Abstract
Summary
In this randomized, controlled trial, treatment with once-weekly subcutaneous injection of teriparatide for 72 weeks was found to be associated with a significant reduction in the incidence of morphometric vertebral fractures compared with alendronate in women with primary osteoporosis who were at high risk of fracture.
Introduction
To determine whether the anti-fracture efficacy of teriparatide is superior to that of alendronate, a prospective, randomized, open-label, blinded-endpoint trial was performed.
Methods
Japanese women aged at least 75 years were eligible for the study if they had primary osteoporosis and were at high risk of fracture. Patients were randomly assigned in a 1:1 ratio to receive sequential therapy (once-weekly subcutaneous injection of teriparatide 56.5 μg for 72 weeks followed by alendronate for 48 weeks) or monotherapy with alendronate for 120 weeks. The primary endpoint was the incidence of morphometric vertebral fractures at 72 weeks (at the end of teriparatide treatment).
Results
Between October 2014 and December 2017, 1011 patients (505 in the teriparatide group and 506 in the alendronate group) were enrolled. Of these, 778 patients (351 and 427, respectively) were included in the primary analysis. The incidence of morphometric vertebral fractures was significantly lower in the teriparatide group (56 per 419.9 person-years, annual incidence rate 0.1334) than in the alendronate group (96 per 553.6 person-years, annual incidence rate 0.1734), with a rate ratio of 0.78 (95% confidence interval 0.61 to 0.99, P = 0.04). In both groups, adverse events were most frequently reported in the following system organ classes: infections and infestations, gastrointestinal disorders, and musculoskeletal and connective tissue disorders.
Conclusion
Once-weekly subcutaneous injection of teriparatide significantly reduced the incidence of morphometric vertebral fractures compared with alendronate in women with primary osteoporosis who were at high risk of fracture.
Trial registration
jRCTs031180235 and UMIN000015573, March 12, 2019
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-021-05996-2.
Keywords: Bone-forming agent, High risk of fracture, Vertebral fracture, Non-vertebral fracture, Weekly teriparatide
Introduction
Pharmacologic treatment is an important component of the management of osteoporosis. According to the recommendations of current guidelines, bone resorption inhibitors such as bisphosphonates and selective estrogen receptor modulators are commonly used as initial treatment in the majority of patients with osteoporosis [1, 2]. At the same time, once-daily subcutaneous injection of teriparatide (recombinant human parathyroid hormone [1-34]) is recommended for patients at high risk of fracture [1, 2].
Teriparatide possesses a potent anti-fracture effect by stimulating bone formation. Results of a network meta-analysis of randomized, controlled trials have shown that teriparatide may be the most effective anti-osteoporosis agent in reducing the risk of fractures [3]. However, its use for more than 2 years is not recommended [4]. Therefore, teriparatide must be switched to another medication once patients have received it for the approved period because of the possibility that bone mineral density (BMD) may decrease after the termination of teriparatide treatment [5, 6].
Recently, once-weekly subcutaneous injection of teriparatide (Teribone, Asahi Kasei Pharma Corporation, Tokyo, Japan) has been approved in Japan. Several non-clinical studies have suggested that the frequency of teriparatide administration may affect the histological pattern of bone formation and that a once-weekly regimen may not increase cortical porosity, in contrast to the once-daily regimen [7–10]. In a randomized, placebo-controlled trial, once-weekly injections of teriparatide significantly reduced the risk of new vertebral fractures [11]. These results indicate that a once-weekly regimen is a promising treatment option, because once-weekly administration is generally more convenient than once-daily administration. However, its use is also restricted to 2 years. Furthermore, no head-to-head clinical trial has compared the anti-fracture efficacy of a once-weekly regimen with that of another anti-osteoporosis agent.
In a follow-up observational study of the placebo-controlled trial mentioned above, patients who received bisphosphonate following once-weekly injections of teriparatide achieved a further gain in BMD [12]. A previous randomized, controlled trial also showed that the sequential therapy with full-length parathyroid hormone (1-84) followed by alendronate significantly increased areal BMD at the lumbar spine compared with parathyroid hormone therapy followed by placebo or monotherapy with alendronate alone [13]. These results suggest that alendronate may be a suitable successor to teriparatide.
Accordingly, the Adequate Treatment of Osteoporosis (A-TOP) research group conducted the Japanese Osteoporosis Intervention Trial-05 (JOINT-05), which compared the efficacy and safety of sequential therapy (once-weekly injection of teriparatide for 72 weeks followed by alendronate for 48 weeks) and monotherapy (alendronate for 120 weeks) in patients at high risk of fracture. This study consisted of 2 parts. In the first part covering the treatment period up to 72 weeks, the efficacy and safety of teriparatide and alendronate were compared, and the hypothesis that teriparatide is superior to alendronate in reducing the incidence of morphometric vertebral fracture was tested. This was the primary objective. In the second part, during the treatment period of 73 to 120 weeks, the plan was to determine whether the efficacy of sequential therapy is superior to that of monotherapy, and this was the secondary objective. The primary results of the study are now reported.
Methods
Study design
This prospective, randomized, open-label, blinded-endpoint, pragmatic effectiveness trial was conducted in accordance with the Declaration of Helsinki and the Clinical Trials Act of the Japanese Ministry of Health, Labour, and Welfare. Patients were recruited from 113 institutions throughout Japan. The protocol was approved by the certified review board of Toranomon Hospital and the central ethics committee of the A-TOP research group. All patients provided written, informed consent. This trial was registered with the Japan Registry of Clinical Trials (number, jRCTs031180235) and the University Hospital Medical Information Network-Clinical Trials Registry (number, UMIN000015573).
