Abstract
This paper evaluated the efficiency of beach hatcheries as a conservation tool for threatened sea turtle clutches. During six nesting seasons (2013 to 2018), several thousand high-risk clutches from loggerhead turtles (Caretta caretta) were relocated to a hatchery constructed on the same beach, within the Sea Turtle Natural Reserve (STNR, Boa Vista Island, Cabo Verde). Some parameters like hatching success; incubation period, hatchlings’ morphology and their behavioral response were compared to in-situ clutches.
-
•
Our findings confirmed that the in-situ nests within the STNR had extremely high egg mortality that was usually over 70 %. Mean hatching success of clutches relocated to hatcheries was significantly higher than in-situ clutches with mean values between 70 to 85 % (p < 0.0001).
-
•
No significant differences were observed in the incubation period (p = 0.786) and morphology of hatchlings (all p > 0.05) between relocated and in-situ clutches.
-
•
This study provided a detailed method and recommendations for sea turtle clutches relocation to the hatchery, that can be beneficial for endangered sea turtle population specially where hatching success is very low.
Keywords: Loggerhead turtle, Egg mortality, Clutch relocation, Conservation, Cabo Verde
Graphical abstract
Specifications table
| Subject Area: | Environmental ScienceEnvironmental Science |
| More specific subject area: | Relocation of doomed sea turtle clutches to hatchery |
| Method name: | Clutches relocation |
| Name and reference of original method: |
Hatchery efficiency as a conservation tool in threatened sea turtle rookeries with high embryonic mortality. The manuscript is accepted for publication in Ocean and Coastal Management (Article reference: OCMA_105807). |
| Resource availability: | None |
Method details
In this study, we evaluate the potential impact of clutch relocation on hatching output. Moreover, we evaluate the impact of the relocation process and the controlled incubation in hatcheries on the phenotype and viability of the offspring, comparing hatchlings from in-situ and relocated clutches. In the study area, Boa Vista Island, Cabo Verde, multiple clutch relocation programs to beach hatcheries consisting of several hundred clutches per season are implemented to maximize hatchling survival due the high mortality by tidal flooding and ghost crab predation [8], [9], [10], [11], [12].
Beach patrols
The beach was monitored nightly during six nesting seasons (2013-2018) to find all clutches laid by the loggerhead turtles. During the beach patrol, standard protocols (Schroeder & Murphy, [7]) were followed.
Nests were marked by experienced staff members during the patrols from mid-June until the end of September. The number of eggs was counted after the turtle began camouflaging. To reduce human influence, the female was not approached until she had finished camouflaging the nest.
To compare the hatchlings’ morphologies between in-situ and hatchery clutches, during the 2015 nesting season, 64 additional nests were protected with a circular plastic mesh to trap hatchlings to enable them to be counted and sampled after emergence.
The nests were monitored every day before sunrise, recording all information of occurrences that could potentially affect the nest, such as ghost crab predation or tidal flooding. Nests were excavated after 60 days or after a large group emergence. The emergence was identified by the tracks of hatchlings in the sand.
Nest Relocation
In the beginning of the nesting season, a hatchery (16 × 27 m; N16.016 W22.741) was built in João Barrosa beach in the same location for each of the different study seasons (2013–2018), in an area easily accessible from the field camp with less than 10 min walking distance. To prevent tidal flooding, an area of higher ground was chosen and the hatchery was constructed in a manner that would prevent ghost crabs and nesting females from entering.
Clutches were carefully relocated to the hatchery after they were laid. During 2013 to 2018 the same clutch relocation method was used following tested standard protocols [2]. The clutches were reburied in standardized hand-dug cavities that resembled in-situ nests in terms of shape, size and sand characteristics. The hole was dug using a ruler to ensure every nest was dug at the same depth, 50 cm which represents the average depth for this population [8]. In the first 30 cm, the hole was 25 cm wide, bellow the depth of 30 cm, a circular chamber was made 35 to 40 cm wide. The sand used to build the hatchery was taken from the same beach area. The priority was to relocate clutches which had been laid in an area with high risk of flooding and also in vegetated or muddy areas. We used the same protocols during all study seasons (2013–2018).
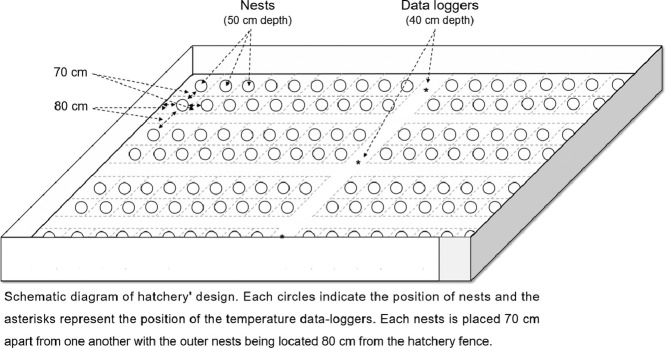
Each clutch was placed 70 cm apart from one another with the outer nests being located 80 cm from the hatchery fence. All nests were marked with sticks that were numbered and dated. A circular plastic mesh was positioned on the surface above each nest, trapping the hatchlings when they emerged to enable them to be counted and sampled. During this period, nests were monitored from dusk until dawn. The total time between nesting and reburying never surpassed 6 hours. Nests were excavated at least 4 days after the last emergence event or after more than 60 days of incubation.
