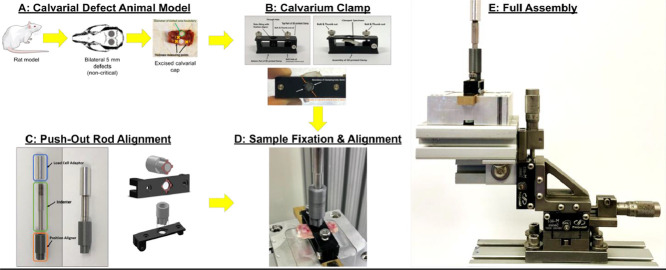
Abstract
Push-out tests are frequently used to evaluate the bone-implant interfacial strength of orthopedic implants, particularly dental and craniomaxillofacial applications. There currently is no standard method for performing push-out tests on calvarial models, leading to a variety of inconsistent approaches. In this study, fixtures and methods were developed to perform push-out tests in accordance with the following design objectives: (i) the system rigidly fixes the explanted calvarial sample, (ii) it minimizes lateral bending, (iii) it positions the defect accurately, and (iv) it permits verification of the coaxial alignment of the defect with the push-out rod. The fixture and method was first validated by completing push-out experiments on 30 explanted murine cranial caps and two explanted leporine cranial caps, all induced with bilateral sub-critical defects (5.0 mm and 8.0 mm nominal diameter for the murine and leporine models, respectively). Defects were treated with an autograft (i.e., excised tissue flap), a shape memory polymer (SMP) scaffold, or a PEEK implant. Additional validation was performed on 24 murine cranial caps induced with a single, unilateral critically-sized defect (8.0 mm nominal diameter) and treated with an autograft or a SMP scaffold.
-
•
A novel fixture was developed for performing push-out mechanical tests to characterize the strength of a bone-implant interface in calvarial defect repair.
-
•
The fixture uses a 3D printed vertical clamp with mating alignment component to fix the sample in place without inducing lateral bending and verify coaxial alignment of push-out rod with the defect.
-
•
The fixture can be scaled to different calvarial defect geometries as validated with 5.0 mm bilateral and 8.0 mm single diameter murine calvarial defect model and 8.0 mm bilateral leporine calvarial defect model.
Keywords: Cranial defect implant, Protocol, Osseointegration, Biomaterial mechanical testing
Graphical abstract
Specifications table
| Subject Area: | Engineering |
| More specific subject area: | Biomechanical evaluation of biomaterials and implants in orthopedic applications |
| Method name: | Push-out test, bilateral cranial defect animal model |
| Name and reference of original method: | Black, J. (1989). " Push-out" tests. J. Biomed. Mater Res., 23(11), 1243-1245. |
| Resource availability: | See included SolidWorks and .STL files |
Background
The push-out test is a common method for assessing the mechanical strength of the bone-implant interface in dental, craniomaxillofacial, and other orthopedic applications. In particular, the cranial defect model poses unique challenges in experimental design. Cranial caps, especially for small animal models, possess significant curvatures which complicate the process of clamping without inducing significant stresses into the specimen. Fig. 1 illustrates the generic push-out test set-up with important geometrical parameters.
Fig. 1.
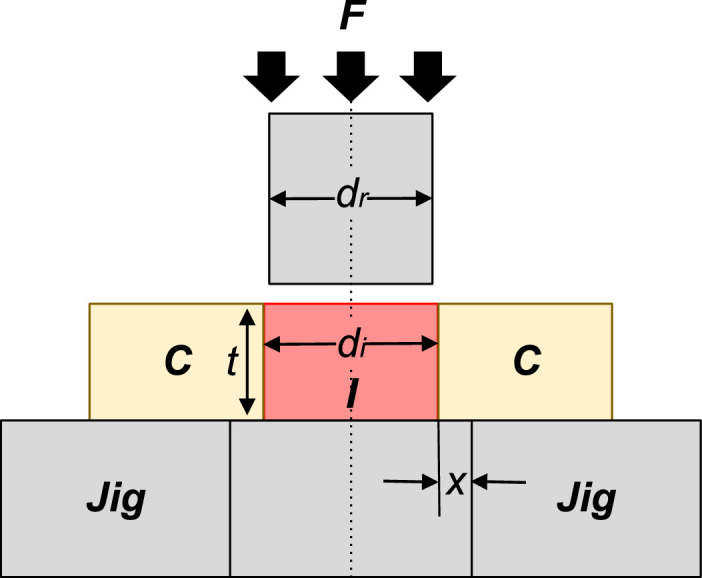
Schematic drawing of a generic pushout test, adapted from Dhert et al. [1]. F = force applied on implant; I = implant; C = cortex of bone; Jig = support jig; x = clearance of hole in support jig, di = implant diameter, dr = push-out rod diameter = cortical thickness.
