Abstract
Anthrax is a zoonosis caused by the spore-forming bacterium Bacillus anthracis, with potential for high fatality rate, especially in herbivores. Upon host death, spores can enter the soil surrounding the carcass and be ingested by other animals feeding in the same location. Accordingly, surveillance to quickly identify and decontaminate anthrax carcasses is crucial to outbreak prevention. In endemic anthrax areas such as Texas and Africa, vultures are used as a surveillance tool for identifying presence and location of dead animals. However, many anthrax outbreaks in the United States have occurred in areas outside the ranges of both black and turkey vultures. Here, we used a longitudinal camera trap survey at carcass sites in southwestern Montana to investigate the utility of facultative avian scavengers on disease and carcass surveillance in a reemerging anthrax risk zone. From August 2016 to September 2018, camera traps at 11 carcass sites were triggered 1996 times by avian scavengers. While the majority were facultative avian scavengers such as corvids and eagles, our results suggest that facultative scavengers cannot replace vultures as a surveillance tool in this ecosystem due to their absence during the anthrax risk period (June to August), reduced search efficiency, or low flight patterns. We found that the conditions in Montana likely parallel systems elsewhere in the continental United States. Using ecological niche models of B. anthracis distribution overlaid with relative abundance maps of turkey vultures, we found that much of North Dakota, South Dakota, Minnesota, Wyoming, Nebraska, and Iowa have areas of anthrax risk, but low or absent turkey vulture populations. Without vultures in these areas, surveillance capacity is reduced, and it becomes more difficult to identify anthrax cases, meaning fewer carcasses are decontaminated, and consequently, outbreaks could become more frequent or severe.
Keywords: vultures, turkey vultures, anthrax, zoonosis, obligate scavengers, corvids
Introduction
Anthrax is a neglected zoonosis caused by the bacterium Bacillus anthracis, meaning that overall burden and economic impacts are poorly described. Epizootics occur annually somewhere in the world, resulting in the deaths of hundreds to thousands of animals (Turnbull 2002, Food and Agriculture Organization of the United Nations et al. 2005, Shadomy et al. 2016). Most mammals are susceptible, but anthrax is primarily a disease of wild and domestic herbivores, which typically become infected when they ingest B. anthracis spores from contaminated soil, water, or vegetation or are bitten by flies carrying bacteria (Turell and Knudson 1987, Gates et al. 1995, Ganeva 2004). Inhalation is another possible route, but with limited evidence (Dragon et al. 1999, Barandongo et al. 2017).
When an animal succumbs to anthrax infection, B. anthracis sporulates and can enter the soil surrounding the dead host. Depending on environmental conditions, the bacteria can persist for decades in spore form (Dragon and Rennie 1995) until infection of another suitable host occurs. During an outbreak, the carcasses of animals dying from anthrax contaminate areas with spores in the soil and vegetation, creating locally infectious zones (LIZs) (Getz 2011). While outbreaks are the subject of ongoing management and control efforts globally, this complex disease cycle produces many management obstacles, including adequate surveillance.
Surveillance is crucial to identifying an outbreak at an early stage and preventing it from scaling up to a major epizootic (Hugh-Jones and de Vos 2002). To detect outbreaks early, land managers typically identify animals with clinical signs indicative of anthrax or find carcasses of suspected anthrax deaths, which are often bloated and without rigor mortis. Quickly locating and decontaminating anthrax carcasses can reduce the number of spores that contaminate the surrounding soil and limit the spread of bacteria associated with scavengers and biting flies (Sharp and Roberts 2006, Mongoh et al. 2008, Blackburn et al. 2010) and thus limit the risk of onward infections. Early identification of an outbreak allows land managers and stakeholders to implement other preventative measures, such as vaccination of healthy animals, limiting herd movement, quarantining animals displaying clinical signs, or treating domestic livestock in the early stages of the disease with antibiotics (Shadomy and Smith 2008).
While surveillance is critical for preventing epizootics, it requires substantial financial and workforce investments (Nishi et al. 2002). Therefore, the majority of surveillance occurs via opportunistic surveys—that is, carcasses are detected while observers are performing other tasks, such as managing livestock, distributing feed, or in routine transportation from one location to another (Bellan et al. 2013a). As one might expect, opportunistic surveys only detect a limited number of carcasses, with the majority of those being near roads, feeders, or other central locations (Bellan 2012). Detection of carcasses can also be accomplished indirectly via the detection of active scavenging (Bellan et al. 2013a).
Vultures are the scavenger species most relied upon for help in carcass detection (Hugh-Jones and de Vos 2002). Their soaring flight pattern can take them thousands of meters into the air, making them easily seen and followed from the ground (Pennycuick 1973, Dhawan 1991, Khosravifard et al. 2018). Further, as obligate scavengers, vultures are adapted to locate carrion quickly—oftentimes within 4 h of death (Prior and Weatherhead 1991, Platt et al. 2016, Harel et al. 2017). Accordingly, vultures are an invaluable surveillance tool for indirectly identifying dead animals and are used in this way in many contexts, including exposing locations of poachers to park rangers in Africa (Burton 2016).
Relative to anthrax, vulture aggregations can direct observers to carcasses. For example, during an anthrax outbreak in East Africa in 2017, radio-tagged white-backed vultures allowed researchers to establish both the location and timing of hippopotamus deaths (Kendall 2017). Vultures without radio tags can also be spotted and followed as a method of identifying anthrax cases as done during outbreaks in West Texas (Blackburn and Goodin 2013, Blackburn et al. 2014a). As Turnbull et al. (2008) state, “For game managers and farmers, circling vultures have always been, and continue to be, the best signal that deaths have occurred in the field” (p. 101).
