Abstract
Background:
The relationship between cerebral autoregulation and outcomes in pediatric complex mild traumatic brain injury (TBI) is unknown, and explored in this study.
Methods:
We conducted a prospective observational study of patients aged 0–18 years hospitalized with complex mild TBI (admission Glasgow Coma Scale score 13–15 with either abnormal computerized tomogram of the head or history of loss of consciousness). Cerebral autoregulation was tested using transcranial Doppler ultrasonography, and impaired autoregulation defined as autoregulation index < 0.4. We collected Glasgow outcome scale extended-pediatrics score and health-related quality of life data at 3, 6, and 12 months after discharge.
Results:
24 patients aged 1.8 to 16.6 years (58.3% male) with complete 12-month outcome data were included in the analysis. Median admission Glasgow Coma Scale score was 15 (range 13–15), median injury severity score was 12 (range 4–29) and 23 patients (96%) had isolated TBI. Overall, 10 (41.7%) patients had impaired cerebral autoregulation. Complete recovery was observed in 6 of 21 (28.6%) children at 3 months, in 4 of 16 (25%) children at 6 months, and in 8 of 24 (33.3%) children at 12 months. There was no difference in median [interquartile range] Glasgow outcome scale extended-pediatrics score (2 [2.3] vs. 2 [IQR 1.3]) or health-related quality of life scores (91.5 [21.1] vs. 90.8 [21.6]) at 12 months between those with intact and impaired autoregulation, respectively. Age-adjusted hypotension occurred in 2/24 (8.3%) patients.
Conclusion:
Two-thirds of children with complex mild TBI experienced incomplete functional recovery at one year. The co-occurrence of hypotension and cerebral autoregulation may be a sufficiency condition needed to affect TBI outcomes.
Keywords: Cerebral autoregulation, pediatrics, complex mild traumatic brain injury, Glasgow outcome scale-extended, health-related quality of life
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in children of all age groups except infants < 1 year of age.1 In 2014, there were 2.87 million TBI-related emergency department visits, hospitalizations, or deaths in the US; over 837,000 TBI-related emergency visits occurred among children, of which 23,000 required hospitalization.2 The majority of hospitalizations were for children with mild TBI.3
Despite the traditional categorization of TBI as mild, moderate, or severe for care and prognostication purposes, clinical TBI severity varies within these stratifications, and there is an overlap in phenotype between categories.4 Additionally, patients do not uniformly achieve complete recovery even within categories, such as mild TBI.5–7 This may be because even within the mild TBI category, there is a more serious subset wherein patients are hospitalized for observation and have abnormalities on neuroimaging; these patients are often deemed to have complex mild TBI. TBI severity is usually categorized into these structurally distinct but functionally overlapping categories by admission Glasgow Coma Scale (GCS) scores. Although patients with complex mild TBI are a subset of those with mild TBI, data on post-discharge outcomes from patients with complex mild TBI have been combined with patients with mild TBI who may be evaluated and discharged without admission to hospital. Additionally, published studies lack quality of life data and/or only examined short-term outcomes.8,9 The largest study of complex mild TBI published in 2011 showed that patients with mild TBI and intracranial hemorrhage had a substantial reduction in quality of life, participation in activities with others, and communication and care for themselves at three months after injury7. Since recovery after mild TBI is variable, with many children having residual long-term needs,10 and since children with complex mild TBI may have more significant TBI, there is an unmet need to understand outcomes of children specifically with complex mild TBI.
Cerebral autoregulation status may affect outcomes after severe TBI.11–13 However, little is known about whether and how cerebral autoregulation affects long-term functional outcomes in children with complex mild TBI. Studies of pediatric TBI show that cerebral autoregulation is often impaired in higher TBI severity14 and low GCS. In moderate-severe pediatric TBI, impaired cerebral autoregulation is common and associated with poor 12-month outcomes.11–13,15,16 Systolic hypotension is also an independent predictor of poor six-month outcomes in moderate-severe TBI in children.15 Although cerebral autoregulation is a protective homeostatic mechanism that typically maintains adequate and near-constant cerebral blood flow, systemic hypotension after TBI can result in cerebral hypoperfusion or ischemia if mechanisms that govern cerebral autoregulation are only minimally or moderately impaired. Prior work has shown that impaired autoregulation occurs commonly in adolescents admitted with a sports-related concussion,17 and in children with complex mild TBI,14 and that there is no relationship between cerebral blood flow velocities and outcomes 4–6 weeks after injury.8 However, the association between autoregulation status and long-term functional outcomes has not been studied. The specific aim of this study was to examine the association between static cerebral autoregulation measured by the autoregulatory index (ARI) during the first week after TBI and long-term outcomes in children with complex mild TBI.
Methods
The University of Washington’s Human Subjects Division approved this prospective observational study on December 1, 2015 (Study number 35291-D). Informed consent was obtained from parents/legal guardians, and assent was obtained from patients.
Children aged 18 years or younger with a diagnosis of complex mild TBI (defined as admission GCS 13–15 with either abnormal head computerized tomography [CT] scan or history of loss of consciousness) and no or mild extracranial injury (non-head abbreviated injury score 0–3) who were admitted consecutively to the pediatric intensive care unit at Harborview medical center (level 1 pediatric trauma center) were eligible for inclusion in this study. Enrollment into the study began in January 2016 and continued until September of 2017. Twelve-month follow-up was completed in September 2018.
