Abstract
Pregnancy and lactation-associated osteoporosis (PLO) with predominantly subsequent vertebral fracture is a rare but severe disease with an estimated incidence of 0.4 in 100,000. In the past, patients with PLO have been predominantly treated with oral and i.v. bisphosphonates to reduce subsequent fracture risk. Hereby, the use of bisphosphonates in premenopausal women is controversial, as bisphosphonates know to persist in bone for many years and can be exposed and circulate in maternal serum and subsequently pass the placenta barrier and may have a detrimental effect on fetal bone health. Here we report the effects of denosumab on the bone mineral density (BMD) and subsequent fracture risk in PLO. In this case presentation, denosumab was administered postpartum with 3000 IE vitamin D and 1000 mg of calcium daily in a patient with PLO and vertebral fracture of L1 and L4. After 18 months of treatment with denosumab, we could demonstrate a clinical significant increase of BMD at the lumbar spine, femoral neck, and total hip of 32.2%, 13.0%, and 11.5% respectively with no further subsequent fractures. As the patient had regular menstrual cycles and considered a further pregnancy, denosumab treatment was terminated and soon a second pregnancy occurred. After the second pregnancy, BMD decreased at the lumbar spine, femur neck, and total hip by −8.8%, −6.9%, and −7.0% respectively compared to the maximum values during treatment with denosumab, but was still significantly higher compared to baseline levels with no further fractures.
Keywords: Denosumab, Fractures, Lactation, Osteoporosis, Pregnancy
Introduction
Lumbopelvic pain (LPP) is one of the most frequent skeletal disabling symptoms in pregnancy with a frequency of around 58–65%. Beside many, one infrequent reason for LLP is pregnancy and lactation-associated osteoporosis (PLO) with predominantly subsequent vertebral and non-vertebral fractures [1, 2]. The incidence of this rare but severe disease is estimated at 0.4 in 100,000 [3]. It is assumed that the number of undiagnosed cases is significantly higher. The pathogenesis of PLO today is still unknown. A recent case-control study including 102 women with PLO and 102 healthy controls matched for age, region, and gravidity reported that women with PLO had excessive dental problems in childhood (p<0.001), performed less frequently sports both before (p<0.002) and after puberty (p < 0.01), and reported twice as often severe diseases during pregnancy (p < 0.029) and frequency of immobilization was twice as often in the PAO group compared to that in the control group (p < 0.005) [4]. The subsequent fracture risk of women with PLO was recently reported in a single-center prospective cohort study including 107 women with PLO with at least one fracture. During a median of 6 years of follow-up, 26 (24.3%) of patients who had a fracture at baseline reported a subsequent fracture. Overall, 30 PLO patients (28%) reported a further pregnancy. In subsequent pregnancies, 6 (20%) of patients reported a subsequent fracture. Patients with up to 1 vs. > 1 fracture at time of diagnosis showed a 3 (10%) and 25 (27%) subsequent fracture rate, respectively (p < 0.047). There was a significant correlation between the number of fractures at time of diagnosis and subsequent fracture risk (p < 0.003). With regard to the clinical course of PLO, it must be emphasized that after weaning, BMD may also improve spontaneously in women with PLO. Today, no consensus on possible risk factors, diagnosis, treatment, and prevention of PLO exists.
Case presentation
Here we present the case of a 33-year-old patient with a height of 167.5 cm, a weight of 62.0 kg, and BMI of 22.1, respectively. The patient’s history comprised a menarche at age of 14 years, with an initially irregular menstrual cycle which normalized after initiation of an oral contraceptive from age 15 to age 30 years. In her adolescence for many years, she performed professional swimming. There was no history of a secondary amenorrhea and she was always at normal weight/BMI. After three abortions in the first trimester, a hemostasiological assessment was performed, which revealed an MTHFR polymorphism (C677C, heterozygous as well as a lipoprotein-a elevation). A treatment with low-molecular heparin was initiated. One year later, a preplanned pregnancy occurred with no complications and spontaneous birth of a healthy child.