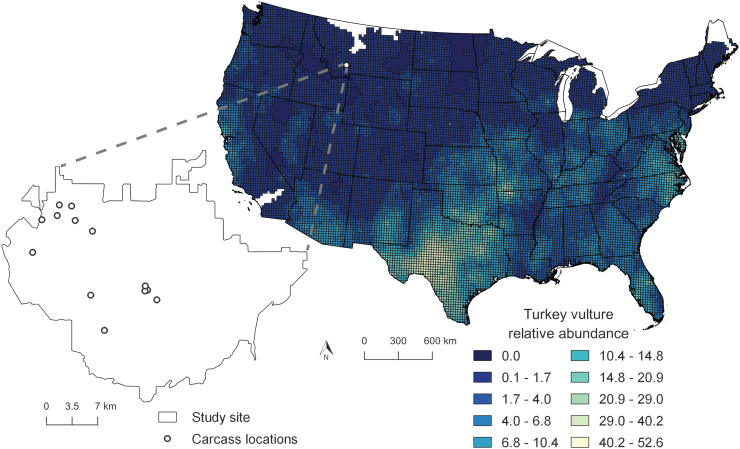
While vultures serve as a surveillance tool in many endemic anthrax areas, for some locations this is impossible, due to regional differences in avian species assemblages. For example, historical anthrax outbreaks have occurred in North Dakota (Centers for Disease Control and Prevention [CDC] 2001), South Dakota (Hugh-Jones 1999), Minnesota (Kanankege et al. 2019), and Montana (Blackburn et al. 2014b), but all four states are completely outside the range of black vultures (Coragyps atratus) and have limited areas where turkey vultures (Cathartes aura) are present (Fig. 1). Instead, scavenger guilds comprise species of facultative avian and mammalian scavengers in these areas.
FIG. 1.
A map showing turkey vulture relative abundance in the continental United States, based on the mean number of birds seen on North American Breeding Bird Survey routes from 2011 to 2015. The magnified inset shows the boundaries of our study site within southwestern Montana and the carcass camera trap locations.
No previous research has been conducted to investigate the effects of a limited or absent vulture population on disease and carcass surveillance or if alternative avian species—in particular, facultative avian scavengers—could substitute for vultures in carcass surveillance. Thus, the goals of this study were (1) to investigate the behavior and search efficiency of facultative avian scavengers in an endemic anthrax zone and (2) to examine the potential implications of a low vulture population on opportunistic anthrax surveillance across the continental U.S. Toward these goals, we used (1) data from a longitudinal camera trap study of animals interacting with carcasses and carcass sites in southwestern Montana, an area that has experienced anthrax outbreaks as recently as 2010 (Blackburn et al. 2014b) and (2) ecological niche model (ENM)-based estimates of the B. anthracis range overlaid with relative abundance maps of turkey vultures for the continental U.S.
Materials and Methods
Camera trap study of avian scavengers in southwest Montana
The field study was conducted on a privately owned ranch (∼300 km2) in southwestern Montana (Fig. 1 inset). Vegetation varies from mixed shrub and grassland to coniferous forest, and the ranch manages domestic plains bison (Bison bison bison) as livestock. The bison herd is monitored daily throughout the year by ranch staff using all-terrain vehicles (ATVs). Low fencing limits bison movements but does not restrict other species inhabiting the ranch, including elk (Cervus canadensis), white-tailed deer (Odocoileus virginianus), and mule deer (Odocoileus hemionus). A diverse carnivore and scavenger guild capitalizes on the presence of these herbivores and includes black bears (Ursus americanus), brown bears (Ursus arctos), gray wolves (Canis lupus), coyotes (Canis latrans), turkey vultures, American crows (Corvus brachyrhynchos) bald eagles (Haliaeetus leucocephalus), golden eagles (Aquila chrysaetos), common ravens (Corvus corax), and black-billed magpies (Pica hudsonia).
A major anthrax epizootic affected this study site in 2008, when at least 300 plains bison and 43 male elk succumbed to the disease (Blackburn et al. 2014b). Since 2008, routine surveillance has been conducted, and two wolf-killed bison tested positive for active B. anthracis infection in 2010 (Blackburn et al. 2014b). Seroprevalence studies found that ∼30% of male elk and 27% of unvaccinated bison tested were sero-positive for anthrax exposure (based on protective antigen; PAG) (Bagamian et al. 2013), suggesting that low-level exposure and survival of sublethal infections are relatively common in this area (Bagamian et al. 2013, Blackburn et al. 2014b).
For this study, we used camera trap data from 11 carcass sites. Monitoring began at 5 carcasses in the summer and fall of 2016, 1 in the fall of 2017, and 5 in the spring of 2018. Monitoring continued until September of 2018. Camera trap data presented here were “by-catch” from our larger project's primary objective to study ungulate interaction with carcasses and the area immediately nearby. These carcasses served as proxies for LIZs and were determined not to be contaminated with B. anthracis due to the animals dying outside of the anthrax risk period (June to August) and a lack of clinical signs such as incomplete rigor mortis, body position, or absence of blood clotting (Bengis and Frean 2014). Study staff were present for anthrax outbreaks in 2008 and 2010 and aware of signs. Any animals found dead suspected of anthrax are tested through by the State Diagnostic Laboratory, Montana Department of Livestock Health (Blackburn et al. 2014b).
We opportunistically set up motion-triggered camera traps at a combination of bison (9) and elk (2) carcasses within 24 h of animal death. Cameras were placed ∼10 meters away from the carcass at a height of 1–1.5 meters, and they were programmed to take photos without delay, at a rate of one picture every second once motion triggered. Cameras were visited weekly or biweekly to check battery levels and download photos from external data cards.
