Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease affecting both adults and children. Belimumab is the only biologic approved for SLE, and the first in a class of drugs known as B-lymphocyte stimulator-specific inhibitors. The introduction of intravenous belimumab in 2011 was a major advance, being the first new therapy approved for SLE in over 50 years. As of April 2021, more than 7200 people with SLE have received belimumab in clinical studies, and it is approved in over 75 countries for the treatment of adults with SLE. A subcutaneous, self-injectable belimumab formulation was licensed in 2017 by both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). Belimumab was then approved for use in children in Europe, the USA and Japan in 2019, and China and Brazil in 2020. Recently, belimumab became the first FDA-approved drug for the treatment of adults with active lupus nephritis (LN), the most-common severe manifestation of SLE.
Over the past 10 years, belimumab has established its position as a disease modifier in the SLE treatment paradigms. Robust evidence from randomised clinical studies and observational, real-world studies has demonstrated the tolerability and efficacy of belimumab for reducing disease activity and the risk of new, severe SLE flares. This enables patients to taper their glucocorticoid use, which limits damage accumulation. Significantly more patients with active LN met the criteria for renal responses and were at less risk of a renal-related event or death after receiving belimumab plus standard therapy, compared with standard therapy on top of mandatory steroid reduction. Ongoing clinical studies are evaluating belimumab’s effectiveness in various indications beyond SLE. Post-marketing and registry studies are gathering additional data on key areas such as pregnancy outcomes after belimumab exposure and belimumab co-administration with other biologics.
Keywords: Musculoskeletal, renal lupus, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that involves the production of autoantibodies and deposition of immune complexes.1,2 SLE presents with various clinical manifestations, 3 among the most severe being lupus nephritis (LN), and SLE occurs more commonly and with poorer long-term outcomes in people of Asian or Black African ancestry and Hispanic populations than in other populations.4–6 Although rare, childhood-onset SLE is associated with more severe disease and greater morbidity and mortality than adult-onset SLE, and until recently, there were no approved therapies for use in children with SLE, making management of paediatric patients even more challenging.7,8
Belimumab is a recombinant, human, immunoglobulin G1 lambda (IgG1λ) monoclonal antibody (mAb) that binds to and antagonises soluble B-lymphocyte stimulator protein (BLyS); it was developed as a novel biologic treatment for SLE. BLyS promotes the survival and differentiation of B lymphocytes into Ig-producing plasma cells9,10 and appears to play a key role in the pathogenesis of SLE and other autoimmune diseases. 9 Here, we discuss the development and approval of belimumab, and review how clinical studies and real-world experience with belimumab over the last decade have cemented its important role in the treatment armamentarium for SLE.
What did the SLE treatment landscape look like before belimumab?
Prior to the introduction of belimumab, there was a considerable unmet need for therapies to manage SLE, with available treatments involving the use of drugs such as antimalarials, corticosteroids and non-specific immunosuppressants. 11 In 1948, aspirin became the first drug to be approved by the US Food and Drug Administration (FDA) for treatment of lupus. 12 The first documented use of glucocorticoids in lupus involved the administration of cortisone in a single patient in 1950. 13 Chloroquine became available in 1953, followed by the FDA approval of both hydroxychloroquine and corticosteroids for lupus in 1955 (Figure 1).12,14,15
Figure 1.
Key milestones in the development of belimumab and the treatment of SLE and LN.
BLyS: B-lymphocyte stimulator; EMA: European Medicines Agency; FDA: Food and Drug Administration; GSK: GlaxoSmithKline; HGS: Human Genome Sciences; LN: lupus nephritis; SLE: systemic lupus erythematosus; ST: standard therapy for SLE; TIGR: The Institute for Genomics Research.
How did the discovery of BLyS lead to the development of belimumab?
