Abstract
Purpose
To compare the diagnostic performance of 68Ga-PSMA-11 PET/CT and mpMRI for pelvic lymph node staging prior to radical prostatectomy in prostate cancer (PCa) patients based on per patient data.
Methods
PubMed and Embase databases were searched until October 2020 for eligible studies evaluating head-to-head comparison of 68Ga-PSMA-PET/CT and mpMRI for the detection of pelvic lymph node metastases (PLNMs) using pelvic lymph node dissection (PLND) as gold standard. The pooled sensitivity, specificity, and area under the summary receiver-operating characteristics curve (AUC) were determined for the two imaging modalities.
Results
Nine studies with 640 patients were included. The pooled sensitivity, specificity, and AUC for 68Ga-PSMA-11 PET/CT vs. mpMRI were 0.71 (95% CI: 0.48–0.86) vs. 0.40 (95% CI: 0.16–0.71), 0.92 (95% CI: 0.88–0.95) vs. 0.92 (95% CI: 0.80–0.97), and 0.92 (95% CI: 0.88–0.95) vs. 0.82 (95% CI: 0.79–0.86), respectively. There was substantial heterogeneity for both imaging modalities, and meta-regression analysis revealed that the number of patients, prevalence of PLNMs, PSA level, reference standard, and risk classification might be the potential causes of heterogeneity.
Conclusion
This meta-analysis of head-to-head comparison studies confirms that there is a trend toward a higher sensitivity and diagnostic accuracy of 68Ga-PSMA-11 PET/CT compared to mpMRI for the detection of PLNMs in PCa patients. Nevertheless, according to current guidelines, PLND still needs to be recommended in case of negative results from 68Ga-PSMA-11 PET/CT due to significant risk of malignancy.
Keywords: 68Ga-PSMA-11 PET/CT, multiparametric MRI, pelvic lymph node metastases, sensitivity, diagnostic accuracy
Introduction
Correct lymph node staging is crucial to identify prostate cancer (PCa) patients with poor prognosis who would benefit from additional therapies (1, 2). Pelvic lymph node dissection (PLND) represents the gold standard, but it is impeded by increased risk of complications such as lymphedema and venous thromboembolism as well as longer hospital stay (3, 4). Although cross-sectional abdominopelvic imaging has been recommended for patients with intermediate to high-risk PCa across guidelines, conventional imaging techniques only have modest diagnostic accuracy (4–7).
In recent years, positron emission tomography (PET) techniques with PSMA ligands have emerged as a promising tool for PCa detection, tumor staging, and treatment planning (8). Among them, 68Ga-PSMA-11 and 18F-DCFPyL have been consecutively approved by the FDA for patients with primary and recurrent PCa (9, 10). Nevertheless, although 18F-based tracers offer important advantages such as higher production capacity, longer physical half-life, and minimal radiotracer accumulation in the bladder (11–13); up until now, 68Ga-PSMA-11 is still worldwide the most commonly used and provides the absolute majority of evidence in the literature for PSMA imaging. Importantly, many accuracy studies and two previous meta-analyses have reported favorable diagnostic performance of 68Ga-PSMA-11 PET/CT for the detection of pelvic lymph node metastases (PLNMs) in intermediate to high-risk PCa (14–17).
Multiparametric MRI (mpMRI), which combines T2-weighted imaging (T2WI), diffusion weighted imaging (DWI), and dynamic contrast-enhanced (DCE) sequence, has been the leading imaging modality in the primary PCa detection and localization in the last decade. Several previous studies have compared it with 68Ga-PSMA-11 PET/CT for pelvic lymph node staging prior to radical prostatectomy. However, the results were variable and sometimes conflicting (18–32). Therefore, to clarify their relative effectiveness, in the present study, we sought to compare the diagnostic performance of these two imaging modalities by summarizing the most recent evidence in the literature. To reduce interstudy heterogeneity, only studies in which both modalities were performed in the same population were included.
Material and Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (33).
Search Strategy
We comprehensively searched all available literature until October 2020 in the PubMed and Embase databases using an algorithm based on a combination of terms: (1) “Gallium Radioisotopes” (Mesh) OR Ga OR gallium; (2) “68Ga-PSMA” (Supplementary Concept) OR PSMA OR “prostate specific membrane antigen”; (3) “Positron Emission Tomography” (Mesh) OR PET OR “positron emission tomography”; (4) “Multiparametric Magnetic Resonance Imaging” (Mesh) OR mpMRI OR “Magnetic Resonance Imaging” (Mesh) OR “magnetic resonance imaging” OR MRI; (5) prostat*; (6) “Prostatic Neoplasms” (Mesh) OR pCa OR cancer* OR tumor* OR carcinoma; (7) “Lymph Nodes” (Mesh) OR “lymph node*” OR “lymph nodal” OR “locoregional.” The reference lists of identified publications were also hand-searched for potentially relevant studies.
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if all the following criteria applied: (a) the diagnostic performance of 68Ga-PSMA-11 PET/CT and mpMRI for pelvic lymph node staging prior to radical prostatectomy in PCa patients were clearly identified in the study or subset of the study; (b) the data were sufficient (i.e., patient number above 9) to construct a 2×2 contingency table; (c) the reference standard was histopathology confirmation from PLND, which should be clearly stated in the article. The exclusion criteria were (a) duplicated articles; (b) abstract, editorial comments, letters, case reports, review, or meta-analyses; and (c) clearly irrelevant titles and abstracts.
Using the aforementioned inclusion and exclusion criteria, two researchers independently screened titles and abstracts of the retrieved articles and then evaluated the full-text version of the remaining articles to determine their eligibility for inclusion. Disagreements between the researchers were resolved by consensus.