Images were organized and analyzed using the image management software digiKam (Kulzer et al. 2010). Each picture was manually assigned metadata tags classifying the number of animals and species present. All metadata were subsequently extracted from camera trap images using ExifTool, a command-line application for reading and editing metainformation (Harvey 2013); metadata were then imported into R using the camtrapR package (Niedballa et al. 2016). Here, all data pertaining to avian presence were analyzed.
Evaluating turkey vulture and anthrax distribution in the United States
To evaluate the distribution of turkey vultures and potential anthrax risk areas across the continental U.S., we overlaid mean turkey vulture counts from the Breeding Bird Survey (BBS; Fig. 1) over a recently published ENM-based estimate of anthrax suitability from Yang et al. (2020). In brief, that study used the genetic algorithm for rule-set prediction (GARP) to estimate the distribution of the A1.a lineage of B. anthracis, the dominant lineage isolated from anthrax outbreaks in Montana and the Dakotas, using the MERRAclim bioclimatic variables, soils data, and vegetation indices. GARP-based predictions use a subset of high-performing predictions made from a random walk through variable space, with each output a binary map of presence or absence across the landscape (here the continental U.S.). In that study, the GARP best subset procedure was used to select 10 best predicting models following Anderson et al. (2003) and summated [and evaluated with accuracy metrics; see Yang et al. (2020)]. To define areas of potential anthrax persistence, we selected a suitability cutoff of 6 or more models in the best subset agreeing, following the ‘moderate’ definition from Morris et al. (2016), which modeled anthrax risk for the Montana study site.
Turkey vulture counts were downloaded from BBS as a shapefile of relative abundance data from 2011 to 2015 from the USGS North American BBS (https://www.mbr-pwrc.usgs.gov/bbs/; accessed June 3, 2020) and rasterized to ∼4.5 × 4.5 km in Q-GIS version 3.8.2 and snapped to the ENM-based B. anthracis prediction. Data from the BBS are smoothed to create continuous surfaces, especially in remote areas. Consequently, the maps often do not define sharp edges of bird distributions and regions covered by the lowest abundance category may not routinely contain the species.
To consider different scenarios of bird abundance, vulture counts were classified 3 times: 10 or more individuals per cell, 5 or more individuals per cell, or any counts greater than 0 per cell. Each of the 3 vulture count surfaces were recoded as 0 (absent) or 2 (the count selected) and added to the GARP output of anthrax suitability, which was a binary raster of 0 (no anthrax) or 1 (anthrax potential), using the raster calculator in Q-GIS. Areas of overlap were identified when anthrax and vultures were present (score of 3) and color coded and contrasted with pixels of vultures/no anthrax (score of 2), anthrax potential/no vultures (score of 1), or no anthrax/no vultures (score of 0). Turkey vultures were the focus here as black vultures are mostly limited to the southeastern U.S. (Avery 2004) and have less overlap with areas of historical anthrax outbreaks.
Results
Camera trap study of avian scavengers in southwest Montana
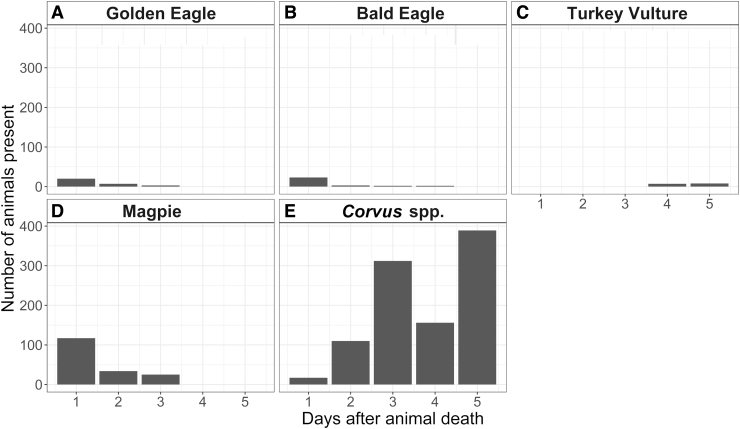
From August 2016 to September 2018, camera traps were triggered 725,000 times. Of these, 120,000 photos contained wildlife. A total of 1996 photos (1.66%) included scavenging avian species, which were golden eagles, bald eagles, turkey vultures, black-billed magpies, common ravens, and American crows (Fig. 2). We grouped common ravens and American crows during data analysis due to difficulty in distinguishing the species from photos and refer to them as Corvus spp. Most camera trap triggers by avian species occurred soon after animal death: of the 1996 total avian photos, 1235 (61.9%) of them were taken in the first 5 days and 1703 (85.3%) in the first 15 days after animal death. When examined by species, Corvus spp. and black-billed magpies were present at carcasses in the largest numbers, respectively, in the initial days following animal death. This was not the case for turkey vultures, which were present in low numbers overall and did not appear at carcasses until at least 4 days after animal death (Fig. 3).
FIG. 2.
Images of avian species captured during a camera trap study in southwestern Montana: golden eagle (A), turkey vulture (B), bald eagle (C), black-billed magpie (D), and Corvus spp. (E).
FIG. 3.
The number of (A) golden eagles, (B) bald eagles, (C) turkey vultures, (D) magpies, and (E) Corvus spp. captured by camera traps at carcass sites from 1 to 5 days after animal death. All avian scavengers were present in the initial days after death (days 1–3) apart from turkey vultures, which is unusual and is a finding unique to this ecosystem.