The treatment of SLE evolved and benefitted from advances in understanding of the inflammatory and immunological pathways involved in this disease. 11 Human Genome Sciences (HGS; Rockville, MD, USA) and The Institute for Genomics Research (TIGR) were established in 1992 with the aim of using human DNA sequences in drug discovery. HGS carried out extensive screening of hundreds of tissue-specific human cDNA libraries. From one search of a library derived from primary human neutrophils, a single cDNA clone (HNEDU15) was identified, which encoded a new member of the tumour necrosis factor (TNF) ligand superfamily. The protein product of the HNEDU15 transcript was BLyS, a type II transmembrane protein that exists in both membrane-bound and soluble forms. 16
The association between BLyS and human SLE was described in 2001 by research demonstrating a frequent increase in circulating BLyS levels in patients with SLE,17,18 thus providing the rationale to investigate the use of BLyS antagonists. 16 HGS in collaboration with Cambridge Antibody Technology (Cambridge, UK; acquired by AstraZeneca in 2006) screened a human single-chain antibody phage-display library to detect clones that bound to human BLyS. One of the identified clones selected for further development, initially named ‘LymphoStat B’, subsequently became belimumab. 16 GlaxoSmithKline (Brentford, UK) collaborated with HGS on belimumab’s development from 1996 until 2012, and acquired HGS in 2012.
Belimumab was first approved as an intravenous (IV) formulation by both the FDA and European Medicines Agency (EMA) in 2011. This constituted a historic moment in the lupus community, because belimumab was the first new therapy approved for this disease in over 50 years. 15 Belimumab is now approved in over 75 countries for adult patients with SLE.
Following the approval of the IV formulation, there have been many milestones for belimumab (Figure 1); most recently, it became the first drug to be approved by the FDA for the treatment of adults with active LN who are receiving standard therapy (ST).
What were the findings from initial clinical studies of belimumab?
Phase 1 clinical study of belimumab
A randomised, double-blind, placebo-controlled, dose-ranging Phase 1 study (NCT00657007) of belimumab in patients with mild-to-moderate SLE was initiated in 2001 by HGS. The study demonstrated a reduction in peripheral blood B cells and circulating anti-double-stranded deoxyribonucleic acid (dsDNA) antibody levels across all doses in patients receiving belimumab, which was not observed in patients receiving placebo. The incidence of adverse events (AEs) with belimumab at all tested doses (1, 4, 10 or 20 mg/kg as a single infusion or two infusions 21 days apart) was similar to that with placebo. As this was a Phase 1 study, it was not possible to demonstrate significant clinical efficacy, due to the small number of patients, and the short treatment schedule and follow-up period. 19 The results of the Phase 1 study were sufficiently robust to prompt initiation of a Phase 2 study in SLE, during which time GlaxoSmithKline exercised its option to support the continued development of belimumab, and committed to conducting a Phase 3 study in partnership with HGS (Figure 1). 16
Phase 2 clinical study of belimumab and the development of the SLE Responder Index-4 (SRI-4)
In late 2003, a 52-week, randomised, double-blind, placebo-controlled, Phase 2 study (NCT00071487) was initiated to evaluate the effects of IV belimumab (1, 4 or 10 mg/kg) or placebo in adult patients with SLE (N = 449). 20 All patients also received ST (i.e. corticosteroids, antimalarials and/or immunosuppressants except IV cyclophosphamide), and belimumab was administered on Days 0, 14 and 28, and every 28 days thereafter. The co-primary endpoints were percent change from baseline in Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score at Week 24 and time to first mild/moderate or severe SLE flare (SELENA-SLEDAI Flare Index [SFI] 21 ) over 52 weeks, neither of which was met. However, post hoc analyses suggested that serologically active patients (as opposed to those who had a history of measurable autoantibodies) responded significantly better to belimumab therapy plus ST than those receiving ST alone. 20 Moreover, in the belimumab-treated group, those patients who had more active disease (SLEDAI scores ≥6, as opposed to ≥4 to <6) and who were autoantibody-positive showed greater improvement compared with all belimumab-treated patients. 22