Quality Assessment
Two researchers independently assessed the quality of the included studies based on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. Each study was evaluated based on the following domains: patient selection, index test, reference standard, and flow and timing. These domains were then evaluated according to the risk of bias and were rated regarding applicability as “high,” “low,” or “unclear.” Disagreements between the researchers were resolved by consensus.
Data Extraction
Two researchers independently conducted data extraction for all included articles. The extracted data included the first author, study characteristics (year, country, study design, prevalence of PLNMs, extracted lymph node number, and reference standard), patient characteristics (number of patients, age, PSA level, and D’Amico risk stratification), and technical aspects (field strength and MRI sequence for mpMRI; injection dose, uptake time, and image analysis for 68Ga-PSMA-11 PET/CT). For each study, the absolute numbers of true-positive, true-negative, false-positive, and false-negative data for mpMRI and 68Ga-PSMA-11 PET/CT were extracted on a per-patient basis. Disagreements between the researchers were resolved by consensus.
Statistical Analysis
The pooled sensitivity and specificity for 68Ga-PSMA-11 PET/CT and mpMRI were presented as estimates with 95% confidence intervals (CIs) by using random-effect analysis. The summary receiver-operating characteristic (SROC) curves were constructed, and the area under the curve (AUC) was calculated.
Heterogeneity among pooled studies was assessed by use of Cochrane Q and I 2 statistics. Values of I 2 equal to 25, 50, and 75% were assumed to represent low, moderate, and high heterogeneity, respectively. In case of substantial heterogeneity, meta-regression analysis was performed to explore the potential source of heterogeneity and the covariates were (1) number of patients included (>40 vs. ≤40); (2) ethnicity (Asian vs. the rest); (3) prevalence of PLNMs (>20% vs. ≤20%); (4) extracted lymph node number (>10 vs. ≤10); (5) reference standard (PLND vs. extended PLND); (6) PSA (>10 vs. ≤10); (7) D’Amico risk stratification (high risk vs. intermediate and high risk); (8) PET image analysis (visual vs. quantitative); (9) field strength (1.5 T vs. 3.0 T); and (10) MRI sequence (T2WI, DWI, and DCE vs. DWI and DCE). Publication bias was assessed by Deeks’ funnel plot. All analyses were conducted with Stata 15.1 (Stata Corporation).
Results
Literature Search and Study Selection
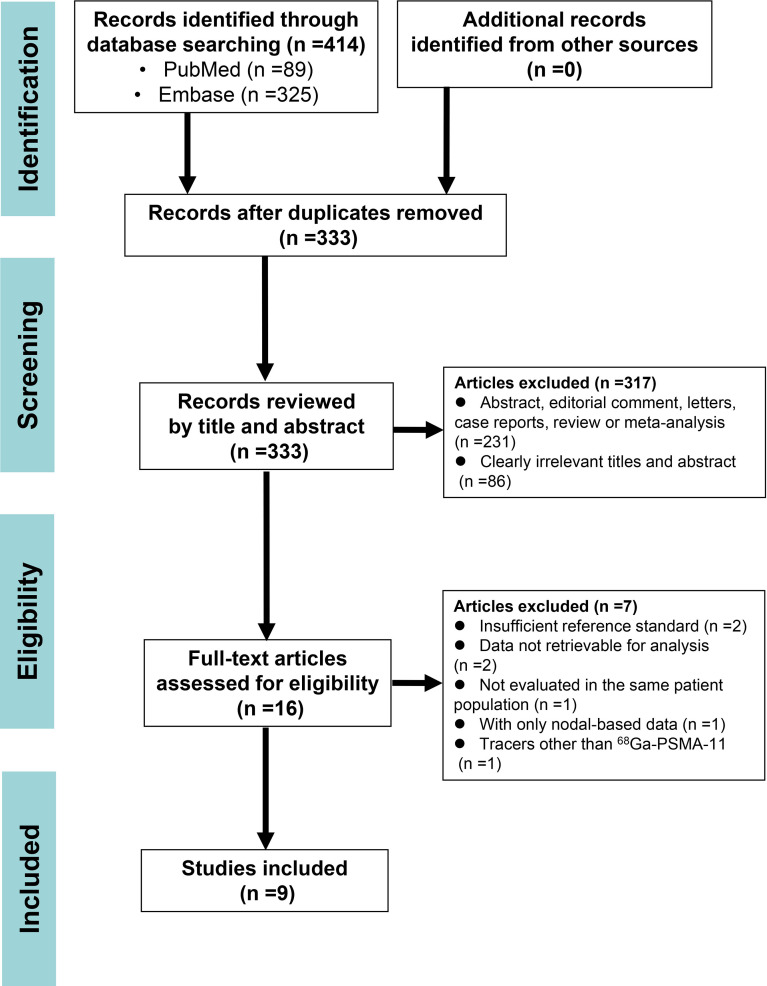
The initial search retrieved 414 articles, and 398 were excluded upon review of titles and abstracts. The remaining 16 articles were carefully assessed by full text, and another seven were excluded for the following reasons: insufficient reference standard (n = 2); data not retrievable for analysis (n = 2); not evaluated in the same patient population (n = 1); with only nodal-based data (n = 1); and tracers other than 68Ga-PSMA-11 (n =1). Finally, nine articles including patient-based data on the head-to-head comparison of diagnostic performance of 68Ga-PSMA-11 PET/CT and mpMRI were eligible for further analysis. A PRISMA flow diagram of the study selection process is shown in Figure 1 .
Figure 1.
The PRISMA flow diagram of study selection.
Study Description and Quality Assessment
The study and patient characteristics of the nine articles comprising 640 patients are summarized in Table 1 . The range of the prevalence of PLNMs for the included studies was 4% to 58.3%, and the median was 25%. The technical aspects of 68Ga-PSMA-11 PET/CT and mpMRI were presented in Table 2 .
Table 1.
