Abstract
Ras-GTPase activating SH3 domain-binding protein 1 (G3BP1) is a multifunctional binding protein involved in the development of a variety of human cancers. However, the role of G3BP1 in breast cancer progression remains largely unknown. In this study, we report that G3BP1 is upregulated and correlated with poor prognosis in breast cancer. Overexpression of G3BP1 promotes breast cancer cell proliferation by stimulating β-catenin signaling, which upregulates a number of proliferation-related genes. We further show that G3BP1 improves the stability of β-catenin by inhibiting its ubiquitin-proteasome degradation rather than affecting the transcription of β-catenin. Mechanistically, elevated G3BP1 interacts with and inactivates GSK-3β to suppress β-catenin phosphorylation and degradation. Disturbing the G3BP1-GSK-3β interaction accelerates the degradation of β-catenin, impairing the proliferative capacity of breast cancer cells. Our study demonstrates that the regulatory mechanism of the G3BP1/GSK-3β/β-catenin axis may be a potential therapeutic target for breast cancer.
Keywords: G3BP1, Wnt/β-catenin signaling pathway, GSK-3β phosphorylation, protein stability, breast cancer, peptide antagonist
Introduction
During mammary gland development and breast tumorigenesis, the Wnt/β-catenin signaling pathway plays an important role in many biological and pathological processes [1, 2]. Acting as a transcriptional coactivator of Wnt target genes, β-catenin is the key to Wnt carcinogenesis [3]. In the absence of Wnt ligand, cytoplasmic β-catenin is recruited and phosphorylated by the β-catenin destruction complex, which consists of Axin, APC, GSK-3β, and CK1α [4, 5]. Phosphorylated β-catenin is easily recognized by β-TrCP and degraded [6]. In the presence of Wnt ligand, activation of the destruction complex is inhibited, increasing β-catenin accumulation and nuclear transport to promote the transcription of target genes [7–10]. In breast cancer, β-catenin is frequently overactivated and predicts poor outcome [11–13]. Elevated β-catenin promotes the stemness, proliferation, metastasis, and invasion of breast cancer cells by regulating molecules downstream of Wnt signaling [14, 15], suggesting that β-catenin is essential in the progression of breast cancer.
G3BP1 is an RNA-binding protein that participates in the regulation of multiple cellular functions, including mRNA stability [16], stress granule formation [17], virus replication [18], DNA pattern recognition [19], and tumor signaling transduction [20, 21]. Studies have shown that G3BP1 is upregulated in a variety of cancers and promotes tumorigenesis and progression [22–24]. However, the role and mechanism of G3BP1 in breast cancer remain elusive. The objective of this study was to investigate the precise role and mechanism of G3BP1 in the progression of breast cancer to provide new strategies for breast cancer therapy.
Materials and methods
Reagents
A CCK-8 kit (FC101-01) was purchased from TransGen (Beijing, China). A Cell-Light EdU Apollo 488 in vitro Kit (C10310-3) was purchased from RiboBio (Guangzhou, China). G3BP1 siRNA (sc-75076) and β-catenin siRNA (sc-29209) were purchased from Santa Cruz Biotechnology (Santa Cruz, Dallas, TX, USA). Cycloheximide (CHX) (01810), MG132 (C2211), and bafilomycin A1 (BAF) (196000) were obtained from Sigma-Aldrich (Sigma, St. Louis, MO, USA). The peptide GAP161 was synthesized by Sangon Biotech (Shanghai, China).
Cell culture
The human breast cell line MCF 10A and human breast cancer cell lines MCF-7, MDA-MB-231, BT-549, BT474, SK-BR-3, and T47D were purchased from the Cell Culture Center of Peking Union Medical College (PUMC, Beijing, China). MCF 10A cells were cultured in DMEM/F12 (1:1) (Gibco, CA, USA) supplemented with 5% horse serum (Gibco), 100 ng/mL cholera toxin (Sigma), 10 μg/mL insulin (Gibco), 0.5 μg/mL hydrocortisone (Sigma), and 20 ng/mL EGF (Gibco). MCF-7 cells were cultured in DMEM (MacGene, Beijing, China) with 10% fetal bovine serum (Gibco). MDA-MB-231 cells were cultured in L-15 (Gibco) with 10% fetal bovine serum. BT-549 and T47D cells were cultured in RPMI-1640 (MacGene) with 10% fetal bovine serum and 0.01 mg/mL insulin. SK-BR-3 and BT474 cells were cultured in RPMI-1640 with 10% fetal bovine serum. All the cells were cultured in medium with 0.1 mg/mL streptomycin and 100 U/mL penicillin (MacGene) at 37 °C in 5% CO2. All cell lines were maintained and used at ≤20 passages.
Plasmid construction and cell transfection
Myc-tagged G3BP1 (GenBank: NM_005754) was inserted into pCDNA3.1-Myc by standard subcloning, and stable cell lines were selected using hygromycin as described previously [25]. In previous studies, our laboratory designed and synthesized three sequences to interfere with G3BP1, from which the target sequence with the highest silencing efficiency was selected. This shRNA sequence targeting G3BP1 was 5′-GGATTGGATTCAAATGGAA-3′; G3BP1-shRNA was subcloned into the pGPU6-shRNA cloning plasmid, and stable cell lines were selected using geneticin (G418) as described previously [26]. Via cell transfection, the G3BP1 overexpression plasmid or G3BP1-shRNA plasmid was transfected into the target cells. After 24 h of transfection, cells were cultured in selective medium containing hygromycin or G418. Through the infinite dilution method, stable single clones were selected and expanded.
Myc-tagged G3BP1 and the truncated G3BP1 mutants Myc-G3BP11-135, Myc-G3BP11-240, Myc-G3BP11-330, Myc-G3BP11-420, and Myc-G3BP11-466 were cloned into the pCDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). Flag-tagged G3BP1 and the G3BP1 mutants Flag-G3BP1135-466, Flag-G3BP11-240, Flag-G3BP1241-466, and Flag-G3BP11-466 were gifts from Dr. Tao Li [19].
The β-catenin siRNA used in this article was purchased from Santa Cruz (sc-29209) and consisted of 3–5 nucleotide sequences with a length of 19–25 nt.
MCF-7 and MDA-MB-231 breast cancer cells were seeded in dishes and transfected with plasmids or siRNA at 70%–80% confluence using Lipofectamine 2000 or Lipofectamine RNAiMAX transfection reagent (Invitrogen).
Oncomine and the cancer genome atlas (TCGA) data analysis
To determine the mRNA expression and survival state associated with G3BP1 in breast cancer, datasets in Oncomine (https://www.oncomine.org) were used. To determine the biological functions in which G3BP1 is involved, Kyoto Encyclopedia of Genes and Genomes (KEGG) gene enrichment analysis was performed using TCGA database at LinkedOmics (https://www.linkedomics.org).
Breast cancer tissue microarray (TMA)
A TMA containing 192 breast diseases was purchased from Alenabio (No. BR2082b, Xi’an, China); the TMA included 32 lymph node metastatic carcinoma samples, 69 invasive ductal carcinoma samples, 21 invasive lobular carcinoma samples, 4 squamous cell carcinoma samples, 17 intraductal carcinoma samples, 1 sample from in situ lobular carcinoma, 9 fibroadenoma samples, 8 hyperplasia samples, 12 inflammatory tissue samples, 17 adjacent breast tissue samples, and 2 autopsy breast tissue samples. Immunohistochemical (IHC) analysis was performed to detect the expression of G3BP1 (1:100; Abcam) and β-catenin (1:100; CST) in the TMA.