Hatchlings and nest excavations
Twenty hatchlings were randomly selected from 39 nests on the beach and 160 nests in the hatchery to measure their straight carapace length (SCL) and straight carapace width (SCW) using a digital calliper (Digital Vernier Calliper 150 mm/6 inch; ± 0.1 mm) and their body mass was weighed with an electronic scale (Mission Quark Pocket Scales - Weigh Darts; ± 0.1 g). Afterwards, a righting response test was conducted once for each hatchling. For the righting response test, the hatchlings were turned over, placed on a smooth sand surface and allowed to turn by themselves while the time was recorded [12,14]. Each hatchling was tested during a maximum time of 60 s. For hatchlings that did not exhibit a righting response after the limit of 60 s, that time was assigned to them. Handling time did not exceed 15 min with the hatchlings being immediately released after sampling.
Sand temperature
During each nesting season, temperature data loggers (n = 3, Stow Away TidbiT® HOBO with accuracy of ± 0.2 °C) were buried in a line along the center of the hatchery 3 m from each other (Supplementary Fig. S1). They were positioned >1 m away from any clutches and at an average depth of 40 cm to record the sand temperature. Additionally, three data loggers were buried in the sand in different areas along the length of the beach (one at either end and one in the centre) at the same depth (40 cm). This corresponds to the mean depth at which the middle of a clutch of eggs is found for this population of loggerheads [2,8]. All dataloggers were calibrated before being buried in the sand. They were programmed to take one measurement every 30 min, from the beginning of July to mid-November. The temperature record allows us to determine if the temperature in the hatchery is too hot or too cool in relation to the beach sand temperature.
Method validation
Our results indicated that in our study area, loggerhead clutches suffered high mortality in in-situ nests by ghost crabs and flooding and relocating the doomed eggs to a hatchery reduced these risks. No significant differences were observed in the incubation period (p = 0.786) and hatchlings morphologies (all p > 0.05) among in-situ and hatchery clutches. We suggest that implementing a hatchery is done based on a case-by-case evaluation as the pressures on a population may differ from one region to another. However, we do not recommend the use of hatcheries without careful consideration of local ecological conditions and conservation priorities.
For better hatchery management, a series of guidelines have been suggested. The first one is the site selection [13] which should preferentially be on the same beach and located at sufficient elevation to prevent damage from flood intrusion and at a safe distance from any plant roots [1,13]. Hatchery fence should be built using wood and plastic material and metal mesh should be avoided. For instance, a study suggested that hatchlings use earth's magnetic field for migration, therefore, the metal material may harm the local magnetic field and disorient the hatchlings [6]. The bottom of the fence should be 40–50 cm deep to prevent the access of predators like ghost crabs.
Previous training is required for all staff or volunteers who will participate in the relocation process in order to standardize the methodology [3]. Extreme care should be taken during egg collection, transport and handling. Throughout egg handling, a high standard of hygiene is necessary and the use of chemicals such as mosquito repellent on hands should be avoided. The relocation can occur at any time, however, sun exposure must be avoided [2]. Clutches that are more than six hours old need to be treated with extreme care. It should be guaranteed that they maintain their original vertical orientation and vibrations should be avoided [2]. In addition, the clutch should be counted and placed at the original nest depth or in the mean nest depth common in the rookery [8]. All the clutches should be fenced by plastic mesh to prevent hatchlings from escaping after the emergence and the hatchery fence should efficiently prevent predators from entering. In areas where human exploitation occurs, the hatchery must be guarded at all times.
It is important to accurately count the number of hatchlings that emerge in each nest to determine the emergence success [13]. During the nest exhumation, all the of the eggshells and unhatched eggs must be removed from the hatchery. The nest exhumation in the hatchery is essential to understanding the hatching success rate of the relocation program and compare it with the productivity of the nests on the beach. Which it is very important to help identify the cause of egg mortality in the hatchery, to reduce the mortality of relocated eggs and improve the effectiveness of the threatened nest in the relocation program.
In cases of rookeries that are vulnerable to high environmental temperatures that lack coastal vegetation, it is recommended to create a shade for the nest or water the sand in the hatchery to mitigate the negative impacts of climate change on the clutch's sex ratio and hatchling's fitness [4], [5]. On the other hand, in the cases of hatcheries with cooler temperatures than the beach it is suggested to deposit a layer of black sand, 2 to 3 cm thick, over the surface of each nest area, immediately after the clutch relocation [12].