Study and patient characteristics of the included studies.
| Author | Year | Study characteristics | Patient characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Study design | Prevalence of PLNMs | No. of extracted lymph node | Reference standard | No. of patients | Age (median, range) | PSA (median, range) | D’Amico risk stratification | ||
| Frumer et al. (28) | 2020 | Israel | Retro | 13.5% | Median 9 Range 6–14 |
PLND | 89 | 67 (64–70) | 8.5 (5–15) | Intermediate 40; high risk 49 |
| Franklin et al. (32) | 2020 | Australia | Retro | 24.5% | Median 16 Range 1–53 |
PLND | 233 | 68 (48–81) | 7.4 (1.5–72.0) | Low risk 2; intermediate 90; high risk 141 |
| Kulkarni et al. (26) | 2020 | India | Retro | 45.7% | NA | PLND | 35 | NA | NA | Intermediate and high risk |
| Pallavi et al. (31) | 2020 | India | Pro | 24.1% | NA | NA | 29 | NA | NA | NA |
| Van Leeuwen et al. (24) | 2019 | Netherlands and Australia | Retro | 36.4% | Median 16 Range 12–21 |
ePLND | 140 | NA | 9.4 | Intermediate 30; high risk 110 |
| Yilmaz et al. (23) | 2019 | Turkey | Retro | 20.0% | NA | rPLND | 10 | NA | NA | Low risk 3; intermediate 15; high risk 6 |
| Berger et al. (21) | 2018 | Australia | Retro | 4% | Median 12 Range 3–22 |
PLND | 50 | 649 ± 5.6 | 10.6 ± 8.1 | NA |
| Gupta et al. (20) | 2017 | India | Retro | 58.3% | Median 20 | ePLND | 12 | 61 (46–76) | 24.3 (8.7–200.6) | High risk 12 |
| Zhang et al. (19) | 2017 | China | Retro | 35.7% | Median 7 Range 2–15 |
PLND | 42 | 69 (55–82) | 37.25 (7.2–348.) | Intermediate 17; high risk 25 |
NA, not available.
Table 2.
Technical aspects of 68Ga-PSMA-11 PET/CT and mpMRI scans.
| Author | Year | mpMRI | 68Ga-PSMA-PET/CT | |||
|---|---|---|---|---|---|---|
| Field strength | MRI sequence | Injection dose | Uptake time (min) | Image analysis | ||
| Frumer et al. (28) | 2020 | 3.0 or 1.5 T | T2WI, DWI, DCE | 3–5 mCi | 50–60 | Visual |
| Franklin et al. (32) | 2020 | 3.0 T | T2WI, DWI, DCE | Mean, 200 MBq | 45–60 | Visual |
| Kulkarni et al. (26) | 2020 | 3.0 T | T2WI, DWI, DCE | 3–4.5 mCi | 60 | Visual |
| Pallavi et al. (31) | 2020 | 3.0 T | T2WI, DWI | Mean, 1.76 MBq/kg | 60 | Visual |
| Van Leeuwen et al. (24) | 2019 | 3.0 or 1.5 T | T2WI, DWI, DCE | 2.0 MBq/kg or 100 MBq | 60 or 45 | NA |
| Yilmaz et al. (23) | 2019 | 3.0 T | T2WI, DWI, DCE | Median, 175 MBq | 60 | Quantitative |
| Berger et al. (21) | 2018 | 3.0 T | T2WI, DWI | NA | 60 | Quantitative |
| Gupta et al. (20) | 2017 | 1.5 T | T2WI, DWI | 2 MBq/kg | 60 | Visual |
| Zhang et al. (19) | 2017 | 3.0 T | T2WI, DWI, DCE | Median 131.7 MBq | 60 | Visual |
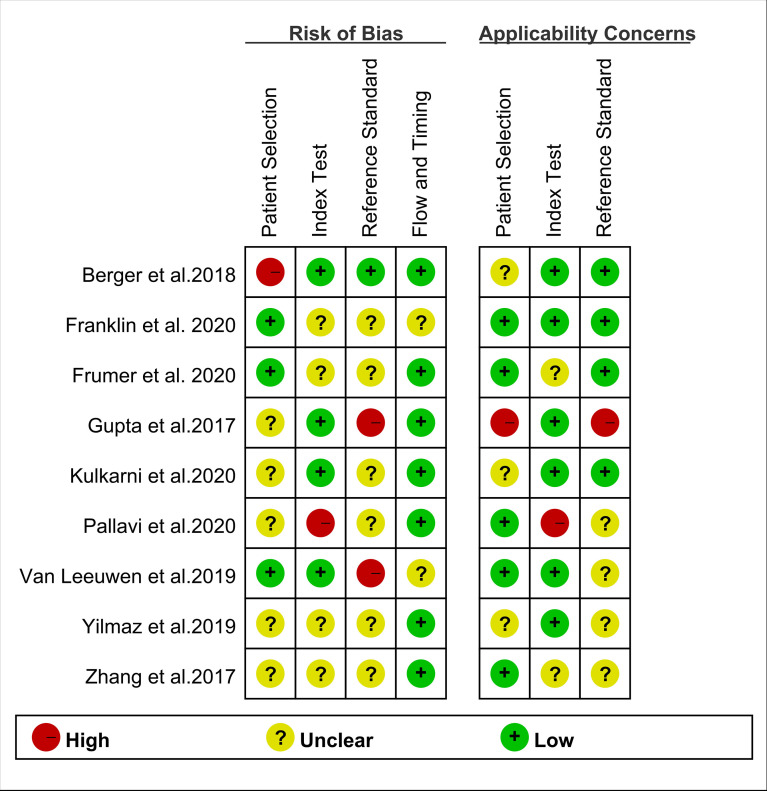
The results of summary risk of bias and applicability concerns of each study are shown in Figure 2 . The quality of the included studies was considered satisfactory.
Figure 2.