IHC scores for the samples were estimated using the following method. The staining intensity of the stained cells was determined as follows: negative staining (−), weak staining (+), moderate staining (++), and strong staining (+++). The number of positive cells was scored as follows: fewer than 25% positive cells (+), 25%–49% positive cells (++), and more than 50% positive cells (+++). Finally, based on comprehensive evaluation of the two scores, a qualitative and semiquantitative coloring intensity score was obtained. At least 5–10 high-power fields were randomly observed, and the average value was counted.
IHC analysis
IHC staining was performed using an UltraSensitive TMS-P kit (KIT-9720, MX Biotechnologies, Fuzhou, China). The TMA slides containing paraffin-embedded tissues were baked at 60 °C for 30 min, and 1 mM Tris-EDTA (pH 8.0) was used to for antigen repair in a microwave. Subsequently, 3% hydrogen peroxide was added to block endogenous peroxidases. The tissues were then blocked with the sera of normal nonimmune animals for 10 min. The tissues were incubated overnight at 4 °C with a mouse monoclonal antibody against G3BP1 (Abcam, Cambridge, MA, USA) or a rabbit polyclonal antibody against β-catenin (CST, Danvers, MA, USA). The slides were incubated with a biotin-labeled goat anti-mouse/rabbit IgG for 10 min. Streptavidin-peroxidase and diaminobenzidine were used to observe the target protein staining.
Cell proliferation assay
Cell growth was determined using the CCK-8 assay. Stably transfected MCF-7 and MDA-MB-231 cells were seeded in 96-well plates at a density of 4000 cells per well and incubated with fresh medium for 24, 48, 72, and 96 h. Subsequently, a 10% CCK-8 solution was added and incubated at 37 °C for 2 h. The optical density at 450 nm was measured using a spectrophotometer. Each assay was replicated three times.
Colony formation assay
For the colony formation assay, stably transfected MCF-7 and MDA-MB-231 cells were seeded in six-well plates at a density of 500 cells per well. After culture with fresh medium for 2 weeks, the cells were fixed in methanol for 10 min and stained with 0.1% crystal violet for 10 min. Colonies containing more than 50 cells were counted and scanned.
5-Ethynyl-2′-deoxyuridine (EdU) assay
An EdU assay was performed to detect active DNA synthesis (RiboBio). Stably transfected MCF-7 and MDA-MB-231 cells were seeded in 24-well coverslips and then incubated in EdU solution. The cells were then fixed and stained with Apollo 488 (RiboBio). Images were acquired using a Leica SP2 confocal microscope (Leica Microsystems, Exton, PA, USA) and analyzed with ImageJ software.
Western blotting and coimmunoprecipitation (co-IP)
Cells were lysed in RIPA buffer (CST), and the protein concentration was quantified using a BCA Protein Assay Kit (Beyotime, Shanghai, China). To 1 mL of the above cell lysate, or approximately 100–1000 µg of total cellular protein, 2 µL of primary antibody was added and incubated for 2 h at 4 °C, after which 20 µL of Protein A/G PLUS-Agarose (Santa Cruz, TX, USA sc-2003) was added. The tubes were capped and incubated at 4 °C on a rocking platform overnight. The cell pellets were collected, and the proteins were separated using SDS-PAGE, transferred to a PVDF membrane (Millipore, Burlington, Massachusetts, USA), and blocked with 5% skimmed milk. The membranes were immunoblotted with the indicated antibodies and developed using the FluorChem HD2 imaging system (Protein Simple, CA, USA). The antibodies used in the article are reported in the supplementary materials (Table S1).
Immunofluorescence assay
MCF-7 and MDA-MB-231 cells were seeded in 24-well coverslips, fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and incubated with 10% goat serum (ZSGQ-BIO Company, Beijing, China). Next, the cells were incubated overnight at 4 °C with antibodies against G3BP1 (1:100, Abcam, Cambridge, UK) and GSK-3β (1:100, CST). Secondary antibodies were incubated with the cells for 1 h in the dark. The slides were stained with DAPI (Vector Labs, CA, USA) to visualize the nuclei. Images were obtained (Olympus, Tokyo, Japan) and analyzed with ImageJ software.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from MCF-7 and MDA-MB-231 cells using TRIzol reagent (Invitrogen). qRT-PCR was performed using SYBR Green Master Mix (Roche, Basel, Switzerland). All samples were quantified using the ABI7500Fast real-time PCR detection system (Applied Biosystems, Foster City, CA, USA). Data were analyzed via the 2−ΔΔCT method using β-Actin as a housekeeping gene. The following primers were used:
G3BP1 forward: 5′-GAGAAGCCTAGTCCCCTGCT-3′
G3BP1 reverse: 5′-CCATTTGAATCCAATCCCCCA-3′
β-catenin forward: 5′-ACCTGGATGCCGTCGTGGAC-3′
β-catenin reverse: 5′-TGTGGCAGCACCAGGGCAGC-3′
β-Actin forward: 5′-CCCAGGCACCAGGGCGTGATGGT-3′
β-Actin reverse: 5′-GGACTCCATGCCCAGGAAGGAA-3′.
Xenograft tumor model
Six-week-old female BALB/c nude mice were obtained from SPF Biotechnology Co., Ltd. (Beijing, China). To determine tumor growth of G3BP1 in vivo, breast cancer cells stably infected with shG3BP1 were resuspended in serum-free medium and injected subcutaneously (107 cells/tumor) into the right axilla of the nude mice (n = 6 per group). Approximately 1 week later, the mice were weighed, and tumor width (W) and length (L) were measured with Vernier calipers every 2 days for 4 weeks. Tumor volume was estimated based on the formula V = 0.5 × L× W2. The animals were sacrificed, and the tumors were excised and weighed. The care and treatment of experimental animals were in accordance with the institutional guidelines of the Experimental Animal Center of the Chinese Academy of Medical Sciences.
Statistical analyses
Statistical analyses were conducted using Student’s t-test. Pearson’s chi-squared test was used to analyze the correlation between G3BP1 expression and the clinical characteristics of breast cancer patients. Kaplan–Meier curves were used for overall survival analysis. The correlation between G3BP1 and β-catenin was analyzed by Spearman’s correlation coefficient. Statistical significance was set at *P < 0.05, **P < 0.01, ***P < 0.001 and ****P< 0.0001. NS indicates that no significance was noted. Each experiment was repeated at least three times, and the data are presented as the means ± standard deviations. Statistical tests were carried out with SPSS 24.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Upregulated G3BP1 in breast cancer corresponds to poor clinical prognosis
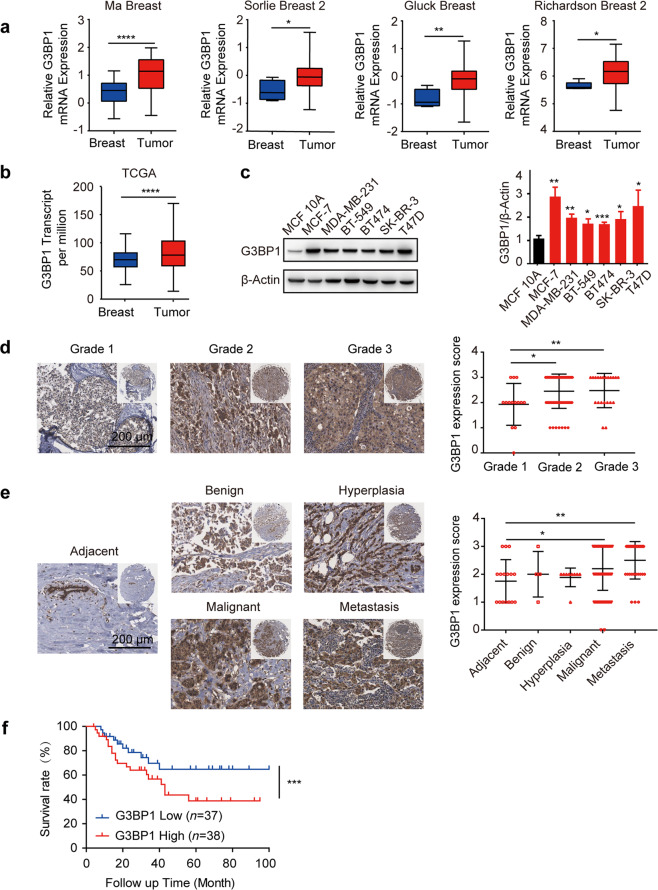
To explore the role of G3BP1 in breast cancer, we first analyzed six independent microarray datasets from Oncomine and TCGA database. Meta-analysis showed that G3BP1 was upregulated in multiple breast cancer types [27] (P = 3.78 × 10−24, Fig. S1a). The mRNA expression level of G3BP1 in tumor tissues was higher than that in normal breast tissues [28–31] (Fig. 1a, b). Consistent with the in vivo results, the expression of G3BP1 was increased in all six breast cancer cell lines compared to MCF 10A normal breast cells (Fig. 1c). Furthermore, a tissue array from 192 patients with breast cancer was tested, and the clinicopathological characteristics of the patients are summarized in Table S2. Compared to adjacent nontumor breast tissues, in which G3BP1 was almost undetectable or expressed at only low levels, the G3BP1 protein was overexpressed in the tumor tissues (Fig. S1b), but its expression was relatively low initially (grade 1) and enhanced in the advanced stages of disease (grades 2–3) (Fig. 1d). Accordingly, more G3BP1 was detected in malignant and metastatic breast cancer (Fig. 1e), implying that G3BP1 may serve as a candidate marker to characterize malignant breast cancer. The results of survival analysis showed that nearly 25% of patients with high G3BP1 expression had lower survival rates than patients with low expression of the protein [32] (P < 0.001, Fig. 1f). Collectively, these results indicate that G3BP1 is upregulated in malignant breast cancer with a poor prognosis.