Study population
The design and rationale for JOINT-05 have been reported previously [14]. In brief, Japanese women aged at least 75 years were eligible for the study if they had primary osteoporosis and if they were at high risk of fracture. Primary osteoporosis was diagnosed according to the revised 2012 Diagnostic Criteria for Primary Osteoporosis of the Japanese Society for Bone and Mineral Research [15]. Patients at high risk of fracture were defined as those who had one of the following: (1) BMD less than 60% of young adult mean (YAM) or less than −3.3 standard deviations (SDs); (2) at least 2 vertebral fractures in the area from the fourth thoracic vertebra (Th4) to the fourth lumbar vertebra (L4); (3) a grade 3 prevalent fracture; or (4) a past hip fracture.
Study treatments
Patients were randomly assigned in a 1:1 ratio to receive the sequential therapy (teriparatide 56.5 μg for 72 weeks followed by alendronate for 48 weeks) or monotherapy with alendronate for 120 weeks. Teriparatide was administered subcutaneously once weekly. Alendronate was administered using the following formulations: 5 mg tablet (orally administered once daily), 35 mg tablet or jelly (orally administered once weekly), or 900 μg infusion bag (administered intravenously once every 4 weeks). When this study was started, the treatment period for once-weekly injection of teriparatide was limited to 72 weeks in Japan. Given this limitation, the treatment period of teriparatide was determined.
Random allocation was implemented by a web-based computerized system with the modified minimization method adjusted for the following prognostic factors: age (75–79 vs. ≥80 years), BMD (<60% vs. ≥60% of YAM), number of prevalent vertebral fractures (0–1 vs. ≥2), presence/absence of prevalent vertebral fractures of grade 3, presence/absence of history of hip fracture, and the study institution. The algorithm for random allocation was concealed from the investigators and the other study personnel.
Outcome measures
The thoracic and lumbar vertebrae were imaged in 2 directions at 0 (baseline), 24, 48, 72, and 120 weeks. For the assessment of prevalent vertebral fractures, anteroposterior and lateral radiographs of the thoracic and lumbar spine were examined by the investigators. They assessed the grade of vertebral fractures from Th4 to L4 according to the semiquantitative (SQ) technique [16]. These assessments were reviewed centrally by one evaluator of the fracture assessment committee blinded to the assigned treatment.
The committee also adjudicated the presence/absence of a new vertebral fracture by comparing radiographs of Th4 to L4 between baseline and post-treatment. After the X-ray films were collected, 2 evaluators blinded to the assigned treatment reviewed the films independently according to the SQ technique mentioned above. If inconsistencies arose between the evaluators, 3 evaluators reviewed the films simultaneously. The presence/absence of the other fractures such as non-vertebral fracture and clinical fracture was assessed by the investigators. Thereafter, 3 evaluators of the fracture assessment committee reviewed the assessment made by the investigators using the collected X-ray films.
BMD at the lumbar spine, proximal femur, radius, and second metacarpal bone was measured in each institution at 0, 24, 48, 72, and 120 weeks by dual-energy X-ray absorptiometry. Blood samples were obtained at 0, 12, 24, 48, 72, and 120 weeks to measure the serum levels of osteocalcin, procollagen type I amino-terminal propeptide (P1NP), and tartrate-resistant acid phosphatase 5b (TRACP-5b). LSI Medience Corporation (Tokyo, Japan) analyzed the levels of osteocalcin and P1NP using a fluorometric enzyme immunoassay and electrochemiluminescence immunoassay, respectively. SB Bioscience Co., Ltd. (Tokyo, Japan) analyzed TRACP-5b levels using an enzyme immunoassay.
The primary endpoint of JOINT-05 was the incidence of morphometric vertebral fracture at 72 weeks. The accumulation of person-years at risk started at the randomization of each patient and ended at the date of the last visit, lost to follow-up, or death. In the first part of the study, the secondary endpoints included the following at 72 weeks: the incidence of any fracture, clinical vertebral fracture, and non-vertebral fracture, fracture at specific skeletal sites, and vertebral fracture progression.
Statistical considerations
Before starting the study, it was assumed that the annualized incidence of vertebral fracture in the alendronate group would be 0.112 and that the hazard ratio of teriparatide relative to alendronate over 72 weeks would be 0.5 [14]. Under these assumptions, a sample size of 500 patients for each group was calculated to detect the difference between the treatment groups in the primary endpoint with a power of 0.80 and a significance level of 0.05.
In the analyses of the primary and secondary endpoints, a multivariable Poisson regression model was fit to calculate the rate ratios of teriparatide to alendronate and their 95% confidence intervals (CIs). This regression model included the minimization factors for random allocation as covariates. For the incidence of morphometric vertebral fracture, any fracture, and clinical vertebral fracture, as well as vertebral fracture progression, the hypothesis that the efficacy of teriparatide is superior to that of alendronate was tested. For the incidence of non-vertebral fractures, the hypothesis that the efficacy of teriparatide is not inferior to that of alendronate, which was defined by the upper limit of the 95% CI for the rate ratio of less than 1.96 (1/0.51), was tested. The margin of non-inferiority was based on the results of the previous meta-analysis for non-vertebral fractures, in which the hazard ratio of alendronate to placebo was 0.51 [17]. Least square means of BMD and bone turnover markers were estimated using mixed models for repeated measures under an assumption of missing at random.