Subsequent post hoc analyses were possible because the Phase 2 study included a large patient population and had a robust design. These highlighted several characteristics of the study that likely contributed to the failure of the study to meet its co-primary endpoints. Firstly, the entry criteria required an SLE diagnosis based on historical presence of measurable autoantibodies, but confirmation at baseline was not required. Secondly, the widespread and poorly controlled use of prednisone and other immunosuppressants during the Phase 2 study, and the ability to make unlimited changes to these medications, confounded assessment of SLE disease activity and response. 20 Thirdly, the SELENA-SLEDAI score comprising the co-primary endpoint was not designed to capture changes over time, and its correct use and interpretation can vary depending on the experience level and expertise of the investigator. 23
Taking all these factors into consideration, it was evident that there was a need to develop a new and robust instrument to measure disease activity and clinical response in clinical studies of SLE. Using the Outcome Measures in Rheumatology Trials (OMERACT), guidance from the European League Against Rheumatism (EULAR), and regulatory requirements of the FDA and EMA as guiding principles, the SRI 24 was developed as a composite instrument that incorporates two disease activity indices (SELENA-SLEDAI and British Isles Lupus Assessment Group [BILAG]) plus the Physician’s Global Assessment (PGA; scored on a 0 to 3 visual analogue scale [VAS]). Using the SRI, a patient with SLE is classified as a responder if they achieve a ≥4-point reduction in SELENA-SLEDAI score, have no new BILAG A or ≥2 new BILAG B organ domain scores, and there is no worsening (i.e. <0.3-point increase) in PGA score versus baseline; 24 because of this requirement for a 4-point improvement, the SRI is also known as the SRI-4. SRI-4 response has been associated with improvements in clinical, laboratory and patient reported outcome measures in patients with moderate to severe SLE. 25
Which pivotal studies led to the approval of belimumab and which trials further support its efficacy and safety?
Phase 3 clinical studies of belimumab
Knowledge of the study limitations identified from the Phase 2 clinical study of belimumab guided the design of the subsequent pivotal Phase 3 clinical studies of belimumab. Based on this knowledge, the pivotal studies included only autoantibody-seropositive patients with confirmed SLE at study baseline, strictly regulated changes to concomitant medications after initiation of belimumab therapy only, and used the SRI-4 as the primary endpoint.26,27
Pivotal studies: BLISS-52 and BLISS-76
The multicentre, randomised, placebo-controlled BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) pivotal Phase 3 studies were initiated by GlaxoSmithKline and HGS in 2007 and conducted in parallel.26,27 Patients with SLE receiving ST (BLISS-52, N = 867; BLISS-76, N = 819) were randomised to either IV belimumab 1 mg/kg or 10 mg/kg, or placebo on Days 0, 14 and 28, and every 28 days thereafter for 48 weeks (BLISS-52) or 72 weeks (BLISS-76) (Figure 1). Belimumab dose selection was based on the combined findings of toxicology studies in cynomolgus monkeys and the safety, efficacy and pharmacokinetics (PK) data from Phase 1 and 2 studies in patients with SLE, and the Phase 2 study in patients with RA. In these studies, both 1 and 10 mg/kg doses were biologically active, well tolerated and appeared safe, producing trough levels of active drug in excess of BLyS concentrations in the peripheral circulation. Inclusion of both doses permitted evaluation of whether the 10 mg/kg dose provided greater or more rapid benefit than the 1 mg/kg dose (there was a trend for faster reduction in SELENA-SLEDAI score and greater reductions in corticosteroid use with the higher dose, observed in the Phase 2 SLE study), and enabled the efficacy of both doses to be confirmed separately. Finally, although the 20 mg/kg belimumab dose used in the Phase 1 study provided valuable safety and exposure data that were used to inform regulatory discussions, there was concern that a higher cost of therapy would limit patient access to this treatment globally; therefore, this dose was not included in Phase 3 studies.
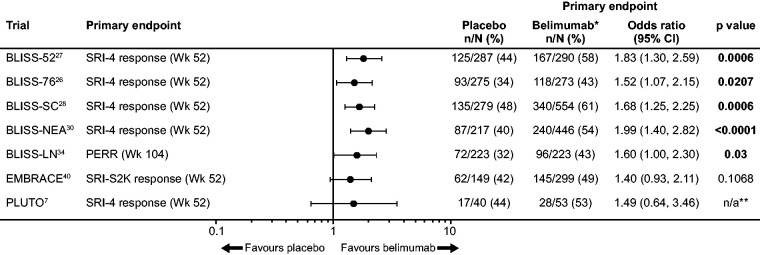
BLISS-52 recruited patients from Latin America, Asia-Pacific and Eastern Europe, while BLISS-76 recruited patients from Western Europe and Northern/Central America, resulting in the latter population including a greater proportion of patients of Black African ancestry.26,27 In both studies, belimumab was associated with a significantly higher SRI-4 response rate at 52 weeks than placebo, denoting a clinically meaningful improvement in SLE disease activity (Figure 2).26,27
Figure 2.
