Abstract
Cholesterol esters, synthesized from cholesterol with long-chain fatty acids, are essential components of plasma lipoproteins and cell membranes that participate in various metabolic processes in the body. Cholesterol can be excreted through the cholesterol reverse transport (RCT) pathway when excessive cholesterol is produced in the extrahepatic cells, which is regulated by the liver X receptor (LXR) and its downstream regulators ATP-binding cassette subfamily A member 1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1) genes. Abnormal cholesterol metabolism is closely associated with the development of diabetic retinopathy (DR). However, the precise underlying mechanism of the RCT pathway in the pathogenesis of DR is still not fully understood. This review focused on cholesterol metabolism, with a particular emphasis on the RCT pathway and its correlation with the development of DR. Particular attention has been paid to the key regulators of the RCT pathway: LXR, ABCA1, and ABCG1 genes and their potential therapeutic targets in the management of DR.
1. Introduction
The association between abnormal lipid metabolism and microvascular complications of diabetes has received considerable attention recently. The imbalance of the reverse transport (RCT) pathway of cholesterol under high glucose pathological conditions is a new focus of research becoming a hot topic. This review summarizes the recent progress on cholesterol metabolism abnormalities, particularly emphasizing the association of the RCT pathway in the pathogenesis of diabetic retinopathy (DR). This novel research direction may lead to an insight into discovering biomarkers and molecular targets for tailored therapies. The key regulators of the RCT pathway will expect to be a frontline in the prevention and treatment of DR.
2. Cholesterol: Biosynthesis and Regulation
2.1. Synthesis and Transportation
Cholesterol is the most common and abundant molecule in the body, comprising approximately 30% of all mammalian cell membranes [1]. In addition to supporting the structure of cells, cholesterol is the precursor of synthetic bile acids, vitamin D, and steroid hormones [2]. Cholesterol further exhibits essential physiological roles in cell transport, signal transduction, and nerve conduction [3, 4]. Exogenous cholesterol is mainly derived from dietary sources, as well as from internal endogenous biosynthetic pathways. The liver and intestinal mucosa are the main organs for cholesterol synthesis, in addition to the brain.
Metabolism of cholesterol includes two main pathways: endogenous synthesis and exogenous absorption. Almost all mammalian cells can synthesize cholesterol, and the most active site of synthesis is the liver. Acetyl-CoA is used in cells to synthesize cholesterol de novo through a multistep enzymatic reaction. Acetyl-CoA reductase is the key enzyme that catalyzes mevalonate production from Acetyl-CoA. Endogenous cholesterol synthesized in hepatocytes is mainly transported by low-density lipoprotein (LDL) to the peripheral tissues in the form of LDL cholesterol (LDL-C). LDL-C found in the blood is recognized by the LDL receptor and transported into the cells [5]. The second pathway involves the exogenous cholesterol pathway: the small intestine directly obtains exogenous cholesterol from dietary sources, which mainly occurs in the upper and middle parts of the small intestine. Niemann-Pick C1-Like protein (a polytopic transmembrane protein of 1332 amino acids) mediates cholesterol absorption and is highly specific and inhibits plant sterol uptake [6].
2.2. Conversion and Efflux
The metabolic pathway of cholesterol includes conversion and efflux. Cholesterol synthesized in the liver is transported to peripheral tissues through circulation to meet the body's regular metabolic needs. Cholesterol is not entirely oxidized and decomposed into CO2 and H2O in mammalian cells. Cholesterol is subsequently converted to other compounds containing a cyclopentane polyhydrophenanthrene nucleus via enzyme catalysis (oxidation and reduction reactions). Under normal circumstances, excessive cholesterol is excreted from cells and transported to the liver through circulation and is metabolized in hepatocytes and finally excreted through bile and feces. Such a process is called reverse cholesterol transport (RCT).
Cholesterol efflux from cells has been proved to be an early step of RCT. The RCT pathway plays a vital role in maintaining the kinetic balance of cholesterol metabolism and is regulated by several genes and proteins. Liver X receptor (LXR) and ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) have been identified as the key regulators in atherosclerosis [7]. It has been reported that serum capacity to induce ABCA1- and SR-BI-mediated cholesterol efflux is impaired in diabetic patients with incipient or overt nephropathy. Such impairment may contribute to the accelerated development of atherosclerosis in these patients, suggesting that the capacity of serum to induce cellular cholesterol efflux is an independent predictor of atherosclerosis and diabetic nephropathy [8].
2.3. Liver X Receptors (LXRs) and Metabolism of Cholesterol
2.3.1. Classification and Distribution of LXR
LXR, a ligand-activated nuclear transcription factor, is a member of the nuclear receptor superfamily and is involved in regulating cholesterol metabolism. LXR is a receptor for cholesterol metabolism and lipid biosynthesis. As an insulin sensitizer, LXR has been found to upregulate glucose transporter type 4 (GLUT4) expression. Furthermore, LXR agonist promotes glucose uptake in adipocyte, suggesting that activation of LXR could alter the expression of genes in liver and adipose tissue, limiting glucose output and improving peripheral glucose uptake [9]. In addition, LXR participates in water-electrolyte balance and immune response, regulating the physiological functions of a variety of apolipoproteins (apos) [10], thereby maintaining cholesterol homeostasis [10].Two subtypes of LXR in humans have been identified: LXRα (NR1H3) and LXRβ (NR1H2). LXRα is mainly expressed in the macrophages from the liver, kidney, adrenal gland, and small intestine, while LXRβ is almost expressed in all of the tissues throughout the body [11]. LXRα and LXRβ share over 75% amino acid sequence identity and are important regulators in the cholesterol and lipid metabolism process. Balasubramanian et al. found that LXRα has a significant role in regulating the expression of GLUT4 in adipocytes and potential targets in treating diabetes [12]. LXRα(-/-), LXRβ(-/-), and LXRα/β(-/-) mice developed acellular capillaries and endothelial progenitor cell dysfunction similar to the streptozotocin-injected DBA/2J mice fed a high-fat Western diet, but activation of LXR with a synthetic ligand reversed those pathological changes [13].
LXR combines with their binding partner retinoid X receptor (RXR) in the nucleus to form an LXR/RXR heterodimer. Upon LXR agonist binding, the LXR/RXR heterodimer releases a corepressor that possesses transcription factor activity and is bound to the target gene-specific gene starter sequence. Such transcription plays a major role in regulating the absorption, storage, excretion, and metabolism of cholesterol [14, 15] (Figure 1).
Figure 1.