Autoregulation testing
Cerebral autoregulation was assessed using transcranial Doppler (TCD) ultrasound and tilt testing during the first week after admission as previously described.14 In brief, a change in head and back position from supine to upright serves as a cerebral autoregulatory stimulus.14 For the relatively upright position, the vertical distance between a noninvasive blood pressure cuff and the external auditory meatus was used to calculate the estimated mean arterial pressure (MAPe) at the Circle of Willis. Mean arterial pressure decreases by 1 mm Hg for every 1.36 cm increase in vertical height, so the change in height from supine to upright position was divided by 1.36 to calculate MAPe in the sitting position.17–19 A target 10 mmHg decrease in MAPe between supine and upright positions served as the autoregulatory stimulus during testing. The ARI for each middle cerebral artery was calculated off-line as previously described.17 Mathematically, cerebral autoregulation was quantified using the ARI as follows:
where eCVR is estimated cerebrovascular resistance calculated as the ratio of mean arterial pressure to middle cerebral artery blood flow velocity as appropriate. Thus, ARI is equal to the percentage of change in estimated cerebrovascular resistance divided by the percentage of change in cerebral perfusion pressure. An ARI of 0 represents absent autoregulation (pressure-dependent middle cerebral artery blood flow velocity),13,20 whereas an ARI of 1.0 represents perfect autoregulation. For the purpose of statistical analysis, we dichotomized ARI into intact and impaired cerebral autoregulation; impaired cerebral autoregulation was defined as unilateral or bilateral ARI less than 0.4.21 Systolic hypotension was defined as systolic blood pressure < (70mmHg + 2 [age]).22
Data collection
Demographic data, including age, sex, Injury Severity Score, admission GCS, intensive care unit and hospital length of stay, GCS at discharge, mechanism of injury, loss of consciousness before admission to hospital, intracranial pathology on cranial CT scan, maximum abbreviated injury score for head and non-head regions, and contemporaneous blood pressure data within 24 hours of TCD examination, were retrospectively abstracted from the electronic medical records.
Outcome measures
Glasgow Outcome Scale Extended-Pediatrics (GOSE-P) score23,24 and health-related quality of life (HRQOL) were assessed at 3, 6, and 12 months after hospital discharge by a trained assessor blinded to the results of the autoregulation status. The assessments were conducted via the telephone or by mail, and responses were provided by the child’s parent or guardian. The GOSE-P is an 8-point score that categorizes outcomes: 1, upper good recovery; 2, lower good recovery; 3, upper moderate disability; 4, lower moderate disability; 5, upper severe disability; 6, lower severe disability; 7, vegetative state, and; 8, death. For the purposes of this study, outcome was dichotomized into complete recovery (GOSE-P =1) and incomplete recovery (GOSE-P ≥ 2).25,26 The Pediatric Quality of Life (PedsQL) Inventory was used to measure HRQOL.27 The PedsQL includes 23 questions that test physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). Three summary scores were computed - psychosocial health summary score, physical health summary score, and total scale score. The psychosocial health summary score is an average of emotional, social, and school functioning. The physical health summary score is calculated as the sum of scores in physical functioning divided by the number of answered items. The total score (ranging from 0 to 100) is an average of all functioning, with a higher score indicating higher function.
Statistical analysis
Stata version 16 was used for statistical analysis.28 Student’s t-test and Fisher exact test were used to analyze differences in patient characteristics, cerebral autoregulation status, clinical data between complete and incomplete recovery groups, and to assess the difference in functional outcomes between impaired and intact autoregulation groups. Data are presented as mean ± standard deviation, and median (range). p <0.05 reflects statistical significance.