Fig. 1. Upregulated G3BP1 in breast cancer correlates with poor prognosis.
a G3BP1 mRNA expression levels were upregulated in tumor tissues compared to normal breast tissues from Oncomine breast cancer datasets. b G3BP1 transcript levels were upregulated in tumor tissues compared to normal breast tissues from TCGA breast cancer datasets. c Western blotting of G3BP1 expression in breast cancer cells and MCF 10A normal breast cells. β-Actin was used as a loading control. d G3BP1 protein expression in breast cancer tissues with different tumor grades. Scale bar, 200 μm. e G3BP1 protein expression in tissues from different breast cancer types. Scale bar, 200 μm. f Kaplan–Meier overall survival curves for all 75 patients with breast cancer stratified by high and low G3BP1 expression from data in the Oncomine database. *P < 0.05; **P < 0.01; ***P < 0.001.
G3BP1 overexpression promotes breast cancer cell proliferation and activates β-catenin signaling
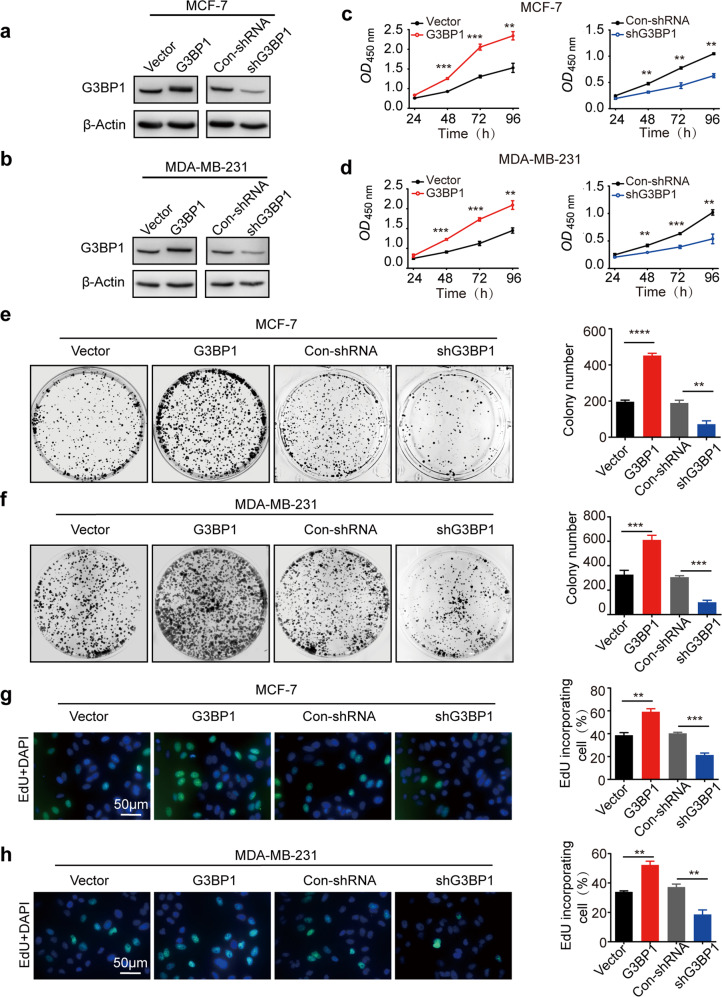
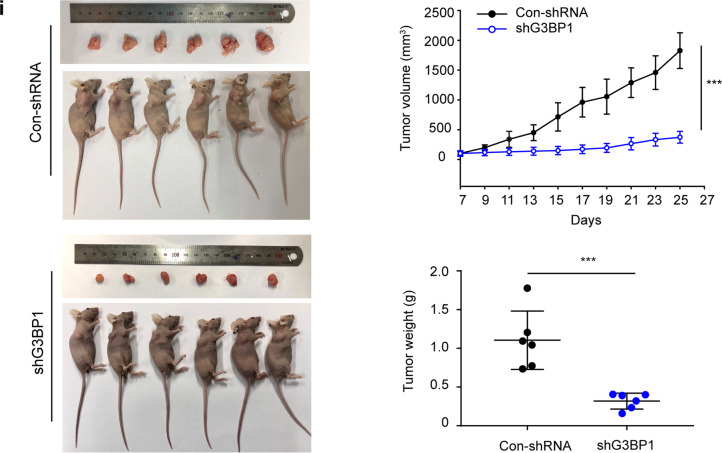
To explore the function of G3BP1 in promoting breast cancer progression, stable cell lines in which G3BP1 was overexpressed and knocked down were constructed. MCF-7 and MDA-MB-231 cells were selected as representative non-TNBC and TNBC cell lines, respectively (Fig. 2a, b). Indeed, overexpression of G3BP1 promoted cell growth (Fig. 2c, d), increased colony formation (Fig. 2e, f), and induced DNA synthesis (Fig. 2g, h), whereas the knockdown of G3BP1 correspondingly weakened the proliferative capacity of MCF-7 and MDA-MB-231 cells (Fig. 2c–h). To further validate the role of G3BP1 in breast cancer cell proliferation, we generated a xenograft model in BALB/c nude mice using shG3BP1 and Con-shRNA MCF-7 cells. Indeed, tumorigenesis was significantly decreased in G3BP1-silenced cells compared to Con-shRNA-transfected cells in vivo (Fig. 2i).
Fig. 2. G3BP1 promotes breast cancer cell proliferation.
a, b The expression of G3BP1 in stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. c, d CCK-8 assays were performed to detect cell growth in stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. e, f Colony formation assays assessed the colony formation capacity of stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. g, h EdU incorporation assays were performed to detect cell proliferation in stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. Scale bar, 50 μm. i Images of tumors excised from six BALB/c nude mice at 28 days after injection with Con-shRNA-transfected cells or shG3BP1 cells. *P < 0.05; **P < 0.01; ***P < 0.001.
