence such as race or geographic region are nonmodifiable, our work highlighted a modifiable risk factor for early nonadherence: lacking a neurologist. These data may guide future interventions aimed at improving ASM adherence, in terms of both timing and target populations.
Keywords: adherence, antiseizure medications, epilepsy, group-based trajectory modeling
1 |. INTRODUCTION
Up to 50% of people with epilepsy are nonadherent to antiseizure medications (ASMs).1 Because nonadherence to ASMs is associated with adverse consequences (i.e., mortality,2 cost,3–5 acute care visits3), suboptimal adherence represents a critical opportunity to improve outcomes.
Prior work has explored ASM nonadherence as a binary variable by a single point in time.1,5–10 However, adherence is a continuous (e.g., partial adherence) and dynamic process whereby a patient may begin as adherent and then later become nonadherent, or vice versa. Being able to predict an individual’s future adherence trajectory from baseline data would allow more personalized self-management interventions targeted to individuals at their highest risk points in time. Prior work demonstrated five adherence patterns in children with newly diagnosed epilepsy11 (e.g., severe early nonadherence, severe delayed nonadherence), which were correlated with whether seizures improved or worsened over time.12 However, it is difficult to generalize such work in children to adult populations directly, given potentially vastly different barriers and facilitators of adherence such as family or social structures assisting with medications, comorbidities, psychosocial consequences of seizures, and/or beliefs toward medications.
In this study, we used Medicare Part D data to (1) characterize trajectories of ASM adherence in adults with newly treated epilepsy and (2) determine predictors of such adherence trajectories.
2 |. MATERIALS AND METHODS
2.1 |. Study design and dataset
We performed a retrospective cohort study of beneficiaries in fee-for-service Medicare.
2.2 |. Procedures involving human subjects
This study was deemed exempt by the University of Michigan Institutional Review Board.
2.3 |. Patient selection
Similar to prior work,13 we included patients with newly treated epilepsy defined as filling a prescription for one or more ASM, plus International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) criteria for inpatient, outpatient, or emergency evaluation and management, or the following consultation codes: (1) one or more codes for epilepsy (ICD-9-CM 345.xx) or (2) two or more codes for convulsions (ICD-9-CM 780.3x) at least 30 days apart, in 2010–2013. Recent work in Medicare14 demonstrated good performance of combining ICD codes plus ASM to identify patients with epilepsy (area under the curve = .93, sensitivity = 88%, and specificity = 98%). We included all individuals qualifying for Medicare (age > 65 years, and/or disability, and/or end-stage renal disease). To identify newly treated epilepsy, we required that the first ASM in this period occurred after the first ICD code, and that beneficiaries had at least 2 years of continuous Part D eligibility prior to their first ASM fill during 2010–2013 with no ASM fill during that 2-year window. To follow adherence over time, we included beneficiaries who also were continuously enrolled in Part D for 2 years after their first ASM fill or until death. To calculate comorbidities and acute care utilization, we also restricted analyses to beneficiaries who were continuously enrolled in Parts A/B (and not C, given their claims would not appear in our dataset) during the year they filled their first ASM.
2.4 |. Variables
Adherence was quantified using the proportion of days covered (PDC) for each beneficiary for each of 8 calendar quarters starting with the first ASM fill, or until death if sooner. The PDC represents the proportion of days with medication supply during an observation period and ranges from 0% to 100%. It is a widely accepted measure for claims-based analysis of medication adherence.3,5–7,15–17 The numerator for each quarter was the number of days’ supply with at least one ASM. If a patient filled more than one ASM at a time during the observation period (polytherapy), the numerator remained the number of days in a quarter with any ASM supply. The denominator for each quarter was either the entire quarter, the days from first fill until the end of the quarter (relevant to the first quarter only), or days until death (relevant to the last quarter of follow-up if death occurred prior to 8 quarters).
We described baseline variables including age, sex, race, Medicaid dual eligibility, cost-sharing by Medicare, reason for entitlement, region of the United States according to state, rural ZIP code,18 whether there was at least one neurology visit in the year of the first ASM fill, whether the first ASM fill included an older generation medication (carbamazepine, phenobarbital, phenytoin, primidone, valproate), whether the first ASM fill was for maximally once-daily dosing versus more than once daily dosing, total out-of-pocket prescription expense in the year of the first ASM fill, and Charlson Comorbidity Index (a weighted sum of 22 comorbidities where higher numbers indicate greater comorbidity) in the year of the first ASM fill.19–21
2.5 |. Statistical analysis
We used group-based trajectory modeling22–24 to characterize distinct groups of individuals with similar trajectories of medication adherence (measured by the PDC) over time (each quarter starting in the quarter of the first ASM fill, up to 8 quarters or else censored after death). Trajectory modeling has been shown to have more accurate classification of PDCs than conventional approaches.25 In a trajectory model, a separate regression is fit for each group simultaneously through maximum likelihood estimation that combines information from all models. Each individual is assumed to fall into only one unique group. Because dichotomizing adherence can reduce power and introduce bias,26 we instead used a censored normal probability distribution for continuous PDC ranging 0%–100% in our main analysis unless stated otherwise. Similar to methods used by prior investigators,11 we selected the number of groups for our final model that demonstrated the Bayesian information criteria (BIC) closest to 0 (which would favor a greater number of groups) while also ensuring that each group contained at least 10% of our sample, given that increasingly small groups may be less statistically stable and more difficult to interpret.23 We evaluated two- to five-group solutions, each allowing cubic trajectories. Data management was performed using SAS 9.4, and models were estimated using the “traj” and “proc traj” commands (http://www.andrew.cmu.edu/user/bjones/index.htm) in SAS and Stata 16.0.
After identifying the optimal number of groups, we conducted a multivariate multinomial logistic regression adjusted for all baseline variables listed above to identify predictors correlated with group membership. A model with four outcome groups produces a separate odds ratio (OR) assessing the effect of a 1-unit change of the predictor on being classified into each of the three comparison groups versus the chosen reference group. Our analyses focused on the “early nonadherent” versus “early adherent” ORs, because we felt comparing the two extreme groups was most informative, although we also displayed ORs comparing “late adherent” versus “early adherent” groups and “late nonadherent” versus “early adherent” for the main analysis. We repeated this analysis restricted to beneficiaries whose first ASM was one of the top five most common ASMs in our sample to display marginal adjusted predicted probabilities of being in each group. Then, to address the complexity of potentially switching medications over time, we repeated this analysis restricted to beneficiaries filling only one ASM throughout the observation period.