Efficacy of belimumab in Phase 3 studies, the EMBRACE Phase 3/4 study and the paediatric PLUTO Phase 2 study.
*10 mg/kg IV dose, except for BLISS-SC (200 mg SC dose); **Because of the difficulty in recruiting sufficient numbers of paediatric patients with cSLE, statistical significance testing was not possible in the PLUTO study; therefore, all data were analysed descriptively.
CI: confidence interval; cSLE: childhood-onset SLE; IV: intravenous; n/a: not assessed; n/N: patient number/total population; PERR: primary efficacy renal response; SC: subcutaneous; SLE: systemic lupus erythematosus; S2K: SLE Disease Activity Index 2000; SRI-4: SLE Responder Index.
Compared with placebo, belimumab treatment also reduced the number of severe SLE flares and the risk of having a new severe flare over a year, as measured by the SFI and BILAG domain scores.26,27 The safety profiles of both belimumab doses were similar to that of placebo, with similar rates of serious and severe AEs observed across treatment groups.26,27
Pivotal study: BLISS-SC
The 52-week, randomised, double-blind, placebo-controlled BLISS-SC (NCT01484496) study was designed to evaluate the efficacy, safety and tolerability of a weekly fixed-dose formulation of belimumab 200 mg, administered via SC injection using a prefilled syringe, in patients with SLE also receiving ST (N = 839). 28 The SC formulation of belimumab was developed prior to completion of the Phase 3 IV program, with the aim of improving patient convenience and independence, and to reduce the healthcare burden of IV belimumab. 29 The 200 mg dose was derived from a prior post hoc population PK and simulation analysis demonstrating that the PK profile, after weekly SC belimumab 200 mg dosing, was consistent with that of monthly IV belimumab 10 mg/kg dosing, resulting in steady state average plasma belimumab concentrations. 29 A maximum of ∼1–1.2 mL can be injected per administration, and increasing the belimumab concentration within this volume likewise increases the viscosity of the solution. Therefore, this 200 mg/mL belimumab dose represents the limiting factor and resulted in a weekly dosing schedule to match the average plasma concentrations of IV belimumab. Placebo was the comparator, despite the availability of the IV belimumab formulation, because the resources and investment required to conduct a non-inferiority study of IV versus SC belimumab formulations made that option unfeasible; this was acknowledged and accepted by both the FDA and EMA.
In BLISS-SC, belimumab when added to ST demonstrated a significantly greater SRI-4 response (Figure 2), significant improvements in both time to, and risk of severe SFI flare, and a numerically greater steroid-sparing effect, compared with placebo plus ST. Overall, patients in the SC belimumab group experienced fewer renal flares, and significantly fewer patients with baseline proteinuria >0.5 g/24 h who were receiving belimumab experienced a renal flare, compared with the placebo group. Incidences of AEs and serious AEs were similar between treatment groups. 28
SC belimumab can be given using either a prefilled syringe or an autoinjector, thus providing healthcare professionals (HCPs) and patients with options for belimumab administration that best suit their individual needs.