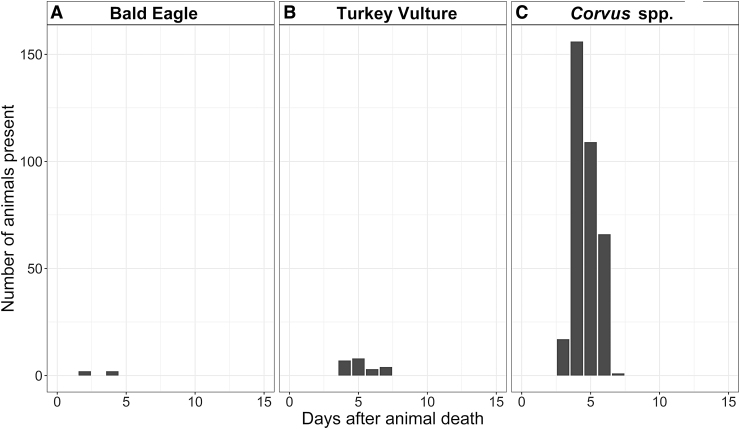
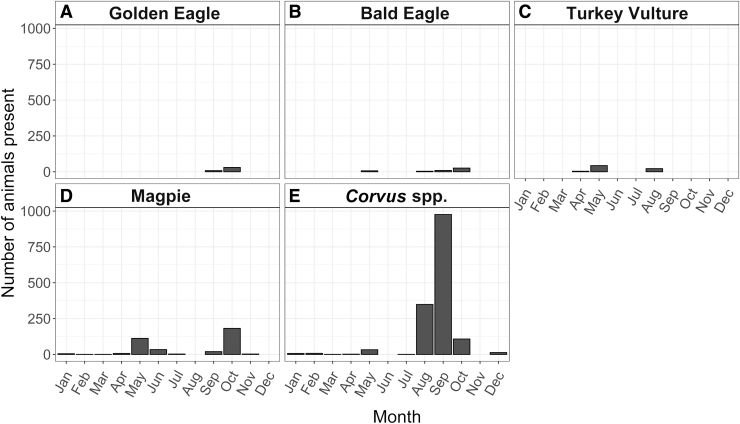
In this ecosystem, the “anthrax risk period” is currently defined as June to August—the months when anthrax was previously reported (Blackburn et al. 2014b, Morris et al. 2016). To determine which avian species were present at carcasses during these months, we examined the results from four of the carcasses initially discovered during the anthrax risk period—three carcasses that were discovered August 12, 2016 and one carcass from September 1, 2016. Only four avian species were present at these sites in the first 15 days after animal death: bald eagles, turkey vultures, and Corvus spp. (Fig. 4). Corvus spp. were present in the largest numbers during the anthrax risk period but did not arrive until 3 to 4 days following animal death. From a seasonal perspective, the only avian scavenger species present at the study site year-round were Corvus spp. and magpies (Fig. 5). Eagles were mostly present in late summer and early fall. Turkey vultures were present from April to August only, corresponding to their breeding season.
FIG. 4.
The counts of four avian species (A) bald eagles, (B) turkey vultures, and (C) Corvus spp. captured by camera traps at carcass sites from 1 to 15 days after animal death, when the animal died during the anthrax risk period (June to August). Corvus spp. (ravens and crows) are present in the largest numbers. Turkey vultures are absent in the initial days after death and are present only in low numbers from 4 days after death to 7 days after death. Magpies and golden eagles were absent; surprisingly, magpies are present at carcass sites during the anthrax risk period but were only seen 15 or more days after animal death.
FIG. 5.
Monthly totals of the number of (A) golden eagles, (B) bald eagles, (C) turkey vultures, (D) magpies, and (E) Corvus spp. present at carcass sites. Magpies and Corvus spp. are present throughout the year, and Corvus spp. have higher numbers in late summer and early fall (August to October). Turkey vultures are only present from April to August, a reflection that this location is likely part of their summer breeding grounds.
Evaluating turkey vulture and anthrax distribution in the United States
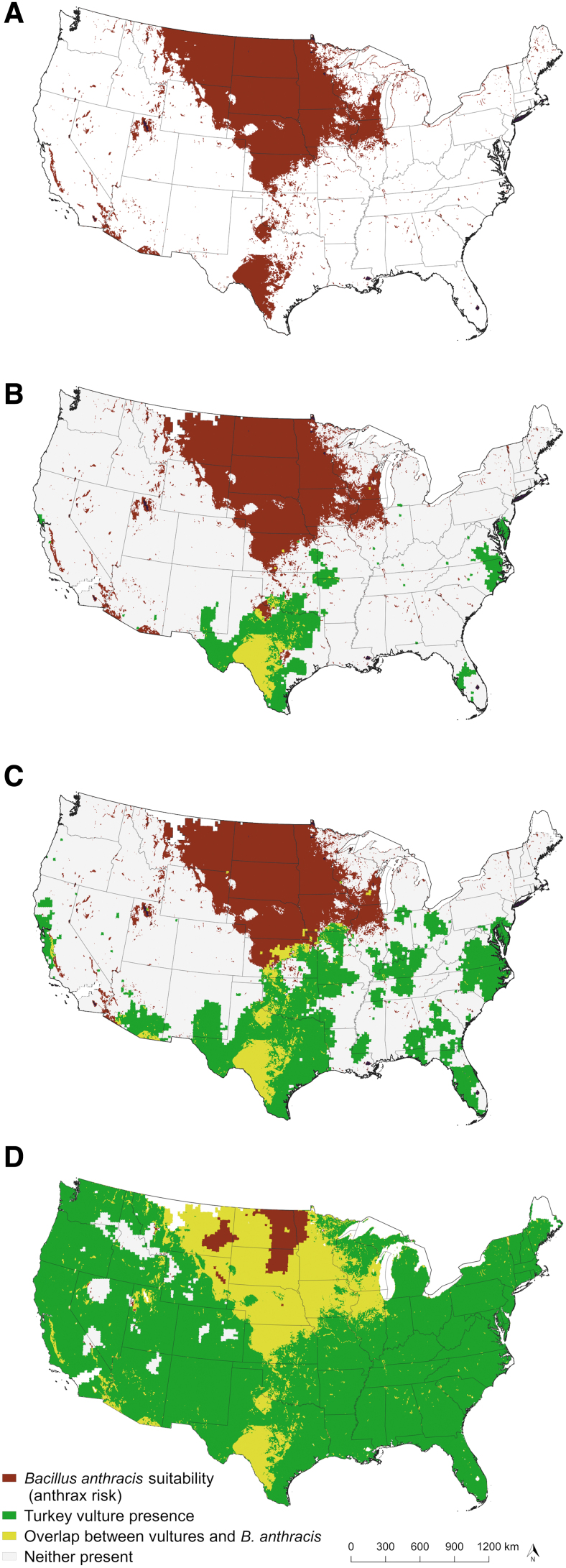
Figure 6 illustrates the potential anthrax risk (red areas), turkey vultures (green areas), and areas of overlap (yellow areas) across the continental U.S. for each vulture abundance cutoff: relative abundance of 10 or more vultures per count area (Fig. 6B), 5 or more per count area (Fig. 6C), or relative abundance above 0 (Fig. 6D). Based on our conservative (Fig. 6B) and moderate (Fig. 6C) cutoffs of turkey vulture abundance, in many northern Midwest states, including Montana, the Dakotas, and Minnesota (all states with recent and/or frequently reported anthrax), turkey vultures are nearly absent. Turkey vultures are in higher abundances in the southern extent of anthrax risk, in and around the enzootic zone of Texas as defined by Blackburn et al. (2014a)—an area with annual anthrax reports. Further, there was no overlap with turkey vultures and the potential anthrax zone in Montana based on the conservative and moderate relative abundance estimates.
FIG. 6.
Predicted overlap of turkey vulture (Cathartes aura) and Bacillus anthracis distribution in the continental United States based on three turkey vulture relative abundance cutoffs. (A) Prediction of B. anthracis suitability without vulture overlay; (B) prediction based on turkey vulture relative abundance of 10 individuals or higher; (C) prediction based on relative abundance of 5 individuals or higher; and (D) prediction based on relative abundance above 0.
Discussion
