Abstract
Background and aims
The aim of the present study was to assess the risk factor burden and stroke etiology of young stroke patients in Estonia and to compare the results with similar cohorts from other countries.
Methods
This study includes ischemic stroke patients aged 18–54 years from the prospective Estonian Young Stroke Registry between 2013 and 2020. All patients were managed in a stroke unit following a prespecified detailed protocol. Data on stroke risk factors, etiology, and stroke severity were analyzed.
Results
A total of 437 patients (mean age 44.7 ± 8.3 years; 62% males) were included in the registry during the 8-year study period. A total of 50.2% of patients had ≥ 3 well-documented risk factors (higher for men: odds ratio (OR) 3.8; 95% cardiac index confidence interval (CI) 1.8–8.3; p < .001) and 6.2% of patients had ≥ 3 less well-documented risk factors. While 42% of patients had undetermined cause of stroke (34% of them cryptogenic), the second most frequent etiologies were large-artery atherosclerosis and cardioembolism (both 19%). 60 percent of cardioembolic strokes were due to high-risk causes. Large-artery atherosclerosis was more prevalent in men (OR 1.8; 95% CI 1–3.3; p = .05) and among older patients (OR 6.2; 95% CI 1.8–21.4; p = .008). The median National Institutes of Health Stroke Scale score on admission was 3 (interquartile ranges 2–6), stroke was more severe in men (p = .05).
Conclusions
Our study revealed that young patients with stroke in Estonia have higher burden of well-documented risk factors, higher prevalence of high-risk cardioembolic causes and higher prevalence of large-artery stroke compared to other young stroke cohorts.
Keywords: young stroke; stroke etiology; trial of org 10,172 in acute stroke treatment classification; risk factors
Introduction
Stroke in young adults is a rare condition. Despite the growing knowledge, still 20–70% of young patients are diagnosed as cryptogenic strokes.1–9 However, the majority of young stroke patients have well-documented modifiable vascular risk factors explaining about 80% of all strokes.8,10–12 As the epidemiology, etiology, and risk factors differ between the sexes and age-groups across nations and continents, 11 data from all over the world, especially from countries reporting high incidence rates, are needed to ascertain these differences and to find specific solutions to decrease the burden of stroke worldwide.
The incidence of stroke in Estonia has been higher among young and middle-aged population compared to many other European countries13,14 and this trend continues. 15 The aim of the study was to assess the risk factor burden and stroke etiology of young stroke patients in Estonia using prospective design and profound clinical assessment and to compare the results with similar cohorts from other countries.
Patients and methods
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Estonian Young Stroke registry is a prospective ongoing hospital-based registry of all consecutive patients aged 18–54 years hospitalized to Tartu University Hospital, the second largest hospital in Estonia, with discharge diagnosis of acute ischemic stroke since 1 January 2013. From 2013 to 2015 also patients hospitalized to North Estonia Medical Center, the largest hospital in Estonia in Tallinn, were included in the registry. The current analysis includes patients recruited from 1 January 2013 to 31 December 2020.
Ischemic stroke was defined as a focal neurological deficit of acute onset lasting more than 24 h or with evidence of acute brain ischemia on neuroimaging, when symptoms lasted < 24 h. All patients were managed by stroke neurologists and evaluated for etiology following a prespecified detailed protocol (see Supplementary Material 1).
The stroke risk factors were divided into well-documented and less well-documented risk factors. 16
Stroke subtypes were defined according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria 17 and according to The Causative Classification of ischemic stroke (CCS). 18 The definitions of stroke risk factors and criteria for etiologic classification are shown in Supplementary Material 1. Stroke severity was assessed according to the National Institutes of Health Stroke Scale (NIHSS) on admission.
Statistical analysis
Patient characteristics were described by relative frequencies for categorical data and means, standard deviations, medians and interquartile ranges (IQR) for continuous data. Differences in continuous variables were examined using Kruskal–Wallis test and Wilcoxon rank-sum test, categorical data were compared by chi-squared test or by Fishers’s exact test if expected frequencies were < 5. All statistical tests were conducted at a significance level of 0.05. Statistically significant differences were further adjusted to age-group and sex. Adjusted odds ratios with 95% CI were calculated using logistic regression analysis for binary risk factors and multinomial logistic regression for etiology. Adjusted differences in median NIHSS scores were calculated using quantile regression. Statistical analysis was performed in Stata 14.2. This study was approved by the Research Ethics Committee of the University of Tartu (license 302/M-23).
