Abstract
Objective
We aimed to assess the impact of early versus late third-trimester maternal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination on transplacental transfer and neonatal levels of SARS-CoV-2 antibodies.
Methods
Maternal and cord blood sera were collected following term delivery after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination, with the first vaccine dose administered between 27 and 36 weeks of gestation. SARS-CoV-2 spike protein (S) and receptor-binding domain (RBD) -specific, IgG levels and neutralizing potency were evaluated in maternal and cord blood samples.
Results
The study cohort consisted of 171 parturients—median age 31 years (interquartile range (IQR) 27–35 years); median gestational age 39+5 weeks (IQR 38+5–40+4 weeks)–83 (48.5%) were immunized in early thrird-trimester (first dose at 27–31 weeks) and 88 (51.5%) were immunized in late third trimester (first dose at 32–36 weeks). All mother–infant paired sera were positive for anti S- and anti-RBD-specific IgG. Anti-RBD-specific IgG concentrations in neonatal sera were higher following early versus late third-trimester vaccination (median 9620 AU/mL (IQR 5131–15332 AU/mL) versus 6697 AU/mL (IQR 3157–14731 AU/mL), p 0.02), and were positively correlated with increasing time since vaccination (r = 0.26; p 0.001). Median antibody placental transfer ratios were increased following early versus late third-trimester immunization (anti-S ratio: 1.3 (IQR 1.1–1.6) versus 0.9 (IQR 0.6–1.1); anti-RBD-specific ratio: 2.3 (IQR 1.7–3.0) versus 0.7 (IQR 0.5–1.2), p < 0.001). Neutralizing antibodies placental transfer ratio was greater following early versus late third-trimester immunization (median 1.9 (IQR 1.7–2.5) versus 0.8 (IQR 0.5–1.1), p < 0.001), and was positively associated with longer duration from vaccination (r = 0.77; p < 0.001).
Conclusions
Early compared with late third-trimester maternal SARS-CoV-2 immunization enhanced transplacental antibody transfer and increased neonatal neutralizing antibody levels. Our findings highlight that vaccination of pregnant women early in the third trimester may enhance neonatal seroprotection.
Keywords: Cord blood, Coronavirus disease 2019, Passive immunity, Pregnancy, Serology, Severe acute respiratory syndrome coronavirus 2, Vaccination
Introduction
Pregnant women and their infants, particularly neonates, are at a higher risk for severe disease in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Pregnant women who contract coronavirus disease 2019 (COVID-19) are more likely to be admitted to an intensive care unit and require invasive ventilation because of COVID-19 compared with non-pregnant women [1–4). Furthermore, maternal SARS-CoV-2 infection has been associated with adverse perinatal outcomes including preterm delivery and stillbirth [[1], [2], [3], [4]]. Although children have been shown to be more mildly affected by COVID-19 compared with adults, infants are at significantly higher risk for severe disease course compared with older children [5,6].
The two SARS-CoV-2 mRNA vaccines of Pfizer and Moderna were shown to elicit a robust immune response among pregnant women [7], coupled with data supporting their safety throughout gestation [8]. In addition to their suggested role in preventing maternal illness, recent studies have demonstrated that antenatal vaccination may lead to transplacental transfer of maternally derived anti-SARS-CoV-2 antibodies [7,[9], [10], [11]]. As children, including young infants, are currently not eligible for SARS-CoV-2 vaccination, offering neonatal seroprotection in the early, vulnerable stages of life through maternal immunization is of paramount importance. This principle is well-established for the prevention of other potentially life-threatening respiratory infections such pertussis and influenza [[12], [13], [14], [15], [16]]. In Israel, a nationwide mass vaccination campaign against COVID-19 using the BNT162b2 (Pfizer/BioNTech) mRNA vaccine was started in December 2020.
Defining the optimal timing for maternal SARS-CoV-2 immunization is crucial to maximize maternofetal antibody transfer and infant protection. Given the high clinical relevance, we aimed to determine the impact of maternal SARS-CoV-2 immunization timing on the efficacy of transplacental antibody transfer.
