Abstract
Background
Diabetes results in a rise in blood glucose above normal physiological levels; if untreated this may cause damage to many systems including the cardiovascular and renal systems. Pregnancy increases resistance to insulin action; for those women who have pre‐gestational diabetes, this results in an increasing insulin requirement. There are several methods of administering insulin. Conventionally, insulin has been administered subcutaneously, formally referred to as intensive conventional treatment, but now more usually referred to as multiple daily injections (MDI). An alternative method of insulin administration is the continuous subcutaneous insulin infusion pump (CSII).
Objectives
To compare CSII with MDI of insulin for pregnant women with pre‐existing and gestational diabetes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2016) and reference lists of retrieved studies.
Selection criteria
Randomised trials comparing CSII with MDI for pregnant women with diabetes.
Data collection and analysis
Three review authors independently assessed studies and two review authors extracted data. Disagreements were resolved through discussion with the third author. We assessed the quality of the evidence using the GRADE approach.
Main results
We included five single‐centre trials (undertaken in Italy) with 153 women and 154 pregnancies in this review.
There were no clear differences in the primary outcomes reported between CSII and MDI in the included trials: caesarean section (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.66 to 1.77; three trials, 71 women, evidence graded very low), large‐for‐gestational age (RR 4.15, 95% CI 0.49 to 34.95; three trials, 73 infants; evidence graded very low), and perinatal mortality (RR 2.33, 95% CI 0.38 to 14.32; four trials, 83 infants, evidence graded very low). Other primary outcomes were not reported in these trials (hypertensive disorders of pregnancy, development of type 2 diabetes, composite outcome of serious neonatal outcomes, and neurosensory disability).
There was no clear evidence of differences in the maternal secondary outcomes: maternal weight gain during pregnancy, 24 hour mean blood glucose in each trimester, mean maternal HbA1c in each trimester, maternal hypoglycaemia, and maternal hyperglycaemia. The included studies did not report several GRADE outcomes: perineal trauma, return to pre‐pregnancy weight, postnatal depression, induction of labour. Many maternal secondary outcomes were also not reported.
In two trials, including a total of 61 infants, CSII was associated with an increase in mean birthweight compared with MDI (mean difference (MD) 220.56 g, 95% CI ‐2.09 g to 443.20 g; P = 0.05). However, the large CI including anything from a small reduction to an increase in mean birthweight and the lack of a difference in macrosomia rate (RR 3.20, CI 0.14 to 72.62; two trials, 61 infants) suggests uncertainty. Large‐for‐gestational age (see above), andsmall‐for‐gestational age also suggests uncertainty of effect. No significant differences were found in: gestation at delivery, preterm birth < 37 weeks' gestation, preterm birth < 32 weeks' gestation, neonatal hypoglycaemia (evidence graded very low),respiratory distress syndrome, neonatal hyperbilirubinaemia, and fetal anomaly. There were no data reported on many important infant outcomes, including the GRADE outcomes adiposity and diabetes. There was no follow‐up of infants in childhood or adulthood, so longer‐term outcomes were not reported.
The only outcome reported for use of health service resources wasmaternal days hospitalised, which did not show a difference between groups in the small number of women included (MD 9.40, CI ‐6.04 to 24.84; one trial, 10 women).
The methods used by the trials were poorly reported, for example although blinding of participants and clinicians regarding intervention allocation is impossible, it is possible to blind assessors and this along with other aspects of trial methods was not reported, which means that the trials are at an unclear or high risk of bias. We do not know if the women who participated were representative, and therefore if the results can be generalised. Most GRADE outcomes were not reported. For the GRADE outcomes that were reported, our assessment was that the evidence is very low quality (caesarean section, large‐for‐gestational age, perinatal mortality, andneonatal hypoglycaemia). This was due to design limitations in the included trials, small sample sizes in the trials contributing data, wide CIs crossing both the line of no effect and the line of appreciable benefit and/or harm, and often few events. We are therefore uncertain whether CSII or MDI improves outcomes for pregnant women with diabetes and their infants, and the results of further studies may differ substantially from those presented in this review.
Authors' conclusions
There is no evidence to support the use of one particular form of insulin administration over another for pregnant women with diabetes. There are only a small number of trials appropriate for meta‐analysis, a small number of women included and questionable generalisability of the trial population.
Pump technology has progressed since these trials were undertaken. Well‐designed randomised trials are required to evaluate comparisons such as patch pumps against MDI and more conventional CSII against MDI. These trials should be adequately powered to assess the effect of interventions, and report the core set of outcomes used in Cochrane reviews of diabetes in pregnancy. Trials to assess the effects of pumps on birthweight and macrosomia rates are needed. It would be beneficial for future trials to undertake longer‐term follow‐up of participants and their infants, assess women's preferences, and conduct an economic evaluation.
Keywords: Female; Humans; Pregnancy; Insulin Infusion Systems; Pregnancy in Diabetics; Birth Weight; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/drug therapy; Hypoglycemic Agents; Hypoglycemic Agents/administration & dosage; Injections, Subcutaneous; Insulin; Insulin/administration & dosage; Randomized Controlled Trials as Topic
Plain language summary
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes
What is the issue?
Diabetes is a condition in which glucose (sugar) in the blood is too high because the body does not respond to insulin or not enough insulin is made. Insulin is a hormone made by the pancreas, which allows glucose to enter the cells where it is used as fuel by the body.
Controlling blood sugar levels is important because levels that are too high or too low can affect the brain and other organs of the body. Poor blood sugar control in pregnant women with diabetes can lead to large babies who may then have a difficult birth. It also increases the chance of abnormalities in the baby, miscarriage, or stillbirth.
Traditionally, insulin is given as multiple daily injections (MDI), however a small pump can continuously give insulin through a fine tube under the skin (CSII).
Why is this important?
An insulin pump may help pregnant women keep their blood glucose more stable than multiple injections. It might stop the woman's blood sugar level going too high or too low, which would be better for the mother and her baby and it may be more acceptable to women. This review compared the positive and negative effects of CSII and MDI to work out which is best for mothers and infants.
What evidence did we find?
Five randomised trials involving 153 women (154 pregnancies) were included.
These trials did not report many of the outcomes we had hoped to look at. The evidence was judged to be very low quality for important outcomes (caesarean section, large‐for‐gestational age, perinatal mortality, and neonatal hypoglycaemia). This was because the trials were small, may not have been fair tests, and did not show a clear difference between MDI and CSII.
There were no clear differences in any of the reported outcomes between women who had insulin via a pump rather than as multiple injections. For mothers, this included caesarean section, weight gain during pregnancy, and blood sugar levels. For babies, this included the baby's weight, if they were born premature, and problems such as difficulty breathing, a low Apgar score at birth, low blood sugar, jaundice, or physical abnormalities.
In one small trial, there was no difference in the number of days mothers spent in hospital. This was the only measure of cost or use of health service resources reported.
What does this mean?
The trials did not provide enough information to know whether an insulin pump or multiple injections are better for a pregnant woman with diabetes or her baby. More research is needed, with bigger groups of women, good reporting of how the trials were undertaken, more outcomes assessed and reported, and using the latest pump technology and insulins.
Summary of findings
Summary of findings for the main comparison. CSII versus MDI: maternal outcomes.
| Continuous subcutaneous insulin infusion (CSII) versus multiple daily injections (MDI) of insulin for pregnant women with diabetes | ||||||
| Patient or population: pregnant women with diabetes Setting: 3 studies in Italy Intervention: CSII Comparison: MDI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with MDI | Risk with GRADE CSII | |||||
| Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | (0 studies) | outcome not reported | ||||
| Caesarean section | Study population | RR 1.09 (0.66 to 1.77) | 71 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 444 per 1000 | 484 per 1000 (293 to 787) | |||||
| Moderate | ||||||
| 438 per 1000 | 477 per 1000 (289 to 774) | |||||
| Development of Type 2 diabetes | (0 studies) | outcome not reported | ||||
| Perineal trauma | (0 studies) | outcome not reported | ||||
| Return to pre‐pregnancy weight | (0 studies) | outcome not reported | ||||
| Postnatal depression | (0 studies) | outcome not reported | ||||
| Induction of labour | (0 studies) | outcome not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 All studies contributing data had design limitations.
2 Wide confidence interval crossing the line of no effect, and small sample size.
Summary of findings 2. CSII versus MDI: infant outcomes.
| Continuous subcutaneous insulin infusion (CSII) versus multiple daily injections (MDI) of insulin for pregnant women with diabetes | ||||||
| Patient or population: infants of pregnant women with diabetes Setting: 4 studies in Italy Intervention: GRADE CSII Comparison: MDI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with MDI | Risk with GRADE CSII | |||||
| Large‐for‐gestational age | Study population | RR 4.15 (0.49 to 34.95) | 73 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Study population and moderate risks were not calculated, due to the small sample size, few events, and no events in the MDI group. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Perinatal mortality (stillbirth and neonatal mortality) | Study population | RR 2.33 (0.38 to 14.32) | 83 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Moderate risks were not calculated, due to the small sample size and few events. | |
| 24 per 1000 | 55 per 1000 (9 to 341) | |||||
| Mortality or morbidity composite | (0 studies) | outcome not reported | ||||
| Neonatal hypoglycaemia | Study population | RR 1.00 (0.07 to 14.64) | 32 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | ||
| 63 per 1000 | 63 per 1000 (4 to 915) | |||||
| Adiposity (infant) | (0 studies) | outcome not reported | ||||
| Type 1 and type 2 diabetes (infant) | (0 studies) | outcome not reported | ||||
| Neurosensory disability | (0 studies) | outcome not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 All studies contributing data had design limitations.
2 Wide confidence interval crossing the line of no effect, small sample size and few events.
3 One study with design limitations.
Background
Description of the condition
Diabetes is a major disease affecting a growing proportion of pregnant women. Diabetes occurs when there is insufficient insulin in the blood. Given that insulin controls blood glucose levels, inadequate circulating insulin levels lead to a rise in blood glucose. Increased blood glucose levels can cause damage to many systems, including the cardiovascular and renal systems and during pregnancy, are associated with increased risk of adverse perinatal outcomes. There are two main types of diabetes that exist prior to pregnancy: type 1 diabetes occurs due to a lack of pancreatic islet b cells, caused by autoimmune destruction and resulting in an absence of insulin; type 2 diabetes occurs due to insulin resistance or b cell dysfunction, or both, and is likely to be the result of interactions between genetic, environmental and immunological factors; including diet, physical activity and obesity (Zaccardi 2016).