Results
Of 31 patients enrolled into the study, 24 with complete 12-month GOSE-P and HRQOL outcome data were included in the analysis (Figure 1). All 24 patients were discharged alive. Nineteen of the 24 patients (79.2%) received follow up at three months, 15 (62.5%) at six months, and 24 (100%) were at twelve months.
Figure 1. Post-discharge follow-up at 3, 6, and 12 months in children with complex mild traumatic brain injury undergoing cerebral autoregulation testing.
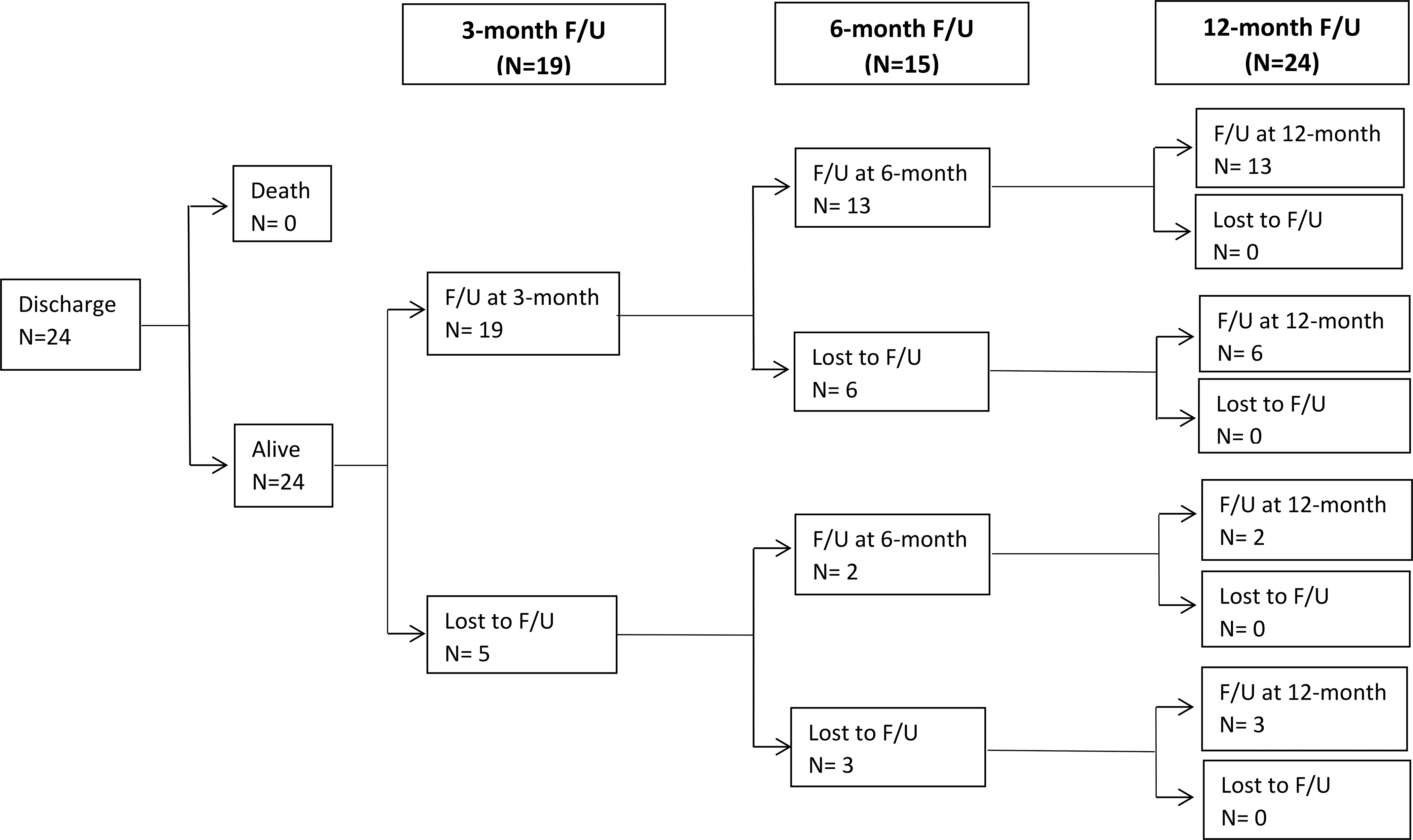
Thirty-one children were enrolled into the study, and 24 with complete 12-month GOSE-P and HRQOL data included in the analysis
F/U, follow up; GOSE-P, Glasgow Outcome Scale Score Extended -Pediatric; HRQOL, health-related quality of life
Patients were aged between 1.8 and 16.6 years, 58.3% (14/24) were males, 96% (23/24), had isolated head injury, median admission GCS was 15 (range 13–15), median Injury Severity Score was 12 (range 4–29), and 45.8% (11/24) experienced a loss of consciousness before admission to hospital. The most common cause of injury was fall (45.8%), and subdural hematoma (45.8%) was the most common intracranial pathology. Patients with complete (GOSE-P = 1) and incomplete (GOSE-P ≥ 2) recovery had similar demographics, injury mechanism and severity, CT head findings, cerebral autoregulation status, and intensive care unit and hospital length of stay (Table 1). Age-adjusted systolic hypotension occurred in 2 of 24 (0.08%) patients.
Table 1.
Baseline characteristics and 12-month outcomes in hospitalized children with mild complex traumatic brain injury
| Overall n= 24 | Complete recovery (GOSE-P = 1) n = 8 | Incomplete recovery (GOSE-P ≥2) n = 16 | |
|---|---|---|---|
| Age, year, median [IQR] | 10.95 [14.8] | 11.45 [10.5] | 10.95 [14.8] |
| Sex, male, n (%) | 14 (58.3) | 7 (87.5) | 7 (43.8) |
| Injury severity score, median [IQR] | 12 [25] | 10 [17] | 14.5 [23] |
| Mechanism of injury, n (%) | |||
| Fall | 11 (45.8) | 5 (62.5) | 6 (37.5) |
| Motor vehicle collision | 1 (4.2) | 1 (12.5) | 0 (0) |
| Stuck by or against | 7 (29.2) | 1 (12.5) | 6 (37.5) |
| Other | 5 (20.8) | 1 (12.5) | 4 (25) |
| Admit Glasgow Coma Scale, n (%) | |||
| 13 | 1 (4.2) | 0 (0) | 1 (6.3) |
| 14 | 5 (20.8) | 2 (25) | 3 (18.8) |
| 15 | 18 (75) | 6 (75) | 12 (75) |
| Loss of consciousness, n (%) | 11 (45.8) | 4 (50) | 7 (43.8) |
| Intracranial pathology on CT head, n (%) | |||
| Subdural hematoma | 11 (45.8) | 4 (50) | 7 (43.8) |
| Epidural hematoma | 6 (25) | 1 (12.5) | 5 (31.3) |
| Intraparenchymal hemorrhage | 4 (4.2) | 1 (12.5) | 3 (18.8) |
| Subarachnoid hemorrhage | 8 (33.3) | 3 (37.5) | 5 (31.3) |
| Skull fracture | 17 (70.8) | 5 (62.5) | 12 (75) |
| Pneumocephalus | 6 (25) | 1 (12.5) | 5 (31.3) |
| Subgaleal hemorrhage | 10 (41.7) | 3 (37.5) | 7 (43.8) |
| AIS head maximum, n (%) | |||
| 1 | 1 (4.2) | 0 (0) | 1 (6.3) |
| 2 | 2 (8.4) | 2 (25) | 0 (0) |
| 3 | 12 (50) | 4 (50) | 8 (50) |
| 4 | 6 (25) | 2 (50) | 4 (25) |
| 5 | 3 (12.5) | 0 (0) | 3 (18.8) |
| Age adjusted hypotension, n (%) | 2 (1) | 0 (0) | 2 (12.5) |
| Impaired autoregulation * , n (%) | 10 (41.7) | 3 (37.5) | 7 (43.8) |
| ICU length of stay (days), median [IQR] | 1.26 [10.6] | 1.12 [0.7] | 1.48 [10] |
| Hospital length of stay (days), median [IQR] | 1.77 [11.5] | 1.12 [2.9] | 1.93 [11.5] |
| Discharge to home, n (%) | 24 (100) | 8 (100) | 16 (100) |
| GCS at discharge, n (%) | |||
| 14 | 2 (8.4) | 2 (25) | 0 (0) |
| 15 | 22 (91.7) | 6 (75) | 16 (100) |
Impaired autoregulation defined as autoregulatory index < 0.4
AIS, abbreviated injury scale score; CT, computerized tomography; GCS, Glasgow Coma Scale Score; GOSE-P, Glasgow Outcome Scale Score Extended -Pediatric; ICU, intensive care unit; IQR, Interquartile range; NS, not statistically significant
A total of 42 TCD examinations were performed during the first week after initial admission to hospital. The median (range) TCD examinations per patient was 2 (1–4), and the first TCD assessment was performed on admission day 2 (1–4).