Results
A total of 437 patients (mean age 44.7 ± 8.3 years) were included in the registry, 273 (62%) of them were men. Men were significantly older than women (mean ages 45.8 ± 7.8 years and 43 ± 9.0 years, respectively (p = .001)).
Stroke risk factors
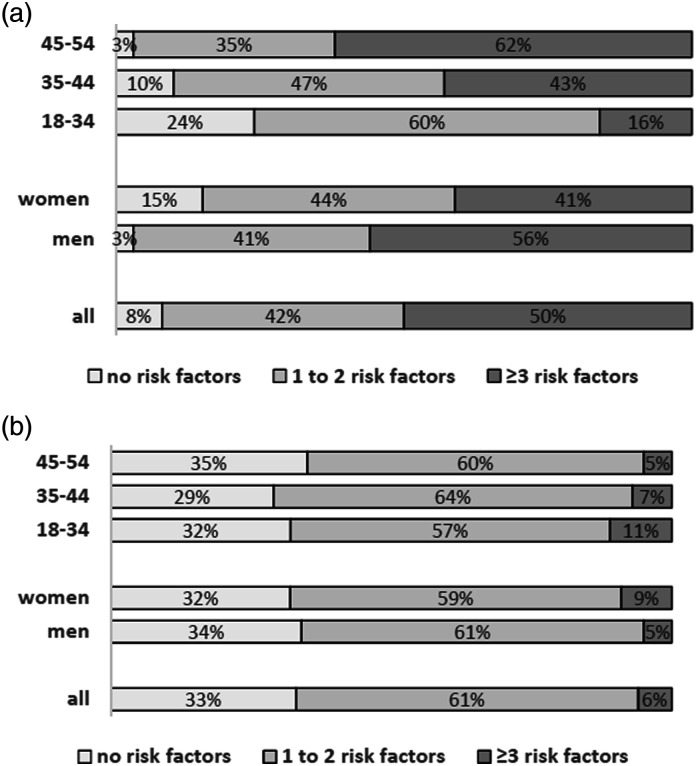
Data on risk factors according to age and sex are shown in Supplementary Table 1 and the burden of risk factors is shown on Figure 1. The overall frequency of well-documented risk factors was higher in men (odds ratio (OR) 3.8; 95% cardiac index confidence interval (CI) 1.8–8.3; p < .001) and in older age-groups compared to the youngest age-group (OR 5.9; 95% CI 2.1–16.5; p = .001 in the age-group 35–44 and OR 21.87; 95% CI 7.8–61.4; p < .001 in the age-group 54–54).
Figure 1.
The burden on well-documented (a) and less well-documented (b) risk factors according to sex and age-group.
The prevalence of most less well-documented risk factors decreased with age, only the prevalence of family history of stroke and chronic heavy drinking increased with age (Supplementary Table 1).
When all risk factors were analyzed together, there were 1.9% of patients with no risk factors and 72.2% of patients ≥ 3 risk factors, while the effect of age remained significant (adjusted OR 8.0; 95% CI 1.7–38.5; p = .01).
Stroke severity
The median NIHSS score on admission was 3 (IQR 2–6) and increased with age: 2 (IQR 1–5) in age-group 18–34, 3 (IQR 1–5.5) in 35–44, and 4 (IQR 2–7.5) in age-group 45–54 (p = .004). Stroke was significantly more severe in men (median 4 (IQR 2–7) vs median three for women (IQR 1–5); p = .05).
