Abstract
Fascioliasis is a zoonotic infection linked to significant economic losses in the livestock industry. Infection prevalence and estimated financial burden vary across locations owing to different diagnostic tests used. The accuracy of liver condemnation to estimate the prevalence and costs of fascioliasis has seldom been evaluated. We performed a pilot study to determine the prevalence and burden of Fasciola hepatica infection among cattle slaughtered at the municipal abattoir in the Anta province of the Cusco highlands in Peru. We compared liver condemnation with bile microscopy for the diagnosis of infection and prediction of carcass weight. Data were collected from 2009 slaughtered cattle for 1 year. The overall prevalence of Fasciola infection by bile microscopy was 62.5% (1247/2009). A higher prevalence was observed after the rainy season from March to August than from September to February (p < 0.01). Fascioliasis prevalence during the first 6 months was 77.4% (714/923), combining the results of condemnation and microscopy. Bile microscopy diagnosed more infections than liver condemnation (62.7% (579/923) versus 55.4% (511/923), McNemar test p < 0.01). The agreement of the bile microscopy testing with liver condemnation was fair (κ = 0.247). Animal age, gender, breed, and liver condemnation predicted carcass weight [F (df 4, 704) = 61.1, p < 0.001]. Liver condemnation and bile microscopy are complementary tools for evaluation of the prevalence and burden of fascioliasis in livestock. Large scale studies are warranted to confirm our results.
Keywords: fascioliasis, livestock, microscopy, liver condemnation, Peru
Introduction
Fascioliasis is a zoonotic infection caused by the foodborne trematodes Fasciola hepatica and Fasciola gigantica. F. hepatica is widely distributed in all continents, whereas F. gigantica is distributed only in Africa, Middle East, and Asia (Carmona and Tort 2017). Fasciola causes high rates of livestock infection in developed and developing countries. Fascioliasis causes significant economic losses in the livestock industry (Charlier et al. 2020). Effects include decreased milk production, decreased carcass weight and composition quality, and decreased fertility (Schweizer et al. 2005, Charlier et al. 2007, Sanchez-Vazquez and Lewis 2013, Arenal et al. 2018, Villa-Mancera and Reynoso-Palomar 2019). Charlier et al. (2020) reported that the direct costs of fascioliasis to the ruminant industry from 18 European countries were 750 million U.S. dollars (USD) a year. Losses in developing countries are more difficult to estimate due to small scale family farming and lack of animal tracking in most regions.
Liver condemnation records are used to estimate the prevalence and burden of infection in developing countries. A study in the highlands of Peru estimated yearly losses of 400,000 USD in a single slaughterhouse associated with liver condemnation and decreased carcass weight due to fascioliasis (Arias-Pacheco et al. 2020). A large study in Zambia evaluating the burden of Fasciola using liver condemnation estimated annual losses of 600,000 USD in three abattoirs (Jaja et al. 2017a). In contrast, a similar study in South Africa estimated annual losses associated with liver condemnation of 3456 USD in three abattoirs (Nyirenda et al. 2019). The wide variations in estimated losses from liver condemnation are probably related to differences in condemnation criteria, intensity of infection, and cost estimations.
Comparing the burden of Fasciola in the livestock industry in different countries is challenging due to the differences in testing strategy used to ascertain infection. Bulk milk tank enzyme-linked immunoassay (ELISA) testing for Fasciola antibodies provides a cost-effective estimate of the herd prevalence among dairy cattle (Charlier et al. 2014, Villa-Mancera and Reynoso-Palomar 2019). Byrne et al. (2018) found Fasciola antibodies in bulk milk tank of 93% of 1494 dairy herds studied in Northern Ireland and 38% of these showed high antibody titers, suggesting high infection burden in the herd. Although this information may guide control decisions for the dairy industry, individual animal information may be needed from meat herds. In Sweden, serum samples of individual cattle were tested for antibodies to estimate a meat herd seropositivity of 10% (Novobilský et al. 2015).