Recovery profiles
Twelve-month GOSE-P scores stratified by cerebral autoregulation status are summarized in Figure 2. Fourteen (58.3%) of the 24 children had intact cerebral autoregulation during the first week after initial hospital admission, of whom 12 had outcome assessments at both 3 and 12 months after discharge. Amongst these 12 patients, three had complete recovery, two had good outcome at both three and 12 months, and one patient’s clinical state deteriorated between three and 12 months. Amongst those with incomplete recovery at three months (n = 9), three patients had recovered completely at 12 months, while the remaining six did not improve after three months.
Figure 2. 12-month Glasgow Outcome Scale Extended-Pediatric scores in children with mild complex traumatic brain injury stratified by cerebral autoregulation status.
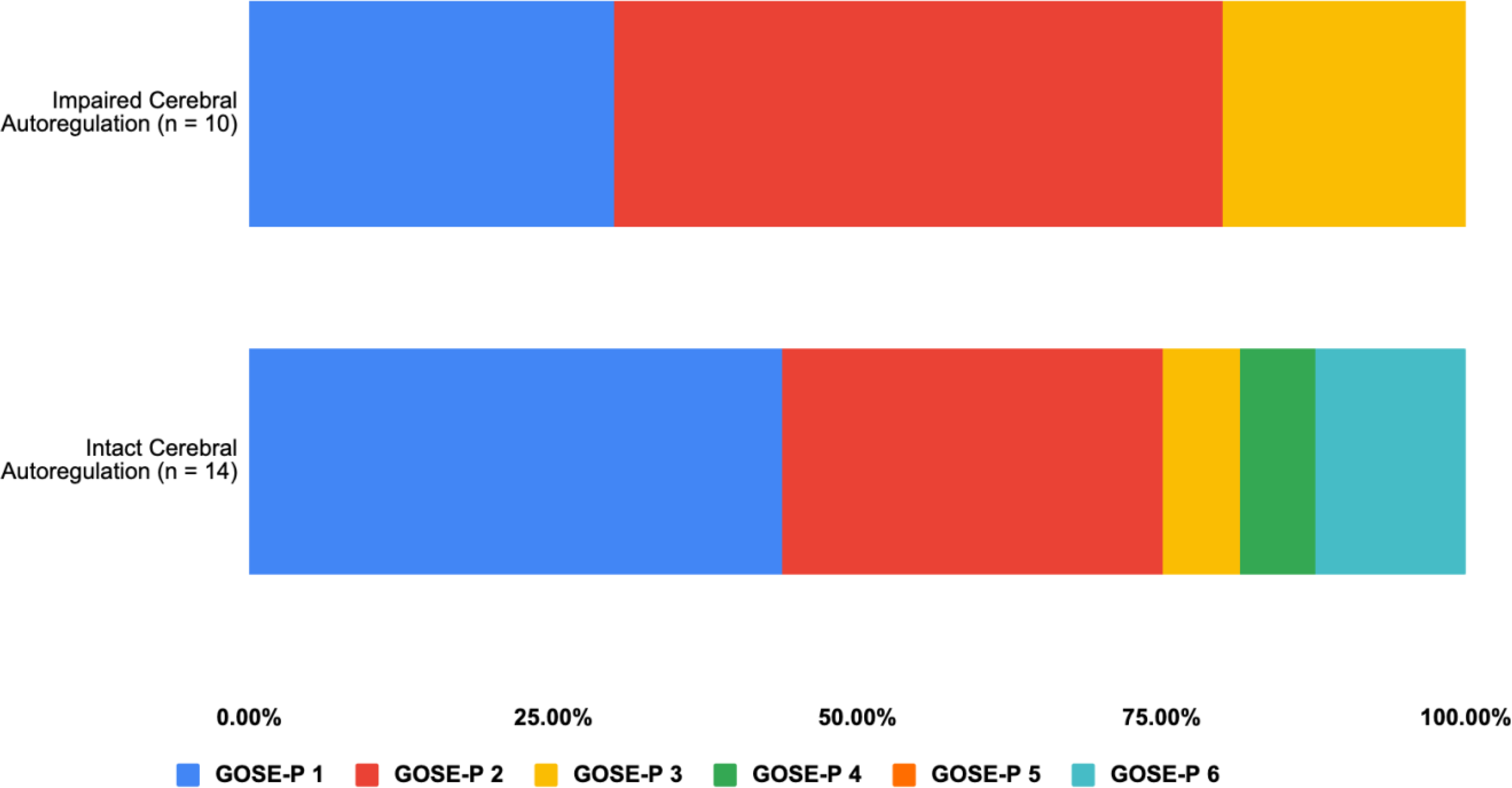
GOSE-P 1, upper good recovery; GOSE-P 2, lower good recovery; GOSE-P 3, upper moderate disability; GOSE-P 4, lower moderate disability; GOSE-P 5, upper severe disability; GOSE-P 6, lower severe disability.
GOSE-P, Glasgow Coma Scale Extended-Pediatric
Ten (41.7%) of the 24 patients had impaired autoregulation during the initial hospitalization, of whom seven patients had follow-up assessments at three and 12 months. Amongst these seven patients, three had complete recovery at three months, two unchanged between three and 12 months, and one patient’s clinical state worsened after three months. Of the four patients with incomplete recovery at three months, one improved while the others did not improve after 3 months.
Two of five (40%) patients with admission GCS 14, and six of 18 (33.3%) patients with admission GCS 15, had complete recovery at 12 months. The single patient with admission GCS 13 did not recover completely. Twelve-month GOSE-P scores stratified by admission GCS are summarized in Figure 3.
Figure 3. 12-month Glasgow Outcome Scale Extended-Pediatric scores in children with mild complex traumatic brain injury stratified by admission Glasgow Coma Scale score.