Stroke etiology
The stroke etiology according to TOAST and CCS classifications in both sexes and different age-groups is shown in Supplementary Table 1. The overall distribution of the etiological subgroups is comparable in both classification systems. The proportion of patients in stroke of undetermined etiology (UND) group with incomplete investigations was 4.1% for CCS and 5.5% for TOAST. Most of the UND etiology groups were cryptogenic strokes (34% of all strokes). In the cardioembolism (CE) group, there were 49 patients (60%) with high-risk and 33 (40%) with low-risk sources according to the TOAST system (Supplementary Table 2). The low-risk causes were more prevalent in younger age-groups, while high-risk causes dominated in the eldest group. The CCS system has classified more patients into the CE group (22% vs 19% in TOAST) and in addition, it includes separately patients with cryptogenic embolism (5%).
The majority of patients in stroke of other determined etiology (OE) group in the TOAST system had dissection (Supplementary Table 2). Four patients from this group were diagnosed as other etiologies by the CCS system. When different etiological subtypes were adjusted for age and sex, the CCS classification showed that men have more large-artery atherosclerosis (LAA; OR 1.8; 95% CI 1–3.3; p = .05). Also, older patients had more LAA compared to younger patients (OR 6.2; 95% CI 1.8–21.4; p = .004). The effect of age on LAA subtype was also shown with TOAST classification (OR 5.4; 95% CI 1.53–18.4; p = .008).
Discussion
Data from our prospective young stroke registry show that young patients with stroke in Estonia have high burden of well-documented risk factors, high prevalence of high-risk cardioembolic causes, and high prevalence of large-artery stroke. We also found that men have significantly more severe strokes and higher risk factor burden compared to women.
Previous young stroke studies have used different age cut-off and thus affect comparisons. The Swiss stroke study and the SIFAP1 study included patients up to 55 years of age, other studies have included patients up to 49–50 years of age 1,4–5,9,19–20 or up to 45 years of age.6–7,21 In addition, most of the published cohorts have been evaluated 10 or more years ago,1,4–7,21,22 some of them started recruitment already in the 1990s when the standards for diagnostic evaluation were somewhat different. Most of the young stroke cohorts demonstrate male predominance, only the German cohort, the American registry, and the FUTURE study have shown equal distribution of sexes or female predominance.1,6,21 In our study, the youngest age-group demonstrated female- and the older age-groups male predominance.
Although it has been known that younger patients have different stroke risk factors compared to older subjects, the recent studies have shown that the proportion of traditional risk factors is increasing among younger patients.8,10-12 The prevalence of hypertension, atrial fibrillation (AF), and dyslipidemia in Estonia are the highest compared to other studies. To some extent, this can be explained by age-difference (most of our AF cases were in the oldest age-group), but probably not thoroughly. The criteria for dyslipidemia vary across studies and very often the criteria for the definition are not provided. Most of the studies use quite strict limits for lipid blood values like we did,2,4,7,20–21 one Italian study used higher cut-off. 5
The proportion of cigarette smokers was also high in our sample, but has been even higher in the SIFAP1 study and in the German and the Greek cohorts reaching up to 59%6–7,22 It is important to note, that the age-adjusted prevalence of cigarette smoking in Estonia is 23%, 23 while in the young stroke cohort it is 55%.
The role of obesity in overall stroke risk has probably been underestimated, but there are some young stroke studies providing these data.4,6,9,20,22 Most of the studies report quite low percentage of obese patients (8–22%), but surprisingly in our cohort it reached 35%. One explanation for this might again be the age-difference between studies. The prevalence of obesity in Estonia is increasing, but in the age-adjusted Estonian population the proportion of obese subjects is twice smaller than in the young stroke cohort. 24
One-third of our patients had stroke family history. The Italian cohort reported family history in 28% of patients, 20 the SIFAP1 study reported it to be as high as 37%. 25 We accounted only first degree relatives with stroke and therefore the proportion could have been higher if we had included all relatives with history of stroke.
Compared to other studies, the proportion of patients with three or more well-documented risk factors was higher for patients aged 35 to 54 in our cohort (43–62% compared to 33–48% in SIFAP1 study and 22–51% in the Swiss Stroke Study).2,22 The Helsinki cohort is somewhat younger and the proportion of patients with the highest risk factor burden is also lower (33%) 26 compared to our study (48%). We demonstrate a sharp increase in traditional risk factors in early 30s when, for example, the proportion of hypertension, dyslipidemia, and smoking are more than twice as high as in late 20 s.