We performed six additional sensitivity analyses. First, because it is possible that an ASM could be discontinued for valid medical reasons such as reclassifying a patient as having an epilepsy mimic, we repeated the multinomial logistic regression excluding beneficiaries with ICD codes for syncope (780.2) or conversion disorder (300.11; 300.15) during the 2 years after the incident ASM fill. Second, because predictors may change over time, we included time-varying covariates for dual Medicaid eligibility (varying by quarter), number of unique medications (varying by quarter), and Charlson Comorbidity Index (varying by year) in the trajectory model. Note that time-varying variables do not produce ORs for group membership. Third, because beneficiaries with dual Medicaid eligibility could potentially fill medications outside of Part D claims, thus causing underestimation of adherence for this group, we excluded beneficiaries with any dual eligibility over the course of the 2 years. Fourth, because many ASMs could have been prescribed primarily for nonepilepsy conditions (i.e., pain, mood), which could affect adherence, we repeated analyses restricting only to the ASMs with >5% frequency in our sample with relatively pure epilepsy indications (levetiracetam, phenytoin, and phenobarbital). Fifth, because beneficiaries in a nursing home or other skilled nursing facility are not directly responsible for medication management, we excluded beneficiaries with any claim in the 8 quarters indicating place of service as skilled nursing facility/nursing facility/custodial care facility, any Current Procedural Terminology codes relating to a facility, or any skilled nursing facility inpatient claims.27 Sixth, because it is possible that an ASM could be started for a single seizure and later appropriately discontinued, and because prior work has shown the positive predictive value of identifying epilepsy in claims data increases with a greater number of ICD codes,28 we excluded beneficiaries without any further ICD codes for epilepsy or convulsions during the 2 years after their first ASM fill.
All models used PDC as a continuous outcome, except the following three models which used a dichotomous outcome (PDC ≥ 80%), given that the linear outcome yielded an unstable covariance matrix: time-varying covariates, restricting to beneficiaries without dual eligibility, and restricting to only the top three relatively pure ASMs.
2.6 |. Data accessibility statement
All datasets are available to purchase at https://www.resdac.org/. Aggregated deidentified data may be shared upon request.
3 |. RESULTS
We included 24 923 beneficiaries (Figure 1). The median age was 70 years (interquartile range [IQR] = 54–80), 57% were female, and 75% were White (Table 1). There were 3992 (16%) who died within 2 years of the first ASM fill; those beneficiaries contributed a median 5 (IQR = 3–6) quarters. The top five first filled ASMs were levetiracetam (41%), phenytoin (15%), gabapentin (11%), valproate (7%), and phenobarbital (6%; Table S1).
FIGURE 1.
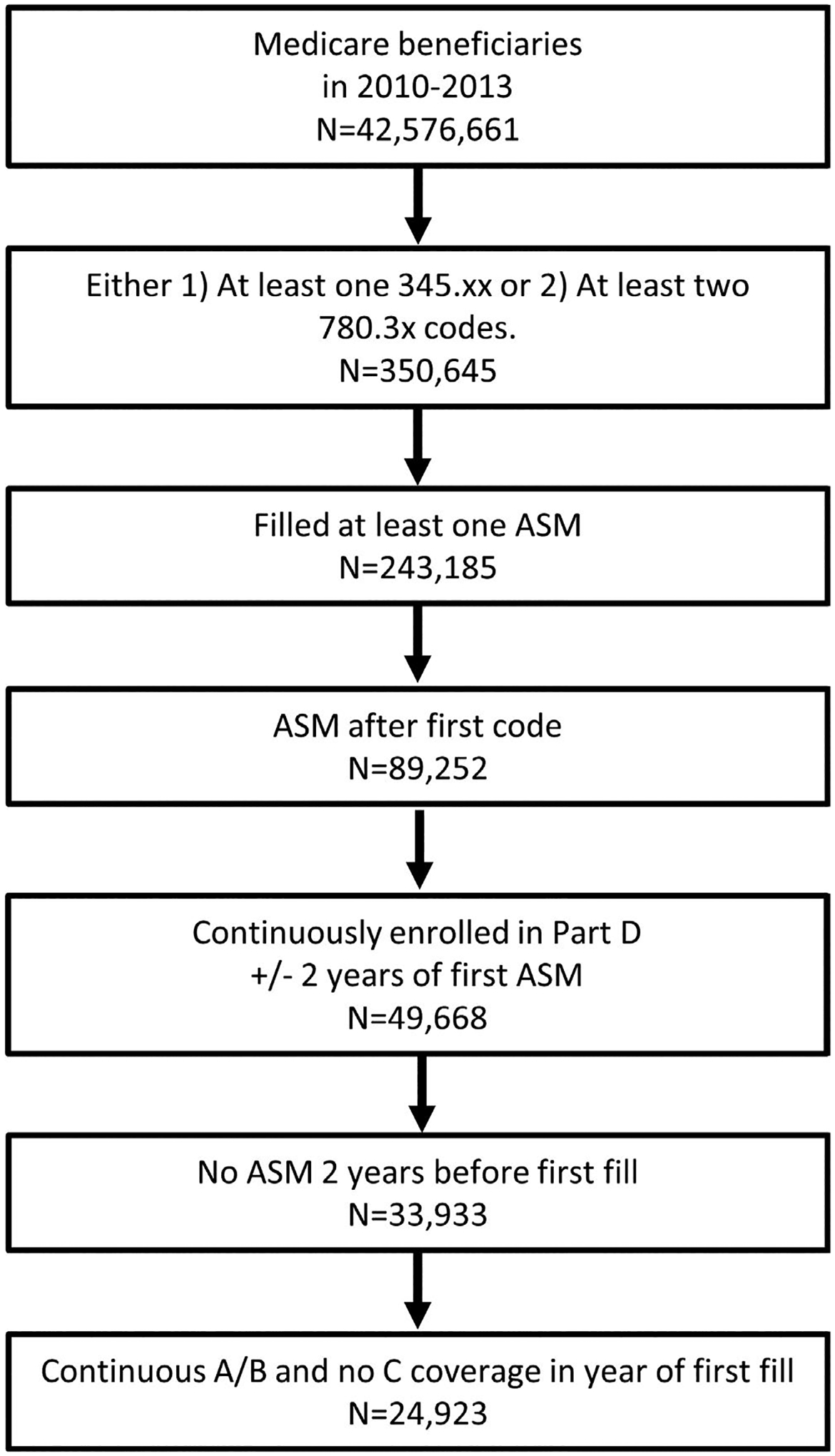
Patient flowchart. ASM, antiseizure medication
TABLE 1.
Population description (N = 24 923)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age, years | 70 (54–80) |
| Female sex | 14 224 (57%) |
| Race | |
| White | 18 746 (75%) |
| Black | 4387 (18%) |
| Asian | 364 (1%) |
| Hispanic | 860 (3%) |
| NAN | 201 (1%) |
| Other | 321 (1%) |
| Dual eligible | |
| None | 9556 (38%) |
| Partial | 2157 (9%) |
| Full | 13 210 (53%) |
| Cost-sharing | |
| None | 8477 (34%) |
| Partial | 978 (4%) |
| Full | 15 468 (62%) |
| Reason for entitlement | |
| Age ≥ 65 years | 14 670 (59%) |
| Disability | 10 067 (40%) |
| ESRD | 583 (2%) |
| Region | |
| Northeast | 4265 (17%) |
| Midwest | 5696 (23%) |
| South | 10 105 (41%) |
| West | 3927 (16%) |
| Other | 930 (4%) |
| Rural ZIP code | 7015 (28%) |
| Neurology visit | 14 307 (57%) |
| Older generation ASM | 8143 (33%) |
| Once daily ASM | 3001 (12%) |
| Unique medications, n | 13 (8–18) |
| Total Part D out-of-pocket cost | $105 ($18–$411) |
| Charlson Comorbidity Index | 2 (0–4) |
Note:: Variables refer to the year of the beneficiary’s first ASM fill. Abbreviations: ASM, antiseizure medication; ESRD, end-stage renal disease; IQR, interquartile range; NAN, North American Native.