Materials and methods
Study population
A prospective study following women admitted for delivery was performed during February–April 2021 at Hadassah Medical Centre, a tertiary-care university-affiliated hospital in Jerusalem, Israel with over 10 000 deliveries annually. Women who received the SARS-CoV-2 BNT162b2 mRNA vaccine during pregnancy were consecutively approached following their admission to the delivery room and offered participation. The institutional review board of the Hadassah Medical Centre approved this study (HMO-0064-21). Those who agreed to participate and provided written informed consent were eligible for this study. The final study cohort included those who received the first vaccine dose at 27–36 weeks of gestation. Parturients who delivered prematurely (before 37 weeks of gestation), who had multifetal gestations and who did not complete the two-dose vaccine series before delivery were excluded. The decision to include only those vaccinated between 27 and 36 weeks of gestation was pre-defined based on data showing that maternal Tdap (tetanus, diphtheria and pertussis) vaccination, during this time period was most protective in terms of pertussis disease prevention among infants [13]. The final cohort was sub-divided into those vaccinated in early third trimester (27–31 weeks) versus late third trimester (32–36 weeks), as early third-trimester Tdap vaccination was previously found to associate with higher neonatal antibody levels and avidity [12,14] and improved clinical effectiveness [13]. The primary outcomes were neonatal SARS-CoV-2 antibody levels and placental transfer ratios. Demographic and clinical data were collected at the time of enrolment.
Laboratory methods
Maternal and cord blood sera were collected following delivery. Spike protein (S) (Liaison SARS-CoV-2 S1/S2 IgG; DiaSorin, Saluggia, Italy) and receptor-binding domain (RBD) -specific (Architect SARS-CoV-2 IgG II Quant assay; Abbott Diagnostics, Chicago, IL, USA) IgG levels were evaluated in maternal and cord blood sera. Maternal and cord blood sera were also tested for SARS-CoV-2 RBD IgM (Liaison, DiaSorin, Saluggia, Italy).
For a subset of mother–newborn dyads (arbitrarily selected), neutralizing antibody titres against SARS-CoV-2 were defined using a wild-type SARS-CoV-2 virus microneutralization assay as previously described [17], with minor modifications. Briefly, serial two-fold dilutions of heat-inactivated serum samples (starting from 1:10; diluted in Dulbecco's modified Eagle's medium in a total volume of 50 μL) were incubated with an equal volume of viral solution, containing 100 median tissue culture infectious doses (TCID50) of SARS-CoV-2 isolate USA-WA1/2020 (NR-52281; obtained from BEI Resources, Manassas, VA, USA), for 1 hour in a 96-well plate (at 37°C in humidified atmosphere with 5% CO2). The serum–virus mixtures (100 μL; eight replicates of each serum dilution) were then added to a 96-well plate containing a semi-confluent VERO E6 cell monolayer (ATCC CRL-1586; maintained as described previously [18]). Following 3 days of incubation (at 37°C in a humidified atmosphere with 5% CO2), the cells in each well were scored for viral cytopathic effect. The median neutralization titre (NT50) was defined as the reciprocal of the highest serum dilution that protected 50% of culture wells from cytopathic effect. Positive and negative serum controls, cell control and a viral back-titration control were included in each assay.
Statistical analysis
Patient characteristics are described as proportions for categorical variables and as medians and interquartile ranges (IQR) for continuous variables. Antibody levels, neutralizing activity and placental transfer ratios are expressed as medians and IQR. Significance between those vaccinated in early (27–31 weeks) versus late (32–36 weeks) third trimester was assessed using the χ2 test and Fisher's exact test for categorical variables, while the Mann–Whitney U test was used for continuous variables. Correlations were reported using the Pearson's test with the correspondent r and p values. A two-sided p value less than 0.05 indicated statistical significance. The data were analysed using the Software Package for Statistics and Simulation (IBM SPSS version 24; IBM Corp, Armonk, NY, USA).