In a seminal 1989 article [2], Jonathan Black listed several factors which should be reported when performing calvarial push-out tests, including: (a) specimen geometry, (b) defect alignment, (c) mounting method, (d) fit of support jig, (e) load-displacement protocol, and (f) push-out rod and clearance hole geometry. While many researchers report the geometry and loading protocol, the alignment and fixation methods were found to be rarely reported in the literature.
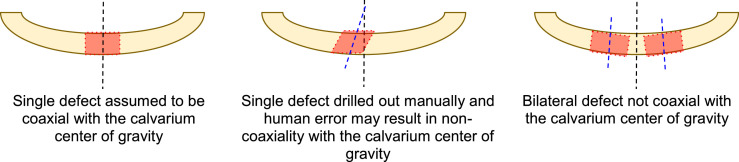
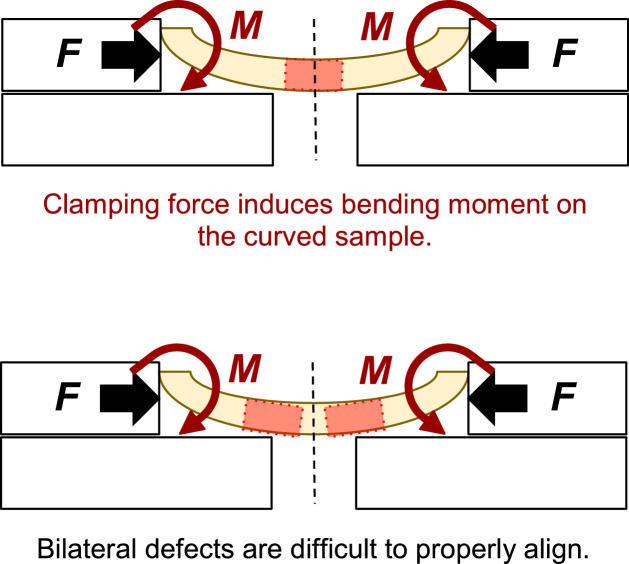
In our own work, we previously attempted a simple method to perform push-out tests of calvarial defects, loosely based on reports in the literature [3,4], and attempted to clamp the two distal ends of a rectangular specimen. However, such a method does not, on its own, ensure coaxial alignment with the push-out rod's line of action. For single critical defect models, coaxial alignment is assumed in virtue of the geometry of the skull; while this assumption is questionable in its own right, it is clearly not accurate in bilateral defect models as we employed in our study design (Fig. 2). Additionally, lateral compressive clamping on the distal ends interacts with the samples' curvature and imposes a significant bending moment on the sample (Fig. 3).
Fig. 2.
Illustration of the geometrical difficulties associated with current single and bilateral defect models.
Fig. 3.
Schematic of a current lateral clamping method with single defect model (top) and bilateral defect model (bottom). Where F = lateral clamping force and M = moment induced due to the curvature of the specimen.
To address these issues in our own testing, we established four design criteria to support an improved calvarial defect pushout testing apparatus. The apparatus should;
-
(1)
rigidly fix the explanted calvarial sample.
-
(2)
minimize lateral bending and the resulting internal stresses.
-
(3)
position the defect accurately.
-
(4)
permit verification of the coaxial alignment of the defect with the push-out rod.