Table S2 demonstrates that the four-group solution provided the BIC closest to 0, while still ensuring that each group still contained at least 10% of our sample. Table S3 displays model coefficients for the four-group solution. Per Table S4, average posterior probabilities that a beneficiary was classified into their assigned group ranged 91%–97% and the odds of correct classification ranged 24–101 (>5 for each group is considered acceptable22).
Based on the trajectory plot (Figure 2), we labeled four groups for interpretation: early adherent (PDCs remained above approximately 80% throughout, 60% of the sample), early nonadherent (PDCs dropped toward 0 within the first several quarters, 18% of the sample), late adherent (PDCs initially decreased then increased, 11% of the sample), and late nonadherent (PDCs more gradually dropped toward 0 by the 8th quarter, 11% of the sample). Note that although the group we labeled late adherent only achieved a final PDC of about 50% in the final studied quarter, we use this nomenclature to refer to the shape of each curve in terms of decreasing then increasing PDC.
FIGURE 2.
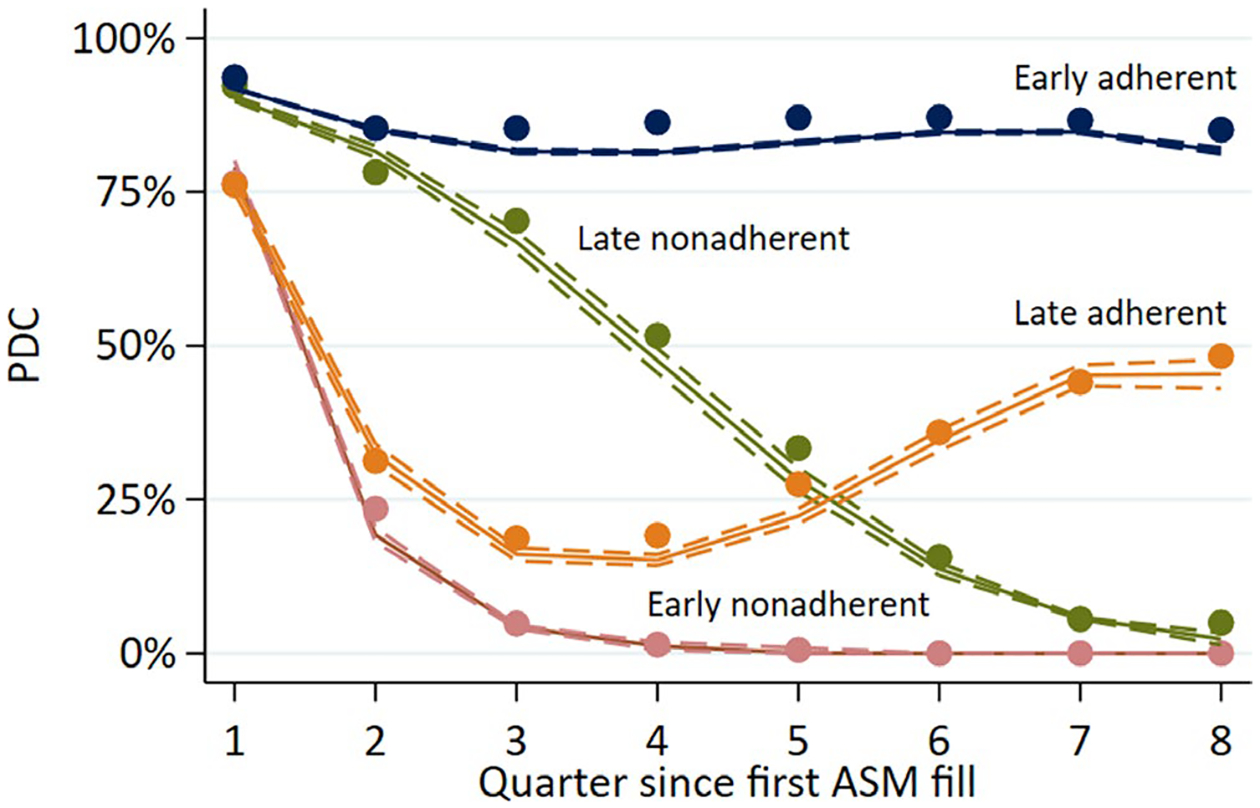
Trajectory plots. Solid lines represent predicted values; dashed lines represent 95% confidence intervals; points represent observed averages. ASM, antiseizure medication; PDC, proportion of days covered
Table 2 displays characteristics according to group. The early adherent group was more likely to be White (78%, vs. 66%–73% for other groups; p < .01), adherent groups were more likely to have any Medicaid full dual eligibility (early: 55%; late: 54%) versus nonadherent groups (early: 48%; late: 49%; p < .01), adherent groups were more likely to have any cost-sharing contribution from Medicare (early: 67%; late: 73%) than the nonadherent groups (early: 62%; late: 63%; p = .02), and groups with higher PDCs in the initial quarters (late nonadherent and early adherent) were more likely to have had a neurologist visit (both: 60%) in the year of the first ASM fill compared with the groups with lower initial PDCs (early nonadherent: 52%; late adherent: 49%; p < .01). The early adherent group had the highest percentage of beneficiaries whose first ASM fill was an older generation medication (35%, vs. 28%–31% for other groups; p < .01).
TABLE 2.