Stool testing or liver inspection in slaughtered animals may also allow the estimation of Fasciola infection burden. In Australia, a study in the Maffra and Bairnsdale districts using coproantigen ELISA found 40% of the cattle herds infected and a mean prevalence of 80% of fascioliasis in individual herds (Elliot et al. 2015). A study in Ireland used postmortem liver fluke status assessed in abattoirs to model herd infection status. The prevalence of Fasciola liver infection was 24% and estimated herd level prevalence was 63% (Byrne et al. 2016).
In most developing countries, small-scale subsistence farming and lack of livestock tracking limit the estimates of the infection distribution and burden. Liver condemnation records are the main source of data to estimate the prevalence of fascioliasis. In southern Brazil, abattoir data on liver inspection revealed a prevalence of Fasciola infection of between <1% and >78% in three states (Dutra et al. 2010). However, the accuracy of liver condemnation to estimate the prevalence and costs associated with fascioliasis has seldom been evaluated. This study aimed at estimating the prevalence and burden of fascioliasis among cattle in the Anta province of Peru comparing liver condemnation with bile microscopy to determine the presence of infection.
Methods
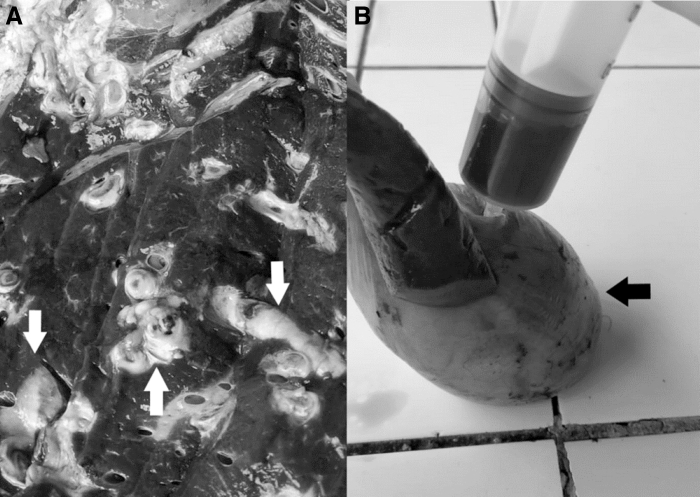
In this pilot study, we evaluated the prevalence and burden of F. hepatica infection among cattle slaughtered at the municipal abattoir in the Anta province of the Cusco highlands (elevation 3350 m) in Peru. This abattoir exclusively slaughters cattle from Anta and other nearby provinces. The abattoir operates 7 days a week but is busier during weekdays than on weekends. Approximately 6000 animals are slaughtered every year. The abattoir is staffed by a veterinarian who performs pre- and postmortem visual inspections on all animals. Cattle carcasses and organs considered to pose a sanitary risk are condemned and destroyed. The reason for condemnation is documented by the abattoir's veterinarian. Livers are condemned when they display the gross fibrotic changes characteristic of advanced Fasciola infection (Fig. 1).
FIG. 1.
Fasciola hepatica infection in the Anta slaughterhouse. (A) Appearance of a cattle liver condemned due to suspected F. hepatica infection. The white arrows show fibrotic and enlarged bile ducts. (B) Gallbladder aspiration to obtain the bile sample for microscopy. The black arrow points to the gallbladder.
Between January and December of 2017, a trained field worker visited the abattoir twice a week, once on a weekday (high-volume day) and once on the weekend (low-volume day). We choose to collect data on high- and low-volume days to capture potential differences in cattle origin and tending practices. Information about age, gender, province of origin, month slaughtered, and carcass weight was collected on all cattle slaughtered on the visit date. The age of the animals was determined by inspection of the number of incisors (American Association of Meat Producers, 2012). Information on liver condemnation due to Fasciola infection, liver appearance, and animal breed was collected during the first 6 months of the study. After that period, that information was no longer available due to changes in management at the abattoir.
Bile samples were collected from all animals to determine the presence of Fasciola eggs. After gently shaking the gallbladder to resuspend parasite eggs, 20 mL of bile was removed from the gallbladders of each slaughtered animal (Fig. 1). Bile specimens were tested for F. hepatica ova at the laboratory of the Universidad Peruana Cayetano Heredia and University of Texas Medical Branch Collaborative Research Center in Cusco City. Eggs were concentrated in the specimen using the rapid sedimentation method (Lumbreras et al. 1962). In brief, bile was sieved through gauze into a 300 mL conical container. The container was then filled with tap water. After 30 min, the top two-thirds of the fluid was discarded and replaced with an equal volume of tap water. After three repetitions, the sediment left in the bottom third of the container was placed in a Petri dish and examined by microscopy at 100 × magnification to detect Fasciola ova.