Acknowledgements
We would like to thank João Barrosa camp staff and volunteers for their support with the fieldwork. We want to express our gratitude to the National Directorate of Environment and Boa Vista Protected Area office for providing us with the necessary permissions to carry out our studies. This research was funded by a Marine Turtle Conservation Fund (MTCF) - US Fisheries and Wildlife Service/NOAA (Grant # F12AS00404) and the Mava Foundation for Nature (Grant # AO1 [17105]).
Declaration of Competing Interest
None.
Footnotes
Direct Submission or Co-Submission: Co-submissions are papers that have been submitted alongside an original research paper accepted for publication by another Elsevier journal
Co-SubmissionCo-Submission: Published in Ocean and Coastal Management. https://doi.org/10.1016/j.ocecoaman.2021.105807
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101518.
Appendix. Supplementary materials
References
- 1.Abd Mutalib A.H., Fadzly N. Assessing hatchery management as a conservation tool for sea turtles: a case study in Setiu, Terengganu. Ocean Coast. Manag. 2015;113:47–53. doi: 10.1016/j.ocecoaman.2015.05.010. [DOI] [Google Scholar]
- 2.Abella E., Marco A., López-Jurado L.F. Success of delayed translocation of loggerhead turtle nests. J. Wildl. Manag. 2007;71:2290–2296. doi: 10.2193/2006-512. [DOI] [Google Scholar]
- 3.Almeida A.P., Mendes S.L. An analysis of the role of local fishermen in the conservation of the loggerhead turtle (Caretta caretta) in Pontal do Ipiranga, Linhares, ES, Brazil. Biol. Conserv. 2007;134:106–112. doi: 10.1016/j.biocon.2006.07.014. [DOI] [Google Scholar]
- 4.Clarke L.J., Elliot R.L., Abella-Perez E., Jenkins S.R., Marco A., Martins S., Hawkes L.A. Low-cost tools mitigate climate change during reproduction in an endangered marine ectotherm. J. Appl. Ecol. 2021;58:1466–1476. doi: 10.1111/1365-2664.13874. [DOI] [Google Scholar]
- 5.Esteban N., Laloë J.O., Kiggen F.S.P.L., Ubels S.M., Becking L.E., Meesters E.H., Berkel J., Hays G.C., Christianen M.J.A. Optimism for mitigation of climate warming impacts for sea turtles through nest shading and relocation. Sci. Rep. 2018;8:17625. doi: 10.1038/s41598-018-35821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmann K., Lohmann C. Orientation and open-sea navigation in sea turtles. J. Exper. Biol. 1996;199(1):73–81. doi: 10.1242/jeb.199.1.73. [DOI] [PubMed] [Google Scholar]
- 7.Marco A., Abella E., Liria-Loza A., Martins S., Loureiro N.de S., López-Jurado L.F. Manual for the monitoring of sea turtles in the Cape Verde Islands. Zool. Caboverdiana. 2012;3:1–23. [Google Scholar]
- 8.Marco A., Abella E., Martins S., López O., Patiño-Martinez J. Female nesting behaviour affects hatchling survival and sex ratio in the loggerhead sea turtle: implications for conservation programmes. Ethol. Ecol. Evol. 2018;30:141–155. doi: 10.1080/03949370.2017.1330291. [DOI] [Google Scholar]
- 9.Marco A., Abella-Pérez E., Tiwari M. Vulnerability of loggerhead turtle eggs to the presence of clay and silt on nesting beaches. J. Exp. Mar. Biol. Ecol. 2017;486:195–203. doi: 10.1016/j.jembe.2016.10.015. [DOI] [Google Scholar]
- 10.Marco A., da Graça J., García-Cerdá R., Abella E., Freitas R. Patterns and intensity of ghost crab predation on the nests of an important endangered loggerhead turtle population. J. Exp. Mar. Biol. Ecol. 2015;468:74–82. doi: 10.1016/j.jembe.2015.03.010. [DOI] [Google Scholar]
- 11.Martins S., Sierra L., Rodrigues E., Oñate-Casado J., Torres Galán I., Clarke L., Marco A. Ecological drivers of the high predation of sea turtle hatchlings on the beach. Mar. Ecol. Prog. Ser. 2021 doi: 10.3354/meps13751. [DOI] [Google Scholar]
- 12.Martins S., Silva E., Abella E., de Santos Loureiro N., Marco A. Warmer incubation temperature influences sea turtle survival and nullifies the benefit of a female-biased sex ratio. Clim. Change. 2020;163:689–704. doi: 10.1007/s10584-020-02933-w. [DOI] [Google Scholar]
- 13.Mortimer J.A. In: Research and Management Techniques for the Conservation of Sea Turtles. Eckert K.L., Bjorndal K.A., Abreu-Grobois F.A., Donnelly M., editors. IUCN/SSC Marine turtle Specialist Group Publication; Pennsylvania (USA): 1999. Reducing threats to eggs and hatchlings: hatcheries; pp. 175–178. [Google Scholar]
- 14.Vieira S., Martins S., Hawkes L.A., Marco A., Teodósio M.A. Biochemical indices and life traits of loggerhead turtles (Caretta caretta) from Cape Verde Islands. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0112181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.