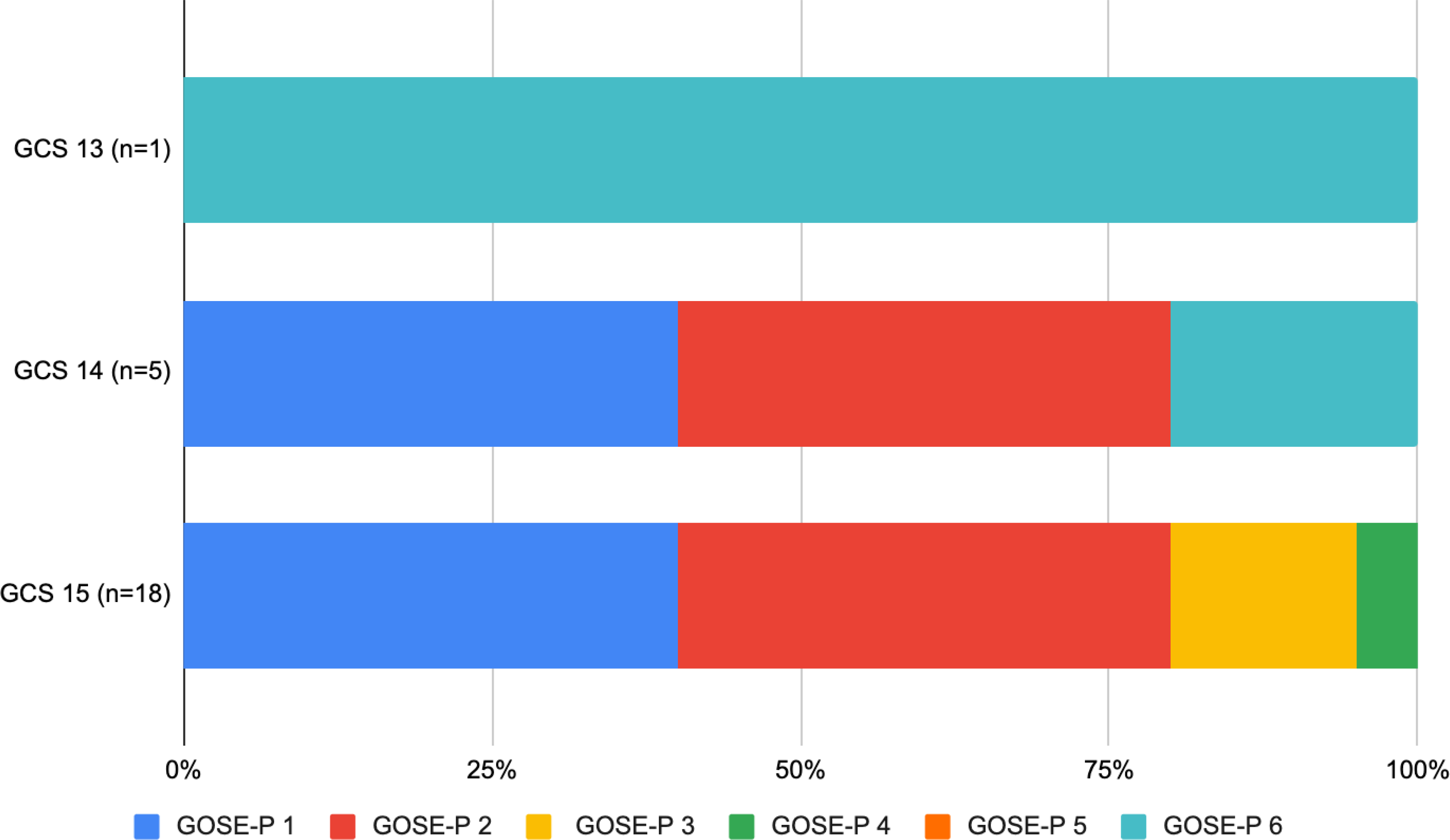
GOSE-P 1, upper good recovery; GOSE-P 2, lower good recovery; GOSE-P 3, upper moderate disability; GOSE-P 4, lower moderate disability; GOSE-P 5, upper severe disability; GOSE-P 6, lower severe disability.
GCS, Glasgow Coma Scale score; GOSE-P, Glasgow Coma Scale Extended-Pediatric
Outcomes and cerebral autoregulation status
There were no differences in physical health summary score, psychosocial health summary score, and total scale score between impaired and intact autoregulation groups at three, six, and 12 months (Table 2). Emotional, school, and social functioning at three, six, and 12 months were also similar between both groups.
Table 2.