Fascioliasis was defined as either having the liver condemned for presumed fascioliasis or demonstration of Fasciola ova in bile samples. This definition was used to include animals who had liver damage due to Fasciola, but who may have been treated with triclabendazole before slaughter, which is a common practice during the fattening period in small feedlots. This definition also includes cases wherein the infection may not have led to severe fibrotic changes leading to condemnation.
The rainy season in this region typically lasts from November until March. There is a typical delay of 12 weeks from infection to onset of shedding of ova. Thus, infections acquired during the rainy season would be detected between March and August, whereas infections acquired during the dry season would become patent from September until February.
Data were analyzed using the Statistical Package for the Social Sciences version 25.0 (SPSS; IBM Corp., New York). First, we analyzed the entire 12 months period of the study when data on a limited set of variables were collected. We performed a separate analysis of the first 6 months when data on all variables were collected. The distribution of the variables was described calculating frequencies, means (±standard deviations), and medians with interquartile range (IQR). The frequencies were compared using the chi-squared test, the means using the Student's t test, and the medians using the Mann–Whitney U test. The analysis of variance (ANOVA) test was used to describe the daily distribution of animals during the study period. The Cohen's kappa statistic was calculated to evaluate the agreement between the determinations of Fasciola infection using the liver condemnation and bile microscopy methods. The McNemar chi-squared test was used to compare the sensitivity of both tests. A stepwise multiple regression model was created with relevant independent variables to predict the carcass weight. A p value <0.05 was considered statistically significant. This study did not include experimentation or manipulation of animals. Bile samples were obtained from organs of animals already slaughtered according to local guidelines. Information on livestock was obtained from slaughterhosue records.
Results
The abattoir was visited on a total of 102 days with data collected from 2009 slaughtered cattle. The mean age of the cattle was 3.4 years (±1.9) and 51.9% (1042/2009) were male. Most were from Anta province (1381/2009, 68.7%), with fewer from Abancay (32/2009, 1.6%), Urubamba (24/2009, 1.2%), Paruro (21/2009, 1%), and other provinces (45/2009, 2.2%). The province of origin was unknown for 506 animals. The median number of cattle slaughtered per month was 166.5 (IQR: 151.2–189.5). Significantly less animals were slaughtered on Sundays compared with other days of the week (one-way ANOVA F: 49.6, p < 0.01).
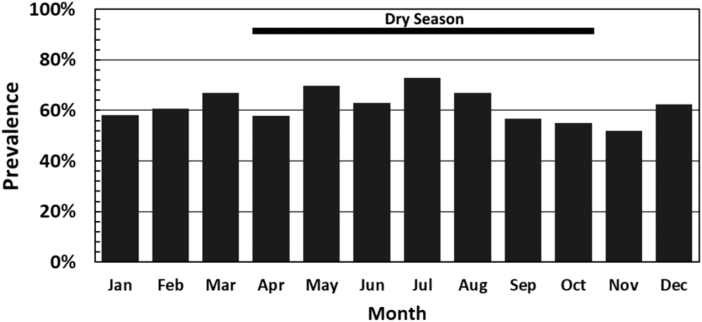
Overall, 62.1% (1247/2009) of the bile samples tested positive for Fasciola eggs by microscopy. There was no difference in prevalence between male (635/1042, 60.9%) and female (612/967, 63.3%, p = 0.27) cattle. However, cattle originating from Paruro and Abancay had a higher prevalence of infection than cattle originating from other provinces (p = 0.04) (Table 1) The highest prevalence was observed after the rainy season from March to August compared with from September to February (p < 0.01) (Fig. 2). Infected cattle had a higher mean age than uninfected cattle (Student's t = 3.2, p < 0.01).
Table 1.