Results
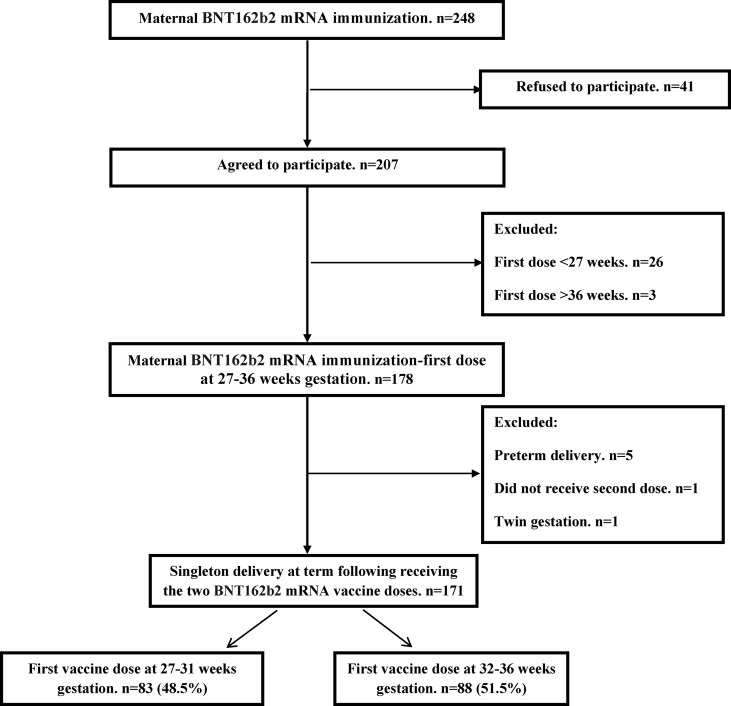
During the study period, samples were collected from 207 parturients who had received the SARS-CoV-2 BNT162b2 mRNA during gestation and agreed to participate. Of them, 36 (17.4%) were excluded—first vaccine dose before 27 weeks (n = 26), first vaccine dose after 36 weeks (n = 3), preterm delivery (n = 5), did not complete the two-dose vaccine series before delivery (n = 1), twin gestation (n = 1). As a result, the final study cohort comprised 171 women including 83 (48.5%) who received the first vaccine dose at early third trimester (27–31 weeks of gestation), and 88 (51.5%) who received the first vaccine dose in late third trimester (32–36 weeks of gestation) (Fig. 1 ).
Fig. 1.
Schematic flow chart of patient inclusion in the study.
Maternal and neonatal characteristics are shown in Table 1 . Median maternal age was 31 years (IQR 27–35 years) with a median gestational age of 39+5 weeks (IQR 38+5–40+4 weeks) at the time of delivery. Demographic, pregnancy and delivery characteristics did not differ in relation to the time of vaccination. The median time elapsed from the first vaccine dose administration until delivery was 71 days (IQR 63–79 days) and 41 days ([IQR 34–50 days), in those immunized in the early third trimester and late third trimester, respectively (p < 0.001).
Table 1.
Maternal and neonatal characteristics among SARS-CoV-2 BNT162b2 immunized pregnant women in relation to vaccination timing
| Characteristics | Early third-trimester vaccination n = 83 (48.5%) | Late third-trimester vaccination n = 88 (51.5%) |
|---|---|---|
| Age (years) | 30 (27–35) (31)a | 32 (29–35) (32) |
| >35 years | 20 (24.1%) | 17 (19.3%) |
| Parity | 2 (1–4) (3) | 3 (2–5) (3) |
| Nulliparous | 28 (33.7%) | 24 (27.3%) |
| Maternal weight (kg) | 75 (69–84) (76) | 74 (66–82) (76) |
| Maternal body mass index (kg/m2) | 28 (26–31) (29) | 28 (26–31) (28) |
| Gestational age at delivery (weeks) | 39+5 (38+6–40+3) (39+4) | 39+6 (38+5–40+6) (39+6) |
| Gestation diabetes mellitus | 6 (7.2%) | 7 (8.0%) |
| Gestational hypertensive disorders | 1 (1.2%) | 2 (2.3%) |
| Received antenatal anti-D immunoglobulin | 9 (10.8%) | 6 (6.8%) |
| Gestational age at first dose immunization (weeks) | ||
| 27 | 15 (18.1%) | — |
| 28 | 18 (21.7%) | — |
| 29 | 18 (21.7%) | — |
| 30 | 17 (20.5%) | — |
| 31 | 15 (18.1%) | — |
| 32 | — | 27 (30.7%) |
| 33 | — | 26 (29.5%) |
| 34 | — | 15 (17.0%) |
| 35 | — | 9 (10.2%) |
| 36 | — | 11 (12.5%) |
| First vaccine dose-to-delivery interval (days) | 71 (63–79) (71) | 41 (34–50) (42) |
| Second vaccine dose-to-delivery interval (days) | 50 (42–58) (50) | 20 (13–29) (21) |
| Mode of delivery | ||
| Vaginal | 77 (92.8%) | 81 (92.0%) |
| Pre-labour caesarean | 3 (3.6%) | 5 (5.7%) |
| In-labour caesarean | 3 (3.6%) | 2 (2.3%) |
| Neonatal birthweight (grams) | 3275 (2985–3730) (3345) | 3440 (3051–3700) (3374) |
| Male gender (%) | 39 (47.0%) | 50 (56.8%) |
All continuous variables are expressed as medians (interquartile range) (means).