Efficacy outcomes were analyzed in the full analysis set, which included randomly assigned patients who received at least one dose of the study medication and had at least one evaluable post-treatment efficacy datum. All data were analyzed with the use of SAS software version 9.4 (SAS institute, Cary, NC). All reported P values are 2-tailed without multiplicity adjustment, with a P value of less than 0.05 indicating a significant difference.
Results
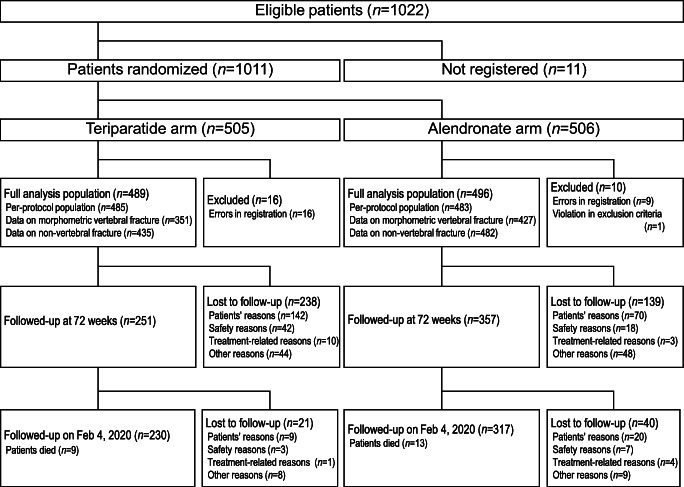
Between October 2014 and December 2017, 1011 patients (505 in the teriparatide group and 506 in the alendronate group) were enrolled (Fig. 1). Of these, 26 patients (16 and 10, respectively) were excluded from the analysis, and the remaining 985 patients (489 and 496, respectively) were included in the full analysis set. In the alendronate group, 222 patients took the 35 mg/week tablet, 179 patients received 900 μg/4 weeks administration, 79 patients took 35 mg/week oral jelly formulation, 3 patients took the 5 mg/day tablet, and the administration routes of 13 patients were unknown. Data on morphometric vertebral fractures were obtained from 778 patients (351 and 427, respectively) and were included in the primary analysis.
Fig. 1.
Flowchart of the patients enrolled in the Japanese Osteoporosis Intervention Trial-05
During the study, 238 patients in the teriparatide group and 139 in the alendronate group discontinued the study. Of these, 142 and 70 patients in the teriparatide and alendronate groups did not wish to continue the study treatment, respectively. Furthermore, 42 and 18 patients in the teriparatide and alendronate groups, respectively, discontinued the study treatment because of safety reasons. However, most of the adverse events leading to treatment discontinuation were mild or moderate in intensity and resolved after treatment discontinuation.
By the end of 72 weeks, 5 and 7 patients in the teriparatide and alendronate groups, respectively, died. Of these, 3 deaths (2 in the teriparatide group and 1 in the alendronate group) were considered possibly related to the treatment (Online Resource Supplementary Table S1). The mean (SD) percentage adherence throughout the 72 weeks was 29.0% (45.2) in the teriparatide group and 64.2% (44.6) in the alendronate group, which was calculated under the assumption that patients not reporting adherence did not receive the study medication at all.
Baseline characteristics were well balanced between the treatment groups (Table 1). The mean (SD) age was 81.4 (4.5) years in the teriparatide group and 81.5 (4.7) years in the alendronate group. Approximately 40% of the patients in each treatment group had at least 2 prevalent vertebral fractures and grade 3 prevalent vertebral fractures. Baseline characteristics were also similar between patients with and without radiographs at 72 weeks (Online Resource Supplementary Table S2).
Table 1.
Baseline characteristics of postmenopausal women with severe osteoporosis included in the full analysis set
| Teriparatide (N = 489) | Alendronate (N = 496) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (y) | 81.4 | 4.5 | 81.5 | 4.7 |
| Age at menopause (y) | 49.6 | 4.4 | 49.2 | 4.4 |
| Time from menopause (y) | 31.8 | 6.5 | 32.3 | 6.4 |
| No. of prevalent vertebral fractures | 1.6 | 1.9 | 1.8 | 2.0 |
| Proportion of patients with the following no. of prevalent vertebral fractures, % | ||||
| 0 | 32.4% | 32.1% | ||
| 1 | 26.8% | 27.1% | ||
| 2 | 16.8% | 15.2% | ||
| 3 | 10.9% | 9.5% | ||
| 4 | 4.9% | 6.1% | ||
| 5 or more | 8.2% | 10.1% | ||
| Maximum grade of prevalent vertebral fractures, % | ||||
| Grade 1 | 9.2% | 9.5% | ||
| Grade 2 | 15.8% | 17.8% | ||
| Grade 3 | 42.6% | 40.6% | ||
| History of hip fractures | 14.1% | 13.5% | ||
| Prior treatment | 53.8% | 54.4% | ||
| Prior use of bisphosphonates | 29.7% | 30.2% | ||
| Comorbidities, % | ||||
| Hypertension | 37.8% | 37.5% | ||
| Diabetes | 8.2% | 9.1% | ||
| Dyslipidemia | 15.7% | 15.3% | ||
| Rheumatoid arthritis | 0.4% | 1.2% | ||
| Osteoarthritis | 0.0% | 0.2% | ||
| Others | 27.0% | 28.0% | ||
| Height (cm) | 146.7 | 6.5 | 146.2 | 6.2 |
