Abstract
Background
Optimal blood pressure is not well established during endovascular therapy of acute ischemic stroke. Applying standardized blood pressure target values for every stroke patient might be a suboptimal approach.
Aim
To assess whether an individualized intraprocedural blood pressure management with individualized blood pressure target ranges might pose a better strategy for the outcome of the patients than standardized blood pressure targets.
Sample size: Randomization of 250 patients 1:1 to receive either standard or individualized blood pressure management approach.
Methods and design
We conduct an explorative single-center randomized controlled trial with a PROBE (parallel-group, open-label randomized controlled trial with blinded endpoint evaluation) design. In the control group, intraprocedural systolic blood pressure target range is 140–180 mmHg. The intervention group is the individualized approach, which is maintaining the intraprocedural systolic blood pressure at the level on presentation (±10 mmHg).
Study outcomes: The primary endpoint is the modified Rankin scale assessed 90 days +/− 2 weeks after stroke onset, dichotomized by 0–2 (favorable outcome) to 3–6 (unfavorable outcome). Secondary endpoints include early neurological improvement, infarction size, and systemic physiology monitor parameters.
Discussion
An individualized approach for blood pressure management during thrombectomy could lead to a better outcome for stroke patients. The trial is registered at clinicaltrials.gov as ‘Individualized Blood Pressure Management During Endovascular Stroke Treatment (INDIVIDUATE)’ under NCT04578288.
Keywords: Ischemic stroke, endovascular therapy, blood pressure
Introduction and rationale
Optimal blood pressure (BP) management during acute endovascular treatment (EVT) for acute ischemic stroke is not well established. Current international guidelines recommend maintaining the systolic blood pressure (SBP) under 180–185 mmHg and over 140 mmHg, as well as avoiding excessive BP drops during thrombectomy with low to moderate level of evidence.1–4 Extreme hypo- as well as hypertensive blood pressures during an acute ischemic stroke may have a harmful influence with a U-shaped relationship between blood pressure and functional outcome.5–14
Substantial decreases of BP during the endovascular procedure are associated with worse functional outcome as a decrease in systemic blood pressure might lead to to larger final infarction sizes.15–18
The Society for Neuroscience in Anesthesiology and Critical Care Expert recommend maintaining SBP >140 mmHg with moderate level of evidence during EVT, based on retrospective data. 3 However, one study suggested that intraprocedural SBP between 100–140 mmHg was not resulting in different functional outcomes and only values <100 mmHg had fewer patients with good functional outcome. 19 Additionally, a post-hoc analysis of the EVT trial (MR CLEAN) showed that 16.2% patients had an SBP of <120 mmHg on presentation and there was no significantly different functional outcome than those who had an SBP >120 mmHg. 9
Current guidelines suggest, that in patients who are eligible for IV thrombolytic and endovascular therapy, BP should be lowered to <185/110 mmHg before treatment and to <180/105 mmHg after treatment with low to moderate evidence. 20 However, in patients with a BP of <220/110 mmHg who did not receive reperfusion therapy (i.e. IV fibrinolytic therapy and/or endovascular thrombectomy) initiating or reinitiating antihypertensive medication is not effective to prevent death or dependency with level A evidence. 20
In summary, there is evidence for association of worse functional outcome for extremes of blood pressure levels at presentation. For intraprocedural intra-individual blood pressure variation the evidence is largely limited for blood pressure drops, while some evidence14,21 also showed negative effects of prolonged high blood pressures. As there are considerable inter-individual differences of necessary systemic blood pressure levels to maintain a sufficient penumbral perfusion, managing blood pressure via absolute targets independent of the individual needs might be a suboptimal approach. Lower BP than necessary might lead to reduced penumbral hypoperfusion and thus larger infarction, higher values might be associated with adverse effects like edema and hemorrhage. The admission blood pressure might represent the lowest necessary compensatory blood pressure to maintain penumbral perfusion. Thus, it could be reasonable to maintain intraprocedural systolic blood pressure before reperfusion at the presentation level, if higher and lower bounds for extreme values are established.
Methods
Design
INDIVIDUATE (NCT04578288) is an exploratory single-center, prospective, parallel-group, open-labeled randomized controlled trial with blinded endpoint evaluation (PROBE). We plan to enroll 250 patients in two years. Stroke patients with vessel occlusion of the anterior circulation undergoing endovascular treatment are eligible, if they have an National Institute of Health Stroke Scale (NIHSS) ≥8 and are stable enough for procedural sedation. The exclusion criteria hemodynamic instability comprise e.g. need for continuous bolus of high doses of vasopressors or arrhythmias with severe blood pressure instability before endovascular treatment, which would compromise patient safety. Exclusion of these patients will ultimately be at the discretion of the treating physician.