Summary risk of bias and applicability concerns of the included studies.
Diagnostic Performance of 68Ga-PSMA-11 PET/CT for PLNMs
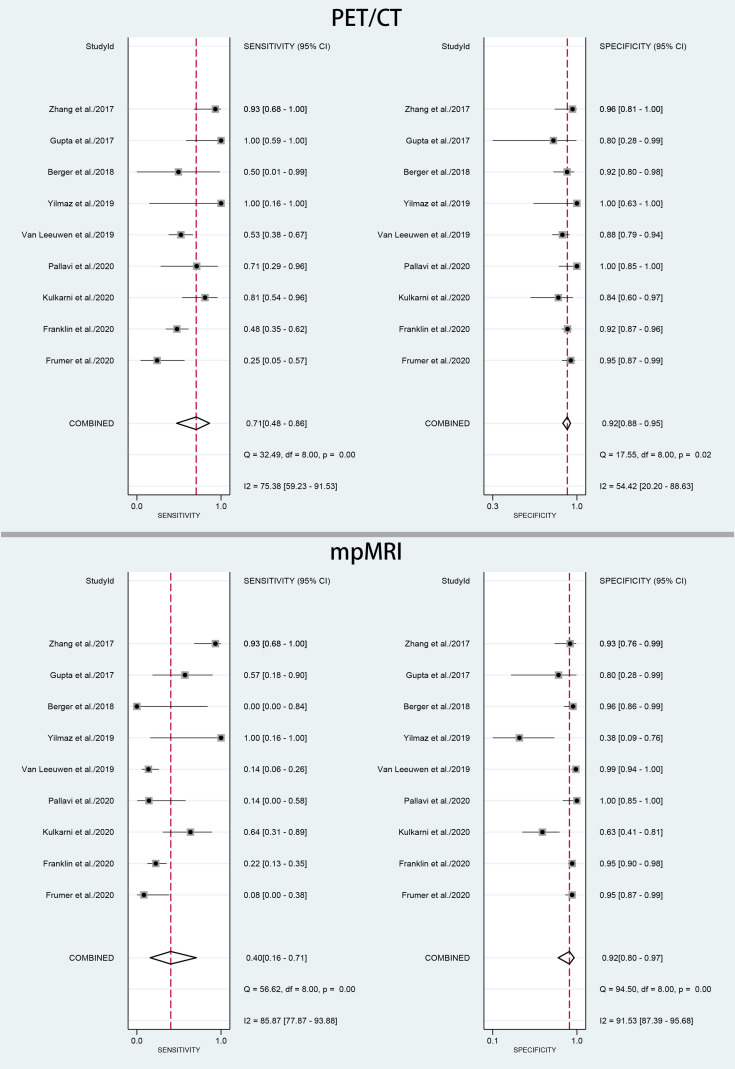
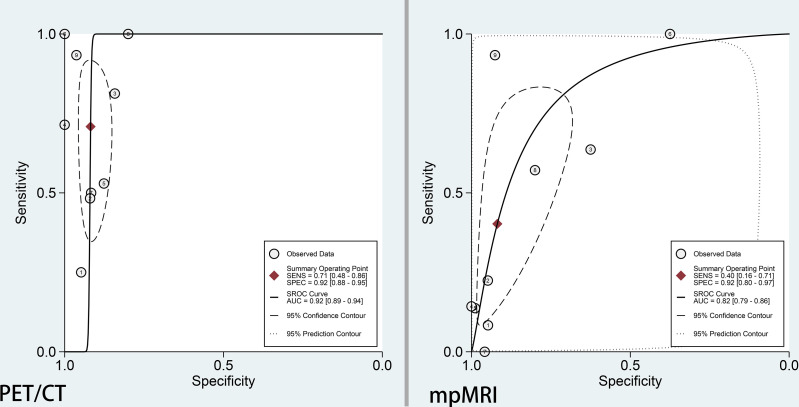
The pooled sensitivity and specificity for 68Ga-PSMA-11 PET/CT were 0.71 (95% CI: 0.48–0.86) with moderate heterogeneity (75%) and 0.92 (95% CI: 0.88–0.95) with moderate heterogeneity (54%), respectively ( Figure 3 ). Figure 4 shows the SROC curve and the AUC for 68Ga-PSMA-11 PET/CT was 0.92 (95% CI: 0.89–0.94).
Figure 3.
Forest plot of pooled sensitivity and specificity of 68Ga-PSMA-PET/CT and mpMRI for the detection of pelvic lymph node metastases prior to radical prostatectomy in PCa patients.
Figure 4.
SROC curve of 68Ga-PSMA-PET/CT and mpMRI for the detection of pelvic lymph node metastases prior to radical prostatectomy in PCa patients.
Meta-regression analysis was performed to explore the sources of heterogeneity, and we identified that prevalence of PLNMs (p = 0.01 for specificity), PSA level (p < 0.001 for sensitivity and p < 0.001 for specificity), risk classification (p < 0.001 for sensitivity), and reference standard (p < 0.001 for specificity) were possible causes of heterogeneity for 68Ga-PSMA-11 PET/CT. No publication bias was found (p = 0.15).
Diagnostic Performance of mpMRI for PLNMs
The pooled sensitivity and specificity for mpMRI were 0.40 (95% CI: 0.16–0.71) with high heterogeneity (86%) and 0.92 (95% CI: 0.80–0.97) with high heterogeneity (92%), respectively ( Figure 3 ). Figure 4 shows the SROC curve and the AUC for mpMRI was 0.82 (95% CI: 0.79–0.86).
Meta-regression analysis revealed that number of patients (p < 0.001 for specificity) and PSA level (p < 0.001 for sensitivity) were possible causes of heterogeneity. No publication bias was found (p = 0.87).