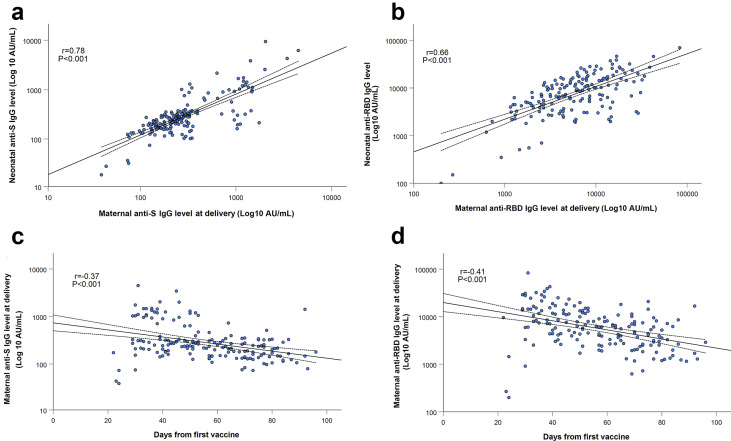
All 171 mother–infant pairs were positive for SARS-CoV-2 anti S- and anti-RBD-specific IgG, with a positive correlation between maternal and neonatal cord blood concentrations (r = 0.78; p < 0.001 and r = 0.66; p < 0.001, respectively; Figs. 2 a,b).
Fig. 2.
SARS-CoV-2 anti-S (a) and anti-RBD-specific (b) IgG levels in maternal sera were positively correlated to their respective concentrations in cord blood (r = 0.78; P < 0.001 and r= 0.66; P < 0.001, respectively). Maternal anti-S (c) and anti-RBD-specific (d) IgG concentrations were negatively associated with the time lapsed since immunization (r = −0.37; P < 0.001 and r = −0.41; P < 0.001, respectively). Correlations, as well as correspondent R and P values were calculated by Pearson's test, as shown in each panel. The dotted lines are the 95% confidence intervals..
SARS-CoV-2 IgM antibodies were not detected in any of the neonates, and were detected in 30 (17.5%) parturients, all of whom were vaccinated late in the third trimester.
Median anti-S-specific and anti-RBD-specific IgG concentrations in maternal sera at the time of delivery were lower in those vaccinated in early third trimester compared with late third trimester—anti-S IgG: 200 AU/mL (IQR 143–296 AU/mL) versus 292 AU/mL (IQR 209–963 AU/mL), anti-RBD-specific IgG: 3980 AU/mL (IQR 2414–7022 AU/mL) versus 8506 AU/mL (IQR 4601–15 094 AU/mL),; p < 0.001 for both comparisons). There was a negative association between maternal anti-S-specific and anti-RBD-specific IgG concentrations and the time elapsed since immunization (r = –0.37; p < 0.001 and r = –0.41; p < 0.001, respectively; Figs. 2c,d).