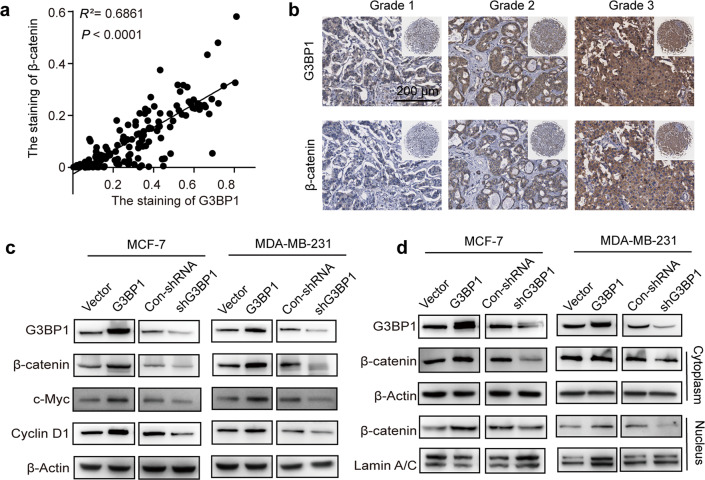
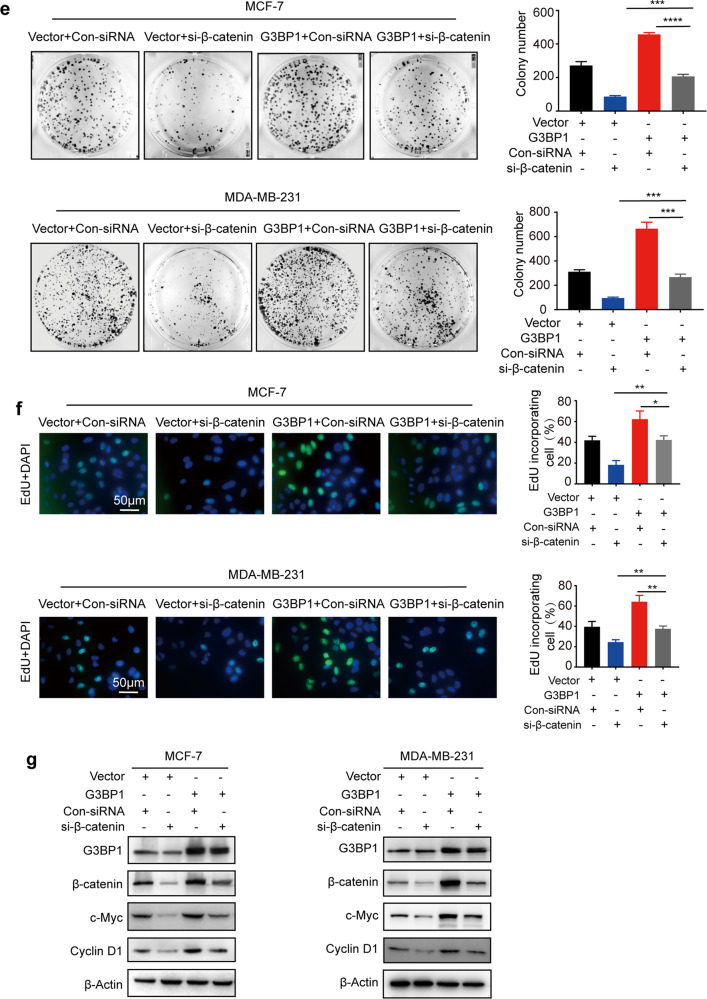
To investigate how G3BP1 regulates breast cancer cell proliferation, KEGG pathway enrichment analysis was performed, and the Wnt/β-catenin signaling pathway was found to be enriched (Fig. S2a). Consistent with the results of an analysis with the Gene Expression Profiling Interactive Analysis database (R = 0.5, P < 0.001, Fig. S2b), the expression of G3BP1 was positively correlated with β-catenin in the breast cancer tissue array (R2 = 0.6861, P < 0.0001, Fig. 3a), and both proteins were associated with breast cancer malignancy grade (Fig. 3b). Furthermore, we explored whether G3BP1 induces cell proliferation through this signaling pathway. The results showed that the upregulation of G3BP1 increased β-catenin expression (Fig. 3c) and supported β-catenin nuclear localization (Fig. 3d). Downstream genes (c-Myc and Cyclin D1) regulated by the Wnt/β-catenin signaling pathway were also elevated in G3BP1-overexpressing cells (Fig. 3c). In contrast, knockdown of G3BP1 suppressed β-catenin signaling (Fig. 3c, d). Notably, silencing β-catenin decreased the increases in colony formation and DNA synthesis caused by G3BP1 overexpression (Fig. 3e, f). In addition, β-catenin knockdown protected against the G3BP1-induced expression of genes regulated by Wnt/β-catenin signaling (Fig. 3g). These data suggest that β-catenin is critical for the proliferative effect of G3BP1 in breast cancer cells.
Fig. 3. G3BP1 promotes breast cancer cell proliferation by regulating β-catenin signaling.
a Correlation analysis following quantitative immunohistochemical staining of G3BP1 and β-catenin in normal breast tissues and breast cancer tissues. b Representative immunohistochemical staining images of breast cancer tissues with different grades. c Western blot analysis of G3BP1, β-catenin, c-Myc, and Cyclin D1 expression in stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. d Expression of β-catenin in the cytoplasm and nucleus after G3BP1 overexpression or knockdown in MCF-7 and MDA-MB-231 cells. β-Actin was used as a cytoplasmic protein loading control, and Lamin A/C was used as a nuclear protein loading control. e Silencing of β-catenin decreased clone formation in G3BP1-overexpressing MCF-7 and MDA-MB-231 cells. f Silencing of β-catenin decreased DNA synthesis in G3BP1-overexpressing MCF-7 and MDA-MB-231 cells. Scale bar, 50 μm. g Silencing of β-catenin decreased the expression of β-catenin, c-Myc, and cyclin D1 in G3BP1-overexpressing MCF-7 and MDA-MB-231 cells. *P < 0.05; **P < 0.01; ***P < 0.001.
G3BP1 improves the stability of β-catenin by inhibiting β-catenin proteasome pathway degradation
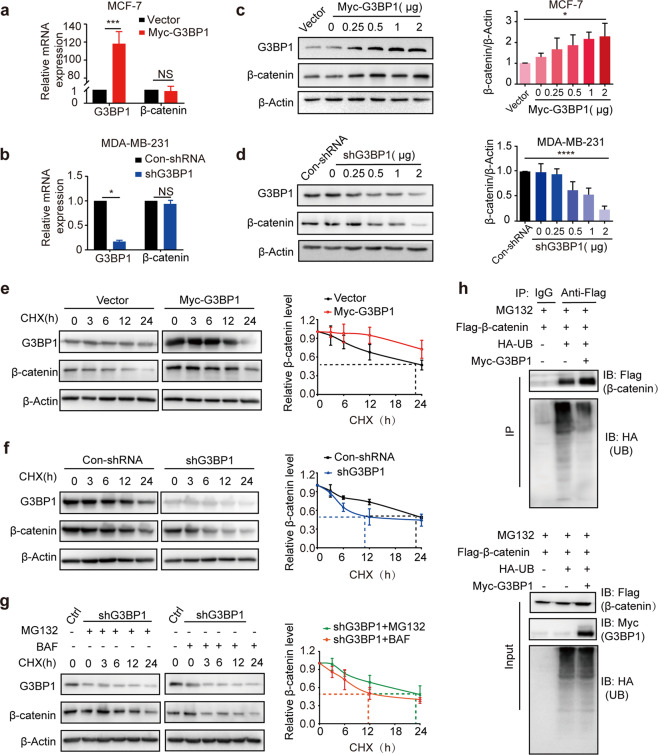
Because G3BP1 was found to promote breast cancer cell proliferation through β-catenin signaling, we further explored the internal mechanism by which G3BP1 regulates β-catenin. Because β-catenin functions as a transcription factor of the Wnt signaling pathway, we first examined the transcriptional activity of β-catenin. Neither overexpression nor knockdown of G3BP1 affected β-catenin mRNA expression levels (Fig. 4a, b), indicating that G3BP1 does not regulate the transcription of β-catenin. However, the protein expression level of β-catenin changed along with that of Myc-G3BP1/shG3BP1 in a dose-dependent manner (Fig. 4c, d), implying that G3BP1 may affect β-catenin protein stability. In CHX-treated MCF-7 cells, G3BP1 overexpression prolonged the half-life of β-catenin (Fig. 4e), while the knockdown of G3BP1 accelerated β-catenin degradation, reducing the half-life of β-catenin from 23 to 8 h in MCF-7 cells (Fig. 4f). Moreover, the degradation of β-catenin could be reversed by treatment with the proteasome inhibitor MG132, whereas the autophagy inhibitor bafilomycin had little effect (Fig. 4g). Indeed, G3BP1 overexpression decreased β-catenin ubiquitination (Fig. 4h). These data demonstrate that G3BP1 inhibits β-catenin degradation through the ubiquitin-proteasome pathway.