Comparison of the Characteristics of Fasciola-Infected Cattle and Cattle Without the Infection
| 12 Months |
First 6 Monthsa |
|||||
|---|---|---|---|---|---|---|
| Fasciola (+) | Fasciola (−) | p | Fasciola (+) | Fasciola (−) | p | |
| Prevalence | ||||||
| Microscopy | 1247/2009 (62.1) | 762/2009 (37.9) | 579/923 (62.7) | 344/923 (37.3) | <0.001b | |
| Condemnation | NP | NP | 511/923 (55.4) | 412/923 (44.6) | ||
| Age in years | ||||||
| Mean (± SD) | 3.5 (1.9) | 3.2 (1.8) | 0.001 | 4.4 (2.4) | 3.4 (2.2) | <0.001 |
| Season | ||||||
| March to August | 496/733 (67.7) | 237/733 (32.3) | <0.001 | 507/646 (78.5) | 139/646 (21.5) | 0.21c |
| September to February | 751/1276 (58.9) | 525/1276 (41.1) | 207/277 (74.7) | 70/277 (25.3) | ||
| Province | ||||||
| Paruro/Abancay | 40/53 (75.5) | 13/53 (24.5) | 0.04 | 12/15 (80) | 3/15 (20) | 0.8 |
| Other | 1207/1956 (61.7) | 749/1956 (38.3) | 702/908 (77.3) | 206/908 (22.7) | ||
| Gender | ||||||
| Female | 612/967 (63.3) | 355/967 (36.7) | 0.278 | 335/430 (77.9) | 95/430 (22.1) | 0.7 |
| Male | 635/1042 (60.9) | 407/1042 (39.1) | 379/493 (76.9) | 114/493 (23.1) | ||
Fasciola infection determined by combination of microscopy and condemnation (714/923, 77.4%).
McNemar chi-squared comparing microscopy and liver condemnation.
Comparing March to June with January to February.
NP, not performed in all the cattle; SD, standard deviation.
FIG. 2.
Prevalence of Fasciola infection by month using bile microscopy (p < 0.01).
Complete data were available from 923 cattle slaughtered during the first 6 months of the study. Compared with the second 6 months, animals included in the first 6 months were older (mean age 4.2 ± 2.3 years compared with 2.6 ± 0.93 years, Student's t = 19.6, p < 0.01). More animals in the first 6 months had an unknown origin (446/923, 48.6% vs. 60/1086, 5.5%, p < 0.01) with fewer coming from Anta (435/923, 47.1% vs. 946/1086, 87.1%). The most common cattle breed was Creole (386/712, 54.2%), followed by Brown-Swiss (213/712, 29.9%) and Holstein (79/712, 11.1%).
The overall prevalence of Fasciola infection during the first 6 months was 77.4% (714/923). Bile microscopy diagnosed more infections than liver condemnation (McNemar test p < 0.01) (Table 1). Of those with livers condemned, Fasciola ova were identified in 376 of 511 cattle (73.6%). The agreement of the bile microscopy testing with the liver condemnation by inspection was fair (Cohen's κ = 0.247). Adult Fasciola parasites were evident in the liver upon inspection in 220 of 900 (24%) cattle.
The median carcass weight of cattle infected with Fasciola determined by bile microscopy was 150 kg (IQR 116–194) and that determined by liver condemnation was 143 kg (IQR: 114–186). There was a significant difference in carcass weight between animals with a positive bile test and animals with negative tests (median 157 kg [IQR 124–202] vs. 150 kg [IQR 116–194], Mann–Whitney U test 90934.5, p = 0.03). Similarly, there was a significant difference in carcass weight between animals with condemned livers and animals without condemned livers (median 166 kg [IQR 128–208] vs. 143 kg [IQR 114–186], Mann–Whitney U test 85,706, p < 0.001). The multiple regression model to predict the carcass weight used animal age, gender, breed, month of slaughter, liver condemnation, and positive bile microscopy as predictors. Animal age, gender, breed, and liver condemnation predicted carcass weight [F (df 4, 704) 61.1, p < 0.001] (Table 2).
Table 2.