Pivotal study: BLISS-NEA
BLISS-NEA (NCT01345253), like the BLISS-52 and -76 studies, was a 52-week, multicentre, randomised, double-blind, placebo-controlled study of the efficacy and safety of IV belimumab 10 mg/kg treatment among patients with clinically active SLE (defined as a SELENA-SLEDAI score ≥8 at screening) receiving ST located in China, Japan and South Korea (N = 677). 30 This study was conducted to obtain more data on patients from North East Asia, a group under-represented in the BLISS-52 and -76 studies, and to support regulatory approvals of belimumab in this region. Moreover, patients with SLE from China are known to show important differences from their European counterparts in clinical manifestations of SLE, including an increase in haematological disorders and kidney disease, and fewer neurological manifestations.30,31 Compared with the placebo group, significantly more belimumab-treated patients achieved the primary endpoint of SRI-4 response at Week 52 (Figure 2) and halved their risk of experiencing a severe SLE flare. Among patients with baseline prednisone dose >7.5 mg/day, a significant steroid-sparing effect favouring belimumab was noted. Safety profiles were similar between treatment groups and the efficacy and safety results were consistent with those from previous BLISS-52 and -76 studies. 30
Pivotal study: BLISS-LN
Post hoc analyses of patients from the BLISS studies who had SELENA-SLEDAI renal involvement at baseline (N = 267) and from a systematic review of prior belimumab studies (N = 234) suggested that belimumab treatment could improve renal parameters in these patients with SLE.32,33 However, in the pre-registration Phase 3 studies (BLISS-52 and BLISS-76), patients with severe, active LN were excluded, and these studies were not designed to assess the effect of belimumab on renal parameters or active LN.
BLISS-LN (NCT01639339) was a post-approval commitment study representing the largest and longest Phase 3 study conducted in patients with active LN. This 104-week, randomised, double-blind, placebo-controlled study involving 448 adult patients with active LN evaluated the efficacy and safety of IV belimumab plus ST compared to placebo plus ST. 34 Because individuals with increased proteinuria also have increased renal clearance, a conditional dosing regimen (every 2 weeks instead of every 4 weeks) was considered for BLISS-LN, based on observations from a mechanistic study of belimumab in patients with idiopathic membranous nephritis, a disease with even more severe proteinuria than that seen in LN. 35 Ultimately, the IV belimumab 10 mg/kg dose used in previous BLISS studies was retained for BLISS-LN because it was considered sufficient to accommodate the level of proteinuria seen in LN.
This study met its primary endpoint of primary efficacy renal response (RR; defined as a urinary protein to creatinine ratio (uPCR) of ≤0.7, an estimated glomerular filtration rate (eGFR) ≥20% below the pre-flare value or ≥60 mL/minute/1.73 m2, and no use of rescue therapy for treatment failure) at 104 weeks (Figure 2) and all four major secondary endpoints including complete renal response at 104 weeks (CRR; defined as eGFR ≥10% below pre-flare value or ≥90 mL/min/1.73 m2, inactive urinary sediment [<5 red blood cells and <5 white blood cells/high power field or within laboratory reference range], no red/white blood cell casts, uPCR <0.5, and no use of rescue therapy for treatment failure), RR at 52 weeks, and time to renal-related event or death.
The RR definition was selected based on evidence that a reduction in proteinuria to <0.8– <0.7 g/day was the best predictor of favourable long-term prognosis in patients with LN, in terms of development of chronic kidney disease and end-stage kidney disease-free survival,36,37 and this target is supported by EULAR recommendations. 38
Additionally, the BLISS-LN study demonstrated that amongst patients receiving belimumab plus ST the risk of a renal-related event or death was approximately halved. 34 This endpoint is an important and clinically relevant outcome as it evaluates kidney-related events associated with long-term progression to kidney failure. The safety results from BLISS-LN were consistent with the known safety profile of belimumab. 34
Phase 3/4 study: EMBRACE
Pooled results from the BLISS-52 and -76 studies, suggested that the subgroup of patients of Black African ancestry did not appear to benefit from IV belimumab treatment, whereas in the Phase 2 study, IV belimumab did demonstrate a benefit in post hoc subgroup analyses of patients of Black African ancestry.16,39 The Efficacy of BeliMumab in Subjects of Black RACE (EMBRACE) study was a 52-week, randomised, double-blind, placebo-controlled, Phase 3/4, post-approval commitment study that evaluated the efficacy and safety of IV belimumab in patients of Black African ancestry with SLE (N = 448). 40 The primary endpoint was SRI-4 response rate with modified SLE Disease Activity Index 2000 (SLEDAI-2K) scoring for proteinuria (SRI-S2K) at Week 52, defined as a ≥4-point reduction in SELENA-SLEDAI-S2K score versus baseline, no new BILAG A or two new BILAG B organ domain scores, and no worsening (i.e. <0.30-point increase) in PGA score (on a 0 to 3 VAS). EMBRACE failed to achieve its primary endpoint, although numerical trends in favour of belimumab were observed (Figure 2). Significant improvements with belimumab versus placebo were observed in subgroups of patients with characteristics of high disease activity, including those with baseline SELENA-SLEDAI-S2K score ≥10, low baseline C3 and/or C4 levels or low C3 and/or C4 levels and anti-dsDNA ≥30 IU/mL. As in the previous BLISS studies, the rates of AEs or serious AEs were similar between treatment groups. 40
Phase 4 clinical study to assess safety outcomes: BASE
Although belimumab had been approved by the FDA based on a favourable benefit:risk profile in the treatment of adult patients with SLE, numerical differences in the incidence of mortality, hypersensitivity reactions, some psychiatric events, and the potential for malignancy and infections based on its mechanism of action warranted a large safety study to assess these events as a post-approval commitment.