| Weight (cm) | 47.7 | 9.0 | 47.3 | 8.1 |
| BMI (kg/m2) | 22.2 | 3.8 | 22.1 | 3.5 |
| HbA1c (%) | 5.9 | 0.5 | 5.9 | 0.7 |
| BMD at L2-L4 (T-score) | −2.3 | 1.4 | −2.4 | 1.4 |
| Corrected pentosidine (pmol/mL) | 45.7 | 25.8 | 44.1 | 19.1 |
| Timed up and go test (sec) | 13.1 | 8.1 | 13.7 | 11.1 |
| 25OHVD (ng/mL) | 17.6 | 5.9 | 17.5 | 5.6 |
| Total cholesterol (mg/dL) | 203.3 | 36.9 | 200.4 | 36.0 |
| HDL-cholesterol (mg/dL) | 63.1 | 16.9 | 60.5 | 16.5 |
| LDL-cholesterol (mg/dL) | 114.8 | 31.6 | 113.2 | 30.4 |
| Triglycerides (mg/dL) | 115.1 | 58.3 | 120.5 | 66.2 |
| eGFR (mL/min/1.73 m2) | 63.6 | 17.5 | 62.8 | 16.7 |
| Creatinine (mg/dL) | 0.7 | 0.2 | 0.7 | 0.2 |
| Urine creatinine (mg/dL) | 77.5 | 58.2 | 73.0 | 50.6 |
| Albumin (g/dL) | 4.1 | 0.4 | 4.1 | 0.4 |
| Ca (mg/dL) | 9.5 | 0.5 | 9.5 | 0.4 |
| Urine Ca (mg/dL) | 11.4 | 8.7 | 11.3 | 8.1 |
Data are summarized as means with standard deviation unless otherwise specified
Abbreviations: SD standard deviation, BMI body mass index, HbA1c hemoglobin A1c, BMD bone mineral density, L2 second lumbar vertebra, L4 fourth lumbar vertebra, 25OHVD 25-hydroxy vitamin D, HDL high-density lipoprotein, LDL low-density lipoprotein, eGFR estimated glomerular filtration rate
In the analysis of the primary endpoint (Table 2), the incidence of morphometric vertebral fracture was significantly lower in the teriparatide group (56 per 419.9 person-years, annual incidence rate 0.1334) than in the alendronate group (96 per 553.6 person-years, annual incidence rate 0.1734) with a rate ratio of 0.78 (95% CI 0.61 to 0.99, P = 0.04). The results of the sensitivity analyses also showed the superiority of teriparatide over alendronate (Online Resource Supplementary Table S3).
Table 2.
Incidences of the primary and secondary endpoints in each treatment group and their rate ratios between the treatment groups at 72 weeks
| Teriparatide | Alendronate | Rate ratio* | 95% CI | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Person | Person-years | Annual incidence rate | Count | Person | Person-years | Annual incidence rate | ||||
| Primary endpoint | |||||||||||
| Morphometric vertebral fracture | 56 | 45 | 419.9 | 0.1334 | 96 | 73 | 553.6 | 0.1734 | 0.78 | 0.61 to 0.99 | 0.04 |
| Secondary endpoints | |||||||||||
| Any fracture | 71 | 55 | 469.2 | 0.1513 | 116 | 87 | 600.2 | 0.1933 | 0.83 | 0.60 to 1.16 | 0.28 |
| Clinical vertebral fracture | 6 | 5 | 419.9 | 0.0143 | 12 | 8 | 553.6 | 0.0217 | 0.70 | 0.23 to 2.09 | 0.52 |
| Progression of vertebral fracture | 30 | 28 | 419.9 | 0.0714 | 38 | 34 | 553.6 | 0.0686 | 1.11 | 0.76 to 1.64 | 0.59 |
| Non-vertebral fracture | 15 | 12 | 469.2 | 0.0320 | 20 | 20 | 600.2 | 0.0333 | 1.09 | 0.68 to 1.75 | <0.01† |
| Secondary endpoints (fractures at specific skeletal sites) | |||||||||||
| Forearm | 4 | 4 | 469.2 | 0.0085 | 3 | 3 | 600.2 | 0.0050 | |||
| Humerus | 0 | 0 | 469.2 | 0.0000 | 0 | 0 | 600.2 | 0.0000 | |||
| Femur | 3 | 3 | 469.2 | 0.0064 | 6 | 6 | 600.2 | 0.0100 | |||
| Lower leg | 0 | 0 | 469.2 | 0.0000 | 0 | 0 | 600.2 | 0.0000 | |||
| Clavicle | 1 | 1 | 469.2 | 0.0021 | 0 | 0 | 600.2 | 0.0000 | |||
| Pelvis | 1 | 1 | 469.2 | 0.0021 | 1 | 1 | 600.2 | 0.0017 | |||
| Rib | 2 | 1 | 469.2 | 0.0043 | 4 | 4 | 600.2 | 0.0067 | |||
| Other | 4 | 4 | 469.2 | 0.0085 | 6 | 6 | 600.2 | 0.0100 | |||
*Generalized estimating equation-Poisson regression adjusted for age, counts, and maximum grade of prevalent vertebral fractures, history of hip fractures, and bone mineral density as covariates and individual and institute as cluster
†Non-inferiority P-value with a pre-specified non-inferiority margin of 1.96
Abbreviation: CI confidence interval
The incidence of non-vertebral fractures in the teriparatide group was 15 per 469.2 person-years (annual incidence rate 0.0320), whereas that in the alendronate group was 20 per 600.2 person-years (annual incidence rate 0.0333), with a rate ratio of 1.09 (95% CI 0.68 to 1.75). The upper limit of the 95% CI did not exceed the prespecified margin of 1.96 (P < 0.01 for non-inferiority). Analyses of the other secondary endpoints did not show significant treatment effects.