Three months after birth of her first child, the patient suddenly experienced severe back pain without any trauma and visited her general practitioner, who indicated an MRI examination of the lumbar spine. The radiological diagnosis included a compression fracture of L1 and L4, both with accompanying edema indicating recent fractures.
The patient was referred to the German reference center for pregnancy and lactation-associated osteoporosis (GRCPLO) 3 weeks later for further diagnosis and treatment initiation. To confirm the diagnosis, a dual X-ray absorptiometry (DXA) examination including a trabecular bone score (TBS) and a quantitative ultrasonometry (QUS) assessment were performed and a severe case PLO could be confirmed. The results are shown in Fig. 1. For the evaluation of the DXA, we used the t-score, although the z-score is also recommended for premenopausal women. There were only marginal differences between the t-score and the z-score in our patient, so we remained using the t-score. An overview of all results of the DXA measurements (t-score, z-score, BMD, and TBS) is shown in Table 1. A secondary osteoporosis could be excluded by specific laboratory assessment proposed by the German osteoporosis guidelines [5].
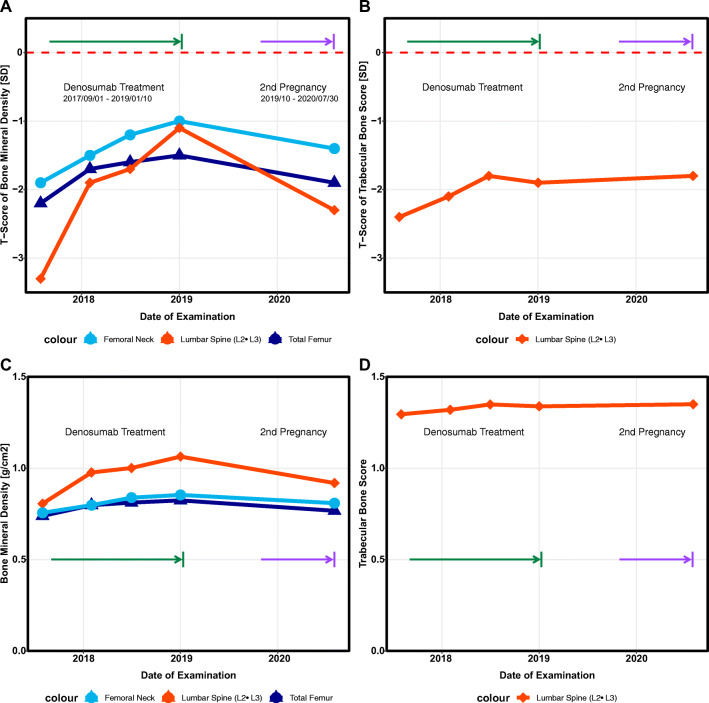
Fig. 1.
A T-score trend of bone mineral density (BMD) of the femoral neck (FN) and lumbar spine (lumbar spine body 2 and 3, L2-3), during treatment period. B T-score trend of trabecular bone score (TBS) at lumbar spine (L2-3) during treatment period. C Trend of absolute values of bone mineral density of the femoral neck and lumbar spine (L2-3) during treatment period. D Trend of absolute values of trabecular bone score (L2-3), during treatment period
Table 1.
Overview of bone mineral density measured by dual X-ray absorptiometry
| Date | BMD (L2-3) | BMD T-score (L2-3) | BMD Z-score (L2-3) | BMD femoral neck | BMD T-score femoral neck | BMD Z-score femoral neck | BMD total femur | BMD T-score total femur | BMD Z-score total femur | Trabecular bone score (L2-3) | Trabecular bone score T-score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 08-2020 | 0.919 | −2.3 | −2.6 | 0.809 | −1.4 | −1.5 | 0.767 | −1.9 | −2.1 | 1.350 | −1.8 |
| 01-2019 | 1.064 | −1.1 | −1.0 | 0.854 | −1.0 | −0.9 | 0.824 | −1.5 | −1.3 | 1.339 | −1.9 |
| 07-2018 | 1.001 | −1.7 | −1.6 | 0.839 | −1.2 | −1.0 | 0.812 | −1.6 | −1.5 | 1.349 | −1.8 |
| 02-2018 | 0.977 | −1.9 | −1.8 | 0.798 | −1.5 | −1.4 | 0.798 | −1.7 | −1.6 | 1.320 | −2.1 |
| 08-2017 | 0.806 | −3.3 | −3.2 | 0.756 | −1.9 | −1.8 | 0.739 | −2.2 | −2.1 | 1.295 | −2.4 |
At first, lactation was terminated and regular menstrual cycles reassumed. Additionally, a healthy lifestyle and diet, including a supplementation of 3000 IE vitamin D and 1000 mg of calcium daily, was initiated [6]. Due to the severity of PLO with fractures of L1 and L4 and the increased subsequent fracture risk, an indication for specific treatment was apparent.