Patient consent is obtained before randomization if they are capable of giving informed consent. If they are incapacitated, their legal representative is consulted. If neither is possible, a deferred consent will be obtained after the procedure within 72 h. Data will be retained, if they die during that time frame or there is a decision for transition to palliative care and withdrawal/withholding of further therapies. The intervention is maintaining the intraprocedural SBP at presentation level, the comparator is maintaining intraprocedural SBP between 140–180 mmHg. Patients are randomized 1:1. The primary endpoint modified Rankin Scale (mRS) at 3 months will be obtained via telephone interview in a blinded fashion. Secondary outcomes will be recorded during the hospital stay.
The study was approved by the local institutional review board (Ethikkommission Medizinische Fakultät Heidelberg, ID S-511/2020).
Inclusion and exclusion criteria
Inclusion criteria
Participants have to meet all of the following criteria to be considered for inclusion in the trial:
Decision for thrombectomy according to local protocol for acute recanalizing stroke treatment
Age 18 years or older, either sex
National Institutes of Health Stroke Scale (NIHSS) ≥8
Acute ischemic stroke in the anterior circulation with isolated or combined occlusion of: Internal carotid artery (ICA) and/or middle cerebral artery (MCA)
Informed consent by the patient him-/herself or his/her legal representative obtainable within 72 h of treatment
Exclusion criteria
Subjects presenting with any of the following criteria will not be included in the trial:
Intracerebral hemorrhage
Coma on admission (Glasgow Coma Scale ≤8)
Severe respiratory instability, loss of airway protective reflexes or vomiting on admission, where primary intubation and general anesthesia is deemed necessary
Intubated state before randomization
Severe hemodynamic instability (e.g. due to decompensated heart insufficiency)
Randomization
To achieve comparable intervention groups, patients will be allocated in a concealed fashion in a 1:1 ratio by means of randomization using concealed envelopes, which are used in a sequential order. The blocked randomization list will be created using the service of Sealed Envelope™. 22
Treatment or intervention
The comparator standard blood pressure management is maintenance of intraprocedural pre-recanalization SBP between 140–180 mmHg for all patients who receive endovascular thrombectomy for acute ischemic stroke in anterior circulation. The intraprocedural pre-recanalization time frame begins with the groin puncture and the last thrombectomy attempt leading to the final reperfusion result.
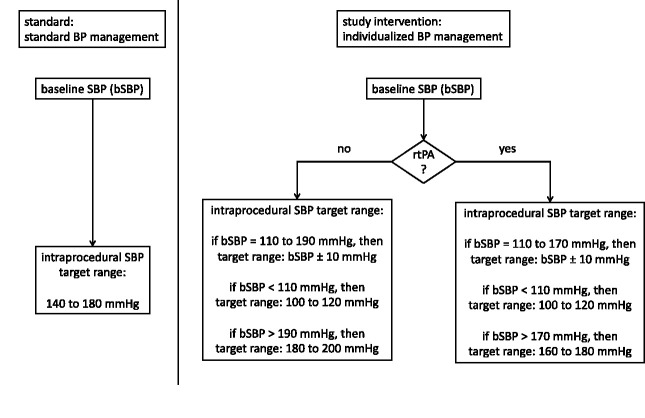
The study intervention would be maintaining the intraprocedural pre-recanalization blood pressure in individualized SBP target ranges depending on the systolic blood pressure of the patient on presentation (=baseline SBP or bSBP). The presentation SBP is defined as the first measured value in the emergency room or in the angiography suite, depending on where the first BP is measured. The individual target range is defined as: bSBP ± 10 mmHg. The lowest possible SBP target range is 100–120 mmHg. The highest SBP target range is determined on the basis of whether patients receive concurrent IV fibrinolytic therapy or not. In patients where IV fibrinolytic therapy is applied, the highest SBP target range is 160–180 mmHg, in patients without concurrent fibrinolytic therapy the highest SBP target range is 180–200 mmHg (see Figure 1). Augmenting the blood pressure will be achieved with crystalloid fluid infusion and additionally via norepinephrine with a perfusor therapy. The main specific antihypertensive drug will be urapidil via bolus and/or continuous infusion.
Figure 1.
Schema of standard vs. individualized blood pressure management. BP: baseline blood pressure; SBP: systolic blood pressure; bSBP: baseline systolic blood pressure; rtPA: recombinant tissue plasminogen activator.