Both the risk factor burden and the etiologic classification of stroke are dependent on the amount of investigations provided in each patient. Different studies have used different criteria for “complete investigations.” Studies do not always provide data on how many patients received all the investigations described. Our prospective sample has reached very high numbers of thoroughly investigated patients, including 24-h cardiac rhythm Holter monitoring which is not always accounted as essential in young stroke studies.
The TOAST classification has been the main tool defining stroke etiology in most of the studies, we have chosen to use also the CCS classification which is more profound. These classification systems are somewhat different with CCS comprising more detailed data on investigations done and providing a more detailed insight to the UND subgroup. To our knowledge, there is only one retrospective study on young stroke patients using the CCS classification algorithm 27 ; therefore, from here on, we compare our results on stroke etiology with studies providing subtyping by TOAST criteria.
The proportion of LAA subgroup (19%) is one of the highest compared to previous studies, only the FUTURE study has reported higher values (26%). 1 Also, increase by 4% since our previous study is noted. 8 One explanation for this could be the high burden of modifiable risk factors in our cohort. Surprisingly, in the Greek study where the proportion of cigarette smokers and dyslipidemia was quite high, the LAA etiologic subgroup is only modest (7%). 7 Low proportion of LAA subgroups is also seen in the Helsinki (8%), the Norwegian (3%), and the American registry (2%).4,19,21 As expected, the proportion of LAA subtype increased with age and was more prevalent among men as shown by several previous studies.3,4,7,28
Small-artery occlusion (SAO) is relatively uniquely represented across studies (7–17%).1–7,19,21,28 As hypertension and diabetes are the main causes of lacunar strokes, it would be expected that also higher burden of these risk factors is present in the cohorts of higher proportion of SAO. However, quite opposite is reported by several of these studies.5,7,19 The diagnosis of SAO might also be controversial: many of our patients in this subgroup had lacunar strokes on imaging, but no other signs of small vessel disease and some of them did not have expected risk factors. Therefore, those patients may have cryptogenic stroke instead, but the classification system puts them into SAO category both in TOAST as well as in CCS classifications.
Our cohort has the highest rate of high-risk cardioembolic causes with AF comprising 37%, while in other studies it is 10–15%.2–4,6–7,20,28 Estonian patients are somewhat older and that might explain the difference, however, we have also reported before that the prevalence of AF among Estonian stroke cohort is higher compared to many other countries.8,14 Another important factor is that most of the patients in our cohort had 24-h cardiac rhythm Holter monitoring and thus the probability of diagnosing AF was high. In young stroke cohorts, the main source of cardioembolic stroke is patent foramen ovale (PFO), comprising 8–49% of all strokes and 44–76% of cardioembolic subtype.3,6–7,20–21,28 In our study, 12% of all strokes and 40% of cardioembolic subtype had PFO despite high rate of transesophageal echocardiography. The OE subgroup has always been of special interest in young stroke studies and usually accounts for 13–34% of all strokes,1-7,19–21,28 but was only 9% in our study despite extensive investigations. It is widely known that arterial dissections are the most frequent specific cause of young stroke and this was also confirmed in our sample (45% of dissections in OE subgroup); however, other studies often report much higher proportions ranging from 25 to 80% of the OE subgroup.2–4,6–7,21,28 As all of our patients were evaluated using either carotid ultrasonography or carotid angiography, it is very unlikely that we have missed many of these cases and the reason for low proportion of dissections remains unsolved.
The OE subgroup is also dependent on the methodology of the study, for example, we did not account single gene mutations or blood analyses’ deviations as OE but rather as UND. There was a relatively high proportion of patients with active cancer (15%) among the OE subtype in our cohort. It is important to mention that despite high risk–factor burden, the etiology of stroke remained unknown for as many as 34% of patients. This indicates that there is much unknown about the etiology and causes of stroke in the young and continuous efforts should be made to reduce this gap in knowledge.