Physiological insulin resistance increases during pregnancy to facilitate glucose transfer across the placenta to the fetus, to ensure growth and development. This physiological insulin resistance leads to increasing Insulin requirements for women who have pre‐gestational diabetes and who manage their diabetes with insulin; for those who manage their diabetes with diet and/or oral anti‐diabetic agents, insulin may be required and for some women without pre‐existing diabetes, gestational diabetes may develop. Recent studies have reported graded linear associations between maternal glucose and adverse perinatal outcomes across the whole glucose spectrum, with no clear threshold where risk increases (HAPO 2008; Farrar 2015), therefore interventions to reduce glucose levels for women with lower glucose levels, than were previously diagnostic of gestational diabetes, are likely to reduce associated perinatal risks. Similarly to maternal glucose, maternal obesity, which is a common 'co‐condition' with type 2 diabetes, has been identified as being independently associated with adverse perinatal outcomes, however when maternal obesity is combined with hyperglycaemia (high blood sugar) the adverse impact on perinatal outcomes is greater than either one alone (HAPO 2010; Catalano 2012).
Since the introduction of insulin as a treatment for diabetes, maternal mortality and morbidity rates have improved; however, they remain significantly higher than those of the general obstetric population (Knight 2014). Perinatal mortality rates amongst babies of women with pre‐existing diabetes have also declined over recent years, however population‐based studies suggest that rates are still 2.5 to nine times higher than in the general obstetric population (Melamed 2009). In 2010 CEMACE reported that 27% of mothers with recorded pre‐existing medical conditions had a stillbirth and that 3% of these had diabetes. Twenty‐eight per cent of mothers whose infants died in the neonatal period also had a pre‐existing medical condition and 2% of these had diabetes, making diabetes one of the commonest conditions associated with stillbirth and neonatal death (CMACE 2010). Complications associated with pre‐existing diabetes include: higher rates of miscarriage, stillbirth and congenital anomaly. Recent congenital anomaly rates reported for infants of women with diabetes range from 42 to 94 per 1000 births, compared to a rate of between 10 and 21 per 1000 births in the general population (Hawthorne 1997; Penney 2003; CEMACH 2005).
A potential problem in the assessment of the management of diabetes has been reported by John 1997; Kilpatrick 1997; Kilpatrick 1998 and Marshall 2000, who suggest that differences have been found in the ranges and results produced by laboratories using the same methods of assessment to measure the same glycaemic index. Thus, a degree of caution should be used when interpreting multi‐centre studies using several laboratories to analyse specimens.
Continuous glucose monitoring systems are used selectively and provide a dynamic picture of interstitial glucose levels, converting these levels to an electrical signal, which produces an average recording of glucose level every five minutes. Studies using the continuous glucose monitoring system in conjunction with other methods of assessment such as glycated haemoglobin (HbA1c) and intermittent glucose monitoring, have found it useful in providing additional information in relation to hypo/hyperglycaemia, which is of particular importance during pregnancy (Buhling 2004; Kerssen 2004; Porter 2004; Hirsch 2005). The addition of continuous glucose monitoring should provide a more accurate picture of control over a 24‐hour period and reduce the impact of anomalies associated with HbA1c monitoring, leading to improved glucose control.
Many women manage their type 2 diabetes with oral anti‐diabetic agents such as metformin. Metformin improves insulin sensitivity and is not associated with hypoglycaemia (low blood sugar). During pregnancy, the use of oral anti‐diabetic agents may be supplemented or substituted with insulin in order to achieve optimum glucose control. There is limited evidence evaluating the effects of oral anti‐diabetic agents on maternal and infant outcomes. Results from the metformin in gestational diabetes (MIG) trial however reported no increase in perinatal complications with metformin compared with insulin (Rowan 2008). A Cochrane protocol has recently been published which aims to examine oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes (Brown 2015). A Cochrane review investigating the use of oral anti‐diabetic agents for women with pre‐existing diabetes/impaired glucose tolerance or previous gestational diabetes however was unable to include any trials, though one trial is ongoing (Tieu 2010). The current Cochrane review evaluating treatments for gestational diabetes (which has now been divided into types of treatments, with protocols being published: Brown 2015; Brown 2015a; Brown 2016) reported that treatment of gestational diabetes reduces the risk of a range of perinatal adverse outcomes (Alwan 2009). Alwan 2009 found that women with gestational diabetes mellitus (GDM) when treated with oral hypoglycaemics compared with insulin were significantly less likely to have a caesarean section and their infants significantly less likely to develop neonatal hypoglycaemia; there were however, no differences in other important outcomes including induction of labour and shoulder dystocia (difficulty in delivering a baby's shoulders).
There remains controversy regarding the degree to which blood glucose level should be controlled in pregnancies complicated by diabetes. Though it is generally agreed that the optimal HbA1c level (retrospective glucose measure) should be between 4 mmol/L and 8 mmol/L (Hawthorne 2002; Williams 2003), though this may be difficult to achieve for women with refractory pre‐existing or gestational diabetes (Maresh 2001). A Cochrane review has examined different intensities of glycaemic control for pregnant women with pre‐existing diabetes (Middleton 2016). This review includes three trials (223 women with type 1 diabetes and their infants) and concluded that although evidence is limited and the included trials were assessed as being at high risk of bias, there is little difference in outcomes between very tight and tight‐to‐moderate glycaemic control. There is however evidence of harm with loose control. A recently published Cochrane review (Martis 2016) has examined trials evaluating different intensities of glycaemic control for women with gestational diabetes. The one eligible trial was published as a conference abstract, includes 171 women, methods were unclear and outcomes reported were few, therefore evidence is insufficient to suggest what intensity of glyaemic control is superior.
Description of the intervention
There are several methods of administering insulin to women who require it in pregnancy. Conventionally, insulin has been administered subcutaneously in the form of a basal/bolus regimen, often referred to as multiple daily injections (MDI). This consists of pre‐meal boluses of rapid‐acting insulin (RAI) and a later evening basal injection of long‐acting insulin, usually given with pen injection devices. The advantage of MDI is that blood sugar levels can be tightly controlled by frequent, self‐regulated adjustment of dose, necessary because of the dynamic insulin requirements of pregnancy, but frequent injections can be painful and may interfere with daily living activities. An alternative insulin administration method is the continuous subcutaneous insulin infusion pump (CSII). Modern pumps are small and lightweight, are battery operated and hold enough insulin for several days (Pozzilli 2016). A more recent advance is the development of the patch pump (tubing‐free pumps in which the reservoir and integrated infusion set adhere to the skin) (Thabit 2012). Different basal rates can be preset and boluses given as required. Potentially CSII maintains the basal rate of insulin and reduces the risk of hypoglycaemia, decreases the risk of fasting hyperglycaemia (the dawn phenomenon) and can improve compliance, as frequent injection of insulin is not required (as with MDI) (Gonzalez 2002; Pozzilli 2016). Hadden 1996, however, suggests that although insulin pumps offer the treatment effects of conventional regimens (and may be preferred by some women), they may be overly complex for routine use. Selective use of pumps for those who are motivated, or with refractory diabetes is an alternative to widespread use.
How the intervention might work
Despite widespread use of CSII pumps in some high‐income countries such as Norway, Germany, France and the USA, they are used infrequently in the other high‐income countries such as the UK and Spain and most low‐ to middle‐income countries (Pozzilli 2016). Reasons for this are unclear, but may be due to conservatism, costs and lack of evidence on effectiveness and safety (Colquitt 2004). Brink 1986, Knight 1985 and Mecklenburg 1984 report improved glycaemic control with CSII compared to MDI, however both Knight 1985 and Mecklenburg 1984, also report an increased incidence of ketoacidosis. Group allocation was not random in the Knight 1985 study and generally ketoacidosis was precipitated by illness. Brink 1986 found no increased incidence in diabetic ketoacidosis using CSII compared to MDI. Both studies (Knight 1985; Brink 1986) report the incidence of ketoacidosis reduced with time, suggesting learning or familiarity played a part. A recent review and meta‐analyses comparing CSII compared with MDI reported that more women in the CSII group had hypoglycaemic spells and ketoacidotic episodes (requiring treatment), however the differences for both these outcomes were non‐significant (Mukhopadhyay 2007).
Both rapid‐acting insulin analogues (RAIA) and regular human insulin can be given via CSII and MDI. RAIAs provide a more physiological time course of action. It has been suggested by some authors that the use of RAIAs, compared to regular human insulin, reduces episodes of hypoglycaemia and improves metabolic control (Anderson 1997; Johansson 2000). This improved control is thought to be due to RAIAs' ability to achieve peak plasma concentrations approximately twice as high and in approximately half the time compared to regular insulin (Siebenhofer 2004). A Cochrane review of RAIA versus regular human insulin in patients with diabetes (Siebenhofer 2006) however, concluded (and taking into account the low quality of trials included in the meta‐analysis), that there seemed to be only a negligible benefit with RAIA over regular human insulin. The authors suggest a cautious response to the vigorous promotion of RAIAs and that more robust trials needed to be carried with longer‐term follow‐up of both mother and child.
Why it is important to do this review
Diabetes affects a significant and growing proportion of women in pregnancy. Hyperglycaemia is associated with increased risk of important perinatal outcomes across the whole spectrum of glucose. Treatments to reduce glucose levels however, in both women with pre‐existing diabetes and gestational diabetes reduce adverse outcome risk. Different methods of administering insulin may have different effects in terms of achieving optimal glucose control and may influence the risk of maternal and infant outcomes. This review will examine the effects of CSII and MDI for women with diabetes in pregnancy.
Objectives
The objective was to conduct a systematic review of randomised trials comparing continuous subcutaneous insulin infusion (CSII) with multiple daily injections (MDI) of insulin for pregnant women with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised trials comparing CSII with MDI of insulin for pregnant women with diabetes. We excluded quasi‐randomised trials and cross‐over trials. Cluster‐randomised trials were eligible for inclusion.