The 3, 6, and 12-Month Glasgow Outcome Scale Extended-Pediatrics Scores and Psychological Health Summary Scores in 24 Hospitalized Children with Mild Complex Traumatic Brain Injury by Cerebral Autoregulation Status During Initial Hospital Admission
| Outcome variable | 3 months | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=19) | Impaired autoregulation* (n=7) | Intact autoregulation (n=12) | Overall (n=15) | Impaired autoregulation* (n=7) | Intact autoregulation (n=8) | Overall (n=24) | Impaired autoregulation* (n=10) | Intact autoregulation (n=14) | |
| GOSE-P median [range] | 2 [3] | 2 [3] | 2.5 [2] | 2 [5] | 1 [2] | 2.5 [4] | 5 [2] | 2 [5] | 2 [5] |
| Complete recovery GOSE-P 1, n (%) | 6 (31.58) | 3 (42.85) | 3 (25) | 4 (26.67) | 4 (57.14) | 0 (0) | 8 (33.3) | 3 (30) | 5 (35.7) |
| Incomplete recovery GOSE-P 2–6, n (%) | 13 (68.42) | 4 (57.14) | 9 (75) | 11 (73.33) | 3 (42.86) | 8 (100) | 16 (66.7) | 7 (70) | 9 (64.3) |
| Physical health summary score | 90.13 (9.67) | 90.18 (10.89) | 90.1 (9.41) | 87.71 (14.58) | 91.52 (12.59) | 84.38 (16.19) | 92.1 (8.2) | 92.2 (8.1) | 92 (8.6) |
| Psychological health summary score | 84.1 (17.83) | 87.77 (13.05) | 81.96 (20.35) | 76.05 (21.11) | 85.71 (13.08) | 67.6 (23.86) | 84.6 (17.1) | 84.9 (19.1) | 84.3 (16.3) |
| Emotion functioning | 77.1 (23.05) | 79.28 (20.09) | 75.83 (25.39) | 70.67 (21.86) | 79.28 (17.67) | 63.12 (23.44) | 81.3 (18.9) | 83.5 (21.1) | 79.6 (17.8) |
| Social functioning | 88.68 (17.3) | 95.71 (6.07) | 84.58 (20.5) | 79.33 (26.18) | 91.43 (12.49) | 68.75 (31.02) | 88.1 (21.5) | 88.5 (22) | 87.6 (22) |
| School functioning | 85.28 (21.11) | 85.71 (21.49) | 85 (21.9) | 78.22 (20.98) | 86.43 (16.26) | 71.04 (22.97) | 92.5 (10.6) | 91.3 (13.8) | 85 (17.3) |
| Total scale score | 86.3 (13.92) | 88.76 (11.81) | 84.86 (15.32) | 80.13(18.07) | 87.73 (12.59) | 73.47 (20.22) | 87.5 (12.5) | 88 (12.8) | 87 (12.7) |
Impaired cerebral autoregulation defined as autoregulation index < 0.4
GOSE-P, Glasgow Outcome Score Extended-Pediatric
Discussion
We examined the association between static cerebral autoregulation measured by the ARI during the first week after TBI and long-term outcomes in children with complex mild TBI. The main findings of this study are: 1) the majority of children had incomplete recovery after one year; 2) recovery trajectories varied, and; 3) there was no association between cerebral autoregulation and neurological recovery. This study is the first to examine and understand the implications of impaired cerebral autoregulation and long-term outcomes in children with complex mild TBI, and hypothesizes that the co-occurrence of hypotension and impaired cerebral autoregulation may be a sufficiency condition needed to affect TBI outcomes.
While the children with complex mild TBI in this study had reassuring GCS both at hospital admission and discharge, intermediate and long functional outcomes varied between patients and over the 12 months of follow-up. The fact that some children and adolescents continued to deteriorate over time may be due to a myriad of reasons, such as failure of recovery response mechanisms, evolving brain injury, and/or lack of access to ongoing medical treatment or rehabilitation. Discharge to home or reassuring discharge GCS may not be reliable markers of long-term outcomes in children and adolescents with complex mild TBI as the observed variability in recovery trajectory29 adds to the argument that there is significant heterogeneity in course and outcomes within the mild TBI severity category.30–35
While the mean total PedsQL inventory score was higher in this study compared with previously reported work,7 we included patients hospitalized with skull fractures which may have contributed to higher HRQOL scores. However, this is not a limitation per se because patients with skull fractures may be discharged from the emergency department or admitted to hospital based on fracture characteristics and clinical status, and may also have incomplete long-term recovery.
Recovery after complex mild TBI has previously been reported but not in relation to cerebral autoregulation status.30–35 Reports suggest that cerebral autoregulation is impaired in over 40% of children and adolescents hospitalized with TBI, whether complex mild TBI,14 moderate TBI, or severe TBI. Unlike prior studies which report an association between impaired cerebral autoregulation and poor outcomes after moderate-severe pediatric TBI,11–13 we did not find an association in our patient cohort with complex mild TBI. There may be several reasons for this. First, age < 1 year has been implicated as a risk factor for both impaired cerebral autoregulation and poor functional outcomes, but our cohort did not end up enrolling infants. Second, although we restricted the study to children with no or relatively minor extracranial trauma, mechanisms other than impaired autoregulation4 may have contributed to incomplete long-term recovery. Third, we excluded patients with severe extracranial injuries which are more common in those with moderate-severe TBI and which may contribute to second insults such as hypotension that can exacerbate brain injury due to cerebral ischemia. Fourth, our sample size may be too small to show an association between impaired cerebral autoregulation in isolation as a risk factor for poor long-term outcomes.
There is another potential explanation for our observed lack of association between impaired cerebral autoregulation and poor outcomes that merits discussion. Hypotension, which is more common in moderate-severe TBI, and impaired cerebral autoregulation may need to occur together to impact TBI outcomes. In other words, as previous work has shown, hypotension36 and impaired cerebral autoregulation37 may both contribute to poor outcomes if each is severe enough and of sufficient duration. Hypotension and impaired cerebral autoregulation may also be individually necessary conditions for cerebral ischemia and poor outcomes to occur.15 However, the presence of each alone may fail to impact long-term outcomes if not sufficiently severe or prolonged. In this case, two hits may be required as described in other clinical scenarios.38 Unlike in moderate-severe TBI, hypotension is rare in mild TBI, especially if extracranial injuries are also mild or absent. The intensity and duration of impaired cerebral autoregulation and hypotension occur for a longer time in moderate-severe TBI than in complex mild TBI.39 Given that multiple studies have documented impaired cerebral autoregulation across TBI severity cohorts,15 we propose that the absence of an association between impaired cerebral autoregulation and poor long-term outcomes in this study likely reflects the absence of hypotension. Since the tilt test measures static autoregulation, and this is not always correlated dynamic autoregulation, it is also unclear whether abnormal dynamic autoregulation may have a similar effect.40
This study allowed us uniquely to examine cerebral autoregulation and long-term outcomes in complex mild TBI and posit a new sufficiency framework for studying factors affecting TBI outcomes. To date, clinical studies have not been large enough to examine or establish the necessary or sufficient systemic or cerebral conditions that contribute to poor pediatric TBI outcomes by age and sex. Yet, existing studies suggest the need to examine the various and contemporaneous scenarios that are likely needed to exist for improving long-term outcomes. Clinical studies are also limited in their ability to study counterfactual scenarios to prove the tested hypotheses that long-term outcomes are favored in the absence of impaired cerebral autoregulation and hypotension. Bidirectional translational studies using animal models and/or data science models are likely needed to answer the more mechanistic and complex what-if scenarios that need to occur together to impact TBI outcomes.30,41
This study has some limitations. It is a single-center study including a small convenience sample size. To examine outcomes (complete recovery) in patients based on their cerebral autoregulation status, the current study is underpowered (post-hoc power = 4.6%). To achieve 80% power, and assuming similar prevalence of impaired cerebral autoregulation (37.5% in the complete recovery group and 43.8% in the impaired autoregulation group), the estimated sample size would be 1000 patients in each group. In addition, we did not collect functional outcomes at discharge from hospital. Despite these limitations, what is apparent is that further research is needed to understand better the systems biology that contributes to poor TBI outcomes so that clinicians can develop prognostication models and treatments to improve TBI outcomes.