Herein, we present a test method and apparatus for performing push-out tests on cranial defects in small animal models that meets these requirements and can be used to evaluate novel calvarial defect therapies in comparison to gold standard therapies.
Fixture design
Clamping without deforming under load
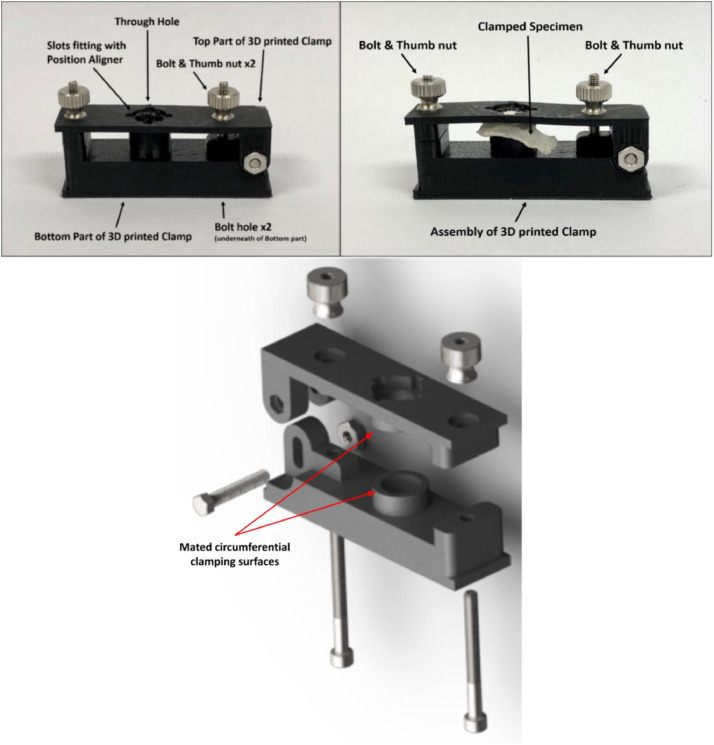
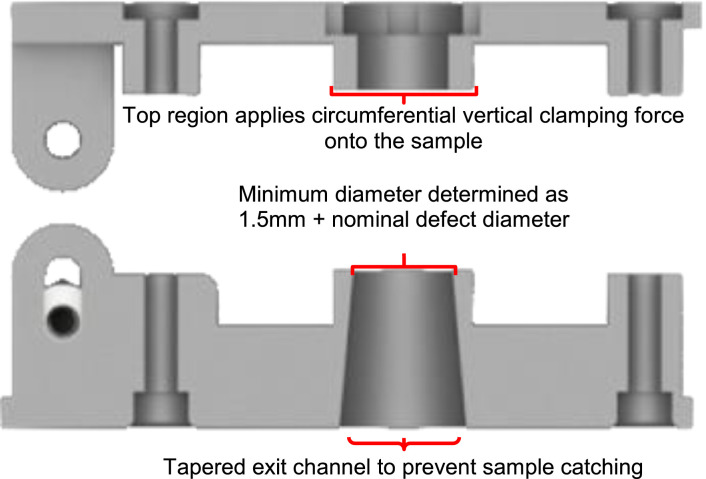
The core component of our fixtures is a custom 3D printed hinged clamp with a through-hole which circumferentially clamps onto the calvarial sample (Fig. 4). The circumferential clamping permits fixation of the specimen while minimizing the bending that is induced in the skull as a result of skull curvature; the circumferential clamping surface is relatively narrow to introduce minimal bending. In practice, these clamps were 3D printed on a MakerBot Replicator 2 × 3D printer. Acrylonitrile butadiene styrene (ABS) plastic was printed at 80% infill. Although poly(lactic acid) (PLA) filament may also work, it is better practice to use ABS when printed parts are used in a mechanical application, especially if exposed to moisture as is the case here. The fixture applies a veritcal clamping force by thumb tightened nuts. Since during the pushout test the sample is loaded in downward compression, it was found that only a moderate compression of the clamp was required to achieve specimen fixation during handling.
Fig. 4.