Types of participants
Women with pre‐existing and gestational diabetes and randomised to receive either CSII or MDI.
Types of interventions
Any comparisons of CSII with MDI of insulin for pregnant women with diabetes.
Types of outcome measures
For this update, we used the core outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus (GDM) and pre‐existing diabetes.
Primary outcomes
Mother
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia
Caesarean section
Development of type 2 diabetes
Neonatal/infant
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Mortality or morbidity composite (e.g. perinatal mortality, shoulder dystocia, bone fracture, and admission to the neonatal unit)
Neurosensory disability
Secondary outcomes
Mother
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention
Behaviour changes associated with the intervention
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin)
Sense of well‐being and quality of life
Views of the intervention
Breastfeeding (e.g. at discharge, six weeks postpartum)
Use of additional pharmacotherapy
Glycaemic control during/end of treated (as defined by trialists)
Maternal hypoglycaemia
Maternal mortality
Long‐term maternal outcomes
Postnatal depression
Postnatal weight retention or return to pre‐pregnancy weight
Body mass index (BMI)
Gestational diabetes mellitus in a subsequent pregnancy
Type I diabetes
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Return to pre‐pregnancy weight (not pre‐specified)
Neonatal/infant
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia (birthweight greater than 4000 g and birthweight greater than 4500 g)
Small‐for‐gestational age
Birthweight and z score
Head circumference and z score
Length and z score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia
Hyperbilirubinaemia
Neonatal hypocalcaemia
Polycythaemia
Relevant biomarker changes associated with the intervention (e.g. cord c peptide, cord insulin)
Fetal anomaly
Later infant and childhood secondary outcomes
Weight and z scores
Height and z scores
Head circumference and z scores
Adiposity (e.g. as measured by BMI, skinfold thickness)
Blood pressure
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Educational achievement
Child as an adult secondary outcomes
Weight
Height
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome)
Type I diabetes
Type 2 diabetes
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Health service use
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (31 March 2016).
The Register is a database containing over 21,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeFarrar 2007.
For this update, we planned to use the following methods to assess the four reports that we identified as a result of the updated search. Unfortunately, no new studies were included in this update.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors planned to extract the data using the agreed form. We would have resolved discrepancies through discussion or, if required, consulted the third review author. We planned to enter data into Review Manager software (RevMan 2014) and to check for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors planned to assess independently the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
For each included study we planned to describe the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We would have assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we planned to describe the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We would have assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We planned to describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
We would have considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results.
We would have assessed blinding separately for different outcomes or classes of outcomes.
We would have assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We planned to describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We would have assessed blinding separately for different outcomes or classes of outcomes.
We would have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included trial and for each outcome or class of outcomes, we planned to describe the completeness of data including attrition and exclusions from the analysis. We would have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We would have assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis carried out with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We planned to describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We would have assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included trial we planned to describe any important concerns we had about other possible sources of bias.
We would have assessed whether each trial was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We intended to make explicit judgements about whether trials were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We intended to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis but there were insufficient data to do so.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison. We used the Cochrane Pregnancy and Childbirth GRADE core outcome set for reviews of diabetes in pregnancy.
Mother
Hypertensive disorders
Caesarean section
Development of type 2 diabetes
Perineal trauma
Return to pre‐pregnancy weight
Postnatal depression
Induction of labour
Infant
Large‐for‐gestational age
Stillbirth and neonatal mortality
Mortality or morbidity composite
Neonatal hypoglycaemia
Adiposity
Type 1 and type 2 diabetes
Neurosensory disability
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference where outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were included in this update. In future updates if eligible and included, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion.
Other unit of analysis issues
Multiple pregnancy
In future updates, if women are included in a trial randomised with a multiple pregnancy, we will present maternal data as per woman randomised and neonatal data as per infant.
Multiple‐arm studies
In future updates, where a trial has multiple intervention arms we will avoid ’double counting’ of participants by combining groups, if appropriate, to create a single pair‐wise comparison. Where this is not possible, we will split the ’shared’ group (often the 'control' group) into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would have been treated as the average of the range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials. If we had used random‐effects analyses, the results would have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses and to consider whether an overall summary was meaningful, and if it was, to use the random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Pre‐existing versus gestational diabetes
We were unable to carry out subgroup analysis because of insufficient data. We will perform this subgroup analysis in future updates of this review if more data become available. We will use the primary outcomes in subgroup analysis.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out the following sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. However, there were insufficient data to allow us to carry out any sensitivity analyses.
Trial quality (if rated 'high risk of bias')
Treatment started before and during pregnancy
Results
Description of studies
We included five trials with 153 women and 154 pregnancies. All five trials were carried out in single centres.
Results of the search
The updated search in March 2016 identified four new reports of three trials (Murphy 2011; Stewart 2014; Thompson 2014). Murphy 2011 was excluded because it does not compare continuous subcutaneous insulin infusion pump (CSII) with multiple daily injections (MDI). Stewart 2014 and Thompson 2014 are currently ongoing.
This updated review is therefore comprised of five trials (involving 153 women and 154 pregnancies) (Trossarelli 1984; Botta 1986; Carta 1986; Nosari 1993; Mello 2005). A total of seven trials have been excluded, and two are ongoing. See Figure 1.
1.
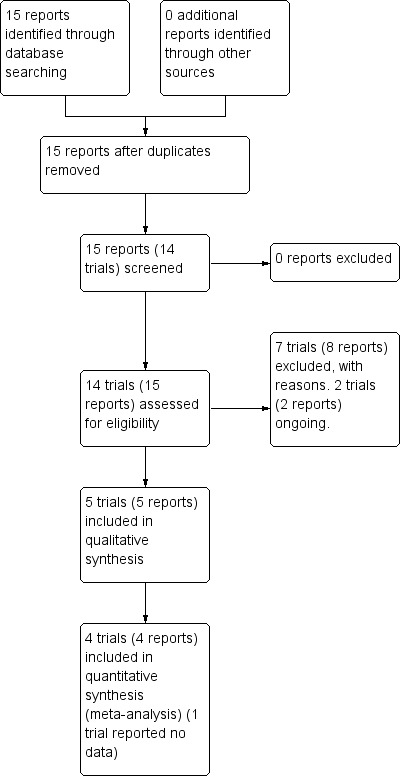
Study flow diagram.
Included studies
Botta 1986 conducted a single‐centre trial in Italy. The paper was translated to English prior to inclusion. Ten participants were included and 'divided randomly' into two groups with no other information reported. Five participants were given 'optimised traditional insulin therapy' and five CSII using a micropump (Lilly CPI 9100). There were no losses to follow‐up, which was carried out until birth. Outcomes were: gestational age at birth, caesarean section, weight gain during pregnancy, preterm birth, hyperbilirubinaemia, and maternal days hospitalised.
Carta 1986 in a single‐centre trial in Italy included 29 women, 15 women with type 1 diabetes (13 on conventional insulin therapy, two on continuous insulin therapy) and 14 women with type 2 diabetes (four were on oral hypoglycaemics, 10 were diet‐controlled). Recruitment was undertaken in the first trimester, two women allocated to CSII had been using a CSII pump pre‐conceptually. For women randomised to CSII, a Microject MC 20 portable syringe pump was used with porcine insulin (Actrapid MC 40 U/ml); adjustments were made to the dosage in order to obtain strict glycaemic control (fasting blood glucose (BG) < 80 + 10 mg/dL, postprandial BG < 120 mg/dL). Participants randomised to MDI, were given Actrapid MC split into four boluses. Outcomes were: maternal and neonatal mortality, large‐for‐gestational age, fetal anomaly and hypoglycaemia, weight gain during pregnancy, mean 24‐hour BG, mean HbA1c, gestational age at delivery, preterm birth, birthweight and rate of instrumental delivery.
Mello 2005 was a single‐centre trial in Italy and included 71 women with type 1 diabetes. The women were randomised in their first trimester, although the trial fulfilled eligibility criteria for inclusion, data were described rather than reported as raw or mean results and therefore could not be included in the meta‐analysis.
Nosari 1993 conducted a single‐centre trial in Italy, included 31 women with type 1 diabetes undergoing 32 pregnancies. Using sealed envelopes, the women were allocated to receive either CSII or MDI. Four women were recruited in the pre‐conception period and 28 recruited during the first trimester. Participants were described by the authors as 'highly motivated'. Microject MC 20 and Daedi B.V. portable battery‐powered syringe infusion pumps were used. Participants receiving MDI had four daily insulin injections (regular insulin at each meal and intermediate acting insulin at night; type of insulin used was not reported). Outcomes were: maternal and neonatal mortality, large‐for‐gestational age, fetal anomaly and hypoglycaemia, mean 24‐hour BG, mean HbA1c, gestational age at delivery, preterm birth, Apgar score less than seven at five minutes, respiratory distress syndrome, birthweight and rate of instrumental delivery. Trossarelli 1984 was a single‐centre trial in Italy that included 12 women with type 1 diabetes recruited in their first trimester, no information was provided regarding the method of insulin administration other than that the trial compared CSII with MDI. Results were mainly reported descriptively. Outcomes were: perinatal mortality, large‐for‐gestational age, maternal weight gain during pregnancy, mean 24‐hour BG in each trimester, and respiratory distress syndrome.
For details of included trials, see the tables of Characteristics of included studies.
Excluded studies
We excluded seven trials (Burkart 1988; Collaborative 1993; Coustan 1986; Ignatova 2007, Laatikainen 1987; Murphy 2011; Zoupas 1991). Burkart 1988 excluded participants from analysis if normoglycaemia was not achieved. Collaborative 1993 compared conventional with intensive therapy, so was the wrong comparison. Coustan 1986 did not report at what gestation the women recruited in pregnancy commenced therapy, and some women were recruited up to a year pre‐conceptually making assessment of treatment effects difficult. Ignatova 2007 is probably not a randomised trial as participants were 'divided' into two groups. Laatikainen 1987 recruited 30 women, nine declined the allocated CSII intervention, no woman declined MDI. Only measures of retinopathy were reported. Murphy 2011 did not evaluate CSII and MDI; they carried out a two‐arm cross‐over trial comparing closed‐loop versus conventional CSII. Zoupas 1991 is a trial registered on the Oxford Database of Perinatal Trials that remains unpublished, no information is available on trial methodology or outcomes and attempts to contact the author have been unsuccessful. For further details see Characteristics of excluded studies.