In the setting where emergent endotracheal intubation is deemed necessary after study inclusion, aforementioned respective blood pressure targets are maintained for each treatment arm during general anesthesia.
Primary outcomes
In patients who receive endovascular thrombectomy for acute ischemic stroke in anterior circulation (according to inclusion and exclusion criteria), the primary objective is the difference in rates of favorable functional outcome 90 days after stroke onset [measured by modified Rankin scale (mRS) assessed 90 days ±2 weeks after onset, dichotomized 0–2 (favorable outcome) to 3–6 (unfavorable outcome)] between individual BP management compared with standard BP management, regardless of treatment discontinuation. Modified RS is assessed by telephone interview and by a rater blinded to the treatment arm.
Secondary outcomes
Early neurological improvement indicated by change of NIHSS 24 hours after admission [NIHSS on admission – NIHSS after 24 h], after 72 h [NIHSS on admission – NIHSS after 72 h] and at discharge [NIHSS on admission – NIHSS at discharge]
Infarction size, determined with MRI or CT scan on a post-interventional follow up scan 12–36h after EVT [mL]
Time of intraprocedural SBP in target range [percentage of time in target range between groin puncture and reperfusion]
Time of intraprocedural SBP spent in target range ± 10 mmHg [percentage of time in range between groin puncture and reperfusion]
Systemic physiology monitor parameters: means, minimal, maximal values of SBP (mmHg), DBP (mmHg), Blood pressure variability, HR (/min), SaO2 (%), etCO2 (mmHg)
Degree of recanalization [modified Thrombolysis in Cerebral Infarction Scale (mTICI)]
Number of EVT attempts
- Times
- Door-to-groin puncture time [time from admission to groin puncture, min]
- Door-to-recanalization time [time from groin puncture to the last thrombectomy attempt leading to the final reperfusion result, min]
- Duration of EVT [from groin puncture to last thrombectomy attempt leading to the final reperfusion result, min]
- Length of stay in hospital [days from admission to discharge]
- Safety endpoints
- e. Critical hyper- or hypotension (SBP >210 mmHg in the population without fibrinolytic therapy and SBP >190 mmHg with fibrinolytic therapy or <90 mmHg) [yes/no],
- f. Post-interventional (symptomatic) intracerebral hemorrhage (using the Heidelberg Bleeding Classification) [yes/no]
- g. Intrahospital mortality [yes/no, cause of death]
- h. Mortality 3 months after onset [yes/no, cause of death]
Data monitoring board
There is no external Data Monitoring Board and data validation aspects (control of completeness, consistency and plausibility of data) is at the responsibility of the principal investigators (S. Schönenberger and M. Chen). The PI will have access to the final trial dataset. Data management, validation, supervising procedures will be performed by the PI according to SOPs, to ICH-GCP guidelines and the declaration of Helsinki in their recent versions.
Sample size
A sample size of 250 patients will be used for analysis which will be randomized with an allocation ratio of 1:1 to intervention and control group (125 per group). Since this is an exploratory study and there is no information about a potential treatment effect available, a formal sample size calculation based on a confirmatory hypothesis testing approach is not feasible. Instead, we base the sample size on the degree of evidence that can be achieved in this trial in terms of the width of the 95% confidence interval for the favourable outcome rate difference between the two groups and the feasibility of recruitment where we estimate to achieve the numbers of patients in two years. Assuming a proportion of 0.3 patients resulting in favorable outcomes in the control group and using the additional assumption of 125 patients per group, the maximal 95% confidence interval (Wilson Score Interval) width for the difference in proportions (between intervention and control group) will be 0.235. The calculation was done using PASS version 16.0.3.
Statistical analyses
All endpoints and baseline variables are descriptively summarized using mean and standard deviation, as well as median, interquartile range, minimum and maximum for continuous variables, and absolute and relative frequencies for categorical variables.
Missing values are documented per variable as absolute frequencies.