A literature search revealed no RCT but case reports and retrospective analysis on treatment effect in women with PLO. Hereby, several authors suggested that a bone-forming treatment using teriparatide could be an effective treatment option [1, 4, 7–10]. As this is an off-label treatment and the health care provider was not willing to accept the high expenses of a teriparatide treatment in our patient, an off-label antiresorptive treatment with denosumab (Prolia, 60 mg/every 6 months s.c.) was initiated.
The first follow-up visit was 1 year after the treatment initiation. The patient consistently continued vitamin D and calcium supplementation. No side effects occurred and no further osteoporosis-associated fractures were observed. Results of the BMD measurement by DXA increased at the lumbar spine (L2-L3, fracture L1 and L4) from a T-sore of −3.3 to a T-score of −1.9 which indicates an increase compared to baseline of +21.2%. Femoral neck and total hip BMD (mead from both sides) showed an increased T-score from −1.9 and −2.2 to −1.5 and −1.7 indicating an increase of +5.6% and +8.0%. The TBS score also increased from 1.295 at baseline to 1.320 after 12 months. Due to the clinical meaningful increase of BMD at all sites with no further fracture, a treatment continuation for a second year was agreed upon.
After 18 months of treatment, with no therapy-associated side effects and no subsequent fractures and regular menstrual cycles, an interim examination was performed as the patient wanted to discuss the option of a second pregnancy. Compared to baseline, DXA at the lumbar spine (L2-L3), femoral neck, and total hip showed an increase vs. baseline of 32.0%, 13.0%, and 11.5%. TBS also showed an increase vs. baseline of 3.4%. In order to examine the current bone density in more detail, a quantitative ultrasonometry (QUS) and an Xtreme-CT® (HRpQCT) were additionally conducted. HRpQCT showed an age-appropriate inconspicuous result. QUS at the Os calcaneus also detected an age-related stiffness index (SI) T-score of −1.0 and −0.8 at the left and right side.
At this visit, while regularly menstruating, the patient expressed her wish for a second child and decided to terminate the treatment with denosumab, while continuing the vitamin D and calcium supplementation. The possibility of excessive decrease of BMD and perhaps also subsequent vertebral fractures (rebound phenomenon) after termination of denosumab treatment was discussed with the patient.
To monitor bone turnover in this post denosumab phase, makers of bone formation (PINP) and of bone resorption (CTX) were assessed 3 and 6 months after the end of the denosumab treatment. CTX (0.274 ng/ml blood serum concentration, reference for premenopausal women: <0.588 ng/ml) and procollagen I-propeptide (23 μg/l blood serum, reference for healthy patients: 15-59 μg/l) showed no increase with no indication of a rebound effect in the regularly menstruating patient.
Ten months after discontinuation of the 18 months of denosumab treatment, the second preplanned pregnancy occurred with no complications during pregnancy and childbirth and no further fractures. To examine changes in BMD, DXA was performed 12 days after childbirth. Results showed a T-score at the lumbar spine (L2-L3), femur neck, and total hip of −2.3, −1.4, and −1.9, which reflects a decrease of −8.8%, −6.9%, and −7.0%, compared to the maximum values during treatment with denosumab, respectively. QUS also showed a decline of −9.2%, while TBS showed no change compared to the maximum values during denosumab treatment. We recommended terminating lactation which led to an immediate recurrence of menstrual cycles as well as a bone-healthy diet and lifestyle including the continuation of the vitamin D supplementation. The patient did not want to restart denosumab treatment, but rather wait for the next BMD measurement.