Risk of bias in included studies
2.
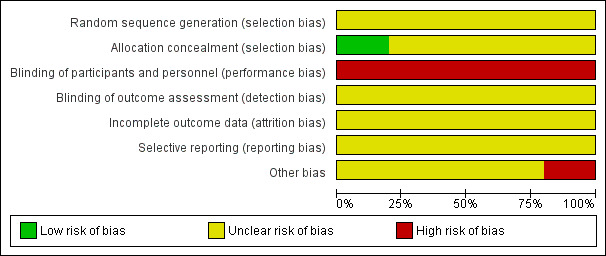
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
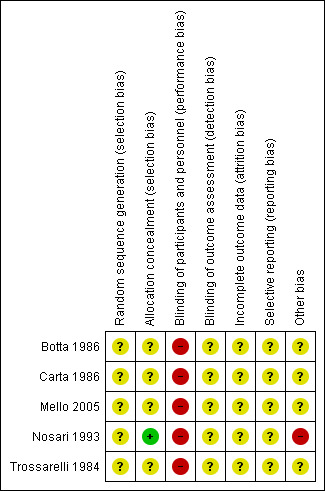
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nosari 1993 used sealed envelopes to conceal allocation of their participants, a method at low risk of bias. However, the risk of bias for method of randomisation is unclear as this is not reported.
All other trials had unclear risk of selection bias for both random sequence generation and allocation concealment. Botta 1986 reported only that their participants were "divided randomly". Carta 1986 do not make clear their randomisation procedure, stating only that participants were randomly assigned to either CSII or MDI. They also included in their group two women who were receiving CSII preconceptually and prior to randomisation. Mello 2005 and Trossarelli 1984 are abstracts and therefore provide only short descriptions of the methods. Attempts to contact the authors for clarification of methods and results have been unsuccessful.
Blinding
Blinding of the type of intervention from clinician and participant for the trials is not possible because of the nature of the study, placing studies at high risk of bias. Outcome assessors could however be blinded to the intervention, though this was not reported by any of the trials, so the risk of bias is unclear.
Incomplete outcome data
All trials had unclear risk of attrition bias. The numbers of women included in analysis across pregnancy comparing maternal 24‐hour mean glucose and mean HbA1c varied in Carta 1986 from 13 to 15 in the CSII group and from eight to 14 in the MDI group.
Carta 1986 only report that 15 women with type 1 and 14 women with type 2 diabetes were included (plus 10 non‐diabetic controls). Carta 1986 do not report any withdrawals or analysis exclusions. Similarly, Nosari 1993 only reported the number of women randomised (31 women who had 32 pregnancies during the study period). The number of women included in each table of results presented (16 MDI and 16 CSII) suggests no attrition, apart from for the maternal and fetal or neonatal complications table. Follow‐up of study participants continued into the early postnatal period and there were no losses to follow‐up. Insufficient information is provided by Mello 2005 and Trossarelli 1984 to draw conclusions regarding attrition. No information regarding attrition was reported by Botta 1986.
Selective reporting
Outcomes were not prespecified by any of the included trials, therefore it is possible that selective reporting could have taken place.
Mello 2005 although eligible and therefore included in this review, does not report data in a format that can be used within the meta‐analysis.
Other potential sources of bias
All trials were small, with limited reporting of methods. Due to this, they were judged to be at unclear of high risk of bias.
Additionally, Nosari 1993 describes the study population as highly motivated, and therefore arguably a biased sample and not a generalisable population. This was judged to introduce high risk of bias.
Effects of interventions
Results from one trial, Carta 1986, were presented as type 1 and 2 diabetes. For most outcomes (excluding weight gain), we combined these results for analysis to ease comparison between the trials. Nosari 1993 and Trossarelli 1984 expressed their mean results with standard errors (SE); these were converted to standard deviations (SD) to enable analysis in Review Manager (RevMan 2014). For the purposes of the meta‐analysis, CSII was classified as the intervention group and MDI as the control.
Primary outcomes
There were no differences between CSII and MDI in the primary outcomes reported in the included studies: caesarean section (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.66 to 1.77; three trials, 71 women, Analysis 1.2, evidence graded very low), large‐for‐gestational age (RR 4.15, 95% CI 0.49 to 34.95; three trials, 73 infants; Analysis 1.4; evidence graded very low), and perinatal mortality (RR 2.33, 95% CI 0.38 to 14.32; four trials, 83 infants, Analysis 1.5, evidence graded very low).
1.2. Analysis.
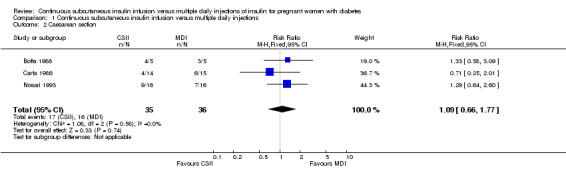
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 2 Caesarean section.
1.4. Analysis.
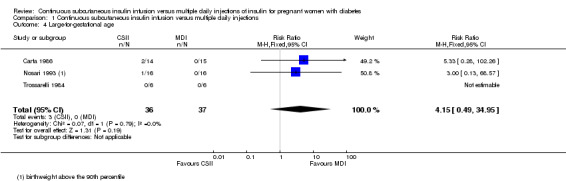
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 4 Large‐for‐gestational age.
1.5. Analysis.
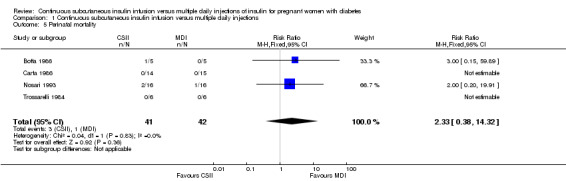
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 5 Perinatal mortality.
Hypertensive disorders of pregnancy and development of type 2 diabetes were not reported for mothers in the included studies. A composite outcome of serious neonatal outcomes, and neurosensory disability were not reported for infants in the included studies.
Secondary outcomes
Maternal outcomes
There was no evidence of differences between CSII and MDI in the maternal secondary outcomes reported. This may reflect the few outcomes and participants included in the limited number of small trials we were able to include. No differences were found in: maternal weight gain during pregnancy (mean difference (MD) 0.52, 95% CI ‐1.12 to 2.17; three trials, 51 women; Analysis 1.10), 24‐hour mean BG in each trimester (first trimester: MD 0.12, 95% CI ‐7.19 to 7.43; three trials, 67 women; Analysis 1.11; second trimester: MD 1.77, 95% CI ‐5.02 to 8.56; three trials, 73 women; Analysis 1.12; third trimester: MD 0.08, 95% CI ‐5.57 to 5.72; three trials, 69 women; Analysis 1.13), mean maternal HbA1c in each trimester (first trimester: MD ‐0.20, 95% CI ‐2.13 to 1.73; one trial, 32 women; Analysis 1.14; second trimester: MD 0.70, 95% CI ‐2.29 to 3.69; one trial, 32 women; Analysis 1.15; third trimester: MD 0.10, 95% CI ‐2.38 to 2.58; one trial, 32 women; Analysis 1.16), maternal hypoglycaemia (RR 3.00, 95% CI 0.35 to 25.87; two trials, 61 women; Analysis 1.17), and maternal hyperglycaemia (RR 7.00, 95% CI 0.39 to 125.44; two trials, 61 women; Analysis 1.18).
1.10. Analysis.
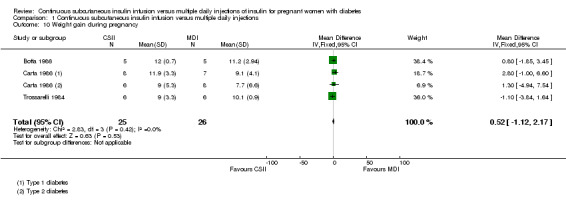
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 10 Weight gain during pregnancy.
1.11. Analysis.
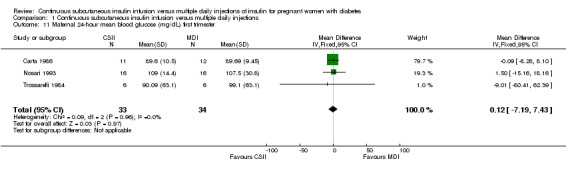
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 11 Maternal 24‐hour mean blood glucose (mg/dL) first trimester.
1.12. Analysis.
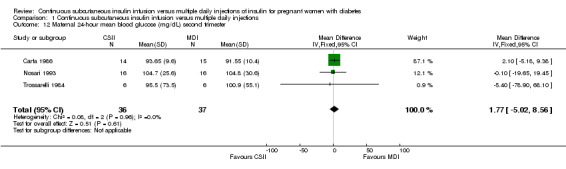
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 12 Maternal 24‐hour mean blood glucose (mg/dL) second trimester.
1.13. Analysis.
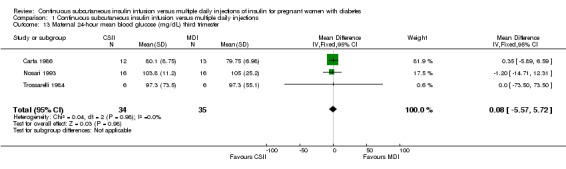
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 13 Maternal 24‐hour mean blood glucose (mg/dL) third trimester.
1.14. Analysis.
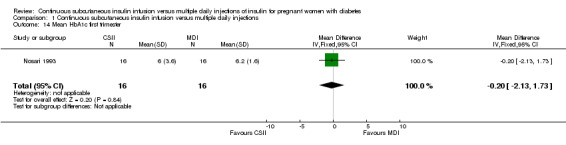
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 14 Mean HbA1c first trimester.
1.15. Analysis.
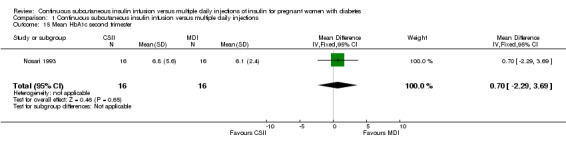
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 15 Mean HbA1c second trimester.