Fig. 4. G3BP1 inhibits the ubiquitination and degradation of β-catenin.
a, b The mRNA levels of β-catenin in stable G3BP1-overexpressing or G3BP1-knockdown MCF-7 and MDA-MB-231 cells. c, d The expression of β-catenin protein levels in MCF-7 and MDA-MB-231 cells transfected with the indicated concentrations of Myc-G3BP1 or shG3BP1 for 48 h. e Overexpression of G3BP1 inhibited the degradation of β-catenin in MCF-7 cells. Cells were transfected with the Myc-G3BP1 plasmid for 24 h and treated with CHX (50 μg/mL) for the indicated durations, after which the cell lysates were assessed by Western blotting. f Knockdown of G3BP1 accelerated the degradation of β-catenin in MCF-7 cells. Cells were transfected with the shG3BP1 plasmid for 24 h and treated with CHX (50 μg/mL) for the indicated durations, after which the cell lysates were assessed by Western blotting. g MG132 inhibition blocked the degradation of β-catenin induced by G3BP1. MCF-7 cells were transfected with control or shG3BP1 plasmid for 24 h and then treated with MG132 (10 μmol/L) and BAF (200 nmol/L) for 2 h before the addition of CHX (50 μg/mL) for the indicated durations. h The effect of G3BP1 on β-catenin ubiquitination. MCF-7 cells were transfected with Flag-β-catenin, HA-ubiquitin, or Myc-G3BP1 expression plasmid for 24 h and then treated with MG132 (10 μmol/L), and the cell lysates were immunoprecipitated using an anti-Flag antibody. Ubiquitinated β-catenin was detected by immunoblotting. Data are presented as the mean ± SD (n = 3). NS nonsignificant, CHX cycloheximide, BAF bafilomycin A1, UB ubiquitin. *P < 0.05; **P < 0.01; ***P < 0.001.
G3BP1 interacts with GSK-3β and regulates its phosphorylation at the S9 and Y216 sites
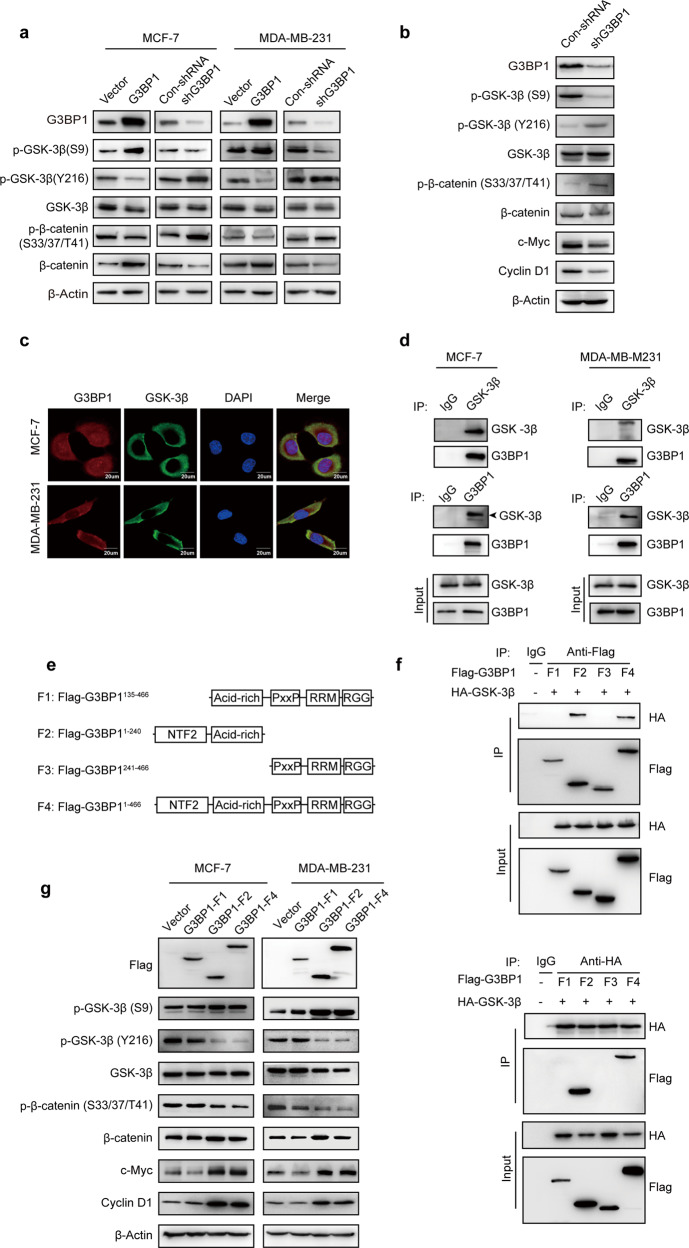
In the canonical Wnt/β-catenin signaling pathway, the stability of cytoplasmic β-catenin is controlled by the destruction complex [33]. Among members of this complex, GSK-3β acts as a negative regulatory factor in this pathway. The activity of GSK-3β is usually affected by its phosphorylation at two sites. S9 phosphorylation inhibits GSK-3β activity [34], while Y216 phosphorylation enhances GSK-3β activity [35]. We found that G3BP1 overexpression not only promoted GSK-3β S9 phosphorylation but also inhibited GSK-3β Y216 phosphorylation (Fig. 5a), ultimately leading to GSK-3β activity inhibition. Moreover, G3BP1 overexpression suppressed the phosphorylation of β-catenin at serine 33 and 37 and threonine 41 (Fig. 5a). The phosphorylation activation of β-catenin at the serine 33 and 37 and threonine 41 sites (phosphorylated by GSK-3β), as well as the serine 45 site (phosphorylated by CK1α), is responsible for β-catenin ubiquitin-mediated degradation [36]. Moreover, the results of the in vivo experiment showed that silencing G3BP1 decreased the expression of β-catenin and its downstream molecules (Fig. 5b). These results suggest that overexpression of G3BP1 protects β-catenin from phosphorylation and ubiquitin degradation by inhibiting the activity of GSK-3β in vitro and in vivo.