Characteristics by group
| Median (IQR) or n (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Early adherent | Early nonadherent | Late adherent | Late nonadherent | Adjusted pa |
| n | 15 101 (60%) | 4606 (18%) | 2738 (11%) | 2478 (11%) | |
| Age, years | 71 (56–80) | 70 (53–80) | 62 (48–75) | 71 (55–80) | <.01 |
| Female | 8757 (58%) | 2525 (55%) | 1507 (55%) | 1435 (58%) | .04 |
| Race | |||||
| White | 11 785 (78%) | 3347 (73%) | 1801 (66%) | 1813 (73%) | <.01 |
| Black | 2343 (16%) | 876 (19%) | 692 (25%) | 476 (19%) | |
| Asian | 193 (1%) | 88 (2%) | 38 (1%) | 45 (2%) | |
| Hispanic | 472 (3%) | 180 (4%) | 131 (5%) | 77 (3%) | |
| NAN | 104 (1%) | 38 (1%) | 33 (1%) | 26 (1%) | |
| Other | 172 (1%) | 70 (2%) | 41 (1%) | 38 (2%) | |
| Dual eligible | |||||
| None | 5688 (38%) | 1952 (42%) | 907 (33%) | 1009 (41%) | <.01 |
| Partial | 1079 (7%) | 466 (10%) | 360 (13%) | 252 (10%) | |
| Full | 8334 (55%) | 2188 (48%) | 1471 (54%) | 1217 (49%) | |
| Cost-sharing | |||||
| None | 5100 (34%) | 1723 (37%) | 738 (27%) | 916 (37%) | .05 |
| Partial | 538 (4%) | 201 (4%) | 146 (5%) | 93 (4%) | |
| Full | 9463 (63%) | 2682 (58%) | 1854 (68%) | 1469 (59%) | |
| Reason for entitlement | |||||
| Disability | 5742 (38%) | 1906 (41%) | 1455 (53%) | 964 (39%) | .66 |
| ESRD | 263 (2%) | 138 (3%) | 92 (3%) | 90 (4%) | <.01 |
| Region | |||||
| Northeast | 2775 (18%) | 704 (15%) | 403 (15%) | 383 (15%) | <.01 |
| Midwest | 3599 (24%) | 975 (21%) | 581 (21%) | 541 (22%) | |
| South | 5890 (39%) | 1942 (42%) | 1220 (45%) | 1053 (42%) | |
| West | 2295 (15%) | 802 (17%) | 432 (16%) | 398 (16%) | |
| Other | 542 (4%) | 183 (4%) | 102 (4%) | 103 (4%) | |
| Rural ZIP code | 4364 (29%) | 1255 (27%) | 729 (27%) | 667 (27%) | .03 |
| Neurology visit | 9100 (60%) | 2386 (52%) | 1337 (49%) | 1484 (60%) | <.01 |
| Older generation ASM | 5293 (35%) | 1283 (28%) | 849 (31%) | 718 (29%) | <.01 |
| Once daily ASM | 1728 (11%) | 614 (13%) | 347 (12%) | 312 (13%) | <.01 |
| Medications, n | 13 (8–18) | 12 (8–18) | 13 (8–18) | 13 (9–19) | <.01 |
| Out of pocket | $105 ($8–$434) | $109 ($30–$409) | $85 ($23–$258) | $125 ($37–$484) | <.01 |
| Charlson index | 2 (0–4) | 2 (0–4) | 2 (0–4) | 2 (1–4) | .12 |
Abbreviations: ASM, antiseizure medication; ESRD, end-stage renal disease; IQR, interquartile range; NAN, North American Native.
Adjusted for all variables listed in the table from a multinomial logistic regression.
From our adjusted multinomial logistic regression just considering the top five ASMs (n = 19 851), Figure 3 demonstrates that beneficiaries whose first filled ASM was phenobarbital demonstrated the highest adjusted proportion in the early adherent group and the lowest adjusted proportion in the early nonadherent group. Gabapentin demonstrated the opposite. We repeated this analysis restricting to only those 12 144 whose first ASM was one of those five medications on monotherapy throughout the entire observation period. Results were similar.
FIGURE 3.
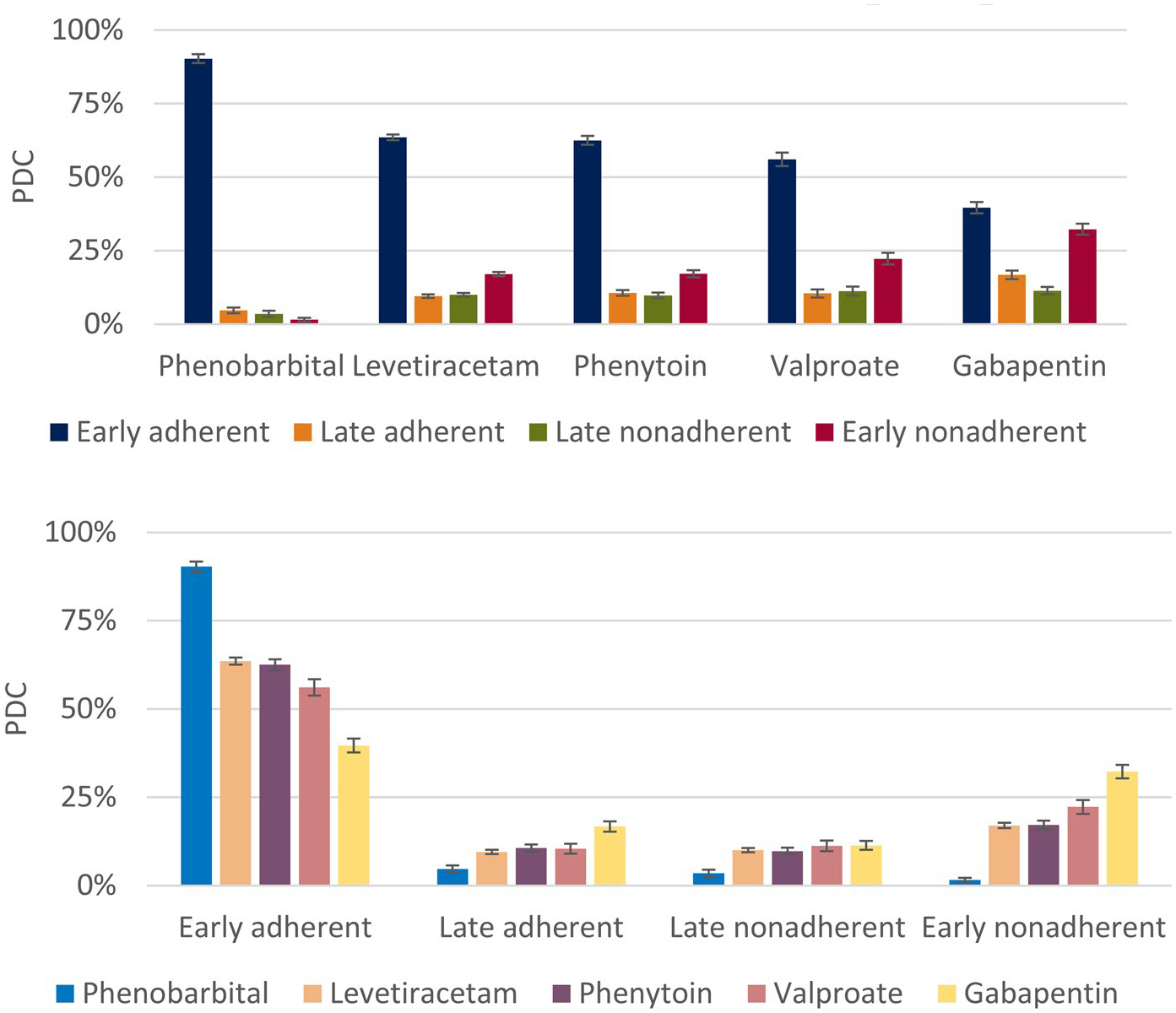
Adjusted group predictions (±95% confidence interval) by first antiseizure medication (ASM). Bars add up to 100% within each ASM, displayed according to medication (top) and group (bottom). This figure demonstrates that phenobarbital had the highest predicted probability of early adherent group membership, whereas gabapentin had the lowest. PDC, proportion of days covered
ORs for being in the early nonadherent versus early adherent group, adjusted for all other displayed variables, are presented in Table 3. For our main analysis, numerous predictors were associated with increased odds of being in the early nonadherent group: non-White race (e.g., Black, OR = 1.7, 95% confidence interval [CI] = 1.5–1.8), region (e.g., South vs. Northeast: OR = 1.2, 95% CI = 1.1–1.4), and beneficiaries whose first filled ASM was once daily (OR = 1.1, 95% CI = 1.0–1.3). Predictors associated with decreased odds of being in the early nonadherent group included older age (OR per decade = .9, 95% CI = .9–.9), female sex (OR = .9, 95% CI = .8–1.0), dual Medicaid eligibility (OR = .6, 95% CI = .4–.8), rural location (OR = .9, 95% CI = .8–1.0), neurologist visit (OR = .6, 95% CI = .6–.7), and initial older generation ASM (OR = .6, 95% CI = .6–.7).