1.16. Analysis.
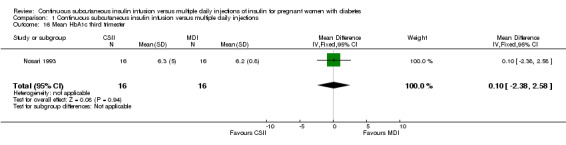
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 16 Mean HbA1c third trimester.
1.17. Analysis.
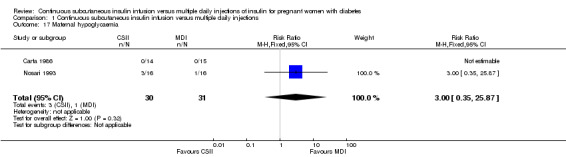
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 17 Maternal hypoglycaemia.
1.18. Analysis.
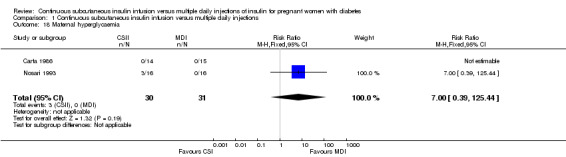
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 18 Maternal hyperglycaemia.
The included studies did not report several GRADE outcomes: perineal trauma, return to pre‐pregnancy weight, postnatal depression, induction of labour. Many maternal secondary outcomes were also not reported: placental abruption, postpartum haemorrhage, postpartum infection, adherence to the intervention, behaviour changes associated with the intervention, relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin), sense of well‐being and quality of life, views of the intervention, breastfeeding (e.g. at discharge, six weeks postpartum), use of additional pharmacotherapy, maternal mortality, body mass index (BMI), gestational diabetes mellitus in a subsequent pregnancy, type I diabetes, impaired glucose tolerance, and cardiovascular health.
Infant outcomes
In two trials, with a total of 61 infants, CSII was associated with an increase in mean birthweight compared with MDI of borderline statistical significance (MD 220.56 g, 95% CI ‐2.09 g to 443.20 g; P = 0.05; Analysis 1.27), however, the large CI and the lack of a difference in macrosomia rate (RR 3.20, CI 0.14 to 72.62; two trials, 61 infants suggests uncertainty; Analysis 1.25). Large‐for‐gestational age (see primary outcomes) and small‐for‐gestational age (average RR 1.40, 95% CI 0.10 to 18.71; I2 = 31%; two trials, 61 infants; Analysis 1.26), suggests uncertainty of effect. No significant differences were found in: gestation at delivery (MD ‐1.18, 95% CI ‐2.92 to 0.57; three trials, 71 infants; Analysis 1.21), preterm birth < 37 weeks' gestation (RR 0.77, 95% CI 0.18 to 3.24; three trials, 71 infants; Analysis 1.22), preterm birth < 32 weeks' gestation (RR 0.33, 95% CI 0.01 to 7.62; three trials, 71 infants; Analysis 1.23), neonatal hypoglycaemia (RR 1.00, 95% CI 0.07 to 14.64; one trial, 32 infants; Analysis 1.30; evidence graded very low),respiratory distress syndrome (RR 3.00, 95% CI 0.13 to 68.57; two studies, 44 infants; Analysis 1.29), neonatal hyperbilirubinaemia (RR 0.33, 95% CI 0.02 to 6.65; one study, 10 infants; Analysis 1.31), and fetal anomaly (RR 1.07, 95% CI 0.07 to 15.54; two trials, 61 infants; Analysis 1.32).
1.27. Analysis.
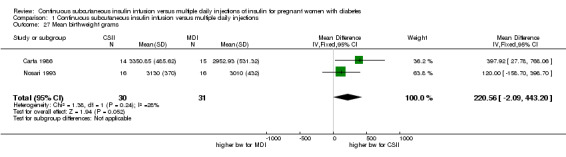
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 27 Mean birthweight grams.
1.25. Analysis.
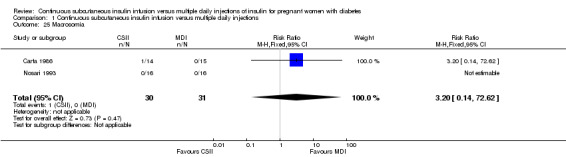
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 25 Macrosomia.
1.26. Analysis.
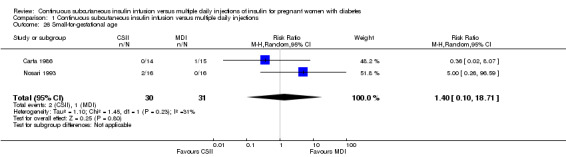
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 26 Small‐for‐gestational age.
1.21. Analysis.
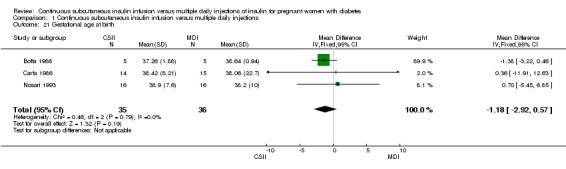
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 21 Gestational age at birth.
1.22. Analysis.
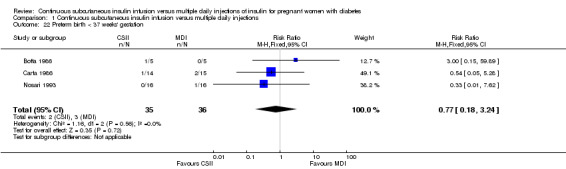
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 22 Preterm birth < 37 weeks' gestation.
1.23. Analysis.
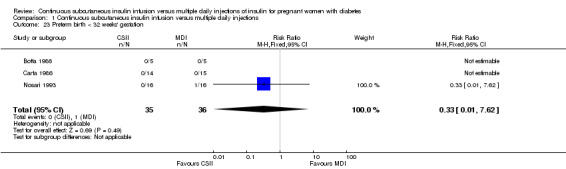
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 23 Preterm birth < 32 weeks' gestation.
1.30. Analysis.
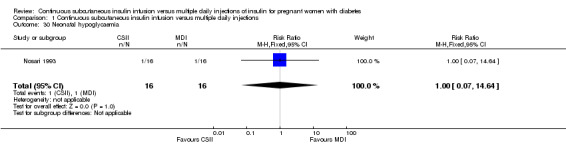
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 30 Neonatal hypoglycaemia.
1.29. Analysis.
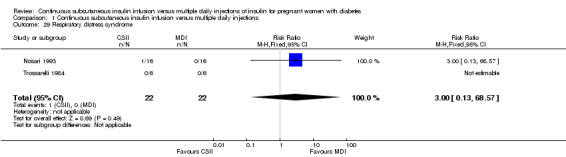
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 29 Respiratory distress syndrome.
1.31. Analysis.
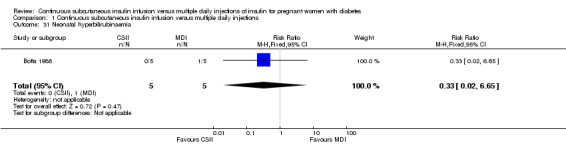
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 31 Neonatal hyperbilirubinaemia.
1.32. Analysis.
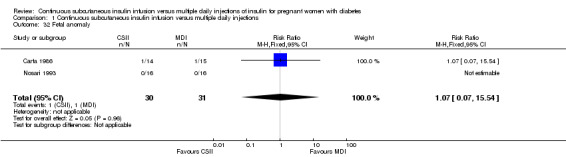
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 32 Fetal anomaly.
There were no data reported on many important infant outcomes. GRADE outcomes not reported were: adiposity, and diabetes. Other neonatal and infant secondary outcomes not reported were: stillbirth, neonatal mortality, head circumference and z score, length and z score, ponderal index, shoulder dystocia, bone fracture nerve palsy, polycythaemia, and relevant biomarker changes associated with the intervention (e.g. cord c peptide, cord insulin). Outcomes from later infant and childhood follow‐up were not reported in any of the trials, thus the following outcomes were not reported: weight and z scores, height and z scores, head circumference and z scores, adiposity (e.g. as measured by BMI, skinfold thickness), blood pressure, type I diabetes, type 2 diabetes, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, and educational achievement. Follow‐up into adulthood was also not carried out, so the following outcomes were not reported: weight, height, adiposity (e.g. as measured by BMI, skinfold thickness), cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome), type I diabetes, type 2 diabetes, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, and employment, education and social status/achievement.
Health service use
The only outcome reported for use of healthcare resources waslength of postnatal stay, which indicates no difference between groups including a small sample of women (MD 9.40, CI ‐6.04 to 24.84; one trial, 10 women; Analysis 1.34).
1.34. Analysis.
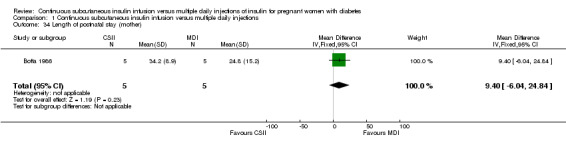
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 34 Length of postnatal stay (mother).
Other outcomes measuring health service use and cost not reported were: number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse), number of antenatal visits or admissions, length of antenatal stay, neonatal intensive care unit admission, length of postnatal stay (baby), costs to families associated with the management provided, costs associated with the intervention, cost of maternal care, and cost of offspring care.
Discussion
Conventionally, insulin has been administered subcutaneously in the form of a basal/bolus regimen, known as multiple daily injections (MDI). This method consists of pre‐meal boluses of short‐acting insulin and a later evening basal injection of long‐acting insulin, usually given with pen injection devices. An alternative insulin administration method is the continuous subcutaneous insulin infusion pump (CSII). Modern pumps are small and lightweight, different basal rates can be preset and boluses given as required and a recent advance is the development of the tubing‐free pump in which the reservoir and integrated infusion set adhere to the skin (patch pump). Potentially, CSII may reduce the risk of hypoglycaemia and ensure a more consistent blood glucose level, leading to a reduction in risk of adverse perinatal outcomes, however CSII was associated with a greater mean birthweight in the two trials reporting this outcome and although there was no significant difference in risk of macrosomia in one small trial, the point estimate suggests MDI may convey greater benefit.