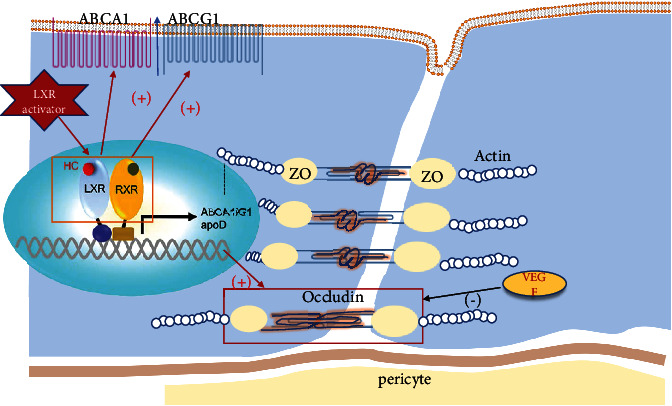
Liver X receptor perturbations of lipid metabolism in diabetic retinopathy. Under normal physiological conditions, transcriptional activity of LXRs is increased in response to elevated cellular levels of cholesterol. A high glucose environment attenuates activation of LXR, inducing the downregulations of transporters ABCA1 and ABCG1 on the cell membrane. Consequence of cellular cholesterol accumulation causes oxidative stress and inflammation in vascular endothelial cells. Furthermore, the increased expression level of VEGF downregulates tight junction proteins ZO-1 and occludin, leading to blood-retinal breakdown. LXR agonists can eventually reverse this pathological process. LXR: liver X receptor; RXR: retinoid X receptor; ABCA1: ATP-binding cassette subfamily A member 1; ABCG1: ATP-binding cassette subfamily G member 1; apo: apolipoprotein; ZO-1: zonula occludens-1.
Endogenous agonists of LRX are mainly derivatives of cholesterol, including hydroxysterols 24(S),25-epoxycholesterol, 22(R)–hydroxycholesterol, and 24(S)-hydroxycholesterol. Exogenous agonists are mainly synthesized, such as T0901317 and GW3965. The majority of these exogenous agonists are nonselective.
2.3.2. Target Genes of LXR associated with Cholesterol Metabolism
Activation of LXR initiates the transcription of multiple genes, participates in the RCT pathway, maintains lipid metabolism balance, and promotes cholesterol outflow. The direct target genes of LXR which have been identified include ABCA1 [16], ABCD2, ATP-binding cassette subfamily G member 1 (ABCG1) [17], SREBP-1c [18], FAS [19], CETP, LPL, CYP7a [20], apoE [21], and GLUT4 [22]. The main downstream regulators are ABCA1, ABCG1, SREBP-1c, apoE genes, and phospholipid transporter [23]. ATP-binding cassette transporters (ABC transporters) are a type of one-way transmembrane transporter, which uses ATP as an energy source. ABC transporters mediate the transmembrane transport of several different substrates, such as amino acids, lipids, ions, and sugars. The target genes of LXR mainly include the four ABC transporters, namely, ABCA1, ABCG1, ABCG5, and ABCG8 [23, 24], all of which are involved in cholesterol transport. Binding of ligand to LXR results in the upregulation of ABCA1 and ABCG1 in cells [25–27].
ABCA1 contains an asymmetric transmembrane domain and is composed of a dimer containing six transmembrane segments. ABCA1 is widely expressed on a variety of cell membranes and binds to apolipoproteins during RCT and participates in HDL formation. ABCA1 promotes the redistribution of cholesterol and sphingomyelin and assists free cholesterol flow to lean apolipoproteins, which are esterified into mature HDL particles [28]. ABCG1 is a semitransporter that mainly transfers intracellular cholesterol to extracellular mature HDL, thereby reducing intracellular cholesterol concentration [29, 30]. ABCG1 mediates intracellular cholesterol transfer to extracellular mature HDL which is the main pathway for cholesterol efflux from endothelial cells, concomitant with a higher level of ABCG1 than ABCA1, which is activated by LXR [31]. In addition, ABCG1 plays a significant role in mediating cholesterol efflux and HDL transport, preventing cellular lipid accumulation in endothelial cells [32, 33]. HDL combines free cholesterol excreted from cells (mainly macrophages and endothelial cells) and promotes its transport to the liver under the action of cholesterol ester transfer protein (CETP). This causes the formation of bile acids and their excretion or the production of hormones by cholesterol in tissues. LXR mediates HDL return to the liver via directly acting on CETP [34]. Furthermore, HDL involves in the RCT pathway through lipid exchange with other apolipoproteins.
Immunochemistry confirms that both ABCA1 and ABCG1 are localized to the ganglion cell layer, outer plexiform layer, and RPE in the retina from primates, suggesting that RCT is an HDL-based intraretinal lipid transport. ABCA1 seems to localize more intensively to the apical side of the RPE, while ABCG1 localizes to the basal side. ABCA1 or ABCG1 is 1.4- or 2.5-folds greater in the human retina than in the liver [35]. Moreover, ABCA1, localized on both sides of the retinal epithelial cells, is modulated by LXR in vitro [36, 37]. However, it remains to be confirmed that the inducer LXR and the impaired ABCA1 and/or ABCG1 contribute to lipid efflux and subsequently lead to DR.
2.3.3. LXR and Apolipoprotein Profiles
Blood lipids, including triglycerides, cholesterol, and lipids (phospholipids, glycolipids, and steroids), are insoluble in water and are mainly transported in the form of lipoproteins. Lipoproteins are mainly classified into chylomicrons (CM), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) particles. The main function of HDL is to participate in the reverse transport of cholesterol. The protein part of the plasma lipoproteins is called apolipoprotein, which is divided into the following five types and several subclasses: apolipoprotein A (apoA1, apoA2, apoA4, and apoA5), apoB (apoB48 and apoB100), apoC (apoC-I, apoC-II, apoC-III, and apoC-IV), apoD, apoE, apoF, apoH, apoL, apoM, and apolipoprotein (a).
Different lipoproteins contain different apolipoproteins that carry lipids and stabilize the lipoprotein structure. Also, certain apolipoproteins possess key enzyme activities that regulate lipoprotein metabolism and participate in lipoprotein receptor recognition. LXR is considered the key regulatory target of lipid metabolism and can directly or indirectly regulate apo mRNA expression, protein synthesis and secretion, and apolipoprotein-mediated cholesterol outflow [38, 39]. Following LXR activation by an agonist, cholesterol efflux is promoted through the RCT pathway and is taken up by apoA-I and apoE to form new HDL molecules, which enter the visceral liver for further transformation and/or decomposition [40].
apoA-I is mainly secreted by the liver and small intestine and is the main structural protein and responsible for the major functions of HDL. apoA-I, a 28 kDa single-chain polypeptide consisting of 243 amino acid residues, possess antioxidant, anti-inflammatory, and antiatherosclerotic activity [41]. apoA-I participates in intracellular cholesterol efflux with ABCA1 [42] to transport blood cholesterol to the liver [43]. apoA4 is a major protein component of lymph chylomicrons, VLDL, and HDL. Both apoA-I and apoA4, essential for lipoprotein metabolism to maintain plasma lipid levels, are modulators of vascular disease [44].