TABLE 3.
Adjusted odds ratios (95% confidence intervals) for early nonadherent versus early adherent
| Characteristic | Main analysis | Exclude syncope/conversion | Time-varying | Exclude dual eligible subjects | Only 3 ASMs | Exclude SNF/NH |
|---|---|---|---|---|---|---|
| n | 24 873 | 18 982 | 24 873 | 9548 | 15 788 | 15 792 |
| Age, decades | .9 (.9–.9)a | .9 (.8–.9)a | .9 (.8–.9)a | .9 (.9–1.0) | .9 (.9–1.0)a | .9 (.9–1.0)a |
| Female | .9 (.8–1.0)a | 1.0 (.9–1.0) | 1.0 (.9–1.0) | .9 (.8–1.0)a | .8 (.8–.9)a | .9 (.8–1.0)a |
| Race | ||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref |
| Black | 1.7 (1.5–1.8)b | 1.8 (1.6–2.0)b | 2.0 (1.8–2.2)b | 2.1 (1.7–2.6)b | 2.3 (2.1–2.6)b | 1.9 (1.7–2.1)b |
| Asian | 1.9 (1.4–2.5)b | 2.2 (1.6–3.0)b | 1.7 (1.3–2.3)b | 3.2 (1.4–7.4)b | 1.9 (1.4–2.6)b | 1.9 (1.4–2.6)b |
| Hispanic | 1.7 (1.4–2.0)b | 1.7 (1.4–2.1)b | 2.1 (1.7–2.5)b | 1.4 (.8–2.6) | 2.3 (1.8–3.0)b | 1.7 (1.4–2.1)b |
| NAN | 1.6 (1.1–2.3)b | 1.7 (1.1–2.5)b | 2.4 (1.6–3.7)b | 2.2 (.7–7.0) | 3.1 (1.8–5.4)b | 1.5 (.9–2.3) |
| Other | 1.6 (1.2–2.1)b | 1.4 (1.0–1.9) | 1.7 (1.3–2.2)b | 1.8 (1.1–2.9)b | 1.9 (1.4–2.8)b | 1.6 (1.2–2.3)b |
| Dual | ||||||
| None | Ref | Ref | — | Ref | Ref | Ref |
| Partial | 1.1 (.8–1.5) | 1.3 (.9–1.8) | — | — | 1.3 (.9–2.0) | .9 (.6–1.3) |
| Full | .6 (.4–.8)a | .6 (.4–.8)a | — | — | .6 (.4–.9)a | .6 (.4–.8)a |
| Cost-sharing | ||||||
| None | Ref | Ref | Ref | Ref | Ref | Ref |
| Partial | .8 (.7–1.0) | .8 (.7–1.0) | 1.0 (.9–1.2) | .9 (.8–1.1) | 1.1 (.9–1.4) | .8 (.6–1.0)a |
| Full | .9 (.6–1.2) | .8 (.6–1.2) | .6 (.6–.7)a | .9 (.6–1.4) | .9 (.6–1.5) | 1.1 (.7–1.6) |
| Reason | ||||||
| Disability | 1.7 (1.4–2.2) | 1.0 (.8–1.1) | .9 (.8–1.0)a | 1.2 (1.0–1.5) | .9 (.8–1.1) | 1.0 (.8–1.1) |
| ESRD | 1.1 (.9–1.2) | 1.8 (1.4–2.3)b | 1.9 (1.5–2.5)b | 1.8 (1.0–3.5) | 1.7 (1.3–2.4)b | 1.6 (1.2–2.1)b |
| Region | ||||||
| NE | Ref | Ref | Ref | Ref | Ref | Ref |
| MW | 1.1 (.9–1.2) | 1.1 (1.0–1.3) | 1.1 (1.0–1.2) | .9 (.8–1.1) | 1.1 (.9–1.2) | 1.0 (.9–1.2) |
| S | 1.2 (1.1–1.4)b | 1.2 (1.1–1.4)b | 1.5 (1.4–1.6)b | 1.3 (1.1–1.5)b | 1.4 (1.2–1.6)b | 1.2 (1.0–1.3)b |
| W | 1.3 (1.2–1.5)b | 1.4 (1.2–1.6)b | 1.4 (1.3–1.6)b | 1.2 (1.0–1.4) | 1.4 (1.2–1.7)b | 1.3 (1.1–1.5)b |
| O | 1.3 (1.1–1.6)b | 1.6 (1.3–2.0)b | 1.6 (1.3–1.9)b | 1.6 (1.2–2.1)b | 1.3 (1.0–1.7)b | 1.7 (1.3–2.1)b |
| Rural | .9 (.8–1.0)a | .9 (.8–1.0)a | .9 (.8–1.0)a | .8 (.7–.9)a | .9 (.8–1.0)a | .9 (.8–1.0)a |
| Neurologist visit | .6 (.6–.7)a | .6 (.6–.7)a | .7 (.6–.7)a | .7 (.6–.7)a | .8 (.7–.8)a | .6 (.5–.6)a |
| Older generation ASM | .6 (.6–.7)a | .6 (.6–.7)a | .6 (.6–.7)a | .7 (.6–.8)a | .7 (.7–.8)a | .6 (.5–.7)a |
| Daily | 1.1 (1.0–1.3)b | 1.2 (1.0–1.3)b | 1.0 (.9–1.1) | 1.2 (1.0–1.4)b | .7 (.6–.8)a | 1.1 (.9–1.2) |
| Unique medications, n | ||||||
| 1–5 | Ref | Ref | — | Ref | Ref | Ref |
| 6–10 | .9 (.8–1.1) | 1.0 (.8–1.1) | — | 1.0 (.8–1.2) | .9 (.8–1.0) | 1.0 (.9–1.2) |
| 11–15 | 1.0 (.9–1.1) | .9 (.8–1.1) | — | 1.1 (.9–1.4) | .9 (.8–1.1) | 1.1 (.9–1.2) |
| 16+ | 1.0 (.9–1.1) | 1.0 (.9–1.2) | — | 1.3 (1.1–1.6)b | .9 (.8–1.1) | 1.2 (1.0–1.3)b |
| Per $100 | 1.0 (1.0–1.0)a | 1.0 (1.0–1.0)a | 1.0 (1.0–1.0)a | 1.0 (1.0–1.0)a | 1.0 (1.0–1.0)a | 1.0 (1.0–1.0)a |
| Charlson Comorbidity Index | ||||||
| 0 | Ref | Ref | — | Ref | Ref | Ref |
| 1–2 | 1.0 (.9–1.1) | 1.0 (.9–1.1) | — | .9 (.8–1.1) | 1.2 (1.0–1.3)b | 1.1 (1.0–1.2) |
| 3–6 | .9 (.8–1.0) | .9 (.8–1.0) | — | 1.0 (.8–1.1) | 1.2 (1.1–1.4)b | 1.0 (.9–1.2) |
| 7+ | .9 (.8–1.1) | .9 (.7–1.0) | — | 1.0 (.8–1.3) | 1.7 (1.4–2.0)b | 1.0 (.8–1.2) |
| Characteristic | Subsequent ICD code |
|---|---|
| n | 20 854 |
| Decades | 1.0 (.9–1.0)a |
| Female | .9 (.8–1.0)a |
| Race | |
| White | Ref |
| Black | 1.5 (1.3–1.7)b |
| Asian | 1.8 (1.3–2.6)b |
| Hispanic | 1.5 (1.2–2.0)b |
| NAN | 1.3 (.8–2.2) |
| Other | 1.5 (1.1–2.2)b |
| Dual | |
| None | Ref |
| Partial | 1.2 (.8–1.9) |
| Full | .7 (.4–1.1) |
| Cost-sharing | |
| None | Ref |
| Partial | .9 (.7–1.1) |
| Full | .8 (.5–1.2) |
| Reason | |
| Disability | 1.1 (.9–1.3) |
| ESRD | 1.9 (1.4–2.6)b |
| Region | |
| NE | Ref |
| MW | 1.0 (.8–1.1) |
| S | 1.1 (1.0–1.3)b |
| W | 1.2 (1.1–1.5)b |
| O | 1.3 (1.0–1.7) |
| Rural | .9 (.8–1.0) |
| Neurologist visit | .7 (.6–.8)a |
| Older generation ASM | .7 (.6–.8)a |
| Daily | 1.2 (1.0–1.3)b |
| Unique medications, n | |
| 1–5 | Ref |
| 6–10 | .9 (.8–1.1) |
| 11–15 | .9 (.8–1.1) |
| 16+ | .9 (.8–1.1) |
| Per $100 | 1.0 (1.0–1.0)a |
| Charlson Comorbidity Index | |
| 0 | Ref |
| 1–2 | 1.0 (.9–1.1) |
| 3–6 | 1.0 (.8–1.1) |
| 7+ | 1.0 (.8–1.2) |
Note:: Values without footnotes are nonsignificant.
Abbreviations: ASM, antiseizure medication; ESRD, end-stage renal disease; ICD, International Classification of Diseases; MW, Midwest; NAN, North American Native; NE, Northeast; NH, nursing home; O, other; Ref, reference; S, South; SNF, skilled nursing facility; W, West.
<1.
>1.