At present, the insulin administration method used by most women is driven by their own and their medical teams' preferences and with what is available in a particular area of the country, rather than evidence of effectiveness. Given that funding for pump therapy may come from a variety of sources: personal, hospital or community, funding influences availability of CSII pumps for many women. Disappointingly none of the trials assessed the economic implications of CSII versus MDI use and only one reported any measure of health service resource use, therefore this is an area that requires investigation in the future.
Because theoretically CSII should result in less fluctuation in glucose level, some women may be offered CSII pumps as an alternative to MDI, particularly if their diabetes is difficult to control. The majority of women in many countries have no choice in how they administer insulin however, and this may be due to the lack of good quality evidence indicating superiority of one method of insulin administration over another. Large high‐quality trials are therefore needed that evaluate the important outcomes indicated in this review, particularly the unexpected finding of increased mean birthweight with CSII use compared to MDI. The population included in future trials should include women with gestational diabetes mellitus (GDM), as well as pre‐existing diabetes, those recruited should as far as possible reflect the general obstetric population of women with diabetes (rather than just motivated women or women with difficult to treat diabetes) and reporting of methods and findings should be comprehensive and transparent. As all the trials were single‐centre and undertaken in Italy, generalisability of findings to other populations may be inappropriate, therefore multi‐centre trials or trials in centres with multi‐ethnic populations would be helpful.
Summary of main results
This review identified five small trials, with 153 women and 154 pregnancies. There were no clear differences found in any of the primary outcomes reported between CSII and MDI in the included trials: caesarean section, large‐for‐gestational age, and perinatal mortality. There were also no clear differences for the following secondary outcomes reported: maternal weight gain during pregnancy, maternal 24‐hour mean blood glucose and mean HbA1c in each trimester, maternal hypoglycaemia and hyperglycaemia, gestational age at delivery, preterm birth < 37 weeks' gestation, preterm birth < 32 weeks' gestation, Apgar score less than seven at five minutes, macrosomia, small‐for‐gestational age, mean birthweight, respiratory distress syndrome, neonatal hypoglycaemia, neonatal hyperbilirubinaemia, and fetal anomaly.
In two trials, including a total of 61 infants, CSII was associated with an increase in mean birthweight compared with MDI. However, this result was not repeated for macrosomia rate or large‐for‐gestational age, suggesting uncertainty in effect.
Many important outcomes were not reported, including most GRADE outcomes: hypertensive disorders, maternal development of type 2 diabetes, induction of labour, perineal trauma, return to pre‐pregnancy weight, postnatal depression, mortality or morbidity composite, adiposity, and subsequent offspring type 1 and type 2 diabetes. There was no longer‐term follow‐up of the infants in these studies. Information on health service use is also lacking, with length of postnatal stay reported in only one study of 10 women.
Overall completeness and applicability of evidence
The trials included are small and include few women. Three were undertaken in the 1980s, one in the 1990s and since this time pump technology has advanced. Therefore, adequately powered trials that report all outcomes suggested by this review to evaluate patch pumps compared with MDI and more conventional CSII with MDI or use a multi‐factorial approach are required.
There were no trials of appropriate methodological quality that assessed the use of MDI versus CSII for women with GDM. Prevalence of GDM is increasing and these women may require insulin; this is a group of women who should be included in future trials.
Quality of the evidence
The trials included in this review were small, with few participants, the quality of reporting was poor and therefore generally risk of bias was unclear. It is not possible to blind participants or clinicians to intervention allocation in trials such as those in this review, however outcome assessors can be blinded and this was not reported. We do not know if the women who participated were representative of the general population of women with diabetes, and therefore if the results can be generalised, one trial Nosari 1993 reported that their participants were 'highly motivated'. Researchers should try to ensure by using appropriate methods that their trial population reflects the general obstetric population of women with diabetes as closely as possible.
Most GRADE outcomes were not reported. For the GRADE outcomes that were reported, our assessment was that the evidence is very low quality (caesarean section, large‐for‐gestational age, perinatal mortality, andneonatal hypoglycaemia). This was due to design limitations in the included trials, small sample sizes in those studies contributing data, wide confidence intervals crossing both the line of no effect and the line of appreciable benefit and/or harm, and often few events. These judgements are shown in Table 1 and Table 2. We are therefore uncertain whether CSII or MDI improves outcomes for pregnant women with diabetes and their infants.
Potential biases in the review process
The assessment of risk of bias involves subjective judgements. This potential limitation is minimised by following the procedures in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with two or more review authors independently assessing studies and resolving any disagreement through discussion, and if required involving a third assessor in the decision. We undertook a comprehensive, systematic search of databases to reduce the potential for publication bias, without language or publication status restrictions.
Agreements and disagreements with other studies or reviews
Our finding, that the limited and potentially low‐quality evidence prevents the drawing of meaningful conclusions regarding the effectiveness of one method of insulin administration over another in pregnancy for women requiring insulin supports the findings of another review (Mukhopadhyay 2007). Mukhopadhyay 2007 suggests their results do not demonstrate a clear‐cut benefit of CSII over MDI and that large randomised trials are required comparing newer pumps which use rapid acting insulin analogues with MDI. Similar to our recommendations, the authors suggest cost‐effectiveness analyses are undertaken.
Authors' conclusions
Implications for practice.
At present there are insufficient data to draw conclusions in relation to the effectiveness of one method of insulin administration over another. Both methods appear to have advantages and disadvantages, though these are often not reported by trials. Decisions regarding the use of different administration methods in the context of pregnancy should therefore be made according to individual needs and available resources.
Implications for research.
Large multi‐centre randomised, adequately powered trials are needed to assess the effectiveness of continuous subcutaneous insulin infusion compared with multiple daily injections for women with diabetes (GDM and pre‐existing) in pregnancy who require insulin. It would be beneficial if outcomes were consistent across trials and included women's preferences. Further trials to assess the effects of pumps on birthweight and macrosomia rates are needed. Future trials should undertake longer‐term follow‐up of participants (women and their infants) as well as assessment of associated costs.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2016 | New citation required but conclusions have not changed | No new studies included in this update. |
| 31 March 2016 | New search has been performed | Search updated and four new reports of three trials identified (Murphy 2011; Stewart 2014; Thompson 2014). One trial was excluded (Murphy 2011) and two are currently ongoing (Stewart 2014; Thompson 2014). Two 'Summary of findings' tables have been added in this update and the core outcome set for reviews of diabetes in pregnancy has been incorporated. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 31 July 2011 | New search has been performed | Search update. The updated search identified two new reports; Ignatova 2007 has been excluded, Trossarelli 1984 has been included. Two trials previously awaiting assessment (Botta 1986; Mello 2005) have been included. This updated review now includes five studies (involving 153 women and 154 pregnancies), the results and conclusions have not changed. Division and names of outcomes have been changed from main and additional, to primary and secondary. Primary outcomes are now macrosomia for the infant and operative birth for the mother. Economic evaluation has been added as a secondary outcome. |
| 1 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Denise Atherton for administrative assistance; Lynn Hampson for the literature search.
Thank you to Marie‐Helene Hayles for translating Botta 1986.
Helen West's contribution to this project was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
For this update, we used the Cochrane Pregnancy and Childbirth core outcome set for reviews of diabetes in pregnancy, developed by the Cochrane Pregnancy and Childbirth Australasian satellite.
Data and analyses
Comparison 1. Continuous subcutaneous insulin infusion versus multiple daily injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.77] |
| 3 Development of type 2 diabetes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Large‐for‐gestational age | 3 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [0.49, 34.95] |
| 5 Perinatal mortality | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.38, 14.32] |
| 6 Mortality or morbidity composite | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neurosensory disability | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Induction of labour | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Perineal trauma | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Weight gain during pregnancy | 3 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐1.12, 2.17] |
| 11 Maternal 24‐hour mean blood glucose (mg/dL) first trimester | 3 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐7.19, 7.43] |
| 12 Maternal 24‐hour mean blood glucose (mg/dL) second trimester | 3 | 73 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐5.02, 8.56] |
| 13 Maternal 24‐hour mean blood glucose (mg/dL) third trimester | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐5.57, 5.72] |
| 14 Mean HbA1c first trimester | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.13, 1.73] |
| 15 Mean HbA1c second trimester | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.29, 3.69] |
| 16 Mean HbA1c third trimester | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.38, 2.58] |
| 17 Maternal hypoglycaemia | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.87] |
| 18 Maternal hyperglycaemia | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.39, 125.44] |
| 19 Postnatal depression | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Postnatal weight retention or return to pre‐pregnancy weight | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Gestational age at birth | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.92, 0.57] |
| 22 Preterm birth < 37 weeks' gestation | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.18, 3.24] |
| 23 Preterm birth < 32 weeks' gestation | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.62] |
| 24 Apgar score < 7 at 5 minutes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Macrosomia | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [0.14, 72.62] |
| 26 Small‐for‐gestational age | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.10, 18.71] |
| 27 Mean birthweight grams | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 220.56 [‐2.09, 443.20] |
| 28 Adiposity (infant) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Respiratory distress syndrome | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 30 Neonatal hypoglycaemia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 31 Neonatal hyperbilirubinaemia | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 32 Fetal anomaly | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 15.54] |
| 33 Diabetes (infant) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Length of postnatal stay (mother) | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [‐6.04, 24.84] |
1.24. Analysis.
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 24 Apgar score < 7 at 5 minutes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Botta 1986.
| Methods | "Randomly divided". | |
| Participants | 10 women recruited from 8 weeks to 'term'. | |
| Interventions | CSII versus MDI. | |
| Outcomes | Gestational age at birth, caesarean section, weight gain during pregnancy, preterm birth, hyperbilirubinaemia, and maternal days hospitalised. | |
| Notes | Single‐centre trial in Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the type of intervention from clinician and participant for the trials was not possible because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Other bias | Unclear risk | Small trial group, limited reporting. |
Carta 1986.