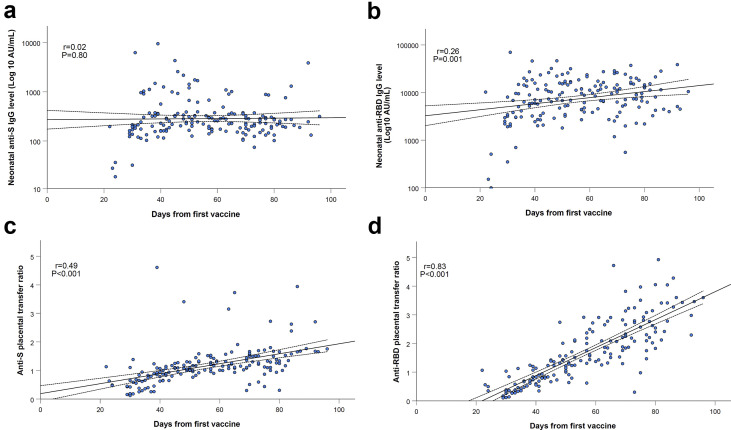
Neonatal concentrations of anti-S IgG antibodies did not differ in relation to maternal third-trimester immunization timing (Fig. 3 a). Anti-RBD-specific IgG concentrations in neonatal sera were significantly higher in those born to mothers vaccinated early in the third trimester compared with late third trimester (median: 9620 AU/mL (IQR 5131–15 332 AU/mL) versus 6697 AU/mL (IQR 3157–14 731 AU/mL), p 0.02), and were positively correlated with increasing time since vaccination (r = 0.26; p 0.001; Fig. 3b). The median placental transfer ratios of anti-S-specific and anti-RBD-specific IgG were significantly increased following early third-trimester immunization compared with late third-trimester immunization (anti-S ratio: 1.3 (IQR 1.1–1.6) versus 0.9 (IQR 0.6–1.1), anti-RBD-specific ratio: 2.3 (IQR 1.7–3.0) versus 0.7 (IQR 0.5–1.2); p < 0.001 for both comparisons), and directly associated with longer duration since immunization (r = 0.49; p < 0.001 and r = 0.83; p < 0.001, respectively; Figs. 3c,d). Higher proportion of maternal–infant dyads had anti-S-specific and anti-RBD-specific IgG placental transfer ratios above 1 following early third-trimester immunization compared with late third-trimester immunization (anti-S ratio >1: 83.1% (69/83) versus 38.6% (34/88), anti-RBD-specific ratio >1: 96.4% (80/83) versus 38.6% (34/88); p < 0.001 for both comparisons). The sequential decrease in anti-RBD-specific IgG neonatal concentrations and placental transfer ratio in relation to later gestational age at the time of vaccination is shown in the online Supplementary material (Fig. S1).
Fig. 3.
Neonatal concentrations of anti-S (a) IgG antibodies did not differ in relation to maternal third trimester immunization timing (P = 0.80). Anti-RBD-specific (b) IgG concentrations in neonatal sera were positively correlated with increasing time since vaccination (r = 0.26; P = 0.001). Placental transfer ratios of anti-S (c) and anti-RBD-specific (d) IgG were directly associated with longer duration since immunization (r = 0.49; P < 0.001 and r= 0.83; P < 0.001, respectively). Correlations, as well as correspondent R and P values were calculated by Pearson's test, as shown in each panel. The dotted lines are the 95% confidence intervals.
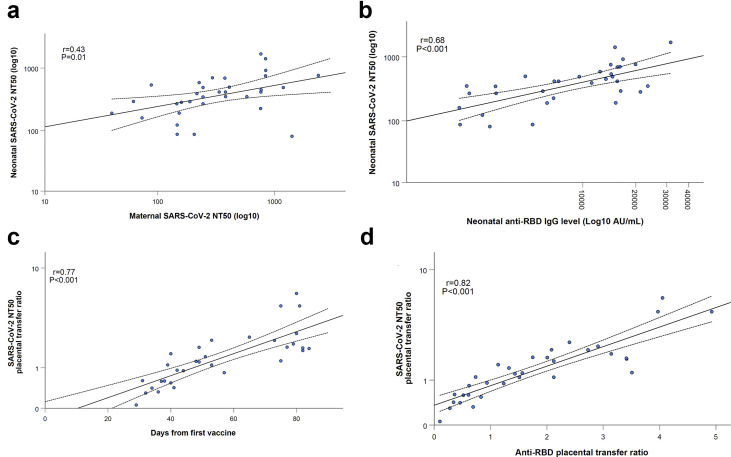
Neutralizing SARS-CoV-2 antibodies were assessed in 33 maternal–infant pairs following early third-trimester vaccination (n = 14) and late third-trimester vaccination (n = 19). Neonatal sera neutralizing activity (as reflected by NT50 values) was positively correlated to maternal sera neutralizing activity (r = 0.43; p 0.01; Fig. 4 a) and neonatal anti-RBD concentrations (r = 0.68; p < 0.001; Fig. 4b). Placental transfer ratio of neutralizing SARS-CoV-2 antibodies was significantly higher following early third-trimester immunization compared with late third-trimester immunization (median 1.9 (IQR 1.7–2.5) versus 0.8 (IQR 0.5–1.1); p < 0.001), and was positively associated with increasing time from vaccination (r = 0.77; p < 0.001; Fig. 4c) and anti-RBD placental transfer ratio (r = 0.82; p < 0.001; Fig. 4d). Neutralizing antibodies placental transfer ratio was above 1 in all 14 (100.0%) mother–neonate dyads vaccinated early in the third trimester and in 7 (36.8%) of those vaccinated in late third trimester (p < 0.001).