Unstandardized B Coefficients for the Multiple Regression Model to Predict Cattle Carcass Weight
| Unstandardized coefficients | 95% CI | p | |
|---|---|---|---|
| Gender | 43.95 | 36.33 to 51.57 | <0.001 |
| Breed | 14.12 | 10.43 to 17.80 | <0.001 |
| Age | 6.44 | 4.89 to 7.98 | <0.001 |
| Liver condemnation | −17.51 | −25.25 to 9.77 | <0.001 |
| Durbin Watson = 1.81 | |||
CI, confidence interval.
Discussion
Fasciola infection is an important cause of decreased productivity in livestock due to decreased carcass weight and quality, liver condemnation, decreased milk production and quality, and decreased fertility (Charlier et al. 2020). Fascioliasis significantly threatens the income and food security among small scale farmers in poor areas of the world (Jaja et al. 2017a, Nyirenda et al. 2019, Arias-Pacheco et al. 2020). Our study documented a high prevalence of F. hepatica infection (77%) in cattle slaughtered in Anta. Some characteristics such as time of the year and animal origin helped predict prevalence and may aid in control strategies. Although bile microscopy detected more infections than liver condemnation, only the latter predicted carcass weight. Thus, these methods likely have a complementary role in the evaluation of the prevalence and burden of fascioliasis in livestock.
The 77% fascioliasis prevalence we documented in the Anta abattoir is significantly higher than the 29% prevalence reported for the Cusco region in official liver condemnation records from 2005 (Espinoza et al. 2010). Importantly, the prevalence we found in Anta is as high as that reported in highly endemic areas of Peru such as Cajamarca and Puno (Claxton et al. 1998). Animals brought from distant places such as Paruro and Abancay tended to have higher prevalence of infection. Valderrama Pome evaluating liver condemnation data reported a prevalence of Fasciola infection of ∼80% in Abancay (Valderrama 2016). Environmental and geographic factors that favor survival of the snail intermediate hosts and their infection with Fasciola are associated with higher prevalence in some regions. This highlights the lack of control on livestock movement between endemic areas despite standing regulations. Poor compliance with livestock movement regulations poses an important challenge to control programs in Peru because introduction of Fasciola or anthelminthic medication resistance to new areas is a possibility.
The prevalence of infection was higher when bile was tested for eggs compared with liver inspection and condemnation. Some condemned livers had no detectable eggs in the bile and some livers with positive bile microscopy were not condemned. The correlation of both diagnostic methods was only fair (Cohen's κ = 0.247). A possible explanation is that cattle with significant liver fibrosis may have received treatment before slaughter. Treatment may have cured the infection but did not reverse these pathologic changes. In this region, animals are commonly treated with triclabendazole during the fattening period before being shipped to slaughterhouses. This could lead to negative microscopic studies in previously infected animals. In contrast, microscopy may have detected low-intensity infections that failed to cause the intense fibrotic changes that lead to condemnation of livers. A study in Algeria found a higher prevalence of Fasciola by bile microscopy compared with postmortem liver inspection. In this study, 77 of 85 (90%) cattle infected with Fasciola were identified by bile microscopy and only 40 of 85 (47%) cattle were identified by liver inspection (Chaouadi et al. 2019). Thus, postmortem bile microscopy is a more sensitive way to detect Fasciola infection and may better reflect prevalence in the community.
In our study, liver condemnation predicted carcass weight. The association between liver condemnation and carcass weight and quality has been reported in several studies (Nyirenda et al. 2019, Arias-Pacheco et al. 2020). A Zambian study showed that leaner cattle had their livers condemned due to Fasciola infection significantly more often than other animals (Nyirenda et al. 2019). A study in South Africa compared premortem stool microscopy by the McMaster quantitative technique with liver condemnation postmortem to detect Fasciola infection. Liver condemnation detected ∼50% more infections than stool microscopy in this study. However, the severity of infection estimated by the number of eggs per gram of stool was associated with the carcass condition score (Jaja et al. 2017b). The carcass condition scores are quantitative tools to assess the muscle and fat composition of cattle regardless of breed for market purposes. Interestingly in our study, the univariate analysis showed that cattle with condemned livers had a higher median carcass weight than the carcass of cattle whose livers were not condemned. This was due to the variability in age and breed of the cattle. The multivariate regression analysis model showed that liver condemnation predicted a significant decrease in carcass weight.