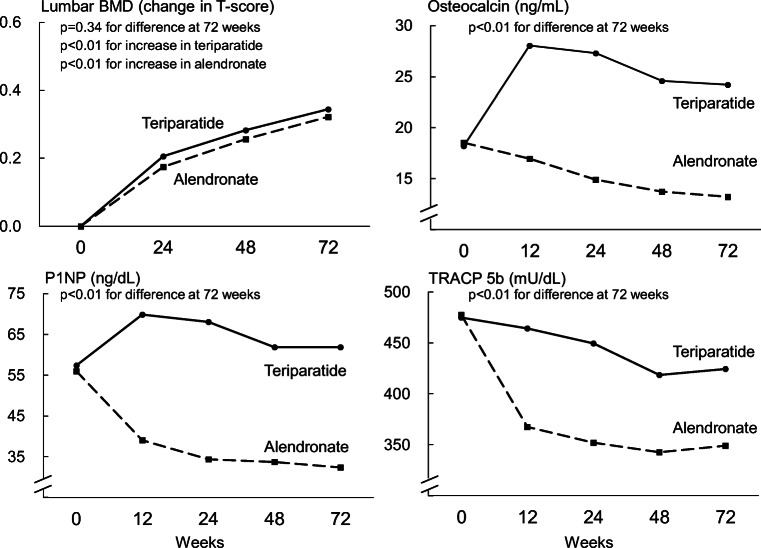
Mean lumbar spine BMD (T-score) was similarly elevated up to 72 weeks in both treatment groups (Fig. 2). Similar trends in BMD were observed at the second lumbar vertebra (L2) to L4, total hip, femoral neck, and forearm (Online Resource Supplementary Table S4). Serum levels of osteocalcin and P1NP rose in the teriparatide group and reached peak values at 12 weeks, whereas they constantly decreased in the alendronate group. As a result, the increases in osteocalcin and P1NP levels were significantly larger in the teriparatide group at 72 weeks (P < 0.01). Serum levels of TRACP-5b decreased in both groups, but the amount of reduction was significantly greater in the alendronate group at 72 weeks (P < 0.01).
Fig. 2.
Changes in lumbar spine bone mineral density and bone turnover markers over 72 weeks by treatment group. BMD, bone mineral density; P1NP, procollagen type I amino-terminal propeptide; TRACP-5b, tartrate-resistant acid phosphatase 5b
The treatment effects of teriparatide on the incidence of morphometric vertebral fractures were generally consistent across various subgroups (Fig. 3). In the subgroups stratified by SQ grade of prevalent vertebral fracture, however, teriparatide showed a good effect in patients with grade 3 fractures, whereas alendronate showed it in those with grades 1–2 fractures (P = 0.08 for interaction). Teriparatide also showed a good effect in patients with T-scores of less than −3.3, whereas alendronate showed it in those with T-scores of at least −3.3 (P = 0.61 for interaction).
Fig. 3.
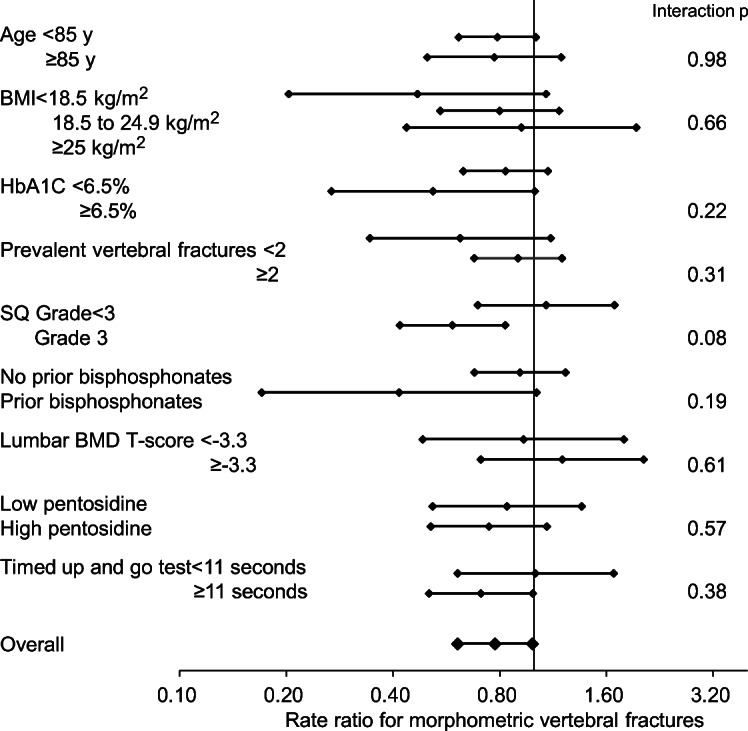
Rate ratio for the incidence of morphometric vertebral fractures stratified by baseline characteristics. BMI, body mass index; HbA1c, hemoglobin A1c; SQ, semiquantitative; BMD, bone mineral density
In both treatment groups, adverse events were most frequently reported in the following system organ classes: infections and infestations, gastrointestinal disorders, and musculoskeletal and connective tissue disorders (Table 3). During the treatment, 6 patients (2 in the teriparatide group and 4 in the alendronate group) experienced severe adverse events that were considered possibly related to the treatment (Online Resource Supplementary Table S1). In the teriparatide group, nausea and pancreatic carcinoma were reported. In the alendronate group, gastric ulcer, herpes zoster, acute heart failure, and death due to unknown cause were reported.