Fig. 5. G3BP1 interacts with GSK-3β and regulates its phosphorylation at S9 and Y216.
a The expression of GSK-3β, p-GSK-3β (S9), p-GSK-3β (Y216), β-catenin, and p-β-catenin (S33/37/T41) was detected in G3BP1-overexpressing or G3BP1-knockdown cells. b Western blot analysis of GSK-3β, p-GSK-3β (S9), p-GSK-3β (Y216), β-catenin, p-β-catenin (S33/37/T41), c-Myc, and Cyclin D1 expression in excised xenograft tumors. c Immunofluorescence staining of G3BP1 and GSK-3β in MCF-7 and MDA-MB-231 cells. Scale bar, 20 μm. d G3BP1 interacted with GSK-3β in MCF-7 and MDA-MB-231 cells. e Deletion mutants of the G3BP1 domain were inserted into a Flag-tagged vector. f Map of the domains of the G3BP1 domain and GSK-3β that interact. g The expression of GSK-3β (S9), GSK-3β (Y216), GSK-3β, β-catenin (S33/37/T41), β-catenin, c-Myc, and Cyclin D1 in MCF-7 and MDA-MB-231 cells transfected with G3BP1 deletion mutants.
Since GSK-3β is a phosphorylation substrate of G3BP1, the two proteins may engage in a protein–protein interaction. Indeed, endogenous G3BP1 was colocalized with GSK-3β in breast cancer cells (Fig. 5c), which was confirmed by coimmunoprecipitation (Fig. 5d), indicating that G3BP1 physically interacts with GSK-3β. Furthermore, mapping the regions of G3BP1 and GSK-3β showed that GSK-3β interacts with the NTF2-like domain of G3BP1 (Fig. 5e, f and S3a, b). Importantly, a G3BP1 mutant without the NTF2-like domain (F1) lost the ability to inhibit GSK-3β activity and increased the expression of β-catenin target genes (Fig. 5g). These data indicate that the NTF2-like domain of G3BP1 is crucial for the interaction between G3BP1 and GSK-3β.
Disrupting the G3BP1-GSK-3β interaction relieves breast cancer proliferation
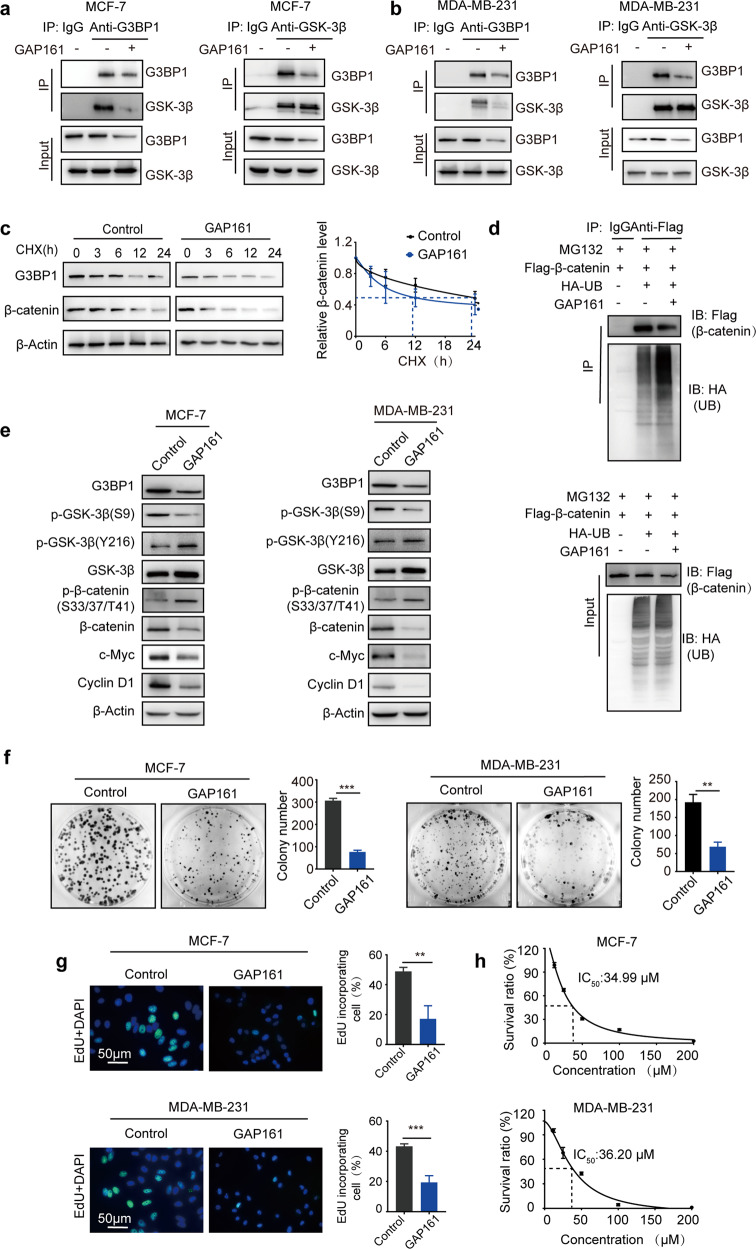
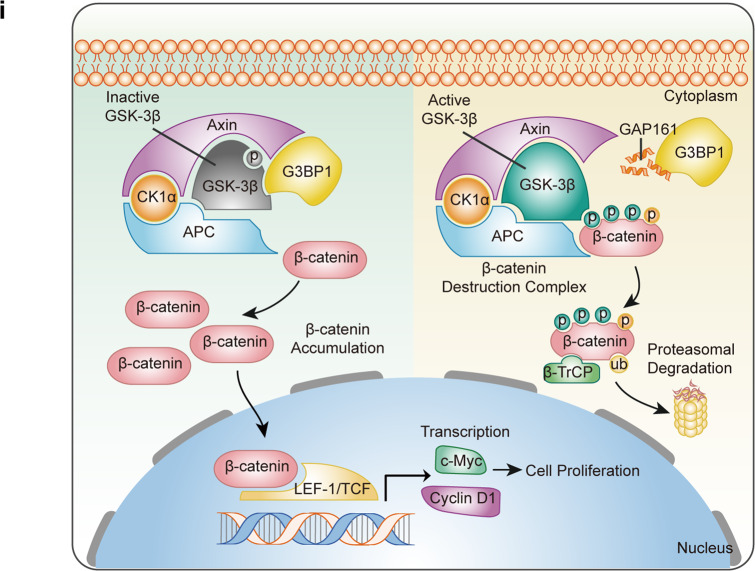
The G3BP1-GSK-3β interaction improves the protein stability of β-catenin, which upregulates the expression of proliferation-related genes in breast cancer cells. Therefore, disrupting the G3BP1-GSK-3β interaction may be a therapeutic option for breast cancer. To verify the role of the G3BP1-GSK-3β interaction in β-catenin signaling activation and cell proliferation, an antagonist peptide of G3BP1 was used to disturb this interaction. GAP161 is a fusion peptide that specifically targets the NTF2-like domain of G3BP1. Our previous results indicated that GAP161 suppresses the progression of colon cancer in vitro and in vivo [37]. Given that GAP161 and GSK-3β share the same G3BP1-binding domain, we explored whether GAP161 affects the interaction of G3BP1-GSK-3β. The immunoprecipitation results suggested that GAP161 effectively disrupted the G3BP1-GSK-3β interaction and moderately decreased G3BP1 expression in MCF-7 and MDA-MB-231 cells (Fig. 6a, b). Furthermore, GAP161 accelerated β-catenin degradation (Fig. 6c) and increased β-catenin ubiquitination in MCF-7 cells (Fig. 6d). The activity of GSK-3β was enhanced, and the expression of target genes regulated by β-catenin was suppressed (Fig. 6e). Moreover, GAP161 not only decreased colony formation (Fig. 6f) and DNA synthesis (Fig. 6g) but also inhibited cell proliferation in MCF-7 and MDA-MB-231 cells (Fig. 6h). These data confirm that the G3BP1-GSK-3β interaction promotes breast cancer cell proliferation by enhancing β-catenin stability; disrupting this interaction may be a promising therapeutic strategy for the treatment of breast cancer (Fig. 6i).