The primary endpoint mRS after 3 months (dichotomized 0–2 versus 3–6) will be evaluated using a logistic regression model including group, premorbid mRS, and NIHSS at baseline as covariates. Odds ratios (OR) and the corresponding 95% confidence intervals, as well as p-values will be reported. The full analysis set (FAS), which is used as primary analysis set, is based on the intention-to-treat principle and including all randomized patients fulfils these requirements. Additionally, the primary outcome will be further evaluated based on the per protocol set. Multiple imputation via predictive mean matching, 23 will be used to deal with missing mRS values. Sensitivity analyses will be performed by applying alternative methods dealing with missing data such as, e.g. complete case analysis and replacement by ICA-r (independent component analysis). 24 Furthermore, multivariable ordinal and binary logistic regression models with elastic net penalty for mRS after 3 months will be performed to identify clinical variables influencing the outcome, if applicable. Secondary outcomes will be evaluated by applying linear or (binary/ordinal) logistic regression methods as appropriate and adjustment will be done according to the primary outcome model. For safety analysis patients are analyzed as-treated. Safety analysis will include the rates of complications (e.g. death, intracerebral hemorrhage) which will be calculated and compared by using a Boschloo’s test which will be based on all randomized patients who were treated with the interventions under investigation.
Exploratory analyses will be performed to identify subgroups and potential moderator variables of patients profiting distinctly from the individual BP management. This will be done by binary logistic regression models including interaction terms between intervention and baseline BP, age, NIHSS, vessel occlusion, pre-morbid mRS, ASPECTS, reperfusion status (mTICI), and thrombolytic therapy respectively.
Additionally, the following subgroups will descriptively be analyzed and compared for the primary endpoint:
NIHSS at admission (8–15, 15–20, >20)
BP strata (SBP targets 100–140 mmHg, 140–160 mmHg, 160–200 mmHg)
Age (<50 y, 50–70 y, >70 y)
Sex
Pre-morbid mRS (0–2, 3–6)
ASPECTS (<4, 5–7, 8–10)
mTICI (0–2a, 2b–3)
Intravenous thrombolytic therapy (yes or no)
Emergent intubation (yes or no)
Localization of occlusion (ICA, M1, M2, ICA + M1/M2, other)
Time since last-seen-well (<12 h and >12 h until admission)
Collateral status (Tan scale: 0–1, 2–3)
Since this is an exploratory data analysis all p-values are of descriptive nature for which there is no accounting for multiplicity. Furthermore, statistical methods are used to assess the quality of data and the homogeneity of intervention groups. Further details of the analysis will be included in the statistical analysis plan (SAP) which will be finalized before data base closure. Statistical analysis will be performed using R version 4.0.2 (or higher).
Study organization and funding
There is no external steering committee for this single-center trial. The PI are responsible for development of the trial protocol, approval of the trial protocol by legal authorities and ethics committees, design of consent forms and obtaining informed consent from the patients/their legal representatives, design of the CRF, organization of a randomization system, any decisions on changes, amendments, communication with the local ethics committee or interruption of the trial. They also supervise the trial conduction. There is no external funding for this trial.
Discussion
The available evidence and pathophysiological considerations argue for an individual approach to manage intraprocedural blood pressure during endovascular stroke treatment. To our knowledge there is one comparable ongoing RCT at the moment: Maier et al. plan to investigate an individualized BP management approach, where the study intervention will be to maintain intraprocedural MAP within ±10% from the first measured value in the angiography suite in a multicenter setting (NCT04352296) in France. Their control is maintaining SBP between 140–180 mmHg.
Because we do not have information about effect sizes due to our novel approach, defining an adequate sample size to confirm superiority is not possible. Thus, our study will be exploratory with the aim to obtain effect sizes, confidence intervals and investigate the feasibility to pursue such highly individualized approach. With this study, we hope to generate data for future confirmatory multicenter trials.
We decide to only include moderately to severely afflicted stroke patients (i.e. NIHSS ≥ 8) as there is evidence that BP drops have a larger effect on functional outcome in more severely afflicted patients. 18
We are aware that the target range for the individualized strategy is very narrow and time of BP spent in treatment range will likely not cover the entire endovascular procedure. However, due to lower intervention thresholds to start antihypertensive or vasopressor therapy the subsequent higher impetus of earlier countermeasures for blood pressure outliers might be a sufficient measure to lead to relevant changes in outcome.
Furthermore, we choose to investigate our intervention strategy in primary procedurally sedated (with monitored anesthesia care) patients, because (1) we follow a primary procedural sedation regimen in our center, (2) we aim to establish a relatively homogeneous study cohort in terms of anesthesia mode and (3) to avoid any confounding influence of primary general anesthesia. We will still include patients, which are emergently converted from PS to GA and further sensitivity analysis will be performed in this subgroup.
We expect a small effect on the functional outcome, as our intervention (i.e. individualized blood pressure manipulation) will be of relatively short duration and other factors are more impactful on outcome. However, altering BP is a relatively non-invasive intervention and can be regarded as a neuroprotective measure during the critical time frame of the hypoperfused state of the ischemic brain. Thus, even if the number needed to treat to obtain a better functional outcome is high, it would still constitute a feasible and convenient strategy.