Fig. 4.
Maternal sera neutralizing activity (a) and neonatal anti-RBD concentrations (b) were positively correlated to neonatal sera neutralizing activity (r = 0.43; P = 0.01 and r = 0.68; P < 0.001, respectively). Duration of time since vaccination (c) and anti-RBD placental transfer ratio (d) were positively associated with neutralizing antibodies placental transfer ratio (r = 0.77; P < 0.001 and r = 0.82; P < 0.001, respectively). Neutralizing activity is reflected by NT50 values (see Methods section).
Discussion
This study investigated transplacental antibody transfer in 171 mother–newborn dyads, following antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Early compared with late third-trimester maternal immunization was associated with higher neonatal anti-SARS-CoV-2 antibody levels and serum neutralizing activity. The current study findings may support the role of vaccination early at the third trimester to enhance maternofetal antibody transfer and neonatal seroprotection.
Pregnant women were excluded from the initial trials evaluating the different SARS-CoV-2 vaccines [19,20]. Nevertheless, although universal recommendations have not yet emerged, given the risk for severe disease course and the accumulating reassuring data for their use in the setting of pregnancy [7,8], the World Health Organization, the US Centers for Disease Control and Prevention and other agencies advocate offering pregnant women the SARS-CoV-2 vaccine following shared decision-making [[21], [22], [23], [24]]. Antenatal SARS-CoV-2 immunization is primarily aimed at preventing maternal illness, considering the low rate of severe disease in the paediatric age group [25]. Nevertheless, the optimal antenatal vaccination regimen to maintain maternal immunity throughout gestation is still unclear. Furthermore, severe COVID-19, while rare, can still occur in infants [25]. Hence, the far-reaching potential to confer neonatal protection against COVID-19 and potentially decrease community transmission through maternal immunization raises critical questions concerning the optimal timing of antenatal vaccination. Physiologically, transplacental antibody transfer is a dynamic process starting in the second trimester; however, it is most efficient in the third trimester [26,27]. For maternal pertussis and influenza vaccination this issue has been extensively studied [[12], [13], [14], [15], [16]], as most women have been previously exposed or immunized against these pathogens, but the findings may not apply for the different SARS-CoV-2 vaccines, which prime a de novo immune response.
In this study, we evaluated pregnant women immunized with the SARS-CoV-2 BNT162b2 mRNA vaccine, with the first dose given between 27 and 36 weeks of gestation. We demonstrated that maternal immunization during the early third trimester (27–31 weeks) yielded higher neonatal antibody concentrations, compared with late third trimester (32–36 weeks). The specific antibody titres required for protection have not been fully defined, but this enhanced neonatal seroprotection was also evident by the increased neonatal serum anti- SARS-CoV-2 neutralizing antibody levels in those vaccinated earlier. Furthermore, the increment noted in neonatal antibody concentrations and placental transfer ratio in association with earlier gestational age at the time of vaccination, was higher for anti-RBD-specific IgG compared with anti-S IgG, implying the preferential transfer of the former, correlating with neutralization potency. These observations indicate that neonatal serum is enriched by the placenta with highly functional antibodies. Our results are in accordance with previous reports evaluating the effect of maternal pertussis and influenza immunization, showing augmented transplacental transfer and neonatal antibody levels along with improved clinical outcomes, following early third-trimester vaccination [[12], [13], [14], [15], [16]]. This is in contrast to two other studies evaluating parturients who delivered following antenatal SARS CoV-2 mRNA vaccination, which did not demonstrate a correlation between the transplacental placental of anti-RBD-specific IgG and neutralizing antibodies [7,28]. The sample size in those studies was limited, including women who received either the Pfizer or Moderna vaccine, and placental antibody transfer was not evaluated according to gestational age at the time of immunization [7,28]. Although the results of the current study are encouraging, future studies should evaluate the kinetics and durability of these passively acquired antibodies in the offspring and their protective effect against clinical COVID-19-related outcomes, as well as the clinical significance of the different titres found in cord blood.