Discussion
Pregnancy and lactation-associated osteoporosis (PLO) is a severe disease including multiple, predominantly vertebral fractures with a prolonged diagnosis. There are no guidelines for diagnosis and treatment and none of the medications is approved for this indication. It has been shown by several case reports that BMD may improve spontaneously after weaning to up to +7.5% at the lumbar spine over 12 months [11] and +11% after 24 months [12] in women with POL who did not receive any treatment with hip BMD which is similarly known to increase significantly over this timeframe. We need to emphasize that our patient had sustained two prevalent vertebral fractures (L1 and L4) with a high subsequent fracture risk and a very low baseline BMD as well as the use of low-molecular weight heparin [13]. In conclusion, the BMD increase in our patient with PLO receiving denosumab was much higher compared to the untreated spontaneous BMD improvement. For technical reasons, serial QUS measurements were performed. This could have helped to better understand the observed changes.
Oral and intravenous bisphosphonates, the application of denosumab, or the use of a bone anabolic treatment with teriparatide are discussed in several case reports [14]. One case report even investigates a sequential treatment of teriparatide followed by denosumab showing a significant improvement of BMD without subsequent fractures [15].
It is important to emphasize that neither bisphosphonates, denosumab, nor teriparatide, an effective bone anabolic treatment, is licensed to be used in patients with PLO and that in the absence of treatment guidelines, every drug treatment remains an individual decision and an off-label treatment [4, 16].
The off-label use of denosumab in cases of PLO has already been reported [14]. Our severe case of PLO included a patient with two vertebral fractures, an increased subsequent fracture risk, and a very low BMD, the use of a low-molecular-weight heparin as well as the existing desire for further children. After a clinically significant increase of BMD in the treatment phase with no further subsequent fractures, we could also observe a decrease in BMD in the post treatment phase including the second pregnancy. The reason for this decrease may be explained by the well-established denosumab rebound effect, but may also be caused by the underling PLO as well as the second pregnancy. Today, we are not able to distinguish to which factor was predominately responsible for the loss of BMD. The decision in favor of denosumab was made on the basis of the rapid onset of action [17, 18], the expected reduction of vertebral fracture risk, and the fact that denosumab—unlike bisphosphonates—does not accumulate in bone and because the reluctance of the health care provider to cover the costs of teriparatide [19]. It will be interesting to further investigate the BMD changes in the 12 months after her 2nd pregnancy without denosumab treatment.
Conclusion
In this case report, we could show the impact of denosumab in a severe case of pregnancy and lactation-associated osteoporosis (PLO) with vertebral fractures in the course of an antiresorptive postpartal treatment of the first pregnancy and the impact of a second pregnancy on the BMD. No further vertebral fractures occurred. RCTs are needed to investigate the efficacy of treatments in this rare form of secondary osteoporosis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflicts of interest
None
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tuna F, Akleylek C, Özdemir H, Demirbağ Kabayel D. Risk factors, fractures, and management of pregnancy-associated osteoporosis: a retrospective study of 14 Turkish patients. Gynecol Endocrinol. 2020;36:238–242. doi: 10.1080/09513590.2019.1648417. [DOI] [PubMed] [Google Scholar]
- 2.Jiao LL, Zhang J, Gao P, et al. Clinical characteristics and bisphosphonates treatment of rare pregnancy- and lactation-associated osteoporosis. Clin Rheumatol. 2018;37:3141–3150. doi: 10.1007/s10067-018-4185-0. [DOI] [PubMed] [Google Scholar]