Several factors were associated with higher prevalence of infection including animal age and month of slaughter. A study in Uruguay found that older cattle had a higher prevalence of Fasciola infection and suggested that older animals have more time to acquire infection by living through several transmission seasons and repeated liver injury due to recurring Fasciola infections (da Costa et al. 2019). We found the highest prevalence of infection between March and August at the beginning of the dry season. Prepatent fascioliasis takes at least 3 months for parasites to mature and start the production of eggs. These infections were likely acquired during the rainy warmer season (November to March) when environmental conditions favor snail and cercariae production (Charlier et al. 2014, Mas-Coma et al. 2018). Snails play a critical role in the lifecycle of Fasciola, generating hundreds of cercariae from a single miracidium hatched from an egg. Thus, snails are an important determinant of transmission and distribution of fascioliasis. Our findings could inform timing and choice of antiparasitic to control the infection among cattle in the area.
There are some limitations of our study. Our data were collected from a small municipal abattoir in a rural area known for its high human fascioliasis prevalence. Thus, our conclusions may not be applicable to other areas in the Cusco region. We also were unable to collect complete information during the second half of the project that limited the comparison between bile microscopy and liver condemnation to a smaller number of animals during the first half of the year. We only collected data from a single year. Thus, seasonality needs to be confirmed with a multiyear study. Our bile microscopy test did not include quantification of eggs. Egg quantification would have allowed evaluating whether heavier infection impacts carcass weight. Nevertheless, our data showed that fascioliasis is a common infection of cattle slaughtered in the Anta abattoir and that it has the predicted effects on decreased production.
Conclusion
The description of Fasciola distribution and transmission dynamics can aid in determining the risk of infection in different areas, expected high transmission seasons, and type of flukicide to use for control (Charlier et al. 2014). Combining bile microscopy and liver condemnation information provided a more accurate estimation of infection prevalence and distribution. Future slaughterhouse studies to evaluate fascioliasis burden in livestock should consider using condemnation and microscopy data for increased accuracy. Standardizing bile microscopy and introducing egg quantitation may improve prediction of infection burden and impact. Larger studies are needed to evaluate the validity of our results in other settings. However, our data may add to Fasciola control efforts in the Anta province.
Acknowledgments
The authors acknowledge the support from the Anta's Municipal Abattoir personnel and the Cusco's Regional Health Directorate.
Data Availability Statement
The data set for this article will be available upon request to the corresponding author.
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Allergy and Infectious Disease.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Part of this study was supported by the National Institute for Allergy and Infectious Diseases at the National Institutes of Health (Grant No. 1R01AI104820). This study was also supported by the Universidad Peruana Cayetano Heredia and University of Texas Medical Branch Collaborative Research Center in Cusco.
References
- American Association of Meat Producers. Using dentition to age cattle. United States Department of Agriculture, Food Safety and Inspection Service. 2012. Available at https://www.aamp.com/wp-content/uploads/Using-Dentation-to-Age-Cattle.pdf
- Arenal A, García Y, Quesada L, Velazquez D, et al. Risk factors for the presence of Fasciola hepatica antibodies in bulk-milk samples and their association with milk production decreases, in Cuban dairy cattle. BMC Vet Res 2018; 14:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Pacheco C, Lucas JR, Rodríguez A, Cordoba D, et al. Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Trop Anim Health Prod 2020; 52:1927–1932. [DOI] [PubMed] [Google Scholar]
- Byrne AW, Graham J, McConville J, Milne G, et al. Seasonal variation of Fasciola hepatica antibodies in dairy herds in Northern Ireland measured by bulk tank milk ELISA. Parasitol Res 2018; 117:2725–2733. [DOI] [PubMed] [Google Scholar]