Results of INDIVIDUATE will provide further evidence regarding the influence of blood pressure during EVT and contribute to the understanding and improvement of peri-interventional stroke management.
Acknowledgements
None.
Footnotes
Trial registration: ClinicalTrials.gov Identifier: NCT04578288.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Peter Ringleb received honoraria for advisory boards and lecture fees from Boehringer Ingelheim, Bayer, Pfizer and Daichii Sankyo, not related to the topic of the manuscript. Markus A Möhlenbruch received honoraria for lectures including service on speakers bureaus from Medtronic, MicroVention, Stryker (money paid to the institution) and has grants/grants pending from Balt, Medtronic and MicroVention, not related to the topic of the manuscript. Simon Nagel consults Brainomix, has a grant from Cerenovus and received payment for lectures including service on Speakers Bureaus from Pfizer and Boehringer Ingelheim, not related to the topic of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent will be obtained from all subjects or their legal representatives during our trial.
Ethical approval: Ethical approval for this study was obtained from Ethikkommission Medizinische Fakultät Heidelberg, ID S-511/2020.
Guarantor: MC.
Contributorship: MC and SS researched literature and conceived the study. MC, DK, PR, SN and SS were involved in protocol development. MC, WW, MB, MK and SS were involved in gaining ethical approval. MC and SS are involved in patient recruitment. MC, DK and SS were involved in statistical planning. MC and DK wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs
Min Chen https://orcid.org/0000-0001-9079-9298
Meinhard Kieser https://orcid.org/0000-0003-2402-4333
References
- 1.Fiehler J, Cognard C, Gallitelli M, et al. European Recommendations on Organisation of Interventional Care in Acute Stroke (EROICAS). Int J Stroke 2016; 11: 701–716. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al.; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Talke PO, Sharma D, Heyer EJ, et al. Society for neuroscience in anesthesiology and critical care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke*: endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J Neurosurg Anesthesiol 2014; 26: 95–108. [DOI] [PubMed] [Google Scholar]
- 4.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. Epub ahead of print 2019. [DOI] [PubMed]
- 5.Fischer U, Cooney MT, Bull LM, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: a population-based study. Lancet Neurol 2014; 13: 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maïer B, Gory B, Taylor G, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J Am Heart Assoc 2017; 6: e006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi-Bee J, Bath PMW, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 8.Goyal N, Tsivgoulis G, Iftikhar S, et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J Neurointerv Surg 2017; 9: 451–454. [DOI] [PubMed] [Google Scholar]
- 9.Mulder MJHL, Ergezen S, Lingsma HF, et al. Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands (MR CLEAN) Investigators. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in The Netherlands). Stroke 2017; 48: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra K, Goyal N, Katsanos AH, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension 2020; 75: 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matusevicius M, Cooray C, Bottai M, et al. Blood pressure after endovascular thrombectomy: modeling for outcomes based on recanalization status. Stroke 2020; 51: 519–525. [DOI] [PubMed] [Google Scholar]
- 12.Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 2010; 41: 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebeskind DS, Jahan R, Nogueira RG, et al. Impact of collaterals on successful revascularization in solitaire FR with the intention for thrombectomy. Stroke 2014; 45: 2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen M, Schönenberger S, Hendèn PL, for the SAGA orators et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke. JAMA Neurol 2020; 77: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treurniet KM, Berkhemer OA, Immink RV, et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg 2018; 10: 107–111. [DOI] [PubMed]
- 16.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke 2015; 46: 2678–2680. [DOI] [PubMed] [Google Scholar]
- 17.Petersen NH, Ortega-Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke 2019; 50: 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whalin MK, Halenda KM, Haussen DC, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol 2017; 38: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Athiraman U, Sultan-Qurraie A, Nair B, et al. Endovascular treatment of acute ischemic stroke under general anesthesia: predictors of good outcome. J Neurosurg Anesthesiol 2018; 30: 223–230. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344. [DOI] [PubMed] [Google Scholar]
- 21.John S, Hazaa W, Uchino K, et al. Lower intraprocedural systolic blood pressure predicts good outcome in patients undergoing endovascular therapy for acute ischemic stroke. Interv Neurol 2016; 4: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sel 2019. Create a blocked randomisation list, https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed 23 April 2020).
- 23.Schenker N, Taylor JMG. Partially parametric techniques for multiple imputation. Comput Stat Data Anal 1996; 22: 425–446. [Google Scholar]
- 24.Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials 2008; 5: 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]