Discussion
The present meta-analysis pooled patient-based data from nine studies which compared 68Ga-PSMA-11 PET/CT and mpMRI in the same population. It was found that the former had higher sensitivity (0.71 vs. 0.40), similar specificity (0.92 vs. 0.92), and higher AUC (0.92 vs. 0.82) as compared with the latter. The resulting relativeness was in agreement with those (sensitivity, 0.65 vs. 0.41; specificity, 0.94 vs. 0.92; AUC, 0.92 vs. 0.83) from a previous meta-analysis, in which indirect comparisons (not in the same population) were made by including 13 studies (29). The higher trend of sensitivity and diagnostic accuracy of 68Ga-PSMA-11 PET/CT over mpMRI for pelvic lymph node staging prior to radical prostatectomy in patients with intermediate to high-risk PCa were thus confirmed based on the most recent evidence. To better illustrate the imaging features of mpMRI and 68Ga-PSMA PET/CT in characterizing lymph node metastases, an example of one patient who had underwent both imaging modalities was shown in Figure 5 .
Figure 5.
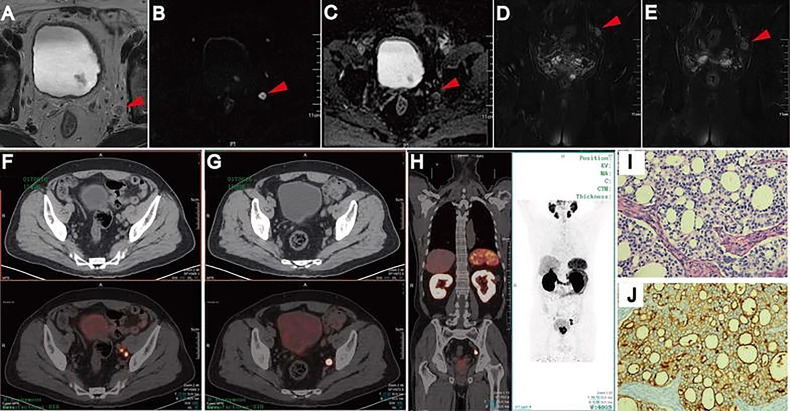
Lymph node metastases on pelvic mpMRI and 68Ga-PSMA PET/CT. Axial T2WI (A), DWI (B), ADC (C), and coronal Fat suppression T2WI (D, E). Fused 68Ga-PSMA PET/CT (F–H) images were taken from left internal iliac and obturator fossa regions with histopathologically proven disease (HE staining, (I) PSMA IHC staining, (J). Reproduced with permission from Figure 2 of Zhang et al. (19).
Different interpreting strategies for small PLNMs between the two imaging modalities across the included studies might help to explain the better performance of 68Ga-PSMA-11 PET/CT. While most of the mpMRI interpretations used the short-axis diameter of more than 10 or 8 mm as a determining factor for malignancy, all PET/CT interpretations decided PLNMs solely based on PSMA uptake, irrespective of the small size of lymph nodes. Thus, some small PLNMs without significant anatomical characteristics might be only detected by PET/CT. In a study of 240 patients, Franklin et al. found that the median diameter of avid lymph nodes on 68Ga-PSMA PET/CT were 7.0 mm (range, 0.5–40 mm), in comparison to 11.7 mm (range, 2.2–20 mm) for mpMRI. The per-patient sensitivity of PET/CT and mpMRI in this study was 48.3% and 22.4%, respectively (32).
Nevertheless, 68Ga-PSMA-11 PET/CT still missed as many as 29% of the PLNMs identified by PLND according to the result of our meta-analysis. In a study of 140 patients, Van Leeuwen et al. reported that no lymph nodes detected < 2 mm and only 27% of the lymph node metastases 2 and 4 mm were detected by preoperative 68Ga-PSMA-PET/CT (24). In a larger study of 208 patients, Yaxley et al. found that 85.4% of histologically positive LNs ≤ 5 mm in maximal diameter were missed by preoperative 68Ga-PSMA PET/CT (34). It seems that the resolution of 68Ga-PSMA PET/CT is still not sufficient to detect many microscopic diseases seen at histopathology, particularly those with a diameter <5 mm. However, since it has been reported that the presence of microscopic diseases is associated with late disease recurrence, similar to PLNMs with large diameter, the clinical impact of these radiographically undetected microscopic diseases could be significant (35, 36). Therefore, despite its known limitations and complications, PLND remains necessary in that it could reveal microscopic diseases that might lead to early initiation of salvage radiotherapy and androgen deprivation therapy, which would eventually result in improved long-term local pelvic control and improved biochemical-free progression (2, 37).
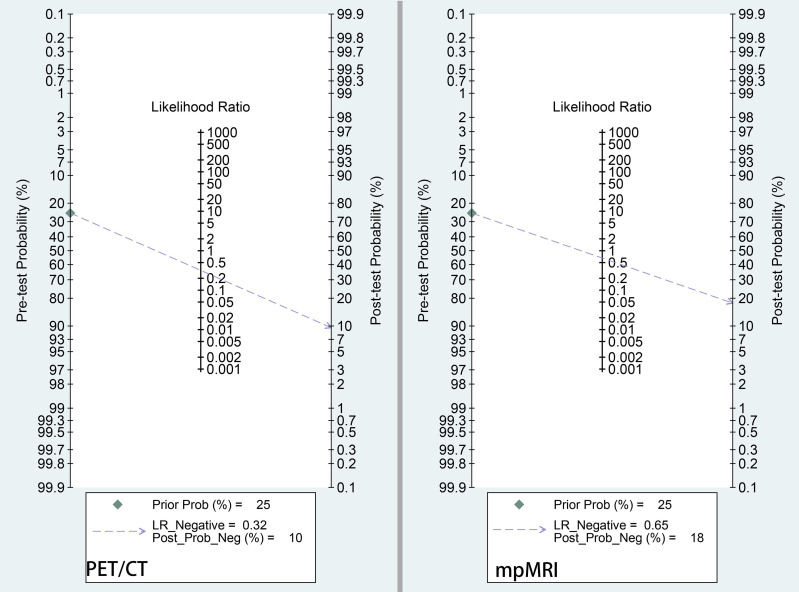
On the other hand, according to the current EAU or NCCN guidelines, if the risk of a PLNM is >5% or >2%, respectively, PLND is recommended at the time of radical prostatectomy (38, 39). Based on the results of this meta-analysis, Fagan’s nomogram indicated that when the pretest probability (prevalence of PLNMs) was assumed to be 25%, which is the medium value of our included studies, the negative posttest probability (the probability of being malignancy when the test is negative) decreased to 10% for 68Ga-PSMA-11 PET/CT and 22% for mpMRI ( Figure 6 ). Thus, negative test results from both imaging modalities leaves a residual malignancy risk of above 5%. In this regard, PLND still needs to be recommended if 68Ga-PSMA PET/CT or mpMRI did not identify any suspicious lymph nodes.