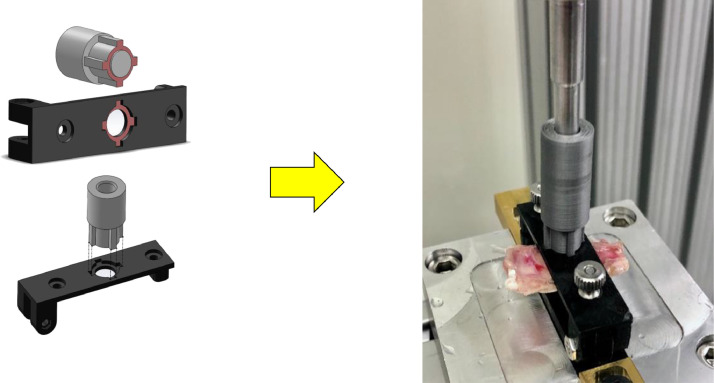
Fully assembled clamp (top left) and with specimen (top right). Exploded view (bottom).
The diameter of the through hole in the clamp (i.e., the hole through which the implant is pushed-out) was determined according to the rule laid out by Dhert, et al. (1992), that the minimum clearance between the defect and the through-hole should be 0.7 mm (i.e., add 0.7 mm to the radius of the defect) (Fig. 5). Additionally, to account for the 100 μm tolerance of the 3D printer, an additional 0.05 mm was added to the nominal radius of the defect in each case; for a mouse model defect with a nominal diameter 5 mm the hole had a diameter of 6.5 mm, and for the rabbit model with a nominal defect diameter of 8 mm the hole diameter was 9.5 mm. For added clearance, the through-hole was printed with a slight downward and outward taper (i.e., the opening at the bottom was larger than the opening in contact with the calvarium) to reduce the opportunity of the dislodged tissue catching on the interior sides of the device.
Fig. 5.
Sectional of the exploded view.
Specimen-fixture alignment
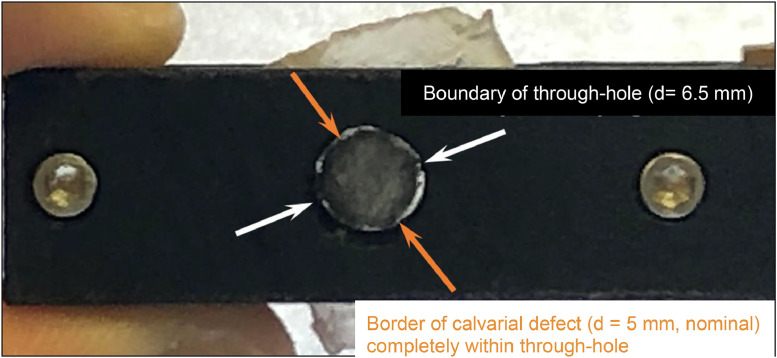
Alignment of the specimen in the fixture is critical; poor alignment results in the implanted device catching the sides of the through hole as it is pushed out, resulting in measured loads that are unrelated to the disengagement of the device from the bone. In this design, defect alignment may be verified by shining a light through the bottom of the clamp and visually confirming the entirety of the defect is within the perimeter of the through hole (Fig. 6). While this may not work for all combinations of implant materials and animal models, in cases reported herein, the alignment was easily verified.
Fig. 6.
The boundary of the defect is verified to be within the boundary of the through-hole by shining a light through the hole. Note: pictured is the murine calvarial defect, hence the 5 mm nominal diameter.