BASE (NCT01705977) was a double-blind, randomised, placebo-controlled study that enrolled 4003 adults with active SLE who were randomised to receive either IV belimumab 10 mg/kg or placebo, plus ST, for 48 weeks. The primary endpoints were incidences of all-cause mortality and AEs of special interest (AESIs) from the first-to-last study drug dose plus 28 days. The incidences of all-cause mortality and most AESIs were similar between belimumab and placebo groups, including malignancies, serious infections, and opportunistic infections. Although the occurrence of the following events was very low, compared with placebo, belimumab was associated with a higher incidence of serious infusion and hypersensitivity reactions (belimumab n = 8 [0.40%], placebo n = 2 [0.10%]), serious depression (belimumab n = 7 [0.35%], placebo n = 1 [0.05%]), treatment-emergent suicidality (belimumab n = 28 [1.42%], placebo n = 23 [1.16%]), and sponsor-adjudicated serious suicide or self-injury (belimumab n = 15 [0.75%], placebo n = 5 [0.25%]). 41
Long-term studies of belimumab
Open-label extension studies of the Phase 2 study, BLISS-52, BLISS-76, BLISS-SC and BLISS-NEA have examined the long-term safety and efficacy of belimumab in patients with SLE. These studies had some limitations, such as a lack of a placebo-controlled arm and possible selection biases; nevertheless, they showed that long-term belimumab use is well tolerated and associated with continued improvements in disease symptoms and control,42–49 as well as in health-related quality of life (HRQoL) and patient-reported outcomes 50 (PROs; see later section). Among the 364 patients with SLE who completed the Phase 2 study of belimumab, 45 345 consented to participate in a 24-week, long-term extension study, of whom 296 continued treatment in a subsequent open-label, long-term continuation study. Disease control was maintained in these patients with active SLE receiving belimumab plus ST, and treatment was well tolerated with no new safety signals apparent for up to 13 years of follow-up (equivalent to 2294 patient-years).44,45,48 These findings were echoed in the long-term extension studies of BLISS-52 and BLISS-76 (n = 268 US patients from BLISS-76, n = 738 non-US patients combined from both studies), in which minimal organ damage progression and no new safety signals were observed during up to 8 years of follow-up.42,43,47
Belimumab safety
In addition to the BASE Phase 4 clinical study described above, the safety of belimumab was assessed using a pooled analysis of data from both Phase 2 and 3 clinical studies; in this combined study population, types and rates of AEs were similar across treatment groups, with serious AEs reported by 18.0% and 16.6% of patients receiving belimumab 10 mg/kg IV or placebo, respectively. 51 A pooled analysis of safety data from Phase 2 and 3 clinical studies and long-term, open-label extension studies of belimumab use in patients with SLE, encompassing up to 13 years of follow-up on-treatment, demonstrated that belimumab is well tolerated and the risk of AEs is similar to ST.42,43,47,48,51 An overview of key safety findings is presented in Table 1.
Table 1.