Table 3.
Incidence of adverse events during the 72-week treatment period
| System organ classa | Teriparatide (N = 489) | Alendronate (N = 496) | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Infections and infestations | 49 | (10.0) | 98 | (20.0) |
| Immune system disorders | 3 | (0.6) | 4 | (0.8) |
| Nervous system disorders | 19 | (3.9) | 21 | (4.3) |
| Eye disorders | 2 | (0.4) | 10 | (2.0) |
| Ear and labyrinth disorders | 9 | (1.8) | 6 | (1.2) |
| Cardiac disorders | 9 | (1.8) | 8 | (1.6) |
| Vascular disorders | 16 | (3.3) | 11 | (2.2) |
| Gastrointestinal disorders | 57 | (11.7) | 46 | (9.4) |
| Hepatobiliary disorders | 4 | (0.8) | 2 | (0.4) |
| Skin and subcutaneous tissue disorders | 38 | (7.8) | 46 | (9.4) |
| Musculoskeletal and connective tissue disordersb | 99 | (20.2) | 84 | (17.2) |
| Renal and urinary disorders | 12 | (2.5) | 9 | (1.8) |
| General disorders and administration site conditions | 43 | (8.8) | 24 | (4.9) |
| Blood and lymphatic system disorders | 5 | (1.0) | 8 | (1.6) |
| Respiratory, thoracic, and mediastinal disorders | 28 | (5.7) | 11 | (2.2) |
| Injury, poisoning, and procedural complications | 0 | (0.0) | 16 | (3.3) |
| Psychiatric disorders | 6 | (1.2) | 5 | (1.0) |
| Metabolism and nutrition disorders | 13 | (2.7) | 8 | (1.6) |
| Neoplasms benign, malignant, and unspecifiedc | 5 | (1.0) | 9 | (1.8) |
| Investigations | 4 | (0.8) | 2 | (0.4) |
| Others | 2 | (0.4) | 1 | (0.2) |
aAdverse events were coded according to the system organ class of the Medical Dictionary for Regulatory Activities
bFractures were excluded
cCysts and polyps were included
Discussion
In the primary analysis of this study, the incidence of morphometric vertebral fractures was significantly lower in the teriparatide group than in the alendronate group. Moreover, the secondary analysis showed the non-inferiority of teriparatide to alendronate in reducing the risk of non-vertebral fractures. Although the vertebral fracture treatment comparisons in osteoporotic women (VERO) study showed the superiority of once-daily injection of teriparatide over risedronate in reducing new radiographic vertebral fractures [18], no randomized, controlled trial has compared the anti-fracture efficacy of a once-weekly regimen and another anti-osteoporosis agent. To the best of our knowledge, this is the first head-to-head comparison trial.
The rate ratio for the incidence of morphometric vertebral fractures was 0.78, which was relatively smaller than that obtained from the VERO study (risk ratio 0.44 vs. risedronate) or a network meta-analysis (hazard ratio 0.46 vs. alendronate) [18, 19]. This smaller effect size may simply imply that the once-daily regimen is more efficacious than the once-weekly regimen, or it may have been derived from the patients’ characteristics in the present study. For example, patients enrolled in this study were older than those in the VERO study by an average of 10 years. Another possibility is the poor treatment adherence. In the present study, the mean percentage adherence in the teriparatide group was 29%, and approximately 30% of the patients did not wish to continue teriparatide treatment. Once-weekly injection of teriparatide, as well as the once-daily regimen, is much more expensive than other therapies. It is notable that this was a pragmatic trial in which study drugs were not provided by the researchers and were open-label. In other words, patients in the present study paid the cost of the study medication. This payment might have led to the poor adherence, although most of the cost was covered by insurance. The cost is an important limitation of teriparatide [2], and effective strategies to improve adherence are needed.
Patients aged at least 75 years were enrolled, and their mean age exceeded 80 years. Because clinical trials usually do not include patients aged 80 years or older, the present findings are important when considering the treatment strategy for such an elderly population. In the subgroup analysis stratified by age (<85 vs. ≥85 years), treatment effects were consistent regardless of age group. This result means that the anti-fracture efficacy of teriparatide is superior to that of alendronate even in elderly persons.
Other subgroup analyses have suggested the superiority of teriparatide in patients with grade 3 prevalent vertebral fractures and those with T-scores less than −3.3. Currently, teriparatide is recommended for patients with severe osteoporosis or those at high risk of fracture worldwide [1, 2, 20]. However, there are no universally accepted criteria for identifying such a population [1]. For example, the European guidance strongly recommends teriparatide for patients with vertebral fractures [1], whereas the Endocrine Society Clinical Practice Guideline recommends it for those with severe or multiple vertebral fractures [2]. Furthermore, the Japanese guideline recommends a once-weekly regimen for those with low BMD, prevalent fractures, older age, or a family history of hip fracture [20]. The results of the subgroup analyses, as well as the inclusion criteria, support the recommendations of the Japanese guideline.