Fig. 6. GAP161 affects the interaction of G3BP1 and GSK-3β.
a, b Effect of GAP161 on the G3BP1-GSK-3β interaction in MCF-7 and MDA-MB-231 cells. Cells were treated with solvent or GAP161 (30 μmol/L) for 24 h; cell extracts were immunoprecipitated with normal rabbit IgG, anti-G3BP1, or anti-GSK-3β antibody and blotted with anti-G3BP1 or anti-GSK-3β antibody. c Effect of GAP161 on β-catenin degradation in MCF-7 cells. d Effect of GAP161 on β-catenin ubiquitination. MCF-7 cells were transfected with Flag-β-catenin and HA-ubiquitin expression plasmids for 24 h and then treated with GAP161 (30 μmol/L), and the cell lysates were immunoprecipitated using an anti-Flag antibody. e The effect of GAP161 on the expression of G3BP1, GSK-3β (S9), GSK-3β (Y216), GSK-3β, β-catenin, p-β-catenin (S33/37/T41), c-Myc, and Cyclin D1 in MCF-7 and MDA-MB-231 cells. f The effect of GAP161 on clone formation in MCF-7 and MDA-MB-231 cells. g The effect of GAP161 on DNA synthesis in MCF-7 and MDA-MB-231 cells. h IC50 of GAP161 in MCF-7 and MDA-MB-231 breast cancer cells after GAP161 treatment. Cells were treated with GAP161 at different concentrations for 48 h, and IC50 values were calculated. i Proposed model of the effect of G3BP1 on breast cancer growth.
Discussion
Previous reports have shown that upregulated G3BP1 predicts poor survival rates in non-small-cell lung cancer and gastric cancer [38, 39], but there is no evidence to verify the function of G3BP1 in breast cancer. In our laboratory’s previous research, we reported the function of G3BP1 in vivo, such as that in lung cancer [26] and breast cancer [25]. We also found that G3BP1 knockdown inhibited breast cancer tumor proliferation in vitro and in vivo, which is consistent with a previous study [25], suggesting that G3BP1 has an important effect on breast cancer proliferation. Although G3BP1 is upregulated in breast cancer cells [40] and may promote proliferation by inhibiting the stability of PMP22 mRNA [41], its mechanism has not been elucidated. In this study, we demonstrate that G3BP1 functions as an oncoprotein that drives breast cancer proliferation in a β-catenin-dependent manner and further verify that G3BP1 activates and stabilizes β-catenin signaling. Through database analysis and the analysis of tumor tissues, coincident increases in G3BP1 and β-catenin were observed in patients with breast cancer along with low survival rates, which indicates that G3BP1 and β-catenin are predictive biomarkers of poor prognosis in breast cancer.
Wnt/β-catenin signaling is critical for cell fate and cancer formation. The key to Wnt carcinogenesis is β-catenin accumulation and nuclear localization [42, 43], suggesting that nuclear β-catenin, rather than cytoplasmic β-catenin, determines cancer progression. Moreover, studies have shown that stabilized β-catenin shuttles in and out the nucleus independent of transport receptors [44]. In this study, we observed that the overexpression of G3BP1 not only elevated the expression of β-catenin in whole-cell lysates but also increased the levels of nuclear β-catenin and Wnt target genes. Mechanistically, the G3BP1-GSK-3β interaction inhibits β-catenin phosphorylation and degradation, thereby improving the stability of β-catenin. Nevertheless, a previous study noted that G3BP1 deficiency increased the mRNA and protein levels of β-catenin [45]. We speculate that this discrepancy may be due to species differences between mouse-derived and human-derived cells. Indeed, a recent study in human esophageal cancer supports this hypothesis [24]. Overall, elevated G3BP1 is necessary for improving β-catenin stability and nuclear localization, and inhibiting the G3BP1-β-catenin axis can alleviate breast cancer cell proliferation.
Due to the complexity of the Wnt signaling pathway, multiple levels of this signaling pathway, such as Wnt secretion [46], ligand–receptor interactions [47], β-catenin stability [48], and β-catenin transcription [49], have become targets of therapeutic intervention. Some small-molecule inhibitors, such as CWP232228, effectively inhibit the growth and progression of breast cancer [50]. In this study, we found that the G3BP1-GSK-3β interaction protects β-catenin from ubiquitin recognition and degradation, enhancing the stability of β-catenin. Furthermore, we found that GSK-3β binds the NTF2-like domain of G3BP1, which has been reported to be involved in interactions with other proteins [51]. Another important domain in G3BP1, the RRM domain, is responsible for RNA binding [52]. In this study, we mainly focused on the interaction between the NTF2-like domain and GSK-3β. Disturbing this interaction effectively inhibited the proliferation of breast cancer cells, implying that the G3BP1/GSK-3β/β-catenin axis is a promising therapeutic target in breast cancer progression.
In addition, we found that G3BP1 interacted with and inactivated GSK-3β by suppressing its phosphorylation at S9 and enhancing Y216 phosphorylation. Usually, the S9 site is phosphorylated in response to insulin stimulation [53]. Y216 phosphorylation is necessary for the maximum activity of GSK-3β [54]. However, we failed to determine which site’s phosphorylation leads to the inhibition of GSK-3β activity because GSK-3β is regulated by a variety of mutual regulatory mechanisms, such as the crosstalk between Wnt signaling and insulin signaling [55–57]. However, both activation and inactivation by phosphorylation at S9 and Y216, respectively decrease the activity of GSK-3β. Our next work will focus on the mechanism by which G3BP1 regulates GSK-3β.
In summary, our study indicates that G3BP1 promotes breast cancer cell growth by activating β-catenin signaling and that the G3BP1-GSK-3β interaction improves β-catenin stability. Disturbing the G3BP1-GSK-3β interaction suppresses breast cancer cell proliferation. Therefore, targeting the G3BP1/GSK-3β/β-catenin axis may be a breast cancer treatment strategy.
Supplementary information
Acknowledgements
We thank Dr. Xue-min Zhang and Tao Li (National Center of Biomedical Analysis, Beijing, China) for gifting the G3BP1 truncation mutant plasmids. This work was supported by the National Key Research and Development Program of China (2016YFA0201504), National Natural Science Foundation of China (No. 81673471, 81102464), the CAMS Initiative for Innovative Medicine (2016-I2M-2-002), and the Drug Innovation Major Project of China (2018ZX09711001-007-002).
Author contributions
RGS: conceptualization, writing-reviewing, and editing, funding acquisition. CHZ: data curation, validation, formal analysis, writing-original draft preparation, and visualization. HL and WLZ: methodology. WXZ and HMZ: Investigation.
Conflict of interest
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41401-020-00598-w) contains supplementary material, which is available to authorized users.