Overview of belimumab safety in SLE clinical studies.
| Adverse event | Experience with belimumab |
|---|---|
| Serious infection 51 | • Similar rate of serious infections for belimumab (1, 4 and 10 mg/kg pooled doses) and placebo: 6.00/100 patient-years vs 5.35/100 patient-years (pooled analysis of Phase 2, BLISS-52 and -76 studies) |
| Opportunistic infection41,51 | • Two opportunistic infections with belimumab (10 mg/kg; Acinetobacter infection, disseminated cytomegalovirus infection) vs none with placebo (pooled analysis of Phase 2, BLISS-52 and -76 studies)• Eleven opportunistic infections with belimumab (10 mg/kg) vs 15 with placebo (BASE) |
| Other infection 51 | • Slight trend toward increased rate of upper and lower respiratory tract infections with belimumab (1, 4 and 10 mg/kg) vs placebo: 52.0–59.5% vs 49.5% (pooled analysis of Phase 2, BLISS-52 and -76 studies)• Increased rate of cellulitis with belimumab (1, 4 and 10 mg/kg) vs placebo: 6.4–8.9% vs 6.7% (pooled analysis of Phase 2, BLISS-52 and -76 studies) |
| Malignancy26,47,48,52 | • Rate: 0.5/100 patient-years (BLISS-76), 0.6/100 patient-years (Phase 2 OLE), and 0.2/100 patient-years (BLISS-52 and BLISS-76 OLE at 8+ years of follow-up) vs background rate for patients with SLE: 0.56/100 patient-years |
| Haematological abnormalities 51 | • Low rate of leukopenia: 4.2% (belimumab 10 mg/kg) vs 3.7% (placebo) (pooled analysis of Phase 2, BLISS-52 and -76 studies)• Low rate of neutropenia: 5.2% (belimumab 10 mg/kg) vs 5.5% (placebo) (pooled analysis of Phase 2, BLISS-52 and -76 studies) |
| Infusion reactions 20 , 26 , 27 , 51 | • n = 1 (severe) (belimumab) (Phase 2 study)• 1% (belimumab 10 mg/kg) vs <1% (placebo) (BLISS-52)• 13.6% (belimumab 10 mg/kg) vs 9.8% (placebo) (BLISS-76)• Most infusion reactions occurred during the first 2 infusions; the rate became similar across treatment groups thereafter (pooled analysis of Phase 2, BLISS-52 and -76 studies) |
| Psychiatric effects and suicide 41 , 51 | • Rates of psychiatric disorders (including suicidal ideation, depression, insomnia and anxiety) were higher with belimumab than placebo (pooled analysis of Phase 2, BLISS-52 and -76 studies)• Rates were higher with belimumab than placebo for serious depression, treatment-emergent suicidality, and sponsor-adjudicated serious suicide or self-injury (BASE) • The ongoing SABLE (NCT01729455) prospective clinical study was designed to evaluate in more detail psychiatric effects with belimumab |
| PML 48 ,53,54 | • Two reported cases of PML in belimumab-treated patients; both patients were also receiving mycophenolate mofetil and corticosteroids• No cases of PML reported in the 13-year follow-up of the Phase 2 study |
| Mortality41,51 | • Similar incidence of death for belimumab (10 mg/kg) and placebo: 0.9% vs 0.4% (pooled analysis of Phase 2, BLISS-52 and -76 studies)• Similar incidence of all-cause mortality with belimumab (10 mg/kg) and placebo: 0.5% vs 0.4% (BASE) |
BLISS: Study of Belimumab in Subjects with SLE; BASE: Belimumab Assessment of Safety in SLE; OLE: open-label extension; PML: progressive multifocal leukoencephalopathy; SABLE: Safety and Effectiveness of Belimumab in SLE; SLE: systemic lupus erythematosus.