Despite the significant difference in the incidence of vertebral fracture between the treatment groups, the mean BMD increased similarly in both groups. In the post hoc analysis of the Fracture Prevention Trial, teriparatide-mediated increases in spine BMD accounted for approximately one-third of the vertebral fracture risk reduction, and the majority of the risk reduction resulted from improvements in non-BMD determinants of bone strength [21]. In another study, total bone mineral content, total and cortical bone areas, periosteal circumference, and polar cross-sectional moment of inertia were significantly higher in patients treated with teriparatide than in those treated with placebo [22]. These results indicate that the anti-fracture efficacy of teriparatide is mainly derived from its effects on bone strength and bone geometry.
As for the changes in bone turnover markers, once-weekly injection of teriparatide increased the serum levels of osteocalcin and P1NP and decreased the levels of TRACP-5b, which indicates that it increases bone formation while decreasing bone resorption. This result is inconsistent with those obtained from the once-daily regimen, which increased bone-resorption markers after increasing bone-formation markers [23, 24]. This may be a unique action of the once-weekly regimen because previous clinical studies have also shown that once-weekly injection of teriparatide induced bone formation without promoting bone resorption [11, 25]. In a non-clinical study in mice, a low frequency of administration of teriparatide induced bone formation by both remodeling and mini-modeling, whereas high-frequency administration induced it predominantly by remodeling [7]. This result may explain its unique action, because bone formation by modeling involves little increase in bone resorption [25].
In the safety assessments, adverse events were frequently reported in both groups because elderly patients were enrolled in the study. However, only 4 severe adverse events were considered possibly related to teriparatide. In addition, similar proportions of patients in both groups died by the end of 72 weeks in the present study, whereas more patients died in the teriparatide group than in the risedronate group in the VERO study [18]. These safety results, as well as the efficacy results, suggest that a once-weekly regimen could be a promising treatment option.
Some limitations should be mentioned. First, the sample size was not large enough to detect the difference between the treatment groups in the incidence of any fracture, the incidence of clinical vertebral fracture, and vertebral fracture progression. We plan to assess the effects of the sequential therapy on these outcomes at 120 weeks. Second, the mean percentage adherence differed between the treatment groups. However, regarding the primary endpoint, the robustness of the findings was examined through three sensitivity analyses, and the results were similar to those of the planned primary analysis, although multiple imputation was not performed. Third, since BMD was measured at the lumbar spine, proximal femur, radius, and second metacarpal bone in each institution, the precise analytical methods might have differed among the institutions. Therefore, changes in T-scores were analyzed in this study.
In conclusion, treatment with once-weekly subcutaneous injection of teriparatide for 72 weeks was associated with a significant reduction in the incidence of morphometric vertebral fractures compared with that with alendronate in women with primary osteoporosis who were at high risk of fracture. However, teriparatide must be switched to another medication once patients have received it for the approved period. Therefore, a suitable successor to teriparatide must be identified. In the second part of this study, we will determine whether the efficacy of sequential therapy with teriparatide followed by alendronate is superior to that of monotherapy with alendronate alone.
Supplementary Information
(PDF 91 kb)
Acknowledgements
The authors would like to thank those who participated as clinical investigators in JOINT-05, the members of the central ethics committee, and the members of the data monitoring committee. The authors would also like to express their sincere thanks to the chairman, Dr. Itsuo Gorai, and the members of the central ethics committee for the JOINT trials. The authors would also like to express their sincere thanks to Mr. Teruhiko Miyazaki and Ms. Yuko Iwata for their secretarial help. Finally, the authors would like to thank FORTE Science Communications (www.forte-science.co.jp) for professional assistance in preparing this manuscript.
Funding
This study was funded by the Public Health Research Foundation and Asahi Kasei Pharma Corp.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest/Competing interests
H Hagino has received lecture fees or grants outside the submitted work from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mitsubishi Tanabe Pharma Corp., Mochida Pharma Co., Ltd, Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd.
T Sugimoto has received research grants from Asahi Kasei Pharma Corp., Astellas Pharma Inc., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Teijin Pharma Co., Ltd., as well as consulting fees from Kissei Pharmaceutical Co., Ltd., Shimadzu Corp., and Takeda Pharmaceutical Co., Ltd.
S Tanaka has received lecture fees from Astra-Zeneca, Taiho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd.; consultation fees from DeNA Life Science Inc. and CanBus; and outsourcing fees from Satt Co., Ltd. and Asahi Kasei Pharma Corp.
T Sone has received research grants from Astellas Pharma Inc., Eisai Co., Ltd., Daiichi-Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Eli Lilly Japan Co., Ltd., as well as consulting and/or lecture fees from Asahi Kasei Pharma Corp., MSD Co., Ltd., and Daiichi-Sankyo Co., Ltd.
T Nakamura has received reports personal fees and other from Asahi Pharma Corp., Teijin Pharma Ltd., Daiichi-Sankyo Pharma Co., Ltd., UCB Japan Co., Ltd., Amgen Inc., Astellas Pharma Inc., and Chugai Pharma Co., Ltd. as well as personal fees and other from Merck & Co., Inc.
S Soen has received consulting fees, speaking fees, and/or honoraria from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mochida Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and UCB Japan Co., Ltd.
K Sasaki and S Mori declare no competing interests.
Ethics approval
The protocol was approved by the certified review board of Toranomon Hospital and the central ethics committee of the Adequate Treatment of Osteoporosis research group.
All procedures performed in this study were in accordance with the Clinical Trials Act of the Japanese Ministry of Health, Labour, and Welfare and with the 1964 Helsinki declaration and its later amendments.
Consent to participate
All patients provided written, informed consent.
Consent for publication
All patients provided written, informed consent.