Conclusions
In conclusion, two-thirds of children with complex mild TBI experienced incomplete functional recovery at one year and recovery trajectories varied. Unlike in moderate-severe TBI, incomplete functional recovery was not associated with early impairment of cerebral autoregulation. The absence of concurrent hypotension in children with complex mild TBI no severe extracranial injury may contribute to this lack of association. The co-occurrence of both hypotension and cerebral autoregulation may be a sufficiency condition needed to affect TBI outcomes. This pilot study positions investigators to develop studies that are sufficiently powered to examine more definitively the relationship between impaired autoregulation and long-term outcomes, and to develop studies that examine tiered hemodynamic treatments based on static and dynamic cerebral autoregulation status.
Acknowledgment:
We acknowledge Crystalyn Clark-Bell, BS, for help with data collection
This study was conducted at Harborview Medical Center
Funding mechanism: National Institutes of Neurological Diseases and Stroke (R21NS095321–02: Vavilala)
Dr. Lele’s institution received one funding from Aqueduct Critical Care (research support from Aqueduct Critical Care for ASSESSED clinical trial and EVD Aware study). Dr. Lele reports receiving salary support from LifeCenter Northwest. Dr. Vavilala received support from the National Institutes of Neurological Diseases and Stroke (R21NS095321–02: Vavilala)
Footnotes
The authors have no financial disclosures to report relevant to this study
References:
- 1.Au AK, Carcillo JA, Clark RS, et al. Brain injuries and neurological system failure are the most common proximate causes of death in children admitted to a pediatric intensive care unit. Pediatr Crit Care Med 2011;12(5):566–571. DOI: 10.1097/PCC.0b013e3181fe3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention National Center for Injury Prevention and Control. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-508.pdf. Published 2014 Accessed November 17, 2020. [Google Scholar]
- 3.Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128(5):946–954. DOI: 10.1542/peds.2010-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lele AV, Alunpipatthanachai B, Qiu Q, et al. Plasma Levels, Temporal Trends and Clinical Associations between Biomarkers of Inflammation and Vascular Homeostasis after Pediatric Traumatic Brain Injury. Dev Neurosci 2019;41(3–4):177–192. DOI: 10.1159/000502276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zonfrillo MR, Durbin DR, Koepsell TD, et al. Prevalence of and risk factors for poor functioning after isolated mild traumatic brain injury in children. J Neurotrauma 2014;31(8):722–727. DOI: 10.1089/neu.2013.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivara FP, Koepsell TD, Wang J, et al. Incidence of disability among children 12 months after traumatic brain injury. Am J Public Health. 2012;102(11):2074–2079. DOI: 10.2105/AJPH.2012.300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivara FP, Koepsell TD, Wang J, et al. Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics. 2011;128(5):e1129–1138. DOI: 10.1542/peds.2011-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deines JJ, Chang J, Reuter-Rice K. Cerebral Blood Flow Velocities and Functional Outcomes in Pediatric Mild Traumatic Brain Injury. J Neurotrauma 2018;36(1):135–141. DOI: 10.1089/neu.2017.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovis JC, Gupta N, Li NY, et al. Assessment of Recovery Following Pediatric Traumatic Brain Injury. Pediatr Crit Care Med 2018;19(4):353–360. DOI: 10.1097/PCC.0000000000001490. [DOI] [PubMed] [Google Scholar]
- 10.Petranovich CL, Smith-Paine J, Wade SL, et al. From Early Childhood to Adolescence: Lessons About Traumatic Brain Injury From the Ohio Head Injury Outcomes Study. J Head Trauma Rehabil 2020;35(3):226–239. DOI: 10.1097/HTR.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavilala MS, Muangman S, Waitayawinyu P, et al. Neurointensive care; impaired cerebral autoregulation in infants and young children early after inflicted traumatic brain injury: a preliminary report. J Neurotrauma 2007;24(1):87–96. DOI: 10.1089/neu.2006.0058. [DOI] [PubMed] [Google Scholar]
- 12.Vavilala MS, Muangman S, Tontisirin N, et al. Impaired cerebral autoregulation and 6-month outcome in children with severe traumatic brain injury: preliminary findings. Dev Neurosci 2006;28(4–5):348–353. DOI: 10.1159/000094161. [DOI] [PubMed] [Google Scholar]
- 13.Vavilala MS, Lee LA, Boddu K, et al. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med 2004;5(3):257–263. [DOI] [PubMed] [Google Scholar]
- 14.Lele AV, Watanitanon A, Lakireddy V, et al. Prevalence, Evolution, and Extent of Impaired Cerebral Autoregulation in Children Hospitalized With Complex Mild Traumatic Brain Injury. Pediatr Crit Care Med 2019;20(4):372–378. DOI: 10.1097/PCC.0000000000001824. [DOI] [PubMed] [Google Scholar]
- 15.Chaiwat O, Sharma D, Udomphorn Y, et al. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J Neurotrauma 2009;26(5):657–663. DOI: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman SS, Udomphorn Y, Armstead WM, et al. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology. 2008;108(4):588–595. DOI: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 17.Vavilala MS, Farr CK, Watanitanon A, et al. Early changes in cerebral autoregulation among youth hospitalized after sports-related traumatic brain injury. Brain Inj 2018;32(2):269–275. DOI: 10.1080/02699052.2017.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts JS, Vavilala MS, Schenkman KA, et al. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med 2006;34(8):2217–2223. DOI: 10.1097/01.CCM.0000227182.51591.21. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Roberts JS, Pihoker C, et al. Transcranial Doppler-based assessment of cerebral autoregulation in critically ill children during diabetic ketoacidosis treatment. Pediatr Crit Care Med 2014;15(8):742–749. DOI: 10.1097/PCC.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 20.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26(6):1014–1019. DOI: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 21.Strebel S, Lam AM, Matta B, et al. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83(1):66–76. DOI: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Humann Services. Pediatric Basic and Advanced Life Support https://chemm.nlm.nih.gov/pals.htm. Published 2020. Accessed May 14, 2020, 2020.