apoA1 can be upregulated by LXR dependent or independent of ABCA1. Endogenous ABC1 gene expression and apolipoprotein A1-mediated cholesterol efflux are upregulated via both receptor ligands, indicating that activation of the LXR-RXR heterodimer by ligands contributes to the ABCA1 pathway therapeutic modulation [9]. The LXR agonist GW3965 has also been shown to increase apoA1 expression independent of ABCA1; apoA1 participates in brain-blood barrier regulation and serves to integrate peripheral and CAN lipid metabolism [25].
apoB is a major component protein of LDL, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), chylomicrons, and LDL. apoB plays a significant role in lipoprotein transport. Plasma levels of apoB and LDL were increased by IDOL (the E3 ubiquitin ligase) which was induced by a synthetic LXR agonist in a nonhuman primate model [45]. Intracellular trafficking of the apical apolipoprotein B was also downregulated by LXR agonists to lower the risk of cardiovascular disorders and T2DM in an in vitro model [46].
Liver secreted apoE in lipid metabolism after entering circulation. apoE is also mainly synthesized in the astrocytes in the brain. apoE has three major allies: apoE-ε2 (cys112, cys158), apoE-ε3 (cys112, arg158), and apo-ε4 (arg112, arg158). apoE-ε2 has both increased and decreased risk for atherosclerosis [47]. apoE-ε3 has been thought to be the “neutral” apoE genotype; apo-ε4 was reported to be involved in the pathogenesis of atherosclerosis [47] and Alzheimer's diseases [48]. LXR directly regulates apoE gene expression in macrophages to control its expression [38]. apoE plays an antiatherosclerotic effect by cooperating with specific apolipoproteins, maybe via maintaining the stability of ABCA1. apoE4 is an independent risk factor for Alzheimer's disease [49].
apoD in the formation of HDL is positively regulated by LXR via reverse transport of cholesterol by promoting cholesterol esterification. apoM is mainly synthesized by the liver and kidneys to regulate the formation of pre-β-HDL via reverse cholesterol transport [50]. apoM also modulates the expression of inflammatory factors and adhesion molecules [49].
Although apoC-II and apoC-III have been implicated in cardiovascular diseases and DM, few literatures reported their link to the RCT pathway. It is interesting to investigate the correlations between other apo members and LXR in future studies.
2.3.4. LXR and Cholesterol Transport under Normal Physiological Conditions
LXR activation promotes cholesterol efflux in cells. In the presence of excess intracellular cholesterol, cholesterol oxidation derivatives activate the LXR/RXR heterodimer, initiate the target gene transcription, increase the protein expression of ABCA1 and ABCG1 on the cell membrane, and promote the excretion of cholesterol from the cell. In addition, LXR indirectly inhibits cholesterol synthesis and small intestine absorption via activating the transcriptional expression of its downstream genes. Cholesterol is synthesized in hepatocytes using Acetyl-CoA. This process is inversely regulated by the RCT pathway via inhibiting the expression of the key enzyme squalene synthase [51]. Following LXR activation, ABCA1, ABCG5, and ABCG8 are upregulated to reduce cholesterol absorption in the small intestine. In addition, LXR activation also promotes liver cholesterol conversion and efflux to maintain the whole-body cholesterol level because the liver is the main organ to be used for cholesterol excretion. Cholesterol is converted into bile acids and excreted in the liver using cholesterol 7α hydroxylase (CY7PA). Activated LXR upregulates the expression levels of ABCG5 and ABCG8 and promotes the secretion and/or excretion of hepatobiliary cholesterol.
3. Cholesterol Metabolism in the Retina
Cholesterol metabolism in the retina includes uptake from the systemic circulation, self-synthesis, and clearance. However, the underlying mechanism is not fully understood. The blood-retinal barrier and blood-brain barrier are the unique structures that represent a functional interface between the bloodstream and neuronal vascular microenvironment, respectively. Cholesterol derived from the brain and retina is mainly found in the form of nonesterified cholesterol, suggesting that the retina may maintain cholesterol metabolism balance in a similar way to that of the brain. Current research indicates that the cholesterol in the brain all relies on its own synthesis and that cholesterol is not obtained from systemic circulation [52]. Previous studies have rarely examined cholesterol metabolism in the retina, and very little is known regarding the balance of cholesterol metabolism in the neuroretina. Cholesterol is found to be synthesized in the retina using radioactive cholesterol precursors and immunohistochemically labelled cholesterol synthesis rate-limiting enzyme HMGCR in rats [53]. Specific compounds were shown to inhibit the last step of cholesterol synthesis which leads to degradation of 7-dehydrocholesterol, suggesting that a large amount of cholesterol precursors is accumulated in the retina [54]. Furthermore, reduction of the cholesterol levels is found to be accompanied by progressive retinal degeneration, suggesting that the retinal tissue requires cholesterol metabolism balance via endogenous cholesterol synthesis in order to maintain the normal structure and function of the retina.However, a controversial finding is also reported, showing that cholesterol synthesis does not occur in the retina by itself but from the systemic circulation. Cholesterol enters the retinal neuroepithelial layer through the RPE layer. Fluorescently labelled cholesterol was detected in the RPE layer, and a small amount of fluorescence is presented in the neural retina following injection of fluorescently labelled low-density lipoproteins in the rhesus monkeys [55]. The strong signal of fluorescently labelled LDL is detected in the RPE layer of the retina and then is found to be gradually spread in the entire retina [56]. Only a small amount of fluorescent label is detected in the HDL cholesterol group. Such finding further illustrates that RPE and neural retinas could rapidly take up cholesterol from the systemic circulation, suggesting that LDL may be an important transporter that uptakes systemic cholesterol to the retina.
4. Correlation between Cholesterol Metabolism, Diabetes, and Diabetic Retinopathy
Elevated serum LDL-C and reduced HDL-C levels are high-risk factors for cardiovascular disease, whereas it has also been shown that excessive cholesterol concentration leads to hypercholesterolemia. Dyslipidemia with microvascular complications has been intensively investigated [57]. However, the associations between traditional lipid markers and DR remain controversial. The full understanding and recognition of the importance of lipid-lowering treatment are considered important for the tertiary prevention of diabetes microvascular complications [58, 59].