References
- 1.Yu QC, Verheyen EM, Zeng YA. Mammary development and breast cancer: a Wnt perspective. Cancers (Basel) 2016;8:65. doi: 10.3390/cancers8070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Schie EH, van Amerongen R. Aberrant WNT/CTNNB1 signaling as a therapeutic target in human breast cancer: weighing the evidence. Front Cell Dev Biol. 2020;8:25. doi: 10.3389/fcell.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin P, Wang W, Zhang Z, Bai Y, Gao J, Zhao C. Wnt signaling in human and mouse breast cancer: Focusing on Wnt ligands, receptors and antagonists. Cancer Sci. 2018;109:3368–75. doi: 10.1111/cas.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimelman D, Xu W. β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–91. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of β-Catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–73. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 9.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 10.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, et al. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–31. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 12.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–20. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of β-catenin in in situ and invasive breast cancer. Eur J Surg Oncol. 2001;27:31–6. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- 14.Jang GB, Kim JY, Cho SD, Park KS, Jung J-Y, Lee HY, et al. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Zhang J, Tian Y, He Y, Xu x, Pan W, et al. The Wnt/β-catenin/VASP positive feedback loop drives cell proliferation and migration in breast cancer. Oncogene. 2020;39:2258–74. doi: 10.1038/s41388-019-1145-3. [DOI] [PubMed] [Google Scholar]
- 16.Laver JD, Ly J, Winn AK, Karaiskakis A, Lin S, Nie K, et al. The RNA-binding protein Rasputin/G3BP enhances the stability and translation of its target mRNAs. Cell Rep. 2020;30:3353–67.e7. doi: 10.1016/j.celrep.2020.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Yang P, Mathieu C, Kolaitis R-M, Zhang P, Messing J, Yurtsever U, et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell. 2020;181:325–45.e28. doi: 10.1016/j.cell.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Ru Y, Ren J, Bai J, Wei J, Fu S, et al. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis. 2019;10:946. doi: 10.1038/s41419-019-2178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZS, Cai H, Xue W, Wang M, Xia T, Li WJ, et al. G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Su J, Wang Y, Fu D, Ideozu JE, Geng H, et al. The interaction of YBX1 with G3BP1 promotes renal cell carcinoma cell metastasis via YBX1/G3BP1-SPP1- NF-κB signaling axis. J Exp Clin Cancer Res. 2019;38:386. doi: 10.1186/s13046-019-1347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam U, Kennedy D. Rasputin a decade on and more promiscuous than ever? A review of G3BPs. Biochim Biophys Acta Mol Cell Res. 2019;1866:360–70. doi: 10.1016/j.bbamcr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Fu D, Chen Y, Su J, Wang Y, Li X, et al. G3BP1 promotes tumor progression and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal cell carcinomas. Cell Death Dis. 2018;9:501. doi: 10.1038/s41419-018-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong R, Gao JL, Yin T. G3BP1 activates the TGF-β/Smad signaling pathway to promote gastric cancer. Onco Targets Ther. 2019;12:7149–56. doi: 10.2147/OTT.S213728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L-N, Zhao L, Yan XL, Huang YH. Loss of G3BP1 suppresses proliferation, migration, and invasion of esophageal cancer cells via Wnt/β-catenin and PI3K/AKT signaling pathways. J Cell Physiol. 2019;234:20469–84. doi: 10.1002/jcp.28648. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Ma Y, Zhang S, Liu H, He H, Li N, et al. Involvement of Ras GTPase-activating protein SH3 domain-binding protein 1 in the epithelial-to-mesenchymal transition-induced metastasis of breast cancer cells via the Smad signaling pathway. Oncotarget. 2015;6:17039–53. doi: 10.18632/oncotarget.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Zhang SH, He HW, Zhang CX, Yu DK, Shao RG. Downregulation of G3BPs inhibits the growth, migration and invasion of human lung carcinoma H1299 cells by suppressing the Src/FAK-associated signaling pathway. Cancer Gene Ther. 2013;20:622–9. doi: 10.1038/cgt.2013.62. [DOI] [PubMed] [Google Scholar]
- 27.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X-J, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glück S, Ross JS, Royce M, McKenna EF, Perou CM, Avisar E, et al. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat. 2012;132:781–91. doi: 10.1007/s10549-011-1412-7. [DOI] [PubMed] [Google Scholar]
- 31.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–32. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 34.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation By phosphorylation. Mol Cell. 2001;7:1321–7. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 35.Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, et al. The role of glycogen synthase kinase 3β in the transformation of epidermal cells. Cancer Res. 2007;67:7756. doi: 10.1158/0008-5472.CAN-06-4665. [DOI] [PubMed] [Google Scholar]
- 36.Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898-a. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Zhang S, He H, Zhao W, Chen J. Shao RG. GAP161 targets and downregulates G3BP to suppress cell growth and potentiate cisplaitin-mediated cytotoxicity to colon carcinoma HCT116 cells. Cancer Sci. 2012;103:1848–56. doi: 10.1111/j.1349-7006.2012.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, Zhan Y, Zhang Y, Liu S, Lu J, Yang Y, et al. Elevated expression of G3BP1 associates with YB1 and p-AKT and predicts poor prognosis in nonsmall cell lung cancer patients after surgical resection. Cancer Med. 2019;8:6894–903. doi: 10.1002/cam4.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min L, Ruan Y, Shen Z, Jia D, Wang X, Zhao J, et al. Overexpression of Ras-GTPase-activating protein SH3 domain-binding protein 1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2015;67:677–88. doi: 10.1111/his.12695. [DOI] [PubMed] [Google Scholar]
- 40.Guitard E, Parker F, Millon R, Abecassis J, Tocqué B. G3BP is overexpressed in human tumors and promotes S phase entry. Cancer Lett. 2001;162:213–21. doi: 10.1016/s0304-3835(00)00638-8. [DOI] [PubMed] [Google Scholar]
- 41.Winslow S, Leandersson K, Larsson C. Regulation of PMP22 mRNA by G3BP1 affects cell proliferation in breast cancer cells. Mol Cancer. 2013;12:156. doi: 10.1186/1476-4598-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes β-catenin nuclear localization and controls wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–42. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133:340–53. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fagotto F, Glück U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr Biol. 1998;8:181–90. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 45.Bikkavilli RK, Malbon CC. Arginine methylation of G3BP1 in response to Wnt3a regulates β-catenin mRNA. J Cell Sci. 2011;124:2310–20. doi: 10.1242/jcs.084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Säfholm A, Tuomela J, Rosenkvist J, Dejmek J, Härkönen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–63. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- 47.Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA. 2010;107:15473. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Faderl S, Pagel JM, Jung CW, Yoon S-S, Pardanani AD, et al. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. 2020;4:2032–43.. doi: 10.1182/bloodadvances.2019000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlösser A, et al. Small molecule inhibitors of Wnt/β-catenin/Lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia. 2010;12:326–IN6. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ, Lee ES. Wnt/β-Catenin small-molecule inhibitor CWP232228 preferentially inhibits the growth of breast cancer stem-like cells. Cancer Res. 2015;75:1691–702. doi: 10.1158/0008-5472.CAN-14-2041. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy D, French J, Guitard E, Ru K, Tocque B, Mattick J. Characterization of G3BPs: tissue specific expression, chromosomal localisation and rasGAP120 binding studies. J Cell Biochem. 2002;84:173–87. doi: 10.1002/jcb.1277. [DOI] [PubMed] [Google Scholar]
- 52.Nagai K, Oubridge C, Ito N, Avis J, Evans P. The RNP domain: a sequence-specific RNA-binding domain involved in processing and transport of RNA. Trends Biochem Sci. 1995;20:235–40. doi: 10.1016/s0968-0004(00)89024-6. [DOI] [PubMed] [Google Scholar]
- 53.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 54.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–8. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng PW, Chen YY, Cheng WH, Lu PJ, Chen HH, Chen BR, et al. Wnt signaling regulates blood pressure by downregulating a GSK-3β–mediated pathway to enhance insulin signaling in the central nervous system. Diabetes. 2015;64:3413. doi: 10.2337/db14-1439. [DOI] [PubMed] [Google Scholar]
- 56.Wang XY, Zhang XZ, Li F, Ji QR. MiR-128-3p accelerates cardiovascular calcification and insulin resistance through ISL1-dependent Wnt pathway in type 2 diabetes mellitus rats. J Cell Physiol. 2019;234:4997–5010. doi: 10.1002/jcp.27300. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Mizukami Y, Zhang X, Jo WS, Chung DC. Oncogenic K-ras stimulates Wnt signaling in colon cancer through inhibition of GSK-3β. Gastroenterology. 2005;128:1907–18. doi: 10.1053/j.gastro.2005.02.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.