Using camera trap data collected at animal carcasses representing anthrax LIZs, we found that facultative avian scavengers do not serve as an adequate replacement for obligate avian scavengers as an anthrax surveillance tool in southwestern Montana. We argue that there are three main criteria for facultative avian scavengers to be used in disease surveillance: (1) facultative avian scavengers would need to be present at carcass sites during the anthrax risk period (June to August); (2) the search efficiency of facultative scavengers would need to be comparable to vultures to ensure carcasses are discovered quickly; and (3) the flight patterns and/or density of facultative scavengers would need to be high enough or in large enough numbers to be noticeable by land and game managers. Based on our results, these conditions were not met by any of the facultative avian scavengers in this system.
There were four facultative species present at carcass sites during the anthrax risk period: Black-billed magpies, bald eagles, and Corvus spp. (Fig. 5). However, magpies do not meet the condition for search efficiency, as they did not arrive at carcasses until at least 15 days after animal death. While bald eagles located carcasses quickly (arrival on the second day after animal death), during the anthrax risk period, a total of only four individuals were ever caught by camera traps. Arguably this low number suggests that bald eagles would easily be missed during opportunistic surveys and would not serve as a useful surveillance tool. Corvus spp. on the contrary, are present at carcasses during the anthrax risk period in large numbers, but their function in surveillance is discounted by their reduced search efficiency (they did not arrive at carcasses until 3 days after animal death) and low flight patterns—since they do not soar, crows and ravens are less likely than vultures to be seen from afar and even more unlikely to be followed by land managers to find carcasses.
The fact that facultative avian scavengers are inadequate as a surveillance tool is a problem compounded by a low population of obligate scavengers in this ecosystem. While the breeding range of turkey vultures extends into southwestern Montana, this is the edge of its northward range, so in some areas, like Gallatin county (where part of our study site is located), the BBS has not recorded sightings of turkey vultures since 2000. This was corroborated by the low number of individuals caught by our camera traps and parallels results from Wilmers et al. (2003) which found magpies, ravens, and eagles feeding at elk carcasses in Yellowstone National Park (∼70 km from our study site), but no vultures. Due to this scarcity, turkey vultures are likely an unreliable method of surveillance during anthrax outbreaks in southwestern Montana. Overall, the assemblage of avian species present in the montane ecosystem makes this means of surveillance difficult and ineffective.
Apart from hindering surveillance, an absence of vultures at carcasses has been associated with longer decomposition times, more individual mammals at carcasses, and mammals spending more time at carcasses (Ogada et al. 2012), all of which could lead to increased anthrax transmission. Prior work in this system has found that grazing ungulates are attracted to LIZs (Turner et al. 2014, Walker et al. 2020), where they may ingest B. anthracis spores and subsequently become infected with anthrax, which is why land managers finding and decontaminating anthrax carcasses during an outbreak is crucial to prevention or reduction of future transmission.
However, the role of vultures in disease transmission is complex, as their potential to disseminate anthrax spores has long been debated (Bullock 1956). Anthrax spores have been found in the feces of scavenging raptors (Saggese et al. 2007) and multiple vulture species (Lindeque and Turnbull 1994). Spores have also been isolated from the feathers, feet, and bills of vultures in Kruger (Pienaar 1967) and Etosha National Parks (Ebedes 1977) (Africa), and West Texas (feathers; Blackburn, unpublished data).