Figure 6.
Fagan nomogram of pretest probability and negative posttest probability for 68Ga-PSMA-PET/CT and mpMRI. The pretest probability was set at 25%.
In recent years, researchers have begun to incorporate 68Ga-PSMA PET/CT and mpMRI parameters into comprehensive preoperative algorithms to evaluate the risk of PLNMs. Franklin et al. found that the combination of a negative 68Ga-PSMA PET/CT, ISUP biopsy grade <4 and PIRADS <4 prostate mpMRI, or an ISUP grade 5 with PIRADS <3 on mpMRI was associated with a <5% risk of PLNMs (32). Ferraro et al. devised a model based on visual lymph node status on 68Ga-PSMA PET/CT, total PSMA uptake of the primary tumor, PSA, and Gleason score, which showed a tendency to improve patient selection for PLND overprediction models using clinical risk factors (40). It is hoped that future nomograms incorporating not only clinical risk factors but also data from modern imaging modalities will help to more appropriately select candidates for PLND. Moreover, hybrid PET/MRI modality may offer incremental value for preoperative detection of PLNMs. In a 2018 study, Thalgott et al. demonstrated that 68Ga-PSMA-11 PET/MRI even had a specificity of 100% in this setting (41).
Major limitations of our study include small sample size and heterogeneous study and patient characteristics and technical aspects of the included studies. We tried our best to perform subgroup analyses and found that number of patients, prevalence of PLNMs, PSA level, reference standard, and risk classification might be the sources of heterogeneity for the two imaging modalities. Besides, we only analyzed patient-based data in the present meta-analysis, because in clinical practice, it is difficult to precisely associate either PET or MRI findings with the histological results in a node-to-node manner and patients with one positive PLNM could provide enough prognostic information to alter patient management (34).
In conclusion, this meta-analysis of head-to-head comparison studies confirms that there is a trend toward a higher sensitivity and diagnostic accuracy of 68Ga-PSMA-11 PET/CT compared to mpMRI for the detection of PLNMs in PCa patients. Nevertheless, according to current guidelines, PLND still needs to be recommended in case of negative results from 68Ga-PSMA-11 PET/CT due to significant risk of malignancy. Hybrid PET/MRI modality exploiting both the superb molecular information from 68Ga-PSMA-11 PET and the high local contrast of MRI may represent a future direction.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
JB and HZ conceived and designed the study, which were proofed by JB. XW, QW, and FT collected and analyzed the data. XW and QW wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate Versus Deferred Androgen Deprivation Treatment in Patients With Node-Positive Prostate Cancer After Radical Prostatectomy and Pelvic Lymphadenectomy. Lancet Oncol (2006) 7(6):472–9. doi: 10.1016/s1470-2045(06)70700-8 [DOI] [PubMed] [Google Scholar]
- 2. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Impact of Adjuvant Radiotherapy on Survival of Patients With Node-Positive Prostate Cancer. J Clin Oncol (2014) 32(35):3939–47. doi: 10.1200/jco.2013.54.7893 [DOI] [PubMed] [Google Scholar]
- 3. Briganti A, Chun FK, Salonia A, Suardi N, Gallina A, Da Pozzo LF, et al. Complications and Other Surgical Outcomes Associated With Extended Pelvic Lymphadenectomy in Men With Localized Prostate Cancer. Eur Urol (2006) 50(5):1006–13. doi: 10.1016/j.eururo.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 4. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent. Eur Urol (2021) 79(2):243–62. doi: 10.1016/j.eururo.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 5. Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw (2018) 16(5s):620–3. doi: 10.6004/jnccn.2018.0036 [DOI] [PubMed] [Google Scholar]
- 6. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol (2018) 199(3):683–90. doi: 10.1016/j.juro.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 7. Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The Diagnostic Accuracy of CT and MRI in the Staging of Pelvic Lymph Nodes in Patients With Prostate Cancer: A Meta-Analysis. Clin Radiol (2008) 63(4):387–95. doi: 10.1016/j.crad.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 8. de Kouchkovsky I, Aggarwal R, Hope TA. Prostate-Specific Membrane Antigen (PSMA)-Based Imaging in Localized and Advanced Prostate Cancer: A Narrative Review. Transl Androl Urol (2021) 10(7):3130–43. doi: 10.21037/tau-20-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hennrich U, Eder M. [(68)Ga]Ga-PSMA-11: The First FDA-Approved (68)Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals (Basel) (2021) 14(8):713. doi: 10.3390/ph14080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song H, Iagaru A, Rowe SP. FDA Approves (18)F-DCFPyL PET Agent in Prostate Cancer. J Nucl Med (2021) 62(8):11n. doi: 10.2967/jnumed.121.262989 [DOI] [Google Scholar]