The severity of stroke has not been studied in many young stroke cohorts, but available data indicate that median NIHSS score on admission is uniquely three points2,6,9,26 and was the same in our study. Interestingly, the stroke was significantly more severe among men which could be derived from the high-risk factor burden, higher prevalence of cardioembolic stroke and stroke due to large-artery atherosclerosis.
The strength of our study is the prospective design and high proportion of thoroughly evaluated patients using profound study protocol. Involving patients up to 54 years of age can also be considered important as these are working-age people and many of the classical stroke risks become important at this age.
The main limitation of our study is hospital-based rather than population-based design. This is mostly associated with the fact that stroke in the young is a rare condition and using population-based design the sample size would have been very small. As Estonia is a small country, we cannot expect large numbers of young stroke patients. Unfortunately, we did not have the possibility to collect data from both largest hospitals in Estonia for the whole study period of 8 years, which could have increased the sample size. However, we included all consecutive patients during that period and the phenotypes of our patients are well described and thoroughly studied.
Summary
The results of our study point out that although we should search for rare causes of stroke in the young, much can be done regarding modifiable risk factors and primary prevention to reduce the impact of stroke among the young. This is also one of the goals of the Stroke Action Plan in Europe 29 and the specific target of young population should be the priority.
Despite the high burden of risk factors, one-third of patients are diagnosed with cryptogenic stroke. There is a need for further studies especially in this area in addition to other continuous efforts in unraveling the causes of stroke in the young and every contribution to this field is invaluable.
Supplemental Material
Supplemental Material, sj-pdf-1-eso-10.1177_23969873211040990 for Estonian young stroke registry: High burden of risk factors and high prevalence of cardiomebolic and large-artery stroke by Riina Vibo, Siim Schneider, Liisa Kõrv, Sandra Mallene, Liisi-Anette Torop and Janika Kõrv in European Stroke Journal
Acknowledgements
We would like to thank Karolin Toompere for statistical analysis and assistance in this research.
Author contributions: JK, RV and SS researched literature and conceived the study and were involved in protocol development, gaining ethical approval, patient recruitment and data analysis. LAT, LK, SM and SS managed data collection. RV wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Riina Vibo https://orcid.org/0000-0001-9138-3227
Janika Kõrv https://orcid.org/0000-0002-6074-0727
References
- 1.van Alebeek ME, Arntz RM, Ekker MS, et al. Risk factors and mechanisms of stroke in young adults: the FUTURE study. J Cereb Blood Flow Metab 2018; 38: 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goeggel SB, Mono ML, Huynh-Do U, et al. Risk factors, aetiology and outcome of ischaemic stroke in young adults: the swiss young stroke study (SYSS). J Neurol 2015; 262: 2025–2032. [DOI] [PubMed] [Google Scholar]
- 3.Rolfs A, Fazekas F, Grittner U, et al. Acute cerebrovascular disease in the young: the stroke in young fabry patients study. Stroke 2013; 44: 340–349. [DOI] [PubMed] [Google Scholar]
- 4.Putaala J, Metso AJ, Metso T M, et al. Analysis of 1008 Consecutive Patients Aged 15 to 49 With first-ever ischemic stroke. Stroke 2009; 40: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 5.Cerrato P, Grasso M, Imperiale D, et al. Stroke in young patients: etiopathogenesis and risk factors in different age classes. Cerebrovasc Dis 2004; 18: 154–159. [DOI] [PubMed] [Google Scholar]
- 6.Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol 2012; 259: 653–659. [DOI] [PubMed] [Google Scholar]