Despite this, other studies have reported no anthrax spores present in bill and mouth swabs of vultures in southern Africa (Mundy and Brand 1978), and did not find higher concentrations of anthrax spores at vulture nests, possibly due to the acidity of vulture feces (Ganz et al. 2012). A study on the exclusion of scavengers from carcass sites revealed that scavengers had no effect on B. anthracis soil spore density, also failing to provide evidence that vultures or other scavengers lead to contamination of carcass sites (Bellan et al. 2013b). Thus, it is difficult to make definitive conclusions about the role of vultures in the epidemiology of anthrax, apart from their benefit as an aid in carcass detection and surveillance. More broadly, vultures provide a critical ecosystem service by removing decomposing organic material from the environment, effectively reducing the risk of infectious disease in humans and other animals (Markandya et al. 2008, Gangoso et al. 2013, Moleón et al. 2014).
A scarcity of obligate scavengers not only impacts the montane ecosystem, but also affects wide swaths of the continental U.S. located in endemic anthrax zones. Using ENM-based predictions of B. anthracis distribution (Fig. 6A) overlaid with relative abundance maps of turkey vultures (yellow areas in Fig. 6B–D), we found much of North Dakota, South Dakota, Minnesota, Wyoming, Nebraska, and Iowa have areas of anthrax risk and low or absent turkey vulture populations. According to our most conservative vulture cutoffs (Fig. 6B), West Texas is the only anthrax risk area in the United States theoretically able to heavily rely on turkey vultures as a surveillance tool.
West Texas is also home to one of the highest densities of white-tailed deer in the United States (Quality Deer Management Association 2009), a species commonly implicated in anthrax outbreaks (Kellogg et al. 1970, Hugh-Jones 1999, Blackburn and Goodin 2013, Blackburn et al. 2014a). The only other suitable area for anthrax with such a high white-tailed deer density is Wisconsin (Quality Deer Management Association 2009), although anthrax is rarely reported in Wisconsin. Likewise, the abundance of turkey vultures in Wisconsin is low, with only our most liberal models (Fig. 6D) indicating any overlap between B. anthracis suitability and vultures.
These areas of the Midwest United States with high B. anthracis suitability likely have not suffered from vulture population declines; in fact, from 1967 to 2004, BBS data reflected an estimated average annual increase in turkey vulture populations of 1.37% and in black vulture populations of 2.99% (Avery 2004). Rather, the breeding range of turkey vultures is discontinuous in much of the western United States and is very local or absent in portions of the Great Plains (Stewart 1975, Johnsgard 1979, Janssen 1987, Peterson 1995), either of which could explain their absence.
Conclusions
The increase in vultures in the United States is an anomaly. Globally, obligate scavengers are experiencing large population declines. Nine of 23 vulture species (39%) are classified as “Critically Endangered” (Buechley and Şekercioğlu 2016). An additional three species are Endangered and four are Near Threatened. Ultimately, regardless of whether a low or absent vulture population is due to natural range limits or ongoing population declines, the cascade of problems created for anthrax prevention are the same: without vultures for surveillance, it becomes more difficult to identify cases; less cases are decontaminated; less decontamination allows more bacteria to sporulate and contaminate the surrounding areas; and consequently, outbreaks could become more frequent or severe.
Areas with high B. anthracis suitability and a scarcity of obligate scavengers should prioritize carcass detection and decontamination efforts that go beyond opportunistic surveys. Alternative methods of surveillance may include aerial surveys via fixed-wing aircraft (Blackburn et al. 2014b), helicopter (Dragon et al. 1999), or drone (Tirado and Cano 2019) combined with the use of thermal imaging equipment (Gates et al. 1995). Additional prevention and management strategies could include vaccination of livestock, increased education of stakeholders, restriction of movement of healthy animals during outbreak periods, and collaboration between private, state, and federal landholders.
Data Depository
Ethics Statement
Camera trap data were analyzed under an observational IACUC approved by the University of Florida (No. 201910563 to J.K.B.).
Acknowledgments
We thank the ranch staff for access, logistical support, local knowledge, and animal/data collection expertise. D. Zincke facilitated sample and data shipments. M. Norris and T. Hadfield provide insights on B. anthracis biology.
Authors' Contributions
M.A.W. and J.K.B. initiated this study. Data were collected by V.A. and M.A.W. M.A.W., M.U., and J.K.B. analyzed the data. J.K.B., W.M.G., S.J.R., and J.M.P. secured project funding. M.A.W. and J.K.B. wrote the article. All authors contributed to the final version and gave approval.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
This work was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under award number R01GM117617 to J.K.B., W.M.G., S.J.R., and J.M.P. Additional support was provided by the Emerging Pathogens Institute at the University of Florida.