The mechanism of dyslipidemia promotion may be at several certain levels. The retinal-specific lipid metabolism contributes to low-grade inflammation resulting in diabetic BRB breakdown [60]. Furthermore, the number of bone marrow-derived (BMD) circulating angiogenic cells (CACs) is decreased in dyslipidemia. Action to Control Cardiovascular Risk in Diabetes found the associations between blood lipid levels and hard retinal exudation [61–63]. The total serum cholesterol or the LDL-C of the patients is increased at baseline, and retinal hard exudation is more likely to occur in the Early Treatment Diabetic Retinopathy Study (ETDRS) [64]. Klein and colleagues evaluate the correlations between the serum level of blood lipids in a five-year followed up cohort. A correlation between DR and the total cholesterol/HDL-C ratio is found, confirming that cholesterol-lowering treatment may delay DR progression [65]. The Hoorn study [66] evaluates the risk factors for DR in 2484 Caucasians aged between 50 and 74 years. The results demonstrate that the prevalence of DR is positively associated with body mass index (BMI), serum cholesterol, and triglyceride levels. In addition, elevated levels of plasma total cholesterol and LDL-C are associated with the occurrence and development of retinal hard exudation. To evaluate the risk factors for diabetic microvascular and macrovascular complications, Nazimek-Siewniak and colleagues [67] investigate the associations between the serum levels of fasting blood glucose, total cholesterol, triglycerides, blood pressure, and BMI with the development of DR in 2175 newly diagnosed type 2 diabetes patients. It is found that elevated total cholesterol significantly increased the risk of developing proliferative retinopathy in diabetic patients. Hadjadj et al. report that high triglycerides are independent predictors of renal and retinal complications in patients with type 1 diabetes without end-stage renal disease [68].
It is further reported by the Diabetes Control and Complications Trial (DCCT) that the total HDL-C ratio and LDL-C concentration are predictors for the development of CSME and hard exudation. Hyperlipidemia may increase the risk of CSME and hard retinal exudation, whereas lipid-lowering treatment in patients with type 1 diabetes may reduce the risk of CSME [69]. On the other hand, LDL is found to be an independent risk factor for DR [70], which is in line with the findings compared with simvastatin alone. HDL-C correlates negatively with the occurrence of DR, PDR, and DME in a cohort study in patients with T2DM [71].
Although traditional lipid profiles are found to be associated with the pathogenesis of DR, the conclusive link is still controversial. The correlation between the serum level of total cholesterol and HDL with DR and retinal hard exudative was not found associated with the serum level of total cholesterol and HDL by the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) [72]. The FIELD study [54] enrolled a total of 9995 T2DM patients aged from 50 to 75 years. The patients are followed up for 5 years to evaluate whether long-term use of fenofibrate, the peroxisome proliferator-activated receptor alpha agonist lipid-lowering therapy, could reduce DR progression and the number of laser treatments. The participants receiving fenofibrate (200 mg/d) exhibited a 31% reduction of reduced incidence of DR or progressed to DME in the first laser treatment requirements compared with the placebo group. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is a randomized, multicenter study. Among the 10,251 middle-aged and older participants with T2MD, a subgroup of 2856 subjects were evaluated for the effects of the intensive or standard treatment for glycemia, dyslipidemia, or systolic blood-pressure control at 4 years on the progression of DR. The results showed that at 4 years, the rate of progression of DR was 6.5% with fenofibrate in comparison with 10.2% of the placebo [73]. However, no conclusive relationship between the lipid effects of fenofibrate and the presence or progression of DR was found in both FIELD and ACCORD studies; Wong et al. suggested that the beneficial effects of fenofibrate on DR may be through regulating the levels of apos [74]. Hadjadj et al. also found that apoA1 is correlated negatively with DR, while apoB and apoB/apoA1 ratios are correlated positively with DR [68], which is consistent with others, showing that serum apoB and apoB/apoA ratios are the most important risk factors for PDR and CSME [59].In summary, current guidelines suggest that the use of lipid-lowering drugs as adjuvant therapy can control diabetes-related microvascular complications [58, 75]. However, in certain large epidemiological studies, such as DCCT, EDIC, and WESDR, there is no direct association between the progression of DR and the levels of peripheral blood lipids. It is speculated that retinal-specific mechanisms may play an important role in the progression of DR caused by cholesterol [76], which remains to be clarified in the future. Although several researchers suggest that LDL, HDL, and apo are related to DR in varying degrees, no clear markers exist for the prediction of the occurrence and development of DR. Further research on the association between dyslipidemia and DR could provide additional evidence for the prevention of the development DR. More recently, the application of the nontraditional lipid marker apo has been continuously explored in the detection of lipid-associated disease, and it is expected that further breakthroughs will be achieved in the near future.
5. LXR and Diabetic Retinopathy
LXR is not only involved in the regulation of lipid metabolism but also considered a part of the insulin signaling pathway, which is involved in the process of glucose metabolism [22]. Activation of LXR regulates the systemic inflammatory response by promoting cholesterol metabolism and inhibiting the inflammatory response. LXRβ polymorphisms with type 2 diabetes mellitus and obesity are also evidenced recently [77]. Figure 1 summarizes the effects of the LXR pathway and LXR agonist in the pathogenesis of DR.
It is speculated that LXR may participate in the development of DR through the following mechanisms (Figure 2).
Figure 2.
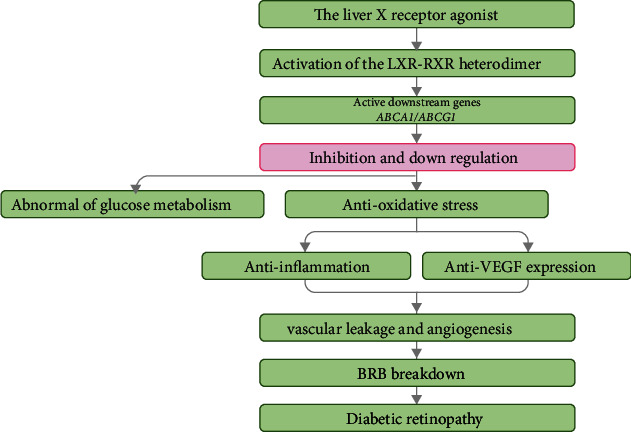
The liver X receptor agonist and diabetic retinopathy. This flow diagram illustrates the regulatory mechanism of activation of LXR signaling by the LXR agonist in DR. LXR: liver X receptor; BRB: blood-retinal breakdown; RXR: retinoid X receptor; DR: diabetic retinopathy.
5.1. Regulation of Glucose Metabolism
LXR participates in the regulation of lipid metabolism and acts as an insulin sensitizer, playing a significant role in glucose metabolism. LXR can be activated with glucose in the liver, which is an endogenous ligand of LXR [78]. Furthermore, LXR inhibits endogenous glucose production, converts excess glucose to glycogen or fatty acids, and stores these molecules in the adipose tissue [79]. Glucose transporter GLUT4 plays a key role in insulin-mediated glucose uptake in adipose tissues. Impairment of GLUT4 is associated with obesity and type 2 diabetes. The promoter of GLUT4 is a direct target gene of LXR. LXR agonists upregulate GLUT4 in adipocytes and promote peripheral glucose uptake [9]. In animal models of diabetes, the LXR agonists GW-3965 and T0901317 significantly reduce blood glucose levels in hyperglycemic mice, improving insulin sensitivity, inhibiting the hepatic synthesis of glycogen, and reducing glucose output [80, 81]. In summary, as a transcription switch, LXR integrates glucose metabolism and lipid metabolism and may be involved in the development of DR.