Fixture-machine alignment
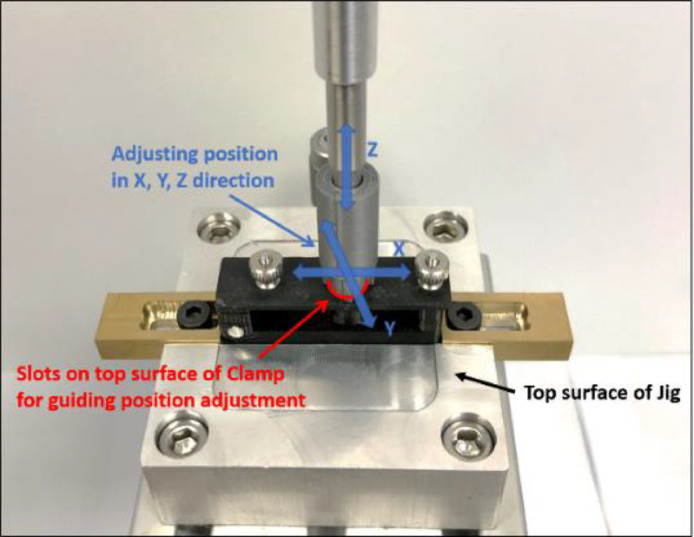
Lastly, the design must enable easy alignment of the clamping fixture to the testing machine axis. In particular, since the specimen is typically clamped while the 3D printed fixture is detached from the machine, alignment must be performed with each use of the clamp and must be fast and accurate. To this end, the top surface of the clamping fixture includes a targeted notch pattern centered on the through-hole (Fig. 7). A removable mating alignment cylinder was 3D printed with the inverse of the targeted notch pattern, which can be attached to the pushout rod. In order to align the clamping fixture with the machine axis, while the specimen is clamped in place, the clamping fixture can be secured with its base which is attached to a Newport M-461 linear stage. The push out rod can have the alignment cylinder attached to its distal end, and the linear stage can be used to adjust the location of the clamping fixture until the mating cylinder mates with the notch pattern of the top surface of the clamping fixture. This verification method is accurate to the precision of the 3D printer used which in our case is on the order of 100 μm.
Fig. 7.
Illustration of the targeted notch mating geometry (left). Verification of the push-out rod's alignment with the clamping fixture with murine bilateral defect calvarial sample (right).
Testing protocol
With a specimen mounted in place and aligned with the machine, the linear actuator is brought down until a compressive preload of 5 N is detected, at which time the position measurement is zeroed out. The push-out rod is then displaced downward at a constant rate of 5 mm/min (0.0083 mm/s) until the region of interest is completely extruded from the bone. Displacement rate was determined following Spicer [3].
Method validation
For device validation, push-out tests were first performed with explanted rat cranial caps (N = 30). This study was approved by the Texas A&M University IACUC (AUP 2016-0348). Fischer rats (N = 60, male, ∼8 weeks old) were each induced with non-critically sized, bilateral cranial defects (5 mm nominal diameter). For each animal, the second likewise treated defect site was reserved for other testing, thereby yielding a total of 30 treated cranial caps for push-out tests. Rats (N = 12) were treated with an “autograft” (i.e., the excised bone flap), yielding 6 specimens for push-out testing. Another group of rats (N = 48) were treated with a novel ”self-fitting” shape memory polymer (SMP) scaffold (d ∼5.5 mm x t ∼1.5 mm), yielding 24 specimens for push-out testing. The SMP scaffolds permitted press-fitting into defects, wherein shape recovery following exposure to warm saline (T ∼55 °C) promoted scaffold expansion to the perimeter. Scaffolds were prepared as semi-interpenetrating networks (semi-IPNs) with crosslinked poly(ε-caprolactone)-diacrylate (PCL-DA, Mn ∼10 k g/mol) and poly(L-lactic acid) (PLLA, Mn ∼15 k g/mol) at a 75:25 wt% as previously reported [5]. Such scaffolds have been shown to exhibit interconnected pores (average size of ∼220 μm) and a compressive modulus of ∼23.8 MPa. All scaffolds were prepared with a cell adhesive peptide (RGD; 1 mM) and half of the scaffold specimens were coated with a bioactive polydopamine as previously reported [6]. Yielding 6 push-out test specimens each, defects were treated with (i) uncoated scaffolds, (ii) uncoated scaffolds pre-seeded with rat-derived bone marrow mesenchymal stem cells [6,7] (BMSCs; 35 k), (iii) coated scaffolds, and (iv) coated scaffolds pre-seeded cells. Scaffolds were sterilized via EtO prior to implantation. For both groups of rats (i.e., autograft- and scaffold-treated), studies were terminated after 4 weeks. Calvaria were removed using a dremel diamond wheel and wrapped in an isotonic saline or 0.9% saline soaked gauze sponges, placed in specimen bags, and placed in a −20 °C freezer until testing.