References
- Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species' distributions: Criteria for selecting optimal models. Ecol Model 2003; 162:211–232. [Google Scholar]
- Avery ML. Trends in North American vulture populations. Proc Vertebr Pest Conf 2004; 21. [Google Scholar]
- Bagamian KH, Alexander KA, Hadfield TL, Blackburn JK. Ante-and postmortem diagnostic techniques for anthrax: Rethinking pathogen exposure and the geographic extent of the disease in wildlife. J Wildl Dis 2013; 49:786–801. [DOI] [PubMed] [Google Scholar]
- Barandongo ZR, Mfune JKE, Turner WC. Dust-bathing behaviors of african herbivores and the potential risk of inhalational anthrax. J Wildl Dis 2017; 54:34–44. [DOI] [PubMed] [Google Scholar]
- Bellan SE. Applications of Data-Driven Modeling to Infectious Diseases in Africa: Anthrax in Wildlife and HIV in Humans. California, USA: UC Berkeley, 2012. [Google Scholar]
- Bellan SE, Gimenez O, Choquet R, Getz WM. A hierarchical distance sampling approach to estimating mortality rates from opportunistic carcass surveillance data. Methods Ecol Evol 2013a; 4:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan SE, Turnbull PCB, Beyer W, Getz WM. Effects of experimental exclusion of scavengers from carcasses of anthrax-infected herbivores on Bacillus anthracis sporulation, survival, and distribution. Appl Environ Microbiol. 2013b; 79:3756–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis RG, Frean J. Anthrax as an example of the One Health concept. Rev Sci Tech 2014; 33:593–604. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Asher V, Stokke S, Hunter DL, et al. . Dances with anthrax: Wolves (Canis lupus) kill anthrax bacteremic plains bison (Bison bison bison) in southwestern Montana. J Wildl Dis 2014b; 50:393–396. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Curtis A, Hadfield TL, O'Shea B, et al. . Confirmation of Bacillus anthracis from flesh-eating flies collected during a West Texas anthrax season. J Wildl Dis 2010; 46:918–922. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Goodin DG. Differentiation of springtime vegetation indices associated with summer anthrax epizootics in West Texas, USA, Deer J Wildl Dis 2013; 49:699–704. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Hadfield TL, Curtis AJ, Hugh-Jones ME. Spatial and temporal patterns of anthrax in white-tailed deer, Odocoileus virginianus, and Hematophagous flies in West Texas during the summertime anthrax risk period. Ann Assoc Am Geogr 2014a; 104:939–958. [Google Scholar]
- Buechley ER, Şekercioğlu ÇH. The avian scavenger crisis: Looming extinctions, trophic cascades, and loss of critical ecosystem functions. Biol Conserv 2016; 198:220–228. [Google Scholar]
- Bullock DS. Vultures as disseminators of anthrax. Auk 1956; 73:283–284. [Google Scholar]
- Burton A. Poaching with poison devastates African vultures. Front Ecol Environ 2016; 14:5–5. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Human anthrax associated with an epizootic among livestock—North Dakota, 2000. MMWR Morb Mortal Wkly Rep 2001; 50:677–680. [PubMed] [Google Scholar]
- Dhawan S. Bird flight. Sadhana 1991; 16:275–352. [Google Scholar]
- Dragon DC, Elkin BT, Nishi JS, Ellsworth TR. A review of anthrax in Canada and implications for research on the disease in northern bison. J Appl Microbiol 1999; 87:208–213. [DOI] [PubMed] [Google Scholar]
- Dragon DC, Rennie RP. The ecology of anthrax spores: Tough but not invincible. Can Vet J 1995; 36:295–301. [PMC free article] [PubMed] [Google Scholar]
- Ebedes H. Anthrax epizootics in Etosha National Park. Madoqua 1977; 1977:99–118. [Google Scholar]
- Food and Agriculture Organization of the United Nations, Great Britain, Department for International Development, Animal Health Programme, et al. The Control of neglected zoonotic diseases: A route to poverty alleviation: Report of a joint WHO/DFID-AHP meeting, WHO Headquarters, Geneva, with the participation of FAO and OIE. Geneva, Switzerland: World Health Organization, 2005. [Google Scholar]
- Ganeva DJ. Analysis of the Bulgarian tabanid fauna with regard to its potential for epidemiological involvement. Bulg J Vet Med 2004; 7:1–8. [Google Scholar]
- Gangoso L, Agudo R, Anadón JD, De la Riva M, et al. . Reinventing mutualism between humans and wild fauna: Insights from vultures as ecosystem services providers. Conserv Lett 2013; 6:172–179. [Google Scholar]
- Ganz H, Karaoz U, Getz W, Versfeld W, et al. . Diversity and structure of soil bacterial communities associated with vultures in an African savanna. Ecosphere 2012; 3:1–18. [Google Scholar]
- Gates CC, Elkin BT, Dragon DC. Investigation, control and epizootiology of anthrax in a geographically isolated, free-roaming bison population in northern Canada. Can J Vet Res 1995; 59:256–264. [PMC free article] [PubMed] [Google Scholar]
- Getz WM. Biomass transformation webs provide a unified approach to consumer–resource modelling. Ecol Lett 2011; 14:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel R, Spiegel O, Getz WM, Nathan R. Social foraging and individual consistency in following behaviour: Testing the information centre hypothesis in free-ranging vultures. Proc Biol Sci 2017; 284:20162654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P. ExifTool by Phil Harvey (Aktualizace December 21, 2013 [cited December 22, 2013]. Dostupné z: Available at http://www. sno. phy …).
- Hugh-Jones M. 1996–97 global anthrax report. J Appl Microbiol 1999; 87:189–191. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones ME, de Vos V. Anthrax and wildlife. Rev Sci Tech 2002; 21:359–383. [DOI] [PubMed] [Google Scholar]
- Janssen RB. Birds in Minnesota. Minneapolis, MN: University of Minnesota Press, 1987. [Google Scholar]
- Johnsgard PA. Birds of the Great Plains: Breeding Species and Their Distribution. Lincoln: University of Nebraska Press, 1979. [Google Scholar]
- Kanankege KS, Abdrakhmanov SK, Alvarez J, Glaser L, et al. . Comparison of spatiotemporal patterns of historic natural Anthrax outbreaks in Minnesota and Kazakhstan. PLoS One 2019; 14:e0217144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg FE, Prestwood AK, Noble RE. Anthrax epizootic in white-tailed deer. J Wildl Dis 1970; 6:226–228. [DOI] [PubMed] [Google Scholar]
- Kendall C. Vultures, Hippos and Anthrax. Scientific American 2017. https://blogs.scientificamerican.com/observations/vultures-hippos-and-anthrax/, accessed February 18, 2021.
- Khosravifard S, Venus V, Skidmore AK, Bouten W, et al. . Identification of griffon vulture's flight types using high-resolution tracking data. Int J Environ Res 2018; 12:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulzer G, Caulier G, Stone E, Doerr O, et al. The digiKam Handbook. 2010. www.digikam.org, accessed July 1, 2019.