- 3.Jia P, Wang RD, Yuan J, Chen H, Bao L, Feng F, Tang H. A case of pregnancy and lactation-associated osteoporosis and a review of the literature. Arch Osteoporos. 2020;15:94. doi: 10.1007/s11657-020-00768-7. [DOI] [PubMed] [Google Scholar]
- 4.Hadji P, Boekhoff J, Hahn M, Hellmeyer L, Hars O, Kyvernitakis I. Pregnancy-associated osteoporosis: a case-control study. Osteoporos Int. 2017;28:1393–1399. doi: 10.1007/s00198-016-3897-8. [DOI] [PubMed] [Google Scholar]
- 5.Dachverband Osteologie e.V. (2017) Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE. In: Prophyl. Diagnostik und Ther. der OSTEOPOROSE. https://www.dv-osteologie.org/uploads/Leitlinie 2017/Finale Version Leitlinie Osteoporose 2017_end.pdf
- 6.Farrah Z, Jawad ASM. Optimising the management of osteoporosis. Clin. Med. J. R. Coll. Physicians London. 2020;20:E196–E201. doi: 10.7861/clinmed.2020-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26:2223–2241. doi: 10.1007/s00198-015-3149-3. [DOI] [PubMed] [Google Scholar]
- 8.Hadgaonkar S, Shah KC, Bhatt H, Shyam A, Sancheti P. Post pregnancy severe spinal osteoporosis with multiple vertebral fractures and kyphoscoliosis in a multigravida: a rare case with management. Asian Spine J. 2015;9:625–628. doi: 10.4184/asj.2015.9.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyvernitakis I, Reuter TC, Hellmeyer L, Hars O, Hadji P. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporos Int. 2018;29:135–142. doi: 10.1007/s00198-017-4239-1. [DOI] [PubMed] [Google Scholar]
- 10.Stumpf UC KAWJFW (2007) Pregnancy-associated osteoporosis: an underestimated and underdiagnosed severe disease. A review of two cases in short- and long-term follow-up. Adv Med Sci 52–94 [PubMed]
- 11.Hong N, Kim JE, Lee SJ, Kim SH, Rhee Y. Changes in bone mineral density and bone turnover markers during treatment with teriparatide in pregnancy- and lactation-associated osteoporosis. Clin Endocrinol. 2018;88:652–658. doi: 10.1111/cen.13557. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17:1008–1012. doi: 10.1007/s00198-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir D, Tam AA, Dirikoc A, Ersoy R, Cakir B. Postpartum osteoporosis and vertebral fractures in two patients treated with enoxaparin during pregnancy. Osteoporos Int. 2015;26:415–418. doi: 10.1007/s00198-014-2852-9. [DOI] [PubMed] [Google Scholar]
- 14.Hardcastle SA (2021) Pregnancy and lactation associated osteoporosis. Calcif Tissue Int. 10.1007/s00223-021-00815-6 [DOI] [PubMed]
- 15.Ijuin A, Yoshikata H, Asano R, Tsuburai T, Kikuchi R, Sakakibara H. Teriparatide and denosumab treatment for pregnancy and lactation-associated osteoporosis with multiple vertebral fractures: a case study. Taiwan J Obstet Gynecol. 2017;56:863–866. doi: 10.1016/j.tjog.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Hadji P, Boekhoff J, Hahn M, Hellmeyer L, Hars O, Kyvernitakis I. Pregnancy-associated transient osteoporosis of the hip: results of a case-control study. Arch Osteoporos. 2017;12:12. doi: 10.1007/s11657-017-0310-y. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Zhang M, Shen Z, Ke J, Zhang D, Yin F. Efficacy and safety of 18 anti-osteoporotic drugs in the treatment of patients with osteoporosis caused by glucocorticoid: a network meta-analysis of randomized controlled trials. PLoS One. 2020;15:e0243851. doi: 10.1371/journal.pone.0243851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesnyak O, Gladkova E, Aleksandrov N, Belaya Z, Belova K, Bezlyudnaya N, Dobrovolskaya O, Dreval A, Ershova O, Grebennikova T, Kryukova I, Mazurenko S, Priymak D, Rozhinskaya L, Samigullina R, Solodovnikov A, Toroptsova N. Treatment of high fracture risk patients in routine clinical practice. Arch Osteoporos. 2020;15:184. doi: 10.1007/s11657-020-00851-z. [DOI] [PubMed] [Google Scholar]
- 19.Deeks ED. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018;35:163–173. doi: 10.1007/s40266-018-0525-7. [DOI] [PubMed] [Google Scholar]