Aside from the strong correlation between the time elapsed from vaccination and transplacental antibody transfer, we also evaluated the influence of other demographic and clinical characteristics. In the setting of third-trimester maternal SARS-CoV-2 infection, relatively low placental transfer ratios were previously reported, possibly relating to an altered antibody glycosylation profile [29], but we observed higher and satisfactory maternofetal antibody transfer. In addition, although mothers with COVID-19 carrying a male fetus were shown by Bordt et al. to exhibit a diminished immune response and compromised neonatal seroprotection [30], this was not observed in our cohort following maternal vaccination. Furthermore, the lack of detrimental effect of maternal anthropometric parameters and the prenatal administration of anti-D immunoglobulin, on the level of anti-SARS-CoV-2 antibodies conveyed to the offspring are reassuring findings.
The major strength of our study is its relatively large cohort size, which allowed for a robust analysis of the superiority of early third-trimester immunization. Nevertheless, this study has several limitations. First, the observational single-centre design of the study may limit the generalizability of our findings. Second, the effects of maternal immunization at an earlier gestational age as well as of the impact of other SARS-CoV-2 vaccines on transplacental antibody transfer dynamics remain to be determined. Earlier immunization could provide maternal protection against COVID-19 through the longer course of pregnancy and may benefit preterm infants. Finally, vaccine-induced maternally derived antibodies might blunt the infant humoral immune response to future SARS-CoV-2 vaccination; although the clinical significance of this interference effect is largely unknown [27], it should be acknowledged and further explored.
Conclusions
The current study results indicate that early third-trimester immunization has the potential to maximize maternofetal transplacental antibody transfer, thereby affording adequate seroprotection during early infancy. As the strategy of maternal SARS-CoV-2 immunization is gaining support, there is a critical need for further scientific evidence to inform the ideal timing for vaccination during pregnancy that would provide mothers and neonates with the highest clinical protection against COVID-19. As the extent of SARS-CoV-2 community spread declines in several parts of the globe, optimizing neonatal immunity may be more heavily weighted by immunization policy-makers.
Author contributions
AR and DGW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SP, AR and DGW were respoinsible for the concept and design; all authors contributed to acquisition, analysis, or interpretation of data. The manuscript was drafted by AR, GZ, sP, GK, EOD and DGW. Laboratory analyses were by EOD, OV and DGW. Statistical analysis was by AR and GK. All authors read and approved the final manuscript.
Transparency declaration
The authors declare that they have no conflicts of interest.
Data sharing
Individual-level data will not be made publicly available with this article. Requests for sharing of de-identified individual-level participant data for scientific research can be directed to the corresponding author. All proposals will be subject to scientific review and institutional review board approval at Hadassah Medical Centre.
Funding
No external funding was used for this study.
Acknowledgements
We thank Dr Doron Kabiri, Dr Shlomi Yahalomi, Prof. Yosef Ezra and Dr Roy Alter for their assistance in patient enrolment. We also thank Rimma Barsuk and Yulia Yachnin for their technical assistance.
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.10.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1Anti-RBD-specific (a) IgG neonatal concentrations and placental transfer ratio (b) in relation to gestational age at the time of vaccination.