- Byrne AW, McBride S, Lahuerta-Marin A, Guelbenzu M, et al. Liver fluke (Fasciola hepatica) infection in cattle in Northern Ireland: A large-scale epidemiological investigation utilising surveillance data. Parasit Vectors 2016; 9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona C, Tort JF. Fasciolosis in South America: Epidemiology and control challenges. J Helminthol 2017; 91:99–109. [DOI] [PubMed] [Google Scholar]
- Chaouadi M, Harhoura K, Aissi M, Zait H, et al. A post-mortem study of bovine fasciolosis in the Mitidja (north center of Algeria): Prevalence, risk factors, and comparison of diagnostic methods. Trop Anim Health Prod 2019; 51:2315–2321. [DOI] [PubMed] [Google Scholar]
- Charlier J, Duchateau L, Claerebout E, Williams D, et al. Associations between anti-Fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev Vet Med 2007; 78:57–66. [DOI] [PubMed] [Google Scholar]
- Charlier J, Rinaldi L, Musella V, Ploeger HW, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med 2020; 182:105103. [DOI] [PubMed] [Google Scholar]
- Charlier J, Vercruysse J, Morgan E, Van Dijk J, et al. Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle. Parasitology 2014; 141:326–335. [DOI] [PubMed] [Google Scholar]
- Claxton JR, Zambrano H, Ortiz P, Delgado E, et al. Strategic control of fasciolosis in the inter-Andean valley of Cajamarca, Peru. Vet Rec 1998; 143:42–45. [DOI] [PubMed] [Google Scholar]
- da Costa RA, Corbellini LG, Castro-Janer E, Riet-Correa F. Evaluation of losses in carcasses of cattle naturally infected with Fasciola hepatica: Effects on weight by age range and on carcass quality parameters. Int J Parasitol 2019; 49:867–872. [DOI] [PubMed] [Google Scholar]
- Dutra LH, Molento MB, Naumann CRC, Biondo AW, et al. Mapping risk of bovine fasciolosis in the south of Brazil using geographic information systems. Vet Parasitol 2010; 169:76–81. [DOI] [PubMed] [Google Scholar]
- Elliott TP, Kelley JM, Rawlin G, Spithill TW. High prevalence of fasciolosis and evaluation of drug efficacy against Fasciola hepatica in dairy cattle in the Maffra and Bairnsdale districts of Gippsland, Victoria, Australia. Vet Parasitol 2015; 209:117–124. [DOI] [PubMed] [Google Scholar]
- Espinoza JR, Terashima A, Herrera-Velit P, Marcos LA. Human and animal fascioliasis in peru: Impact in the economy of endemic zones. Rev Peru Med Exp Salud Publica 2010; 27:604–612. [DOI] [PubMed] [Google Scholar]
- Jaja IF, Mushonga B, Green E, Muchenje V. Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite Epidemiol Control 2017a; 2:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja IF, Mushonga B, Green E, Muchenje V. Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Vet Ani Sci 2017b; 4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbreras H, Cantella R, Burga R. About a rapid sedimentation procedure to evlaute Fasciola hepatica eggs: assessment and field use. [Spanish]. Rev Med Peru 1962; 31:167–174. [Google Scholar]
- Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018; 145:1665–1699. [DOI] [PubMed] [Google Scholar]
- Novobilský A, Novák J, Björkman C, Höglund J.. Impact of meteorological and environmental factors on the spatial distribution of Fasciola hepatica in beef cattle herds in Sweden. BMC Vet Res 2015; 11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda SS, Sakala M, Moonde L, Kayesa E, et al. Prevalence of bovine fascioliasis and economic impact associated with liver condemnation in abattoirs in Mongu district of Zambia. BMC Vet Res 2019; 15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vazquez MJ, Lewis FI. Investigating the impact of fasciolosis on cattle carcase performance. Vet Parasitol 2013; 193:307–311. [DOI] [PubMed] [Google Scholar]
- Schweizer G, Braun U, Deplazes P, Torgerson PR. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet Rec 2005; 157:188–193. [DOI] [PubMed] [Google Scholar]
- Valderrama A. Prevalence of fascioliasis in polygastric animals from Peru, [Spanish]. 1985–2015. Rev Med Vet 2016; 32:121–19. [Google Scholar]
- Villa-Mancera A, Reynoso-Palomar A. High prevalence, potential economic impact, and risk factors of Fasciola hepatica in dairy herds in tropical, dry and temperate climate regions in Mexico. Acta Trop 2019; 193:169–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set for this article will be available upon request to the corresponding author.