To validate design versatility, push-out tests were also performed on rabbit calvaria with the geometrical parameters of the clamp scaled appropriately. This study was approved by the Texas A&M University IACUC (AUP 2015-0240/2018-0403). New Zealand White rabbits (N = 2, male, 6 months old) were induced with non-critically sized, bilateral cranial defects (8 mm nominal diameter). Each animal was treated with one SMP scaffold (d∼9 mm x t∼2 mm) and one polyetheretherketone (PEEK) implant (d∼8.5 mm x t∼2 mm). The scaffolds were prepared from PCL-DA (Mn ∼ 10 k g/mol) per prior reports [5,8]. Scaffolds exhibited interconnected pores (average size of ∼220 μm) and a compressive modulus of ∼18.0 MPa. Scaffolds were sterilized via gamma irradiation and the PEEK specimens were sterilized via EtO. The study was terminated after 16 weeks. Calvaria were removed as above.
The design was further validated with push-out tests performed on another group of rat cranial caps (N = 24). This study was approved by the Texas A&M University IACUC (AUP 2019-0447). Fischer rats (N = 24, male, ∼8 weeks old) were each induced with a critically sized, unilateral cranial defect (8 mm nominal diameter). Rats (N = 8) were treated with an autograft (i.e., the excised bone flap). The remaining rats (N = 16) were divided equally into two groups treated by an SMP scaffold: (i) a PCL-DA/PLLA semi-IPN scaffold (d∼8.6 mm x t∼2 mm) (analogous to that noted above), and (ii) a PCL/polydimethylsiloxane (PDMS) scaffold (d∼8.6 mm x t ∼2 mm). As per a prior report, the PCL/PDMS scaffolds were prepared as a co-network with PCL-DA (Mn ∼ 10 k g/mol) and PDMS-dimethacrylate (PDMS-DMA; Mn ∼5 k g/mol) at a 75:25 wt% ratio [9]. Such PCL/PDMS scaffolds have been shown to exhibit interconnected pores (average size of ∼230 μm) and a compressive modulus of ∼5 MPa. to All scaffolds were prepared with a cell adhesive peptide (RGD; 1 mm) and sterilized via EtO. The study was terminated after 12 weeks. Calvaria were removed as above.
Bill of materials
Materials essential the method
✓ Custom 3D printed clamp (available for download)
✓ Custom 3D printed position aligner (available for download)
✓ 2-56 x ½" hex head screw, 18-8 stainless steel (McMaster 92314A404), QTY:1
✓ 2-56 hex nut, 18-8 stainless steel (McMaster 91841A003), QTY: 1
✓ 2-56 x ⅞" socket head screw, 18-8 stainless steel (McMaster 92196A086), QTY: 2
✓ 2-56 flanged knurled-head thumb nut, 18-8 stainless steel (McMaster 95150A110), QTY: 2
✓ 5 mm diameter stainless steel push-out rod (bilateral murine samples)
✓ 8 mm diameter stainless steel push-out rod (leporine samples and unilateral murine samples)
✓ Load cell
○ NOTE: We used Futek Load Cell, LSB 210, capacity 100 lbf
✓ 1 L of 0.1 M PBS solution heated at 37 °C
○ NOTE: Room temperature (RT) PBS will also work, but heated is preferred.
Materials used in our validation study that can be substituted
✓ Newport M-461 xyz linear stage
○ NOTE: The z component was unnecessary, only movement in the xy plane is needed for alignment verification. Any xy linear stage with micron resolution will work.
✓ Aluminum base block with slot
○ NOTE: This was used to attach the clamp to the linear stage; many alternatives are possible, especially depending on which linear stage is used.
✓ 8020 aluminum extrusions
○ NOTE: These were used to attach and elevate the linear stage on our testing machine. Depending on the testing machine used, this may not be necessary.
Work instructions
Protocol for validation study.