- 11. Grünig H, Maurer A, Thali Y, Kovacs Z, Strobel K, Burger IA, et al. Focal Unspecific Bone Uptake on [(18)F]-PSMA-1007 PET: A Multicenter Retrospective Evaluation of the Distribution, Frequency, and Quantitative Parameters of a Potential Pitfall in Prostate Cancer Imaging. Eur J Nucl Med Mol Imaging (2021). doi: 10.1007/s00259-021-05424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun J, Lin Y, Wei X, Ouyang J, Huang Y, Ling Z. Performance of 18F-DCFPyL PET/CT Imaging in Early Detection of Biochemically Recurrent Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol (2021) 11:649171. doi: 10.3389/fonc.2021.649171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wondergem M, van der Zant FM, Broos WA, Knol RJ. Matched-Pair Comparison of (18)F-DCFPyL PET/CT and (18)F-PSMA-1007 PET/CT in 240 Prostate Cancer Patients; Inter-Reader Agreement and Lesion Detection Rate of Suspected Lesions. J Nucl Med (2021) 62(10):1422–1429. doi: 10.2967/jnumed.120.258574 [DOI] [PubMed] [Google Scholar]
- 14. Erdem S, Simsek DH, Degirmenci E, Aydin R, Bagbudar S, Ozluk Y, et al. How Accurate Is (68)Gallium-Prostate Specific Membrane Antigen Positron Emission Tomography/Computed Tomography [(68)Ga-PSMA PET/CT] on Primary Lymph Node Staging Before Radical Prostatectomy in Intermediate and High Risk Prostate Cancer? A Study of Patient- and Lymph Node- Based Analyses. Urol Oncol (2021) S1078–1439(21):00307–0. doi: 10.1016/j.urolonc.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 15. Esen T, Falay O, Tarim K, Armutlu A, Koseoglu E, Kilic M, et al. (68)Ga-PSMA-11 Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging Before Radical Prostatectomy: Central Review of Imaging and Comparison With Histopathology of Extended Lymphadenectomy. Eur Urol Focus (2021) 7(2):288–93. doi: 10.1016/j.euf.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 16. Tu X, Zhang C, Liu Z, Shen G, Wu X, Nie L, et al. The Role of (68)Ga-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Intermediate/High Risk Patients With Prostate Cancer: A Diagnostic Meta-Analysis. Front Oncol (2020) 10:1365. doi: 10.3389/fonc.2020.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng L, Li J, Meng C, Li J, You C, Tang D, et al. Can (68)Ga-Prostate Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography Provide an Accurate Lymph Node Staging for Patients With Medium/High Risk Prostate Cancer? A Diagnostic Meta-Analysis. Radiat Oncol (2020) 15(1):227. doi: 10.1186/s13014-020-01675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tulsyan S, Das CJ, Tripathi M, Seth A, Kumar R, Bal C. Comparison of 68Ga-PSMA PET/CT and Multiparametric MRI for Staging of High-Risk Prostate Cancer68ga-PSMA PET and MRI in Prostate Cancer. Nucl Med Commun (2017) 38(12):1094–102. doi: 10.1097/mnm.0000000000000749 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q, Zang S, Zhang C, Fu Y, Lv X, Zhang Q, et al. Comparison of (68)Ga-PSMA-11 PET-CT With mpMRI for Preoperative Lymph Node Staging in Patients With Intermediate to High-Risk Prostate Cancer. J Transl Med (2017) 15(1):230. doi: 10.1186/s12967-017-1333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta M, Choudhury PS, Hazarika D, Rawal S. A Comparative Study of (68)Gallium-Prostate Specific Membrane Antigen Positron Emission Tomography-Computed Tomography and Magnetic Resonance Imaging for Lymph Node Staging in High Risk Prostate Cancer Patients: An Initial Experience. World J Nucl Med (2017) 16(3):186–91. doi: 10.4103/1450-1147.207272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F, et al. (68)Ga-PSMA PET/CT vs. mpMRI for Locoregional Prostate Cancer Staging: Correlation With Final Histopathology. Prostate Cancer Prostatic Dis (2018) 21(2):204–11. doi: 10.1038/s41391-018-0048-7 [DOI] [PubMed] [Google Scholar]
- 22. Meißner S, Janssen JC, Prasad V, Diederichs G, Hamm B, Brenner W, et al. Accuracy of Standard Clinical 3T Prostate MRI for Pelvic Lymph Node Staging: Comparison to (68)Ga-PSMA PET-Ct. Sci Rep (2019) 9(1):10727. doi: 10.1038/s41598-019-46386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yilmaz B, Turkay R, Colakoglu Y, Baytekin HF, Ergul N, Sahin S, et al. Comparison of Preoperative Locoregional Ga-68 PSMA-11 PET-CT and mp-MRI Results with Postoperative Histopathology of Prostate Cancer. Prostate (2019) 79(9):1007–17 doi:10.1002/pros.23812 [DOI] [PubMed] [Google Scholar]
- 24. van Leeuwen PJ, Donswijk M, Nandurkar R, Stricker P, Ho B, Heijmink S, et al. Gallium-68-Prostate-Specific Membrane Antigen [(68) Ga-PSMA] Positron Emission Tomography (PET)/computed Tomography (CT) Predicts Complete Biochemical Response From Radical Prostatectomy and Lymph Node Dissection in Intermediate- and High-Risk Prostate Cancer. BJU Int (2019) 124(1):62–8. doi: 10.1111/bju.14506 [DOI] [PubMed] [Google Scholar]
- 25. Çelen S, Gültekin A, Özlülerden Y, Mete A, Sağtaş E, Ufuk F, et al. Comparison of 68Ga-PSMA-I/T PET-CT and Multiparametric MRI for Locoregional Staging of Prostate Cancer Patients: A Pilot Study. Urol Int (2020) 104(9-10):684–91. doi: 10.1159/000509974 [DOI] [PubMed] [Google Scholar]