- 7.Spengos K, Vemmos K. Risk factors, etiology, and outcome of first-ever ischemic stroke in young adults aged 15 to 45 - the Athens young stroke registry. Eur J Neurol 2010; 17: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 8.Schneider S, Kornejeva A, Vibo R, et al. Risk factors and etiology of young ischemic stroke patients in estonia. Stroke Res Treat 2017; 2017: 8075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divisova P, sanak D, Kral M, et al. Young cryptogenic ischemic stroke: a descriptive analysis of clinical and laboratory characteristics, outcomes and stroke recurrence. J Stroke Cerebrovasc Dis 2020; 29: 105046. [DOI] [PubMed] [Google Scholar]
- 10.Aigner A, Grittner U, Rolfs A, et al. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke 2017; 48: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 11.Boot E, Ekker MS, Putaala J, et al. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry 2020; 91: 411–417. [DOI] [PubMed] [Google Scholar]
- 12.Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, et al. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014; 10: 315–325. [DOI] [PubMed] [Google Scholar]
- 13.Kõrv J, Roose M, Kaasik AE. Stroke registry of Tartu, Estonia, from 1991 through 1993. Cerebrovasc Dis 1997; 7: 154–162. [Google Scholar]
- 14.Vibo R, Kõrv J, Roose M. The third stroke registry in Tartu, Estonia, from 2001 to 2003. Acta Neurol Scand 2007; 116: 31–36. [DOI] [PubMed] [Google Scholar]
- 15.Korv Kõrv L, Mallene S, Vibo R, et al. High incidence of stroke in young adults in Tartu, Estonia, 2013-2017: a prospective population-based study. Eur J Neurol 2021: 1984–1991. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke. Stroke 2006; 37: 1583–1633. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 18.Ay H, Benner T, Murat Arsava E, et al. A computerized algorithm for etiologic classification of ischemic stroke. Stroke 2007; 38: 2979–2984. [DOI] [PubMed] [Google Scholar]
- 19.Nacu A, Fromm A, Sand K M, et al. Age dependency of ischaemic stroke subtypes and vascular risk factors in western Norway: the bergen norwegian stroke cooperation study. Acta Neurol Scand 2016; 133: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renna R, Pilato F, Profice P, et al. Risk factor and etiology analysis of ischemic stroke in young adult patients. J Stroke Cerebrovasc Dis 2014; 23: e221–e227. [DOI] [PubMed] [Google Scholar]
- 21.Ji R, Schwamm LH, Pervez MA, et al. Ischemic stroke and transient ischemic attack in young adults. JAMA Neurol 2013; 70: 51–57. [DOI] [PubMed] [Google Scholar]
- 22.Von Sarnowski B, Putaala J, Grittner U, et al. Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the stroke in young fabry patients study. Stroke 2013; 44: 119–125. [DOI] [PubMed] [Google Scholar]
- 23.Reile R, Tekkel M, Veideman T. Milline on eesti inimeste tervis? Ülevaade täiskasvanud rahvastiku tervisekäitumise uuringu 2018. Aasta tulemustest. Eesti Arst 2019; 98: 377–380. [Google Scholar]
- 24.Reile R, Baburin A, Veideman T, et al. Long-term trends in the body mass index and obesity risk in Estonia: an age-period-cohort approach. Int J Public Health 2020; 65: 859–869. [DOI] [PubMed] [Google Scholar]
- 25.Thijs V, Grittner U, Dichgans M, et al. Family history in young patients with stroke. Stroke 2015; 46: 1975–1978. [DOI] [PubMed] [Google Scholar]
- 26.Putaala J, Haapaniemi E, Kaste M, et al. How does number of risk factors affect prognosis in young patients with ischemic stroke? Stroke 2012; 43: 356–361. [DOI] [PubMed] [Google Scholar]
- 27.Gokcal E, Niftaliyev E, Asil T. Etiological classification of ischemic stroke in young patients: a comparative study of TOAST, CCS, and ASCO. Acta Neurol Belgica 2017; 117: 643–648. [DOI] [PubMed] [Google Scholar]
- 28.Yesilot Barlas N, Putaala J, Waje-Andreassen U, et al. Etiology of first-ever ischaemic stroke in European young adults: The 15 cities young stroke study. Eur J Neurol 2013; 20: 1431–1439. [DOI] [PubMed] [Google Scholar]
- 29.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J 2018; 3(4): 309–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-eso-10.1177_23969873211040990 for Estonian young stroke registry: High burden of risk factors and high prevalence of cardiomebolic and large-artery stroke by Riina Vibo, Siim Schneider, Liisa Kõrv, Sandra Mallene, Liisi-Anette Torop and Janika Kõrv in European Stroke Journal