5.2. Anti-inflammation
Inflammation and oxidative stress are considered important pathological mechanisms of DR. Increased retinal or systemic inflammatory factors in DR patients or animals are reported. Oxidative stress is responsible for the inhibition of inflammation and oxidative stress, which prevents the development of DR significantly [82, 83]. Because activation of LXR inhibits inflammatory response via promoting cholesterol metabolism, LXR is considered to be one of the key receptors that regulate systemic inflammation [13, 84]. Anti-inflammatory mechanisms of LXR include transrepression, posttranscriptional level regulation, and inhibition of inflammatory factor expression via regulating lipid metabolism. Activation of LXR inhibits the activity of transcription factors, such as NF-κB, to block NF-κB-dependent inflammatory factors [85], regulating the expression of inflammatory factors via posttranscriptional mechanisms. Activation of LXR further inhibits the expression levels of the inflammatory factors via regulating lipid metabolism [86]. This is supported by the finding that oral administration of LXR agonists significantly prevents DR development and reduces inflammatory cells in the retina via downregulating oxidative stress genes in an STZ-induced diabetic model [13]. LXR is also found to normalize reverse cholesterol transport and prevent diabetes-induced inflammation in retinal cells. Activation of LXR reduces the number of proinflammatory macrophages and prevented DR-like pathology. The aforementioned findings suggest that LXR agonists may prevent diabetic vascular disease by inhibiting the inflammatory response and oxidative stress.
5.3. Inhibition of Angiogenesis
Vascular endothelial growth factor (VEGF) is a specific mitogenic protein secreted by endothelial cells, with the VEGF family of VEGF-A, B, C, D, and E and placental growth factor (PlGF). These VEGFs activate the downstream cascade after binding to VEGF receptors (VEGFR-1, VEGFR-2, and VEGFR-3).
VEGF-A is the most abundant and biologically active form among the VEGF family in tissues. Upregulation of VEGF in retinal vascular endothelial cells leads to an increase in the permeability of capillaries, which subsequently breaks down the blood-retinal barrier and acceleration of neovascularization and eventually exacerbates the progression of DR [87].
LXR agonists improve cholesterol metabolism and inhibit human umbilical vein vascular endothelial cell proliferation and migration, as well as formation of luminal structures via downregulating VEGF2 l [88]. Activation of the LXR receptor is found to suppress angiogenesis via induction of apoD in human umbilical vein endothelial cells [89]. It is also found that LXR activation reduces angiogenesis by impairing lipid raft localization and signaling of VEGF receptor 2. Furthermore, VEGF-A can be upregulated in the presence of LDL, suggesting that VEGF-A synergizes the effects of LDL contributing to the progression of atherosclerosis [90]. Therefore, further exploration of the association between VEGF expression, LXR pathway, and cholesterol metabolism may aid the identification of new targets for DR intervention.
6. LXR Agonist Modulates Diabetic Retinopathy Pathophysiology
LXR has been proved to be the novel therapeutic target in both animal models and humans. Synthetic oxysterol as the gene-selective LXR modulator mediates transcriptional activation of ABCA1 gene expression with minimal effects on SREBP-1c both in vitro and in vivo in mice [91]. Selective LXRα and LXRβ increased HDL cholesterol and increased liver triglycerides in wild-type mice, induced ABCA1 expression, and stimulated cholesterol efflux in macrophages from both LXRα- and LXRβ-deficient mice [92].
Synthetic chemical agonists of LXT have been shown efficacious for inhibition of DR in diabetic animal models. However, due to the adverse effect profile, including hypertriglyceridemia and hepatic steatosis, the trials were halted [93, 94]. N,N-Dimethyl-3β-hydroxy-cholenamide (DMHCA) is a synthetic oxysterol with shortened sidechain and amide moiety which exhibits only limited activity for increasing hepatic SREBP-1c mRNA and does not stimulate triglyceride synthesis and alter circulating plasma triglycerides in comparison with known nonsteroidal LXR agonists [95]. Cell-based studies also indicate that this selective modulator inhibits cholesterol accumulation. DMHCA has been shown to rescue retinal and BM dysfunction in diabetes, thereby restoring retinal structure, function, and cholesterol homeostasis [95]. DMHCA has been tested in patients with advanced solid malignancies and lymphoma and with refractory malignancies [96].
There are also several ongoing clinical trials of DMHCA targeting on DR. An observational study is being carried out to investigate if LXR activation can restore cholesterol homeostasis in the diabetic retina and rescue diabetes-induced bone marrow dysfunction to sustain circulating angiogenic cells (CAC) and macrophages (ClinicalTrials.gov Identifier: NCT03403686).
7. Perspective
Previous studies have confirmed an inextricable link between abnormal cholesterol metabolism and the development of DR. Further research on lipid metabolites, such as the identification of cholesterol and nontraditional lipid markers, will aid the clarification of their role in disease development and provide new intervention directions for the preventive diagnosis and treatment of DR. All of this is expected to become a novel research hotspot with regard to the prevention and treatment of DR.
In summary, the current literature suggests that the RCT pathway plays an important role in the process of cholesterol metabolism. Recently, several studies have examined the interaction of LXR thoroughly with its agonists in an attempt to provide potential directions for clinical transformation. At present, a variety of artificial LXR agonists have been synthesized. However, since LXR agonists are applied to the whole body, they may upregulate SREBP-1c expression and produce related undesired adverse reactions, including induction of adipogenesis, hypertriglyceridemia, and liver steatosis. Although there are no safe and effective LXR agonists for clinical application yet, the development of LXR selective receptor agonists might promote the identification of novel LXR agonists that do not alter liver and plasma triglyceride levels. LXR agonists could be tailored therapeutical targets in the treatment of DR and other diseases with abnormal cholesterol metabolism in the near future.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant 81570850, Grant 81170859, and grant 82070988) and the Ministry of Science and Technology Foundation of China (Grant 2016YFC1305604).