- 26. Kulkarni SC, Sundaram PS, Padma S. In Primary Lymph Nodal Staging of Patients With High-Risk and Intermediate-Risk Prostate Cancer, How Critical Is the Role of Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography-Computed Tomography? Nucl Med Commun (2020) 41(2):139–46. doi: 10.1097/mnm.0000000000001110 [DOI] [PubMed] [Google Scholar]
- 27. Arslan A, Karaarslan E, Güner AL, Sağlıcan Y, Tuna MB, Kural AR. Comparing the Diagnostic Performance of Multiparametric Prostate MRI Versus 68ga-PSMA PET-CT in the Evaluation Lymph Node Involvement and Extraprostatic Extension. Acad Radiol (2020) S1076–6332(20):30427–X. doi: 10.1016/j.acra.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 28. Frumer M, Milk N, Rinott Mizrahi G, Bistritzky S, Sternberg I, Leibovitch I, et al. A Comparison Between (68)Ga-Labeled Prostate-Specific Membrane Antigen-PET/CT and Multiparametric MRI for Excluding Regional Metastases Prior to Radical Prostatectomy. Abdom Radiol (NY) (2020) 45(12):4194–201. doi: 10.1007/s00261-020-02640-1 [DOI] [PubMed] [Google Scholar]
- 29. Wu H, Xu T, Wang X, Yu YB, Fan ZY, Li DX, et al. Diagnostic Performance of (68)Gallium Labelled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Staging the Prostate Cancer With Intermediate or High Risk Prior to Radical Prostatectomy: A Systematic Review and Meta-Analysis. World J Mens Health (2020) 38(2):208–19. doi: 10.5534/wjmh.180124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen LJ, Nielsen JB, Langkilde NC, Petersen A, Afshar-Oromieh A, De Souza NM, et al. (68)Ga-PSMA PET/CT Compared With MRI/CT and Diffusion-Weighted MRI for Primary Lymph Node Staging Prior to Definitive Radiotherapy in Prostate Cancer: A Prospective Diagnostic Test Accuracy Study. World J Urol (2020) 38(4):939–48. doi: 10.1007/s00345-019-02846-z [DOI] [PubMed] [Google Scholar]
- 31. Pallavi UN, Gogoi S, Thakral P, Malasani V, Sharma K, Manda D, et al. Incremental Value of Ga-68 Prostate-Specific Membrane Antigen-11 Positron-Emission Tomography/Computed Tomography Scan for Preoperative Risk Stratification of Prostate Cancer. Indian J Nucl Med (2020) 35(2):93–9. doi: 10.4103/ijnm.IJNM_189_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franklin A, Yaxley WJ, Raveenthiran S, Coughlin G, Gianduzzo T, Kua B, et al. Histological Comparison Between Predictive Value of Preoperative 3-T Multiparametric MRI and (68) Ga-PSMA PET/CT Scan for Pathological Outcomes at Radical Prostatectomy and Pelvic Lymph Node Dissection for Prostate Cancer. BJU Int (2021) 127(1):71–9. doi: 10.1111/bju.15134 [DOI] [PubMed] [Google Scholar]
- 33. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 34. Yaxley JW, Raveenthiran S, Nouhaud FX, Samartunga H, Yaxley AJ, Coughlin G, et al. Outcomes of Primary Lymph Node Staging of Intermediate and High Risk Prostate Cancer With (68)Ga-PSMA Positron Emission Tomography/Computerized Tomography Compared to Histological Correlation of Pelvic Lymph Node Pathology. J Urol (2019) 201(4):815–20. doi: 10.1097/ju.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 35. Pagliarulo V, Hawes D, Brands FH, Groshen S, Cai J, Stein JP, et al. Detection of Occult Lymph Node Metastases in Locally Advanced Node-Negative Prostate Cancer. J Clin Oncol (2006) 24(18):2735–42. doi: 10.1200/jco.2005.05.4767 [DOI] [PubMed] [Google Scholar]
- 36. Conti A, Santoni M, Burattini L, Scarpelli M, Mazzucchelli R, Galosi AB, et al. Update on Histopathological Evaluation of Lymphadenectomy Specimens From Prostate Cancer Patients. World J Urol (2017) 35(4):517–26. doi: 10.1007/s00345-015-1752-8 [DOI] [PubMed] [Google Scholar]
- 37. Touijer KA, Karnes RJ, Passoni N, Sjoberg DD, Assel M, Fossati N, et al. Survival Outcomes of Men With Lymph Node-Positive Prostate Cancer After Radical Prostatectomy: A Comparative Analysis of Different Postoperative Management Strategies. Eur Urol (2018) 73(6):890–6. doi: 10.1016/j.eururo.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent. Eur Urol (2017) 71(4):618–29. doi: 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 39. Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(5):479–505. doi: 10.6004/jnccn.2019.0023 [DOI] [PubMed] [Google Scholar]
- 40. Ferraro DA, Muehlematter UJ, Garcia Schüler HI, Rupp NJ, Huellner M, Messerli M, et al. (68)Ga-PSMA-11 PET has the Potential to Improve Patient Selection for Extended Pelvic Lymph Node Dissection in Intermediate to High-Risk Prostate Cancer. Eur J Nucl Med Mol Imaging (2020) 47(1):147–59. doi: 10.1007/s00259-019-04511-4 [DOI] [PubMed] [Google Scholar]
- 41. Thalgott M, Düwel C, Rauscher I, Heck MM, Haller B, Gafita A, et al. One-Stop-Shop Whole-Body (68)Ga-PSMA-11 PET/MRI Compared With Clinical Nomograms for Preoperative T and N Staging of High-Risk Prostate Cancer. J Nucl Med (2018) 59(12):1850–6. doi: 10.2967/jnumed.117.207696 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.