Footnotes
The original version of this article, published on 17 May 2021, unfortunately contained a mistake. The article was published with incomplete author list. The Author K. Sasaki was missing due to a typesetting mistake. The publisher apologizes for this mistake. The original article has been corrected.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/27/2021
A Correction to this paper has been published: 10.1007/s00198-021-06066-3
Contributor Information
H. Hagino, Email: hagino@tottori-u.ac.jp
T. Sugimoto, Email: sugimoto@med.shimane-u.ac.jp
S. Tanaka, Email: tanaka.shiro.8n@kyoto-u.ac.jp
K. Sasaki, Email: s.kotaro79@gmail.com
T. Sone, Email: tsone@med.kawasaki-m.ac.jp
T. Nakamura, Email: t-nak@utopia.ocn.ne.jp
S. Soen, Email: nra48207@nifty.com
S. Mori, Email: stmori@sis.seirei.or.jp
References
- 1.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 3.Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97:1871–1880. doi: 10.1210/jc.2011-3060. [DOI] [PubMed] [Google Scholar]
- 4.Eli Lilly and Company (2012) FORTEO highlights of prescribing information. https://pi.lilly.com/us/forteo-pi.pdf. Accessed 26 January 2021
- 5.Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS) J Bone Miner Res. 2009;24:726–736. doi: 10.1359/jbmr.081215. [DOI] [PubMed] [Google Scholar]
- 6.Adami S, San Martin J, Muñoz-Torres M, Econs MJ, Xie L, Dalsky GP, McClung M, Felsenberg D, Brown JP, Brandi ML, Sipos A. Effect of raloxifene after recombinant teriparatide [hPTH(1-34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int. 2008;19:87–94. doi: 10.1007/s00198-007-0485-y. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Hasegawa T, Sasaki M, Hongo H, Tsuboi K, Shimizu T, Ota M, Haraguchi M, Takahata M, Oda K, Luiz de Freitas PH, Takakura A, Takao-Kawabata R, Isogai Y, Amizuka N. Frequency of teriparatide administration affects the histological pattern of bone formation in young adult male mice. Endocrinology. 2016;157:2604–2620. doi: 10.1210/en.2015-2028. [DOI] [PubMed] [Google Scholar]
- 8.Takakura A, Lee J-W, Hirano K, Isogai Y, Ishizuya T, Takao-Kawabata R, Iimura T (2017) Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res 5. 10.1038/boneres.2017.2 [DOI] [PMC free article] [PubMed]
- 9.Zebaze R, Takao-Kawabata R, Peng Y, Zadeh AG, Hirano K, Yamane H, Takakura A, Isogai Y, Ishizuya T, Seeman E. Increased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatide. Bone. 2017;99:80–84. doi: 10.1016/j.bone.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Yamane H, Takakura A, Shimadzu Y, Kodama T, Lee JW, Isogai Y, Ishizuya T, Takao-Kawabata R, Iimura T. Acute development of cortical porosity and endosteal naïve bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS One. 2017;12:e0175329. doi: 10.1371/journal.pone.0175329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97:3097–3106. doi: 10.1210/jc.2011-3479. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto T, Shiraki M, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Kuroda T, Nakamura T. Vertebral fracture risk after once-weekly teriparatide injections: follow-up study of Teriparatide Once-Weekly Efficacy Research (TOWER) trial. Curr Med Res Opin. 2013;29:195–203. doi: 10.1185/03007995.2012.761956. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Mori S, Hagino H, Sugimoto T. Design of a randomized trial of teriparatide followed by alendronate: Japanese Osteoporosis Intervention Trial-05 (JOINT-05) J Bone Miner Metab. 2020;38:412–417. doi: 10.1007/s00774-019-01074-0. [DOI] [PubMed] [Google Scholar]
- 15.Soen S, Fukunaga M, Sugimoto T, et al. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab. 2013;31:247–257. doi: 10.1007/s00774-013-0447-8. [DOI] [PubMed] [Google Scholar]
- 16.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 17.Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, The Osteoporosis Methodology Group. The Osteoporosis Research Advisory Group Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 18.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Möricke R, López-Romero P. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–240. doi: 10.1016/S0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 19.Simpson EL, Martyn-St James M, Hamilton J, Wong R, Gittoes N, Selby P, Davis S. Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone. 2020;130:115081. doi: 10.1016/j.bone.2019.115081. [DOI] [PubMed] [Google Scholar]
- 20.Japan Osteoporosis Society (2015) Japanese 2015 guidelines for prevention and treatment of osteoporosis (in Japanese). http://www.josteo.com/ja/index.html. Accessed 26 January 2021
- 21.Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:1785–1790. doi: 10.1359/jbmr.060802. [DOI] [PubMed] [Google Scholar]
- 22.Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, Gaich GA, Dalsky GP, Myers SL. Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18:539–543. doi: 10.1359/jbmr.2003.18.3.539. [DOI] [PubMed] [Google Scholar]
- 23.Shiraki M, Sugimoto T, Nakamura T. Effects of a single injection of teriparatide on bone turnover markers in postmenopausal women. Osteoporos Int. 2013;24:219–226. doi: 10.1007/s00198-012-2159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto T, Shiraki M, Fukunaga M, Hagino H, Sone T, Nakano T, Kishimoto H, Ito M, Yoshikawa H, Kishida M, Irie C, Nakamura T. 24-month open-label teriparatide once-weekly efficacy research trial examining bone mineral density in subjects with primary osteoporosis and high fracture risk. Adv Ther. 2017;34:1727–1740. doi: 10.1007/s12325-017-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 91 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.