- Lindeque PM, Turnbull PC. Ecology and epidemiology of anthrax in the Etosha National Park, Namibia. Onderstepoort J Vet Res 1994; 61:71–83. [PubMed] [Google Scholar]
- Markandya A, Taylor T, Longo A, Murty MN, et al. . Counting the cost of vulture decline—An appraisal of the human health and other benefits of vultures in India. Ecol Econ 2008; 67:194–204. [Google Scholar]
- Moleón M, Sánchez-Zapata JA, Margalida A, Carrete M, et al. . Humans and scavengers: The evolution of interactions and ecosystem services. BioScience 2014; 64:394–403. [Google Scholar]
- Mongoh MN, Dyer NW, Stoltenow CL, Hearne R, et al. . A review of management practices for the control of anthrax in animals: The 2005 anthrax epizootic in North Dakota—case study. Zoonoses Public Health 2008; 55:279–290. [DOI] [PubMed] [Google Scholar]
- Morris LR, Proffitt KM, Asher V, Blackburn JK. Elk resource selection and implications for anthrax management in Montana. J Wildl Manag 2016; 80:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy PJ, Brand FE. Investigation of vultures and anthrax in Southern Africa. Rhod Vet J 1978; 9:36–39. [Google Scholar]
- Niedballa J, Sollmann R, Courtiol A, Wilting A. camtrapR: An R package for efficient camera trap data management. Methods Ecol Evol 2016; 7:1457–1462. [Google Scholar]
- Nishi JS, Dragon DC, Elkin BT, Mitchell J, et al. . Emergency response planning for anthrax outbreaks in bison herds of Northern Canada. Ann N Y Acad Sci 2002; 969:245–250. [DOI] [PubMed] [Google Scholar]
- Ogada DL, Torchin ME, Kinnaird MF, Ezenwa VO. Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv Biol 2012; 26:453–460. [DOI] [PubMed] [Google Scholar]
- Pennycuick CJ. The soaring flight of vultures. Sci Am 1973; 229:102–109.4731796 [Google Scholar]
- Peterson RA. The South Dakota Breeding Bird Atlas. South Dakota Ornithologists' Union. Jamestown, ND: Northern Prairie Wildlife Research Center Online, 1995. [Google Scholar]
- Pienaar UD. Epidemiology of anthrax in wild animals and the control of anthrax epizootics in the Kruger National Park, South Africa. In Federation Proceedings, 1967; 26:1496–1502. [PubMed] [Google Scholar]
- Platt SG, Rainwater TR, Miller SM. An observational study of carrion use by foraging turkey vultures (Cathartes aura) in West Texas. Tex Ornithol Soc 2016; 49:65. [Google Scholar]
- Prior KA, Weatherhead PJ. Competition at the carcass: Opportunities for social foraging by turkey vultures in southern Ontario. Can J Zool 1991; 69:1550–1556. [Google Scholar]
- Quality Deer Management Association. QDMA Whitetail Report. Bogart, GA, 2009:68. https://deerassociation.com/wp-content/uploads/2016/07/2009_Whitetail_Report.pdf, accessed February 18, 2021. [Google Scholar]
- Saggese MD, Noseda RP, Uhart MM, Deem SL, et al. . First detection of Bacillus anthracis in feces of free-ranging raptors from central Argentina. J Wildl Dis 2007; 43:136–141. [DOI] [PubMed] [Google Scholar]
- Shadomy S, El Idrissi A, Raizman E, Bruni M, et al. . Anthrax Outbreaks: A Warning for Improved Prevention, Control and Heightened Awareness. Rome, Italy: FAO, 2016. [Google Scholar]
- Shadomy SV, Smith TL. Anthrax. J Am Vet Med Assoc 2008; 233:63–72. [DOI] [PubMed] [Google Scholar]
- Sharp RJ, Roberts AG. Anthrax: The challenges for decontamination. J Chem Technol Biotechnol 2006; 81:1612–1625. [Google Scholar]
- Stewart RE. Breeding birds of North Dakota (Tri-college center for environmental studies Fargo, ND). 1975. [Google Scholar]
- Tirado F, Cano PT. Drones and epidemiology: A new anatomy for surveillance. BioSocieties 2020; 15:115–133. [Google Scholar]
- Turell MJ, Knudson GB. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus). Infect Immun 1987; 55:1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull PCB. Introduction: Anthrax history, disease and ecology. Curr Top Microbiol Immunol 2002; 271:1–19. [DOI] [PubMed] [Google Scholar]
- Turnbull PCB, Diekmann M, Kilian JW, Versfeld W, et al. . Naturally acquired antibodies to Bacillus anthracis protective antigen in vultures of southern Africa. Onderstepoort J Vet Res 2008; 75:95–102. [DOI] [PubMed] [Google Scholar]
- Turner WC, Kausrud KL, Krishnappa YS, Cromsigt JP, et al. . Fatal attraction: Vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proc Biol Sci 2014; 281:20141785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Uribasterra M, Asher V, Ponciano JM, et al. . Ungulate use of locally infectious zones in a re-emerging anthrax risk area. R Soc Open Sci 2020; 7:200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmers CC, Crabtree RL, Smith DW, Murphy KM, et al. . Trophic facilitation by introduced top predators: Grey wolf subsidies to scavengers in Yellowstone National Park. J Anim Ecol 2003; 72:909–916. [Google Scholar]
- Yang A, Gomez JP, Blackburn JK. Exploring environmental coverages of species: A new variable contribution estimation methodology for rulesets from the genetic algorithm for rule-set prediction. PeerJ 2020; 8:e8968. [DOI] [PMC free article] [PubMed] [Google Scholar]