1. Assemble testing clamp (Fig. 4).
1.1. Print desired number of clamps. We recommend printing one (1) clamp per sample to test.
1.2. Place the 2–56 hex head screw through the slotted hinge of the clamp and secure with the 2–56 thread hex nut.
1.3. Place the long 2–56 socket head screws through the bottom of the clamp such that the threaded end is coming out through the top.
1.4. Loosely screw on the knurled knobs onto the socket head screws.
2. Set up testing fixture
2.1. Initialize testing machine, attach load cell, and configure for testing.
2.2. Mount the push-out rod on the load cell.
2.3. Mount the 3D printed alignment tool to the push-out rod.
2.4. Assemble the 8020 extrusions and aluminum block to the Newport linear stage; mount assembly to the testing machine with the aluminum block centered approximately 10 mm under the push-out rod.
2.5. Place an assembled 3D printed clamp into the aluminum block.
2.6. Adjust the linear stage until the targeted notch of the clamp and push-out rod alignment tool are mated (Fig. 8).
3. Prepare the testing samples
3.1. Take the explanted calvarium and slide it into an assembled clamp with the concave side of the calvarium facing up.
3.2. Shine a bright light through the bottom of the clamp to illuminate the boundary of the defect (Fig. 6).
3.3. Position the explanted calvarium such that the entirety of the defect is within the boundary of the through hole (Fig. 6).
3.4. Tighten the knurled knobs with your thumb until the sample is secured in place. If the top clamp begins to bend, then the knobs are getting too tight.
3.5. Store the assembled test article in the PBS solution until it is time to test.
4. Performing experiment
4.1. Remove the assembled test article from the PBS bath and secure it in the slot of the aluminum block.
4.2. Verify the clamp is aligned with the push-out rod aligner then remove the push-out aligner (Fig. 8).
4.3. Bring the actuator down until the push-out rod registers 5 N of preload, then zero displacement.
4.4. Proceed with the experiment, displacing the push-out rod at 5 mm/min (0.0083 mm/s) until sample failure (Fig. 9).
Fig. 8.
Alignment of the push-out rod with the through-hole by mating the targeted notch patterns.
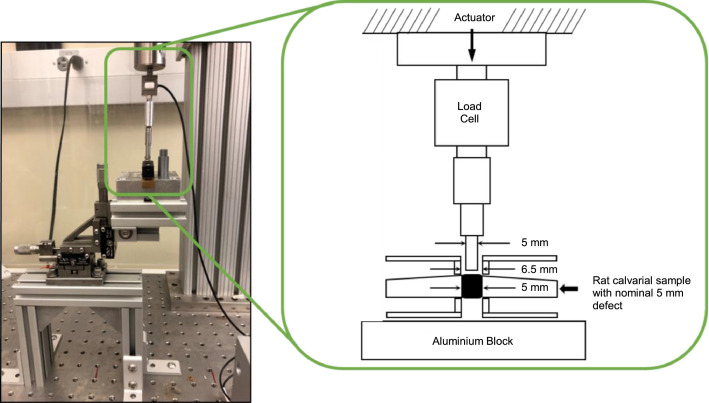
Fig. 9.
Full assembly of the testing setup (left) with schematic (right).
■ NOTE: A constant displacement to twice the thickness of the sample is recommended to ensure the rod has fully pushed through the sample.
Discussion
The method described herein has demonstrated several important benefits for push-out testing of calvarial defect specimens. Firstly, the circumferential clamping modality significantly reduced the bending induced in the cranial cap as compared to lateral compression clamps. Secondly, having a clamp that is easily removed from the testing machine allows visual inspection of specimen alignment in the clamp that is not possible with other designs. Thirdly, the targeted notch pattern ensures the defect, push-out rod, and through-hole are reliably coaxial to sub-millimeter precision. Lastly, 3D printing allows each sample to have its own, inexpensive clamp; this was found to be a significant advantage to improve workflow efficiency. This permitted each sample to be aligned and secured in an individual clamping fixture and then store the clamped specimen in a warm (37 °C) saline bath until ready for testing. This also helped maintain tissue hydration by reducing exposure time to air. Additionally, the reported method translates particularly well to the use of bilateral calvarial defects which are favored to increasing study power while decreasing animal usage. While bilateral defect models are common for histological studies, their use in push-out and other biomechanical studies is rare due in part to the above mentioned geometrical difficulties of laterally located defects. Our method addresses these difficulties, thus allowing the benefits of bilateral models to translate to the mechanical evaluation of calvarial defect repair devices.