References
- 1.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBolt C.A., Bianco A., Limaye M.A., Silverstein J., Penfield C.A., Roman A.S., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.11.022. S0002-9378(20)31312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., et al. CDC COVID-19 response pregnancy and infant linked outcomes team; COVID-19 pregnancy and infant linked outcomes team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy: SET-NET, 16 jurisdictions, March 29-october 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim L., Whitaker M., O’Halloran A., Kambhampati A., Chai S.J., Reingold A., et al. COVID-NET Surveillance Team Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 doi: 10.1101/2021.03.07.21253094. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mithal L.B., Otero S., Shanes E.D., Goldstein J.A., Miller E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhu M., Murphy E.A., Sukhu A.C., Yee J., Singh S., Eng D., et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healy C.M., Rench M.A., Swaim L.S., Smith E.O., Sangi-Haghpeykar H., Mathis M.H., et al. Association between third-trimester Tdap immunization and neonatal pertussis antibody concentration. JAMA. 2018;320:1464–1470. doi: 10.1001/jama.2018.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter K., Nickell S., Powell M., Harriman K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis. 2017;64:3–8. doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 14.Abu Raya B., Bamberger E., Almog M., Peri R., Srugo I., Kessel A. Immunization of pregnant women against pertussis: the effect of timing on antibody avidity. Vaccine. 2015;33:1948–1952. doi: 10.1016/j.vaccine.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 15.Naidu M.A., Muljadi R., Davies-Tuck M.L., Wallace E.M., Giles M.L. The optimal gestation for pertussis vaccination during pregnancy: a prospective cohort study. Am J Obstet Gynecol. 2016;215:237. doi: 10.1016/j.ajog.2016.03.002. e1–6. [DOI] [PubMed] [Google Scholar]
- 16.Cuningham W., Geard N., Fielding J.E., Braat S., Madhi S.A., Nunes M.C., et al. Optimal timing of influenza vaccine during pregnancy: a systematic review and meta-analysis. Influenza Other Respir Virus. 2019;13:438–452. doi: 10.1111/irv.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percivalle E., Cambiè G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the lodi red zone in lombardy, Italy, as at 06 april 2020. Euro Surveill. 2020;25:2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfi O., Yakirevitch A., Wald O., Wandel O., Izhar U., Oiknine-Djian E., et al. Human nasal and lung tissues infected ex vivo with SARS-CoV-2 provide insights into differential tissue-specific and virus-specific innate immune responses in the upper and lower respiratory tract. J Virol. 2021 doi: 10.1128/JVI.00130-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS CoV- 2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention COVID-19 (coronavirus disease): people with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available at:
- 22.Stafford I.A., Parchem J.G., Sibai B.M. The coronavirus disease 2019 vaccine in pregnancy: risks, benefits, and recommendations. Am J Obstet Gynecol. 2021;30 doi: 10.1016/j.ajog.2021.01.022. S0002-9378(21)00077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The American College of Obstetricians and Gynecologists . 2020. Vaccinating pregnant and lactating patients against COVID-19.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 Available at: [Google Scholar]
- 24.Society for maternal-fetal medicine (SMFM) statement: SARS-Co-V-2 vaccination in pregnancy. https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12-1-20_(final).pdf Available at:
- 25.Harwood R., Yan H., Talawila Da Camara N., Smith C., Ward J., Tudur-Smith C., et al. Which children and young people are at higher risk of severe disease and death after SARS-CoV-2 infection: a systematic review and individual patient meta-analysis. Medrxiv. 2021 doi: 10.1016/j.eclinm.2022.101287. 2021.06.30.21259763v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kachikis A., Englund J.A. Maternal immunization: optimizing protection for the mother and infant. J Infect. 2016;72:S83–S90. doi: 10.1016/j.jinf.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Maertens K., Orije M.R.P., Van Damme P., Leuridan E. Vaccination during pregnancy: current and possible future recommendations. Eur J Pediatr. 2020;179:235–242. doi: 10.1007/s00431-019-03563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collier A.Y., McMahan K., Yu J., Tostanoski L.H., Aguayo R, Ansel J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atyeo C., Pullen K.M., Bordt E.A., Fischinger S., Burke J., Michell A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordt E.A., Shook L.L., Atyeo C., Pullen K.M., De Guzman R.M., Meinsohn M.C., et al. Sexually dimorphic placental responses to maternal SARS-CoV-2 infection. bioRxiv. 2021 doi: 10.1126/scitranslmed.abi7428. 03.29.437516. [DOI] [PMC free article] [PubMed] [Google Scholar]