| Methods | "Randomly assigned" no other information reported. | |
| Participants | 15 women with type 1 diabetes (13 on conventional insulin therapy, 2 on continuous insulin therapy) and 14 women with type 2 diabetes (4 were on oral hypoglycaemics, 10 diet‐controlled). Recruitment occurred in the first trimester, 2 women allocated to CSII had already been using a CSII pump pre‐conceptually. | |
| Interventions | CSII versus MDI. Participants were hospitalised initially in order to achieve optimal glycaemic control, diet was prescribed according to individual needs. A Microject MC 20 portable syringe pump was used with porcine insulin (Actrapid MC) 40 U/mL, adjustments were made to the dosage in order to obtain strict glycaemic control (fasting BG < 80 + 10 mg/dL, postprandial BG < 120 mg/dL). Participants randomised to the MDI dose were given Actrapid MC split into 4 boluses. | |
| Outcomes | Maternal and neonatal mortality, large‐for‐gestational age, fetal anomaly and hypoglycaemia, weight gain during pregnancy, mean 24‐hour BG, mean HbA1c, gestational age at delivery, preterm birth, birthweight and rate of instrumental delivery. | |
| Notes | Single‐centre trial in Italy. Participants and their neonates were followed up at delivery and for the first 2 days postnatally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the type of intervention from clinician and participant for the trials was not possible because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The numbers of women included in analysis across pregnancy comparing maternal 24‐hour mean glucose and mean HbA1c varied from 13 to 15 in the CSII group and from 8 to 14 in the MDI group. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Other bias | Unclear risk | Small trial group, limited reporting of methods. |
Mello 2005.
| Methods | "Randomly assigned". | |
| Participants | 71 women with type 1 diabetes. | |
| Interventions | MDI versus CSII. | |
| Outcomes | Daily glucose levels, 24‐hour glycaemic profiles, infant abdominal fat deposition. | |
| Notes | Raw and mean data not reported, therefore could not be included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the type of intervention from clinician and participant for the trials was not possible because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Other bias | Unclear risk | Single‐centre trial in Italy, small trial group, limited reporting of methods. |
Nosari 1993.
| Methods | "Randomly allocated", using sealed envelopes. | |
| Participants | 31 women with type 1 insulin‐dependent diabetes included undergoing 32 pregnancies, 1 woman included twice for separate pregnancies. 4 women were recruited in the pre‐conception period, 28 women recruited during their first trimester. The women were described as highly motivated and referred to their centre for intensive therapy. | |
| Interventions | Allocated either CSII or MDI. Microject MC 20 and Daedi B.V. portable battery‐powered syringe infusion pumps, participants receiving MDI had 4 daily insulin injections (regular insulin at each meal and intermediate acting insulin at night, type of insulin used was not stated). | |
| Outcomes | Maternal and neonatal mortality, large‐for‐gestational age, fetal anomaly and hypoglycaemia, mean 24‐hour BG, mean HbA1c, gestational age at delivery, preterm birth, Apgar score < 7 at 5 minutes, respiratory distress syndrome, birthweight and rate of instrumental delivery. | |
| Notes | No losses to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes to conceal allocation of their participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the type of intervention from clinician and participant for the trials was not possible because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Other bias | High risk | Small trial group, described as "highly motivated". Single‐centre trial in Italy, limited reporting of methods. |
Trossarelli 1984.
| Methods | "Randomly assigned". | |
| Participants | 12 women with type 1 diabetes. | |
| Interventions | MDI versus CSII. | |
| Outcomes | Perinatal mortality, large‐for‐gestational age, maternal weight gain during pregnancy, mean 24‐hour BG in each trimester, and respiratory distress syndrome. | |
| Notes | Several data outcomes described as comparable rather than reported as statistics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the type of intervention from clinician and participant for the trials was not possible because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Other bias | Unclear risk | Not reported. |
BG: blood glucose CSII: continuous subcutaneous insulin infusion HbA1c: glycated haemoglobin MDI: multiple daily injection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burkart 1988 | Exclusion of MDI group participants from analysis if normoglycaemia not achieved (number not reported). |
| Collaborative 1993 | Wrong intervention: conventional versus intensive therapy, rather than CSII versus MDI. Women using CSII and MDI therapy included in the intensive therapy group. |
| Coustan 1986 | 21 women randomised antenatally (1 randomised twice for separate pregnancies), gestation at randomisation not stated, therefore length of treatment may be inconsistent. 7 women randomised in the pre‐conceptual period, conceiving between 2 weeks and 1 year. The differences in time to conceive and treatment length is likely to lead to inconsistency of treatment effects. |
| Ignatova 2007 | Probably not a randomised study: 41 women were "divided" into 2 groups. Number of women included in the study and by study group inconsistently reported (41 women included and 21 in each of the 2 groups). |
| Laatikainen 1987 | 30 women randomised, 9 declined CSII, so did not receive the allocated intervention and were reported as a separate group, thus potentially confounding the results. |
| Murphy 2011 | Wrong intervention: 2‐arm cross‐over trial comparing closed‐loop versus conventional CSII. |
| Zoupas 1991 | No methods or data provided by trial authors, therefore assessment of the trial is not possible. |
CSII: continuous subcutaneous insulin infusion MDI: multiple daily injection
Characteristics of ongoing studies [ordered by study ID]
Stewart 2014.
| Trial name or title | CLIP‐03. |
| Methods | 2‐arm cross‐over trial comparing closed‐loop CSII overnight with not closed‐loop (continuous glucose monitor and insulin pump but not closed‐loop) CSII overnight. |
| Participants | Pregnant women with type 1 diabetes, 8‐24 weeks GA. Setting: UK. |
| Interventions | Closed‐loop CSII overnight versus continuous glucose monitor and insulin pump but not closed‐loop. |
| Outcomes | Overnight time spent in the target glucose range (3.5‐7.8 mmol/L), number of episodes of nocturnal hypoglycaemia, duration and outcome of events. Frequency and duration of use of the closed‐loop system, users’ responses (lifestyle change and diabetes self‐management). Overnight time above and below target range, metabolic control, trends in CGM data. |
| Starting date | 01/04/2014. |
| Contact information | Dr Zoe Stewart. |
| Notes | This study may not be eligible for inclusion, because it does not compare CSII with MDI. |
Thompson 2014.
| Trial name or title | |
| Methods | 2‐arm randomised trial, comparing insulin pump with multiple daily insulin injection. Single‐blind (outcome assessor). The information indicates that allocation is “randomized”, there is no description of the method. |
| Participants | Pregnant women with type 1 or type 2 diabetes prior to pregnancy. First trimester, singleton pregnancy, receiving intensive insulin therapy of less than 100 unit of insulin per day. Setting: Canada. |
| Interventions | Insulin pump versus multiple daily insulin injections. |
| Outcomes | Composite obstetric/perinatal morbidity or mortality, mean maternal HbA1c during pregnancy, number of episodes of severe hypoglycaemia. |
| Starting date | April 2014. |
| Contact information | Dr David Thompson david.thompson@vch.ca |
| Notes |
CGM: continuous glucose monitor CSII: continuous subcutaneous insulin infusion GA: gestational age HbA1c: glycated haemoglobin MDI: multiple daily injection
Differences between protocol and review
For this update, we have used the Cochrane Pregnancy and Childbirth core outcome set for reviews of diabetes in pregnancy, developed by the Cochrane Pregnancy and Childbirth Australasian satellite.
We have added 'Summary of findings' tables and an assessment of the quality of the evidence using the GRADE approach.
'Return to pre‐pregnancy weight' was added as a secondary outcome (long‐term maternal outcomes) in the 2016 update.
Contributions of authors
Diane Farrar drafted the review and update, assessed study quality, undertook data entry and analysis in Review Manager. Jane West assessed study quality and made comments and suggestions on drafts of the review and update. Derek Tuffnell assessed study quality and made comments and suggestions on drafts of the review and update. In the review update, Helen West assessed all trials for inclusion, prepared GRADE tables and commented on the draft.
Sources of support
Internal sources
(HW) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
(HW) NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Helen West is paid to work on Cochrane reviews by a grant to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Botta 1986 {published data only}
- Botta RM, Sinarga D, Angelico MC, Bomplani GD. Intensified conventional insulin therapy as compared to micropump therapy in pregnant women affected by type 1 diabetes mellitus [Confronto fra terapia insulinica tradizionale ottimizzata e con microinfusore in gravide affette da diabete mellito di tipo1]. Minerva Medica 1986;77:657‐61. [PubMed] [Google Scholar]
Carta 1986 {published data only}
- Carta Q, Meriggi E, Trossarelli GF, Catella G, Dal Molin V, Menato G, et al. Continuous subcutaneous insulin infusion versus intensive conventional insulin therapy in type I and type II diabetic pregnancy [Etude comparee de la perfusion sous‐cutanee continue d'insuline et de l'insulinotherapie intensive classique chez des femmes diabetiques de type I ou II enceintes]. Diabete et Metabolisme 1986;12:121‐9. [PubMed] [Google Scholar]
Mello 2005 {published data only}
- Mello G, Parretti E, Tondi F, Riviello C, Borri P, Scarselli G. Impact of two treatment regimens with insulin lispro in post‐prandial glucose excursion patterns and fetal fat mass growth in type 1 diabetic pregnant women [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S36. [Google Scholar]
Nosari 1993 {published data only}
- Nosari I, Maglio ML, Lepore G, Cortinovis F, Pagani G. Is continuous subcutaneous insulin infusion more effective than intensive conventional insulin therapy in the treatment of pregnant diabetic women?. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1993;6:33‐7. [Google Scholar]
Trossarelli 1984 {published data only}
- Trossarelli GF, Cavallo‐Perin P, Meriggi E, Menato G, Dolfin G, Carta Q, et al. Metabolic and obstetrical results in Type 1 (insulin‐dependent) diabetic pregnancy: pump versus optimized conventional insulin therapy [abstract]. Diabetologia 1984;27(2):340A. [Google Scholar]
References to studies excluded from this review
Burkart 1988 {published data only}
- Burkart W, Hanker JP, Schneider HPG. Complications and fetal outcome in diabetic pregnancy ‐ Intensified conventional verses insulin pump therapy. Gynecologic and Obstetric Investigation 1988;26:104‐12. [DOI] [PubMed] [Google Scholar]
Collaborative 1993 {published data only}
- Collaborative. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
Coustan 1986 {published data only}
- Coustan DR, Reece A, Sherwin RS, Rudolf MCJ, Bates SE, Sockin SM, et al. A randomised clinical trial of the insulin pump vs intensive conventional therapy in diabetic pregnancy. JAMA 1986;255:631‐6. [PubMed] [Google Scholar]
Ignatova 2007 {published data only}
- Ignatova N, Arbatskaya N, Melnikova E. Continuous subcutaneous insulin infusion (CSII) reduces the rate of hypoglycaemic episodes throughout pregnancy. Diabetologia 2007;50(Suppl 1):S383‐4. [Google Scholar]
Laatikainen 1987 {published data only}
- Laatikainen L, Teramo K, Hieta‐Heikuraninen H, Koivisto V, Pelkonen R. A controlled study of the influence of continuous subcutaneous insulin infusion treatment on diabetic retinopathy during pregnancy. Acta Medica Scandinavica 1987;221:367‐76. [DOI] [PubMed] [Google Scholar]
Murphy 2011 {published data only}
- Murphy HR. Evaluation of the safety and efficacy of closed loop glucose control during the activities of normal daily living in women with type 1 diabetes during pregnancy: an open label randomised cross‐over study. ISRCTN Registry (http://www.isrctn.com/) [accessed 2 November 2015] 2010.