Table S5 also displays ORs from the main analysis for being classified into the late adherent versus early adherent group, and for being classified into the late nonadherent versus early adherent group. For example, the following variables were associated with decreased odds of being in the early adherent group across all of the three nonreference comparisons: younger age, Black or North American Native race, South/West/Other region, lacking a neurologist, and newer generation initial ASM.
The following variables were associated with being in the early nonadherent group compared with the early adherent group across all sensitivity models (Table 3): Black or Asian race, South region, lacking a neurologist, first filled ASM being a newer generation medication, and lower out-of-pocket drug costs. Other variables were not consistently significant, although the following variables were associated with being in the early nonadherent group compared with the early adherent group in almost all models: younger age, all other non-White race categories, full Medicaid dual eligibility, West or Other regions, and nonrural ZIP code.
4 |. DISCUSSION
We identified four distinct groups of ASM adherence trajectories in Medicare beneficiaries with newly treated epilepsy: early adherent (60%), early nonadherent (18%), late adherent (11%), and late nonadherent (11%). Groups differed according to numerous clinical characteristics; for example, the early adherent group was most likely to be White and older, adherent groups were more likely to have several features (Medicaid dual eligibility, cost-sharing contribution from Medicare, older generation first ASM), and groups with greater initial adherence were more likely to have had a neurologist visit.
One framework that illustrates these distinct phenotypes is the Necessity-Concerns Framework,29,30 which presents adherence as a complex interplay between beliefs regarding need for treatment and concern about potential adverse consequences of medications. The early adherent group may have both high beliefs in the effectiveness of ASMs for themselves and low concern for medication-related harms. The early nonadherent group could be the opposite, where concerns for potential or actual adverse effects outweighed the perceived benefit of ASMs. The late adherent group could start with negative attitudes or patient-level barriers, such as cost or pharmacy access, but then experience additional seizures, which could increase their belief regarding the necessity for adherence, or else could be switching from a poorly tolerated ASM to a better tolerated ASM. The late nonadherent group could begin with positive views about ASMs, but then adverse effects could later reduce adherence or else initial seizure freedom could reduce the future perceived importance of continued treatment. As Medicare data do not inform ASM beliefs and concerns, the identified distinct trajectories should be viewed as hypothesis-generating regarding underlying differences.
We identified one modifiable process of care that could influence adherence consistently across sensitivity models: visiting a neurologist. Beneficiaries in groups with initially higher adherence (early adherent and late nonadherent) were approximately 7%–9% more likely to have had a neurologist visit in the year of their first ASM fill. Specialist visits could simply be a marker for increased health care interactions, but specialists are uniquely positioned to provide expert disease-specific and treatment-specific education, including the prognosis, consequences, and treatment options relevant to epilepsy, which could motivate a patient to remain adherent. Neurologist ambulatory care has been associated with improvements in a wide variety of disease-specific or general outcomes.31,32
Many other variables were significant predictors of trajectory groups, although results were less clear targets for intervention. We found worse adherence in non-White groups. This echoes other literature documenting worse adherence in Hispanics and non-Hispanic Blacks compared with Whites, which was only partially attenuated by adjusting for socioeconomic status,33 pointing to different underlying beliefs or concerns toward medications or socioeconomic variables more difficult to capture in administrative datasets like social support or transportation. Other literature in epilepsy has similarly suggested socioeconomic disparities in terms of occupational11 or geographic status.6 Our findings that adherence was better with greater dual eligibility or cost-sharing provided by Medicare and that adherence differed by region also underscore the relevance of socioeconomic and insurance coverage. Prior work9,34 has highlighted a large number of reasons for ASM nonadherence such as negative beliefs about medications,29,30 poorer medication self-administration strategies, frequent medication dosing, medication side effects, uncontrolled recent seizures, comorbidities (e.g., depression), suboptimal physician–patient relationships, inadequate social support, minority status,10 geographic residence,6 lesser employment and education,35 cost, and forgetfulness.35 Although administrative data only capture a subset of such patient-level variables, those captured above were generally consistent with prior literature.