Abbreviations
- ABCA1:
ATP-binding cassette subfamily A member 1
- ABCG1:
ATP-binding cassette subfamily G member 1
- ACCORD:
Action to Control Cardiovascular Risk in Diabetes
- Apo:
Apolipoproteins
- BMD:
Bone marrow-derived
- BMI:
Body mass index
- CAC:
Circulating angiogenic cells
- CETP:
Cholesterol ester transfer protein
- CM:
Chylomicrons
- CSME:
Clinical significant macular edema
- CY7PA:
Cholesterol 7α hydroxylase
- DMHCA:
N-Dimethyl-3β-hydroxy-cholenamide
- DR:
Diabetic retinopathy
- ETDRS:
Early Treatment Diabetic Retinopathy Study
- GLUT4:
Glucose transporter type 4
- HDL:
High-density lipoprotein
- LDL:
Low-density lipoprotein
- LXR:
Liver X receptor
- PlGF:
Placental growth factor
- RCT:
Cholesterol reverse transport
- RXR:
Retinoid X receptor
- VEGF:
Vascular endothelial growth factor
- VEGFR:
VEGF receptors
- VLDL:
Very low-density lipoprotein
- WESDR:
Wisconsin Epidemiological Study of Diabetic Retinopathy.
Data Availability
This review did not include any original data.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Yeagle P. L. Modulation of membrane function by cholesterol. Biochimie . 1991;73(10):1303–1310. doi: 10.1016/0300-9084(91)90093-G. [DOI] [PubMed] [Google Scholar]
- 2.Payne A. H., Hales D. B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Reviews . 2004;25(6):947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 3.Incardona J. P., Eaton S. Cholesterol in signal transduction. Current Opinion in Cell Biology . 2000;12(2):193–203. doi: 10.1016/S0955-0674(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 4.Czuba E., Steliga A., Lietzau G., Kowiański P. Cholesterol as a modifying agent of the neurovascular unit structure and function under physiological and pathological conditions. Metabolic Brain Disease . 2017;32(4):935–948. doi: 10.1007/s11011-017-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science . 1976;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- 6.Betters J. L., Yu L. NPC1L1 and cholesterol transport. Febs Letters . 2010;584(13):2740–2747. doi: 10.1016/j.febslet.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H., Tan K. C. B., Shiu S. W. M., Wong Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes/Metabolism Research and Reviews . 2008;24(8):617–623. doi: 10.1002/dmrr.895. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi R., Mu H., Wang X., Yao Q., Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. Qjm . 2005;98(12):845–856. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 9.Laffitte B. A., Chao L. C., Li J., et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proceedings of the National Academy of Sciences of the United States of America . 2003;100(9):5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nature Reviews Drug Discovery . 2014;13(6):433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 11.Peet D. J., Janowski B. A., Mangelsdorf D. J. The LXRs: a new class of oxysterol receptors. Current Opinion in Genetics & Development . 1998;8(5):571–575. doi: 10.1016/S0959-437X(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian B., Kim H.-J., Mothana R. A., Kim Y. O., Siddiqui N. A. Role of LXR alpha in regulating expression of glucose transporter 4 in adipocytes -- Investigation on improvement of health of diabetic patients. Journal of Infection and Public Health . 2020;13(2):244–252. doi: 10.1016/j.jiph.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Hazra S., Rasheed A., Bhatwadekar A., et al. Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes . 2012;61(12):3270–3279. doi: 10.2337/db11-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes & Development . 1995;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson T., Treuter E., Gustafsson J.-Å., Steffensen K. R. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends in Pharmacological Sciences . 2012;33(7):394–404. doi: 10.1016/j.tips.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L., Choi H. Y., Li W.-P., Xu F., Herz J. LRP1 controls cPLA2 phosphorylation, ABCA1 expression and cellular cholesterol export. PLoS One . 2009;4(8, article e6853) doi: 10.1371/journal.pone.0006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabol S. L., Brewer H. B., Jr., Santamarina-Fojo S. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. Journal of Lipid Research . 2005;46(10):2151–2167. doi: 10.1194/jlr.M500080-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T., Shimano H., Amemiya-Kudo M., et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Molecular & Cellular Biology . 2001;21(9):2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph S. B., Laffitte B. A., Patel P. H., et al. Direct and Indirect Mechanisms for Regulation of Fatty Acid Synthase Gene Expression by Liver X Receptors. Journal of Biological Chemistry . 2002;277(13):11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 20.Ratliff E. P., Gutierrez A., Davis R. A. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet- induced hypercholesterolemia. Journal of Lipid Research . 2006;47(7):1513–1520. doi: 10.1194/jlr.M600120-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Mak P. A., Laffitte B. A., Desrumaux C., et al. Regulated Expression of the Apolipoprotein E/C-I/C-IV/C-II Gene Cluster in Murine and Human Macrophages: Journal of Biological Chemistry . 2002;277(35):31900–31908. doi: 10.1074/jbc.M202993200. [DOI] [PubMed] [Google Scholar]
- 22.Pettersson A. M. L., Stenson B. M., Lorente-Cebrián S., et al. LXR is a negative regulator of glucose uptake in human adipocytes. Diabetologia . 2013;56(9):2044–2054. doi: 10.1007/s00125-013-2954-5. [DOI] [PubMed] [Google Scholar]
- 23.Edwards P. A., Kennedy M. A., Mak P. A. LXRs;: Oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascular pharmacology . 2002;38(4):249–256. doi: 10.1016/S1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 24.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., Mangelsdorf D. J. Regulation of ATP-binding Cassette Sterol Transporters ABCG5 and ABCG8 by the Liver X Receptors α and β. Journal of Biological Chemistry . 2002;277(21):18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y.-F., Kim H., Kim H. S., Park W. J., Kim J. Y., Chung D. K. Lactobacillus acidophilus K301 inhibits atherogenesis via induction of 24 (S), 25-epoxycholesterol-mediated ABCA1 and ABCG1 production and cholesterol efflux in macrophages. PLoSOne . 2016;11(4, article e0154302) doi: 10.1371/journal.pone.0154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J., Zhao R. Q., Parro C., et al. Small molecule inducers of ABCA1 and apoE that act through indirect activation of the LXR pathway. Journal of Lipid Research . 2018;59(5):830–842. doi: 10.1194/jlr.M081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeree P., Janvilisri T., Kangsamaksin T., Tohtong R., Kumkate S. Downregulation of ABCA1 and ABCG1 transporters by simvastatin in cholangiocarcinoma cells. Oncology Letters . 2019;18(5):5173–5184. doi: 10.3892/ol.2019.10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiriet M. Hyperlipidemias and obesity . 2018.