- 23.Evans E, Cook NE, Iverson GL, et al. Monitoring Outcome after Hospital-Presenting Milder Spectrum Pediatric Traumatic Brain Injury Using the Glasgow Outcome Scale-Extended, Pediatric Revision. J Neurotrauma 2020;37(14):1627–1636. DOI: 10.1089/neu.2019.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma 2012;29(6):1126–1139. DOI: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Naalt J, Timmerman ME, de Koning ME, et al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol 2017;16(7):532–540. DOI: 10.1016/S1474-4422(17)30117-5. [DOI] [PubMed] [Google Scholar]
- 26.Yue JK, Ngwenya LB, Upadhyayula PS, et al. Emergency department blood alcohol level associates with injury factors and six-month outcome after uncomplicated mild traumatic brain injury. J Clin Neurosci 2017;45:293–298. DOI: 10.1016/j.jocn.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Limbers CA. The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatr Clin North Am 2009;56(4):843–863. DOI: 10.1016/j.pcl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Stata Statistical Software, Release 15 [computer program]. College Station, TX, 2017. [Google Scholar]
- 29.Wade SL, Fisher AP, Kaizar EE, et al. Recovery Trajectories of Child and Family Outcomes Following Online Family Problem-Solving Therapy for Children and Adolescents after Traumatic Brain Injury. J Int Neuropsychol Soc 2019;25(9):941–949. DOI: 10.1017/S1355617719000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstead WM, Vavilala MS. Improving Understanding and Outcomes of Traumatic Brain Injury Using Bidirectional Translational Research. J Neurotrauma 2020;37(22):2372–2380. DOI: 10.1089/neu.2018.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KM, Barker-Collo S, Parmar P, et al. Trajectories in health recovery in the 12 months following a mild traumatic brain injury in children: findings from the BIONIC Study. J Prim Health Care. 2018;10(1):81–89. DOI: 10.1071/HC17038. [DOI] [PubMed] [Google Scholar]
- 32.Goreth MB, Palokas M. Association between premorbid neuropsychological conditions and pediatric mild traumatic brain injury/concussion recovery time and symptom severity: a systematic review. JBI Database System Rev Implement Rep 2019;17(7):1464–1493. DOI: 10.11124/JBISRIR-2017-004008. [DOI] [PubMed] [Google Scholar]
- 33.Ledoux AA, Tang K, Yeates KO, et al. Natural Progression of Symptom Change and Recovery From Concussion in a Pediatric Population. JAMA Pediatr 2019;173(1):e183820. DOI: 10.1001/jamapediatrics.2018.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum PE, Locandro C, Chrisman SPD, et al. Characteristics of Pediatric Mild Traumatic Brain Injury and Recovery in a Concussion Clinic Population. JAMA Netw Open. 2020;3(11):e2021463. DOI: 10.1001/jamanetworkopen.2020.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones KM, Prah P, Starkey N, et al. Longitudinal patterns of behavior, cognition, and quality of life after mild traumatic brain injury in children: BIONIC study findings. Brain Inj 2019;33(7):884–893. DOI: 10.1080/02699052.2019.1606445. [DOI] [PubMed] [Google Scholar]
- 36.Erickson SL, Killien EY, Wainwright M, et al. Mean Arterial Pressure and Discharge Outcomes in Severe Pediatric Traumatic Brain Injury. Neurocrit Care. 2020. DOI: 10.1007/s12028-020-01121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hockel K, Diedler J, Neunhoeffer F, et al. Time spent with impaired autoregulation is linked with outcome in severe infant/paediatric traumatic brain injury. Acta Neurochir (Wien). 2017;159(11):2053–2061. DOI: 10.1007/s00701-017-3308-8. [DOI] [PubMed] [Google Scholar]
- 38.Fiebich BL, Akter S, Akundi RS. The two-hit hypothesis for neuroinflammation: role of exogenous ATP in modulating inflammation in the brain. Front Cell Neurosci 2014;8:260. DOI: 10.3389/fncel.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flechet M, Meyfroidt G, Piper I, et al. Visualizing Cerebrovascular Autoregulation Insults and Their Association with Outcome in Adult and Paediatric Traumatic Brain Injury. Acta Neurochir Suppl 2018;126:291–295. DOI: 10.1007/978-3-319-65798-1_57. [DOI] [PubMed] [Google Scholar]
- 40.Peterson E, Chesnut RM. Static autoregulation is intact in majority of patients with severe traumatic brain injury. J Trauma 2009;67(5):944–949. DOI: 10.1097/TA.0b013e3181ae6e6d. [DOI] [PubMed] [Google Scholar]
- 41.Armstead WM, Vavilala MS. Translational approach towards determining the role of cerebral autoregulation in outcome after traumatic brain injury. Exp Neurol 2019;317:291–297. DOI: 10.1016/j.expneurol.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]