One finding was less consistent with prior literature6,8,36: beneficiaries initially filling an older generation ASM (phenobarbital) were more likely to be in the early adherent group. Given a large number of equally effective ASMs,37 and that older ASMs may exert more adverse effects38 or drug interactions, we hypothesized that phenobarbital users would exhibit poorer adherence trajectories. For example, in one large study of older veterans,8 phenobarbital had 52% increased odds of nonadherence and levetiracetam had 31% reduced odds of nonadherence compared to phenytoin. However, that study was cross-sectional, conditioned upon subjects already having taken AED monotherapy for 3 months (biasing toward greater apparent adherence), dichotomized adherence, and had a markedly different ASM composition (70% phenytoin, 2% levetiracetam). In contrast, our study benefitted from longitudinal assessment of more updated ASMs plus continuous outcomes. Furthermore, another study of more than 8000 probable new cases of epilepsy in Medicare beneficiaries10 did not find a clean boundary between older versus newer ASMs; adherence was highest for lamotrigine (71%) and lowest for carbamazepine (59%) or phenytoin, levetiracetam, or pregabalin (all 62%). Other data in indigent populations actually found phenobarbital adherence exceeded all other ASMs.39 Thus, the data are not so clear. We considered numerous mechanisms by which our result could be due to confounding or mismeasurement. The effect of initially filling phenobarbital could be driven by once-daily medications influencing greater adherence,6 although the effect persisted after adjusting for dosing frequency. Understanding the effect of initial ASM selection is further made challenging because clinicians may be appropriately most likely to choose once-daily medication dosing for individuals deemed least likely to adhere for reasons not captured in Medicare data. We considered whether beneficiaries on phenobarbital could be more likely to receive care in a nursing home or skilled nursing facility where adherence does not depend upon the beneficiary’s self-management. After excluding this population, once-daily first ASM dosing no longer predicted early nonadherence as expected, but the effect of phenobarbital remained. Because phenobarbital would be an unusual first choice for newly treated epilepsy in adults, we considered whether these beneficiaries could have actually taken phenobarbital earlier in life before our 2-year ASM washout period, which may inflate their apparent adherence given prior life experience. We note 3% of our sample filled more than one ASM on the first date after the 2-year washout period, which could indicate switching insurance rather than being newly treated. However, these possibilities are untestable within our available data, and we did perform an analysis including only those on monotherapy throughout. We also acknowledge that categorizing individuals based on their first filled ASM does not capture the complexity of subsequent ASM modifications. We entered total number of medications as a time-varying variable as another strategy to unpack this complexity, without changing conclusions. Note that entering number of ASMs or presence of an older generation as a time-varying outcome would risk reverse causation bias (i.e., poor adherence causing fewer ASMs, rather than polytherapy influencing adherence).
One prior study performed group-based trajectory modeling in 124 children with new onset epilepsy, predominantly prescribed carbamazepine (60%) and valproate (40%). Their final model included five groups labeled similarly to ours. However, parental occupation was the only predictor associated with adherence groups (nonsignificant: age, sex, caregiver marital status, convulsive seizures, seizure frequency, total number of ASMs, adverse effects, witnessed seizures). One explanation for differences between studies would be that factors predicting adherence groups truly differ between children with parental supervision seen at an academic institution for new onset epilepsy, versus our adult Medicare sample. It is conceivable that adherence during childhood is determined by parental influence, whereas adults without constant supervision by a caretaker could have a much larger number of salient personal medical and sociodemographic variables that distinguish adherence trajectories. A second explanation for differences between studies could be our substantially larger sample size more highly powered to detect differences; guidelines suggest at least 200 patients for group-based trajectory modeling.11 Moreover, their study used electronic pill cap monitoring, whereas our outcome was based on administrative fills. Administrative claims could overestimate adherence, because they measure fills rather than ingestion.
Our study has numerous additional limitations. In addition to possibly overestimating adherence, claims also could underestimate adherence,40 because it is challenging to distinguish nonadherence from medically directed cessation of ASM treatments. Nonetheless, it is reasonable to assume that patients with newly diagnosed epilepsy have a continuous indication for at least 2 years unless a previously conferred epilepsy diagnosis is rescinded.41 Also, most results did not change substantively when we performed sensitivity analyses excluding those with syncope or conversion disorder as examples of seizure mimics or when excluding those without any subsequent epilepsy or convulsion codes after their first filled ASM, who could have been more likely to have a single seizure rather than epilepsy. Our older or disabled Medicare population also may not generalize to a younger privately insured population without Part D coverage, for whom adherence could be worse or for whom adherence barriers may be different. Also, complex interactions may exist between reason for Medicare eligibility (e.g., age vs. disability) and other covariates. However, whereas many studies using Medicare address this complexity by excluding all beneficiaries eligible due to reasons other than age, in the case of epilepsy this would have excluded 40% of our intended population; hence, we chose to include those eligible due to disability and added this as a key covariate to account for such variability. It is also well known that identifying epilepsy cases in administrative datasets using ICD codes risks some degree of misclassification.42 Nonetheless, recent work has suggested good sensitivity (up to 88%) and specificity (98%) of Medicare data compared with chart review epilepsy diagnoses.14 Also, Medicare encompasses a large, diverse national population, and the PDC represents an accepted policy-driving metric by which the Centers for Medicare and Medicaid evaluate Medicare Advantage and Part D plan performance.43 Finally, complex bidirectional time-dependent dynamics are challenging to disentangle. For example, patients who are less adherent due to more negative health care beliefs may be less likely to be evaluated for comorbidities, which would be underestimated. Although we applied a wide array of sensitivity analyses, these observational data are best considered for prediction rather than to establish causation and may be validated in future external samples.
5 |. Conclusions
We identified four trajectories of ASM adherence in individuals with newly treated epilepsy. Although it was encouraging that the largest group represented early adherent individuals, 40% still were classified as either having initially low (late adherent), eventually low (late nonadherent), or persistently low (early nonadherent) adherence. Whereas many risk factors for nonadherence such as race or geographic region outside of the Northeast are difficult to modify, our work identified that lacking a neurologist could be a modifiable risk factor for early nonadherence. These data may guide future interventions aimed at improving ASM adherence, in terms of both timing and target populations.
Supplementary Material
Key Points.