- 29.Vaughan A. M., Oram J. F. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. Journal of Biological Chemistry . 2005;280(34):30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 30.Langmann T., Klucken J., Reil M., et al. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochemical & Biophysical Research Communications . 1999;257(1):29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 31.Hassan H. H., Denis M., Krimbou L., Marcil M., Genest J. Homeostasie cellulaire du cholesterol dans les cellules endotheliales vasculaires. Canadian Journal of Cardiology . 2006;22(Supplement B):35B–40B. doi: 10.1016/S0828-282X(06)70985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terasaka N., Yu S., Yvan-Charvet L., et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. Journal of Clinical Investigation . 2008;118(11):3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrer L., Ohnsorg P. M., Lehner M., Landolt F., Rinninger F., von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circulation Research . 2009;104(10):1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 34.Fisher E. A., Feig J. E., Hewing B., Hazen S. L., Smith J. D. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arteriosclerosis, Thrombosis, and Vascular Biology . 2012;32(12):2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J. W., Moreira E. F., Rodriguez I. R. Oxysterols induce the expression of ABCA1 and ABCG1 in cultured RPE cells. Investigative Ophthalmology & Visual Science . 2008;49(13) [Google Scholar]
- 36.Storti F., Raphael G., Griesser V., et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human RPE cells. Experimental Eye Research . 2017;165:65–77. doi: 10.1016/j.exer.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Storti F., Klee K., Todorova V., et al. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. Elife . 2019;8, article e45100 doi: 10.7554/eLife.45100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laffitte B. A., Repa J. J., Joseph S. B., et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proceedings of the National Academy of Sciences of the United States of America . 2001;98(2):507–512. doi: 10.1073/pnas.98.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelcer N., Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. Journal of Clinical Investigation . 2006;116(3):607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zannis V. I., Chroni A., Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. Journal of Molecular Medicine . 2006;84(4):276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 41.Saito H., Dhanasekaran P., Nguyen D., et al. α-Helix Formation Is Required for High Affinity Binding of Human Apolipoprotein A-I to Lipids. Journal of Biological Chemistry . 2004;279(20):20974–20981. doi: 10.1074/jbc.M402043200. [DOI] [PubMed] [Google Scholar]
- 42.Ribalta J., Vallvé J.-C., Girona J., Masana L. Apolipoprotein and apolipoprotein receptor genes, blood lipids and disease. Current Opinion in Clinical Nutrition & Metabolic Care . 2003;6(2):177–187. doi: 10.1097/00075197-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Zhu L., Parker M., Enemchukwu N., et al. Combination of apolipoprotein-A-I/apolipoprotein-A-I binding protein and anti-VEGF treatment overcomes anti-VEGF resistance in choroidal neovascularization in mice. Communications Biology . 2020;3(1):p. 386. doi: 10.1038/s42003-020-1113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida A., Srivastava S. K., Manjunath D., et al. Impact of Drusen burden on incidence of subclinical CNV with OCTA. Ophthalmic Surgery, Lasers Imaging Retina . 2020;51(1):22–30. doi: 10.3928/23258160-20191211-03. [DOI] [PubMed] [Google Scholar]
- 45.Lee S. D., Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis . 2015;242(1):29–36. doi: 10.1016/j.atherosclerosis.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briand O., Touche V., Colin S., et al. Liver X Receptor Regulates Triglyceride Absorption Through Intestinal Down- regulation of Scavenger Receptor Class B, Type 1. Gastroenterology . 2016;150(3):650–658. doi: 10.1053/j.gastro.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis . 1988;8(1):1–21. doi: 10.1161/01.ATV.8.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Corder E. H., Saunders A. M., Strittmatter W. J., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science . 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y., Li J., Shuai Z., Xiaochuan S. Apo E4 influences growth cone of neuronal axon through ERK signal pathway. Journal of Third Military Medical University . 2012;34 [Google Scholar]
- 50.Lin C.-I., Chen C.-N., Lin P.-W., Lee H. Sphingosine 1-phosphate regulates inflammation-related genes in human endothelial cells through S1P1 and S1P3. Biochemical & Biophysical Research Communications . 2007;355(4):895–901. doi: 10.1016/j.bbrc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Rogers P. M., Su C., Varga G., Stayrook K. R., Burris T. P. Regulation of Cholesterologenesis by the Oxysterol Receptor, LXRα. Journal of Biological Chemistry . 2008;283(39):26332–26339. doi: 10.1074/jbc.M804808200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arteriosclerosis, Thrombosis, and Vascular Biology . 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 53.Fliesler S. J., Florman R., Rapp L. M., Pittler S. J., Keller R. K. In vivo biosynthesis of cholesterol in the rat retina. Febs Letters . 1993;335(2):234–238. doi: 10.1016/0014-5793(93)80736-E. [DOI] [PubMed] [Google Scholar]
- 54.Fliesler S. J. Retinal Degenerative Diseases . Vol. 664. Springer; 2010. Retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome: thinking beyond cholesterol deficiency; pp. 481–489. (Advances in Experimental Medicine and Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elner V. M. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Transactions of the American Ophthalmological Society . 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- 56.Tserentsoodol N., Sztein J., Campos M., et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Molecular Vision . 2006;12:1306–1318. [PubMed] [Google Scholar]
- 57.Misra A., Kumar S., Kishore Vikram N., Kumar A. The role of lipids in the development of diabetic microvascular complications: implications for therapy. American Journal of Cardiovascular Drugs . 2003;3(5):325–338. doi: 10.2165/00129784-200303050-00004. [DOI] [PubMed] [Google Scholar]
- 58.Keech A., Mitchell P., Summanen P., et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. The Lancet . 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 59.Crosby-Nwaobi R., Chatziralli I., Sergentanis T., Dew T., Forbes A., Sivaprasad S. Cross talk between lipid metabolism and inflammatory markers in patients with diabetic retinopathy. Journal of Diabetes Research . 2015;2015:9. doi: 10.1155/2015/191382.191382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammer S. S., Beli E., Kady N., et al. The mechanism of diabetic retinopathy pathogenesis unifying key lipid regulators, sirtuin 1 and liver X receptor. EBioMedicine . 2017;22:181–190. doi: 10.1016/j.ebiom.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasaki M., Kawasaki R., Noonan J. E., Wong T. Y., Lamoureux E., Wang J. J. Quantitative measurement of hard exudates in patients with diabetes and their associations with serum lipid levels. Investigative Ophthalmology & Visual Science . 2013;54(8):5544–5550. doi: 10.1167/iovs.13-11849. [DOI] [PubMed] [Google Scholar]
- 62.Gupta A., Gupta V., Thapar S., Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. American Journal of Ophthalmology . 2004;137(4):675–682. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Papavasileiou E., Davoudi S., Roohipoor R., et al. Association of serum lipid levels with retinal hard exudate area in African Americans with type 2 diabetes. Graefe's Archive for Clinical and Experimental Ophthalmology . 2017;255(3):509–517. doi: 10.1007/s00417-016-3493-9. [DOI] [PubMed] [Google Scholar]