Despite these advantages to this method, several limitations are noted. First, the clamping bolts are independently tightened which may result in non-uniformity of clamping force and non-defined effects. In this study, a single researcher secured the samples in place after developing proficiency in the method using several pilot samples. In future work, a second iteration will use a single thread to apply the clamping force along the common central axis. A second limitation of this method is the necessity for sufficiently thin or translucent test articles to verify defect alignment with light that may prohibit utility for calvarial push-out tests of larger animal models or other push-out applications (e.g., dental or long-bone). Furthermore, the geometry and material (ABS) have only been validated at relatively small loads (i.e., ≤250 N). Given its high compressive modulus, ABS can withstand much higher compressive loads; however, the 80% print infill sufficient for the present experiment may not be sufficient for higher load applications. While the design could be translated into a different material (e.g., stainless steel), there may be a trade-off in cost and efficiency. A third limitation is the visibility of the experiment. If the push-out rod were visible throughout the entirety of the experiment, the initial contact with the sample could be visually verified, interference with the interior of the through-hole could be detected, and the failure mode of the test article could be analyzed in more detail. Finally, the z-axis on the Newport linear stage was unnecessary as the linear actuator controls the push-out rod's position; in later iterations of this method, we removed the z-axis stage and used x-y linear stages only.
Acknowledgments
This work was supported by NIH NIDCR 1R01DE025886-01A1.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101541.
Appendix. Supplementary materials
References
- 1.Dhert W.J.A., Verheyen C.C.P.M., Braak L.H., De Wijn J.R., Klein C.P.A.T., De Groot K., Rozing P.M. A finite element analysis of the push-out test: influence of test conditions. J. Biomed. Mater. Res. 1992;26(1):119–130. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 2.Black J. Push-out" tests. J. Biomed. Mater Res. 1989;23(11):1243–1245. doi: 10.1002/jbm.820231102. [DOI] [PubMed] [Google Scholar]
- 3.Spicer P.P., Kretlow J.D., Young S., Jansen J.A., Kasper F.K., Mikos A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012;7(10):1918–1929. doi: 10.1038/nprot.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon J., Lee D.J., Kocher M., Kim Y.I., Wu T.J., Whitley J., Ko C. The inhibition of radial and axial micromovement of bone scaffold with gelfoam® and titanium mesh fixation and its effects on osteointegration. Methods Protoc. 2019;2(1):20. doi: 10.3390/mps2010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfau M.A., McKinzey K.G., Roth A.A., Graul L.M., Maitland D.J., Grunlan M.A. Shape memory polymer (SMP) bone scaffolds with improved self-fitting properties. J. Mater. Chem. B. 2021;9:3286–3837. doi: 10.1039/d0tb02987d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabiyat A.A., Pfau M.R., Grunlan M.A., Hahn M.S. Intrinsic osteoinductivity of PCL-DA/PLLA semi-IPN shape memory polymer scaffolds. J. Biomed. Mater. Res. Part A. 2021;109(11):2334–2345. doi: 10.1002/jbm.a.37216. in press online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maniatopoulos C., Sodeck J., Melcher A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D., George O.J., Petersen K.M., Jimenez-Vergara A.C., Hahn M.S., Grunlan M.A. A bioactive “self-fitting” shape memory polymer (SMP) scaffold with potential to treat craniomaxillofacial (CMF) bone defects. Acta Biomater. 2014;10:4597–4605. doi: 10.1016/j.actbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Beltran F.O., Houk C.X., Grunlan M.A. Bioactive siloxane-containing shape memory polymer (SMP) scaffolds with tunable degradation rates. ACS Biomater. Sci. Eng. 2021;7:1631–1639. doi: 10.1021/acsbiomaterials.1c00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.