We identified four antiseizure medication adherence trajectories: early adherent, early nonadherent, late adherent, and late nonadherent
Our work highlighted a modifiable risk factor for early nonadherence: lacking a neurologist
Other disparities included non-White race and geographic region
These data may guide future interventions aimed at improving ASM adherence, in terms of both timing and target populations
ACKNOWLEDGMENTS
S.W.T. is supported by the Susan S. Spencer Clinical Research Training Scholarship and the Michigan Institute for Clinical and Health Research J Award (UL1TR002240). S.W.T was recently supported by the University of Michigan Department of Neurology Training Grant (5T32NS007222-38). W.T.K. is supported by National Institutes of Health (R25NS065723). Z.A.M. is supported by the National Institute of Aging (K76AG059929). L.W. has no funding to report. J.F.B. is supported by the National Institute of Minority Health and Health Disparities (R01 MD008879). All coauthors were substantially involved in the study and the preparation of the manuscript. No undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation. All coauthors have seen and approved the submitted version of the paper and accept responsibility for its content.
Funding information
University of Michigan Department of Neurology Training Grant, Grant/Award Number: 5T32NS007222-38; NIH, Grant/Award Number: R25NS065723; National Institute of Minority Health and Health Disparities, Grant/Award Number: R01 MD008879; National Institute of Aging, Grant/Award Number: K76AG059929; American Epilepsy Society; Michigan Institute for Clinical and Health Research, Grant/Award Number: UL1TR002240
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Faught E Adherence to antiepilepsy drug therapy. Epilepsy Behav. 2012;25(3):297–302. [DOI] [PubMed] [Google Scholar]
- 2.Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology. 2008;71(20):1572–8. [DOI] [PubMed] [Google Scholar]
- 3.Faught RE, Weiner JR, Guerin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501–9. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324–9. [DOI] [PubMed] [Google Scholar]
- 5.Davis KL, Candrilli SD, Edin HM. Prevalence and cost of non-adherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49(3):446–54. [DOI] [PubMed] [Google Scholar]
- 6.Gollwitzer S, Kostev K, Hagge M, Lang J, Graf W, Hamer HM. Nonadherence to antiepileptic drugs in Germany: a retrospective, population-based study. Neurology. 2016;87(5):466–72. [DOI] [PubMed] [Google Scholar]
- 7.Alsous M, Hamdan I, Saleh M, McElnay J, Horne R, Masri A. Predictors of nonadherence in children and adolescents with epilepsy: a multimethod assessment approach. Epilepsy Behav. 2018;85:205–11. [DOI] [PubMed] [Google Scholar]
- 8.Zeber JE, Copeland LA, Pugh MJV. Variation in antiepileptic drug adherence among older patients with new-onset epilepsy. Ann Pharmacother. 2010;44:1896–904. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke G, O’Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160–8. [DOI] [PubMed] [Google Scholar]
- 10.Piper K, Richman J, Faught E, Martin R, Funkhouser E, Sza JP, et al. Adherence to antiepileptic drugs among diverse older Americans on Part D Medicare. Epilepsy Behav. 2017;66:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305(16):1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi AC, Wu YP, Rausch JR, Peugh JL, Glauser TA. Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology. 2014;83(22):2085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faught E, Richman J, Martin R, Funkhouser E, Foushee R, Kratt P, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology. 2012;78(7):448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moura LMVR, Smith JR, Blacker D, Vogeli C, Schwamm LH, Cole AJ, et al. Epilepsy among elderly Medicare beneficiaries. Med Care. 2019;57(4):318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–57. [PubMed] [Google Scholar]
- 16.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–7. [DOI] [PubMed] [Google Scholar]
- 17.Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372–8. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services Zip code to carrier locality file. Accessed January 8, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/FeeScheduleGenInfo
- 19.Charlson M, Pompei P, Ales K, MacKenzie R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Comorbidity SAS Macro. 2014. Accessed January 8, 2021. https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2014.html
- 22.Nagin D Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 23.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 24.Alhazami M, Pontinha VM, Patterson JA, Holdford DA. Medication adherence trajectories: a systematic literature review. J Manag Care Spec Pharm. 2020;26(9):1138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin JM, Shrank WH, Pakes J, Sanfélix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–96. [DOI] [PubMed] [Google Scholar]
- 26.Tueller SJ, Deboeck PR, Van Dorn RA. Getting less of what you want: reductions in statistical power and increased bias when categorizing medication adherence data. J Behav Med. 2016;39(6):969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun H, Kilgore ML, Curtis JR, Delzell E, Gary LC, Saag KG, et al. Identifying types of nursing facility stays using Medicare claims data: an algorithm and validation. Health Serv Outcomes Res Methodol. 2010;10(1–2):100–10. [Google Scholar]
- 28.Holden EW, Grossman E, Nguyen NT, Gunter MJ, Grebosky B, Von WA, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8(1):1–14. [DOI] [PubMed] [Google Scholar]
- 29.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–67. [DOI] [PubMed] [Google Scholar]
- 30.Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ney JP, Johnson B, Knabel T, Craft K, Kaufman J. Neurologist ambulatory care, health care utilization, and costs in a large commercial dataset. Neurology. 2016;86(4):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein LB, Matchar DB, Hoff-Lindquist J, Samsa GP, Horner RD. VA stroke study: neurologist care is associated with increased testing but improved outcomes. Neurology. 2003;61(6):792–6. [DOI] [PubMed] [Google Scholar]
- 33.Xie Z, Clair PS, Goldman DP, Joyce G. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One. 2019;14(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507–15. [DOI] [PubMed] [Google Scholar]
- 35.Paschal AM, Rush SE, Sadler T. Factors associated with medication adherence in patients with epilepsy and recommendations for improvement. Epilepsy Behav. 2014;2014(31):346–50. [DOI] [PubMed] [Google Scholar]
- 36.Nevitt S, Sudell M, Weston J, Turdur Smith C, Marson A. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev. 2017;12(12):CD011412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–63. [DOI] [PubMed] [Google Scholar]
- 38.Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17:413–25. [DOI] [PubMed] [Google Scholar]
- 39.Bautista RED, Rundle-Gonzalez V. Effects of antiepileptic drug characteristics on medication adherence. Epilepsy Behav. 2012;23(4):437–41. [DOI] [PubMed] [Google Scholar]
- 40.Fanaroff AC, Peterson ED, Kaltenbach LA, Cannon CP, Choudhry NK, Henry TD, et al. Agreement and accuracy of medication persistence identified by patient self-report vs pharmacy fill: a secondary analysis of the cluster randomized ARTEMIS trial. JAMA Cardiol. 2020;5(5):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beghi E, Giussani G, Grosso S, Iudice A, La A, Pisani F, et al. Withdrawal of antiepileptic drugs: guidelines of the Italian League Against Epilepsy. Epilepsia. 2013;54(Suppl 7):2–12. [DOI] [PubMed] [Google Scholar]
- 42.Jette N, Beghi E, Hesdorffer D, Moshé SL, Zuberi SM, Medina MT, et al. ICD coding for epilepsy: past, present, and future—a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia. 2015;56(3):348–55. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Medicare and Medicaid Services. Medicare 2021 Part C & D star rating technical notes. 2020. Accessed January 8, 2021. https://www.cms.gov/files/document/2021technotes20201001.pdf-0
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets are available to purchase at https://www.resdac.org/. Aggregated deidentified data may be shared upon request.