- 64.Chew E. Y., Klein M. L., Ferris F. L., 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) report 22. Archives of Ophthalmology . 1996;114(9):1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 65.Klein B., Klein R., Moss S. Is serum cholesterol associated with progression of diabetic retinopathy or macular edema in persons with younger-onset diabetes of long duration? American Journal of Ophthalmology . 1999;128(5):652–654. doi: 10.1016/S0002-9394(99)00222-6. [DOI] [PubMed] [Google Scholar]
- 66.van Leiden H. A., Dekker J. M., Moll A. C., et al. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care . 2002;25(8):1320–1325. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 67.Nazimek-Siewniak B., Moczulski D., Grzeszczak W. Risk of macrovascular and microvascular complications in type 2 diabetes: results of longitudinal study design. Journal of Diabetes & Its Complications . 2002;16(4):271–276. doi: 10.1016/S1056-8727(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 68.Hadjadj S., Duly-Bouhanick B., Bekherraz A., et al. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes & Metabolism . 2004;30(1):43–51. doi: 10.1016/S1262-3636(07)70088-5. [DOI] [PubMed] [Google Scholar]
- 69.Miljanovic B., Glynn R. J., Nathan D. M., Manson J. E., Schaumberg D. A. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes . 2004;53(11):2883–2892. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- 70.Wong T. Y., Cheung N., Tay W. T., et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology . 2008;115(11):1869–1875. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 71.Toth P. P., Simko R. J., Palli S. R., Koselleck D., Quimbo R. A., Cziraky M. J. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovascular Diabetology . 2012;11(1):p. 109. doi: 10.1186/1475-2840-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein B. E., Moss S. E., Klein R., Surawicz T. S. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XIII. Relationship of Serum Cholesterol to Retinopathy and Hard Exudate. Ophthalmology . 1991;98(8):1261–1265. doi: 10.1016/S0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 73.ACCORD Study Group and ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. New England Journal of Medicine . 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong T. Y., Simó R., Mitchell P. Fenofibrate - a potential systemic treatment for diabetic retinopathy? American Journal of Ophthalmology . 2012;154(1):6–12. doi: 10.1016/j.ajo.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Elam M., Lovato L., Ginsberg H. The ACCORD-lipid study: implications for treatment of dyslipidemia in type 2 diabetes mellitus. Clinical Lipidology . 2011;6(1):9–20. doi: 10.2217/clp.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasongko M. B., Wong T. Y., Nguyen T. T., et al. Serum apolipoproteins are associated with systemic and retinal microvascular function in people with diabetes. Diabetes . 2012;61(7):1785–1792. doi: 10.2337/db11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solaas K., Legry V., Retterstol K., et al. Suggestive evidence of associations between liver X receptor β polymorphisms with type 2 diabetes mellitus and obesity in three cohort studies: HUNT2 (Norway), MONICA (France) and HELENA (Europe) BMC Medical Genetics . 2010;11(1):p. 144. doi: 10.1186/1471-2350-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grempler R., Günther S., Steffensen K. R., et al. Evidence for an indirect transcriptional regulation of glucose-6-phosphatase gene expression by liver X receptors. Biochemical & Biophysical Research Communications . 2005;338(2):981–986. doi: 10.1016/j.bbrc.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 79.Mitro N., Mak P. A., Vargas L., et al. The nuclear receptor LXR is a glucose sensor. Nature . 2007;445(7124):219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 80.Cao G., Liang Y., Broderick C. L., et al. Antidiabetic Action of a Liver X Receptor Agonist Mediated By Inhibition of Hepatic Gluconeogenesis. Journal of Biological Chemistry . 2003;278(2):1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 81.Grefhorst A., van Dijk T. H., Hammer A., et al. Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. American Journal of Physiology Endocrinology & Metabolism . 2005;289(5):E829–E838. doi: 10.1152/ajpendo.00165.2005. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W., Liu H., al-Shabrawey M., Caldwell R. W., Caldwell R. B. Inflammation and diabetic retinal microvascular complications. Journal of Cardiovascular Disease Research . 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kowluru R. A., Kanwar M., Chan P.-S., Zhang J. P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Archives of ophthalmology . 2008;126(9):1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faulds M. H., Zhao C., Dahlman-Wright K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Current Opinion in Pharmacology . 2010;10(6):692–697. doi: 10.1016/j.coph.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine . 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 86.de Wit N. M., Vanmol J., Kamermans A., Hendriks J. J. A., de Vries H. E. Inflammation at the blood-brain barrier: The role of liver X receptors. Neurobiology of Disease . 2017;107:57–65. doi: 10.1016/j.nbd.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 87.Romero-Aroca P., Baget-Bernaldiz M., Pareja-Rios A., Lopez-Galvez M., Navarro-Gil R., Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. Journal of Diabetes Research . 2016;2016:17. doi: 10.1155/2016/2156273.2156273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noghero A., Perino A., Seano G., et al. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arteriosclerosis, Thrombosis, and Vascular Biology . 2012;32(9):2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- 89.Lai C.-J., Cheng H.-C., Lin C.-Y., et al. Activation of liver X receptor suppresses angiogenesisviainduction of ApoD. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology . 2017;31(12):5568–5576. doi: 10.1096/fj.201700374R. [DOI] [PubMed] [Google Scholar]
- 90.Kivelä A. M., Dijkstra M. H., Heinonen S. E., et al. Regulation of endothelial lipase and systemic HDL cholesterol levels by SREBPs and VEGF-A. Atherosclerosis . 2012;225(2):335–340. doi: 10.1016/j.atherosclerosis.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 91.Quinet E. M., Savio D. A., Halpern A. R., Chen L., Miller C. P., Nambi P. Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. Journal of Lipid Research . 2004;45(10):1929–1942. doi: 10.1194/jlr.M400257-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Lund E. G., Peterson L. B., Adams A. D., et al. Different roles of liver X receptor α and β in lipid metabolism: Effects of an α-selective and a dual agonist in mice deficient in each subtype. Biochemical Pharmacology . 2006;71(4):453–463. doi: 10.1016/j.bcp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Chisholm J. W., Hong J., Mills S. A., Lawn R. M. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. Journal of Lipid Research . 2003;44(11):2039–2048. doi: 10.1194/jlr.M300135-JLR200. [DOI] [PubMed] [Google Scholar]
- 94.Kirchgessner T. G., Sleph P., Ostrowski J., et al. Beneficial and adverse effects of an LXR agonist on human lipid and lipoprotein metabolism and circulating neutrophils. Cell Metabolism . 2016;24(2):223–233. doi: 10.1016/j.cmet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 95.Vieira C. P., Fortmann S. D., Hossain M., et al. Selective LXR agonist DMHCA corrects retinal and bone marrow dysfunction in type 2 diabetes. JCI Insight . 2020;5(13, article e137230) doi: 10.1172/jci.insight.137230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mita M. M., Mita A. C., Chmielowski B., et al. Pharmacodynamic and clinical activity of RGX-104, a first-in-class immunotherapy targeting the liver-X nuclear hormone receptor (LXR), in patients with refractory malignancies. Journal of Clinical Oncology . 2018;36(15_suppl):3095–3095. doi: 10.1200/JCO.2018.36.15_suppl.3095. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review did not include any original data.