- Murphy HR, Kumareswaran K, Elleri D, Allen JM, Caldwell K, Biagioni M, et al. Safety and efficacy of 24‐h closed‐loop insulin delivery in well‐controlled pregnant women with type 1 diabetes: a randomized crossover case series.[Erratum appears in Diabetes Care. 2012 Jan;35(1):191]. Diabetes Care 2011;34(12):2527‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoupas 1991 {published data only}
- Zoupas C. Insulin infusion pumps in pregnancy. Personal communication 1991.
References to ongoing studies
Stewart 2014 {published data only}
- Stewart Z. Evaluation of the feasibility, utility, safety and efficacy of overnight closed‐loop insulin delivery at home in women with type 1 diabetes during pregnancy. Acronym: CLIP_03. ISRCTN Registry (http://www.isrctn.com/) [accessed 2 November 2015] 2014.
Thompson 2014 {published data only}
- Thompson DM. Comparison of continuous subcutaneous insulin infusion (CSII) with multiple daily injections (MDI) for the treatment of pregestational diabetes during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 1 November 2015] 2014.
Additional references
Alwan 2009
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1997
- Anderson JH, Brundelle RL, Koivisto VA. Reduction of postprandial hyperglycaemia and frequency of hypoglycaemia in IDDM patients on insulin analog treatment. Multicentre insulin lispro study group. Diabetes 1997;46:265‐70. [DOI] [PubMed] [Google Scholar]
Brink 1986
- Brink SJ, Stewart C. Insulin pump treatment in insulin‐dependent diabetes mellitus. JAMA 1986;255(5):617‐21. [PubMed] [Google Scholar]
Brown 2015
- Brown J, Martis R, Hughes B, Rowan J, Crowther CA. Oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015a
- Brown J, Alwan NA, West J, Brown S, McKinlay CJD, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011970] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016
- Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhling 2004
- Buhling KJ, Kurzidim B, Wolhlfarth K, Mahmoudi M, Wascher C, Siebert G, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycaemia by comparing the self monitoring of blood glucose (SMBG) in non‐pregnant women and pregnant women with impaired glucose tolerance and gestational diabetes. Experimental and Clinical Endocrinology & Diabetes 2004;112(10):556‐60. [DOI] [PubMed] [Google Scholar]
Catalano 2012
- Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
CEMACH 2005
- CEMACH. Pregnancy in Women with Type 1 and Type 2 Diabetes 2002‐2003. London: CEMACH, 2005. [Google Scholar]
CMACE 2010
- Centre for Maternal and Child Enquiries (CMACE). Perinatal Mortality 2008: United Kingdom. London: CMACE, 2010. [Google Scholar]
Colquitt 2004
- Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technology Assessment Programme 2004;8(9):43. [DOI] [PubMed] [Google Scholar]
Farrar 2015
- Farrar D, Fairley L, Santorelli G, Tuffnell D, Sheldon TA, Wright J, et al. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes and Endocrinology 2015;3:795‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2002
- Gonzalez JL. Management of diabetes in pregnancy. Clinical Obstetrics and Gynecology 2002;45(1):165‐9. [DOI] [PubMed] [Google Scholar]
Hadden 1996
- Hadden DR. The management of diabetes in pregnancy. Postgraduate Medical Journal 1996;72:525‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
HAPO 2008
- HAPO study cooperative research group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
HAPO 2010
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG: an international journal of obstetrics and gynaecology 2010;117:575‐84. [DOI] [PubMed] [Google Scholar]
Hawthorne 1997
- Hawthorne G, Robson S, Ryall EA, Sen D, Roberts SH, Ward Platt MP. Prospective population based survey of outcome of pregnancy in diabetic women: results of the Northern diabetic pregnancy audit, 1997. BMJ 1997;315(103):279‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawthorne 2002
- Hawthorne G, Modder J. Maternity services for women with diabetes in the UK. BMJ 2002;19(Suppl 4):50‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 2005
- Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28(3):533‐8. [DOI] [PubMed] [Google Scholar]
Johansson 2000
- Johansson UB, Adamson UC, Lins PE, Wredling RA. Improved blood glucose variability, HbA1C insuman infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish Multicentre Lispro Insulin Study. Diabetes and Metabolism 2000;26:192‐6. [PubMed] [Google Scholar]
John 1997
- John WG. Glycated haemoglobin analysis. Annals of Clinical Biochemistry 1997;34:17‐31. [DOI] [PubMed] [Google Scholar]
Kerssen 2004
- Kerssen A, De‐Valk HW, Visser GHA. The continuous monitoring system during pregnancy of women with type 1 diabetes mellitus: accuracy assessment. Diabetes Technology & Therapeutics 2004;6(5):645‐51. [DOI] [PubMed] [Google Scholar]
Kilpatrick 1997
- Kilpatrick ES. Problems in the assessment of glycaemic control in diabetes mellitus. Diabetic Medicine 1997;14:819‐31. [DOI] [PubMed] [Google Scholar]
Kilpatrick 1998
- Kilpatrick ES, Maylor PW, Keevil BG. Biological variation of glycated haemoglobin. Diabetes Care 1998;21(2):261‐4. [DOI] [PubMed] [Google Scholar]
Knight 1985
- Knight G, Jennings AM, Boulton AJM, Tomlinson S, Ward JD. Severe hyperkalaemia and ketoacidosis during routine treatment with an insulin pump. BMJ 1985;291:371‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2014
- Knight MKS, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ (Eds.) on Behalf of MBRRACE‐UK. Saving Lives, Improving Mothers’ Care: Lessons learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009‐2012. National Perinatal Epidemiology Unit, 2014. [Google Scholar]
Maresh 2001
- Maresh M. Diabetes in pregnancy. Current Opinion in Obstetrics and Gynecology 2001;13:103‐7. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall SM, Barth JH. Standardization of HbA1c measurements‐a consensus statement. Diabetic Medicine 2000;17:5‐6. [DOI] [PubMed] [Google Scholar]
Martis 2016
- Martis R, Brown J, Alsweiler J, Crawford TJ, Crowther CA. Different intensities of glycaemic control for women with gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011624.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mecklenburg 1984
- Mecklenburg RS, Benson EA, Benson JW, Fredlund PN, Guinn T, Metz RJ, et al. Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients. JAMA 1984;252(23):3265‐9. [PubMed] [Google Scholar]
Melamed 2009
- Melamed N, Moshe H. Perinatal mortality in pregestational diabetes. International Journal of Gynaecology and Obstetrics 2009;104:S20‐S24. [DOI] [PubMed] [Google Scholar]
Middleton 2016
- Middleton P, Crowther CA, Simmonds L. Different intensities of glycaemic control for pregnant women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD008540.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukhopadhyay 2007
- Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs intensive conventional insulin therapy in pregnant diabetic women: a systematic review and metaanalysis of randomized, controlled trials. American Journal of Obstetrics and Gynecology 2007;197:447‐56. [DOI] [PubMed] [Google Scholar]
Penney 2003
- Penney GC, Mair G, Pearson DWM. On behalf of the Scottish Diabetes in Pregnancy Group. Outcomes of pregnancies in women with type 1 diabetes in Scotland: a national population‐based study. BJOG: an international journal of obstetrics and gynaecology 2003;110:315‐8. [PubMed] [Google Scholar]
Porter 2004
- Porter H, Belfort MA. Evaluation of a new real‐time continuous glucose monitoring system in pregnant women without gestational diabetes: a pilot study. Journal of Perinatal and Neonatal Nursing 2004;18(2):93‐102. [DOI] [PubMed] [Google Scholar]
Pozzilli 2016
- Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz‐Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes/metabolism Research and Reviews 2016;32:21‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowan 2008
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine 2008;358:2003‐15. [DOI] [PubMed] [Google Scholar]
Siebenhofer 2004
- Siebenhofer A, Plank J, Berghold A, Horvath K, Sawicki PT, Beck P, et al. Meta‐analysis of short‐acting insulin analogues in adult patients with type 1 diabetes: continuous subcutaneous insulin infusion versus injection therapy. Diabetologia 2004;47:1895‐905. [DOI] [PubMed] [Google Scholar]
Siebenhofer 2006
- Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003287.pub4] [DOI] [PubMed] [Google Scholar]
Thabit 2012
- Thabit H, Hovorka R. Closed‐loop insulin delivery in type 1 diabetes. Endocrinology and Metabolism Clinics of North America 2012;41:105‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieu 2010
- Tieu J, Coat S, Hague W, Middleton P. Oral anti‐diabetic agents for women with pre‐existing diabetes mellitus/impaired glucose tolerance or previous gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007724.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2003
- Williams J. Overview of the care of pregnant women with pre‐existing diabetes. Journal of Diabetes Nursing 2003;7:12‐6. [Google Scholar]
Zaccardi 2016
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90‐year perspective. Postgraduate Medical Journal 2016;92(1084):63‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Farrar 2007
- Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005542.pub2] [DOI] [PubMed] [Google Scholar]