Belimumab in the paediatric patient
The approval of belimumab as a treatment for paediatric patients with SLE was supported by data from the PLUTO study (NCT01649765), a Phase 2, randomised, placebo-controlled, double-blind, post-approval commitment study. 7 PLUTO evaluated the efficacy, safety and PK of IV belimumab plus ST in children aged 5–17 years with active childhood-onset SLE (cSLE; N = 93). Recruitment for this study was particularly challenging, with a high rate of screening failures meaning that it took more than 5 years to achieve sufficient patient numbers. Efficacy, PK, pharmacodynamics (PD) and safety profiles of belimumab were consistent with the results of the clinical studies in adults with SLE. At Week 52, numerically higher proportions of patients receiving belimumab achieved a clinically meaningful improvement in disease activity according to the SRI-4, compared with placebo (Figure 2). 7 An across-study comparison of PLUTO with the BLISS pivotal studies confirmed that the efficacy and safety results of IV belimumab (plus ST) in the paediatric population were consistent with those in the adult population, supporting the favourable benefit:risk profile of IV belimumab in patients aged ≥5 years with cSLE/SLE. 55 Following the results of the PLUTO study, belimumab became the first biologic treatment to be approved in children 5 years and older. 56
A Phase 2 bridging PK study (NCT04179032) is currently ongoing to investigate the safety, PK and PD of repeated doses of 200 mg/mL SC belimumab in paediatric patients aged 5–17 years with SLE who are also receiving ST. This study has an estimated primary completion date of March 2023.
The effect of belimumab on organ damage
Organ damage in SLE is frequently a consequence of the disease process and chronic corticosteroid use, with 32–50% of patients accumulating organ damage within the first 5 years after SLE diagnosis, despite disease activity decreasing during this time.57–60 Risk factors for organ damage accumulation include older age and/or high disease activity at diagnosis, longer disease duration, Black African ancestry, Hispanic ethnicity, or Asian race, and greater overall disease activity during the course of disease.4,61,62 Existing organ damage is associated with a greater likelihood of accumulating further organ damage, increased morbidity and mortality, and decreased HRQoL and daily functioning. 60 The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI) measures irreversible damage across 12 organ systems as a result of SLE disease activity and its treatment, 61 and SDI scores are shown to increase in a linear fashion over time in patients with SLE, reflecting organ damage accumulation. 62
In the long-term extension studies of BLISS-52 and -76, approximately 85% of the belimumab-treated patients demonstrated no organ damage progression over 5–6 years of follow-up.42,47 A post hoc propensity score-matched analysis focused on a pooled population of patients with SLE receiving IV belimumab plus ST from the BLISS long-term extension studies (n = 99), compared to the Toronto Lupus Cohort (n = 99). This type of analysis matches patients from different cohorts according to a series of predetermined characteristics, in order to compare their outcomes. In this study, belimumab use was associated with a statistically significant smaller increase in SDI over 5 years than ST alone, and patients receiving belimumab were 61% less likely to progress to a higher SDI score than those receiving ST alone. 63 A year later, these results were replicated in a larger, more generalisable population using the pooled US and non-US data. 64
Corticosteroid use is a key contributor to organ damage; 59 therefore, the apparent ability of belimumab to delay organ damage accumulation in patients with SLE may be attributed in part to its steroid-sparing effect.8,65,66
Belimumab and patient reported outcomes
Fatigue affects 50–86% of patients with SLE67–69 and has a considerable negative impact on HRQoL and work disability.68,70,71 Additionally, patients rank fatigue and functional measures (such as reduced ability to perform usual activities) as their highest concern. 72 In a pooled analysis of the BLISS-52 and -76 studies, significant and clinically meaningful improvements in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores were observed at Weeks 8, 12 and 52 of treatment in patients with SLE who were receiving IV belimumab, compared with placebo. There were also significantly greater improvements versus placebo in HRQoL as measured with the Short Form 36 (SF-36) physical component summary (PCS) and SF-36 vitality domain scores at Week 52 in favour of belimumab. 73 A subsequent analysis of patients with SLE from the BLISS-76 extension study (NCT00724867; N = 268) who were followed for up to 6 years showed continued, clinically meaningful and significant improvements in fatigue versus baseline, assessed by FACIT-Fatigue score and HRQoL, assessed using the SF-36. 50
A post hoc analysis of BLISS-52 and -76 that stratified patients with SLE by SRI-4 response showed that at Week 52, mean improvements in FACIT-Fatigue scores, and SF-36 physical and mental component summary scores, as well as all individual domain scores, were significantly greater in SRI-4 responders versus non-responders. The magnitude of improvements amongst SRI-4 responders exceeded the minimum clinically important difference for the FACIT-Fatigue (4 points), SF-36 physical and mental component summary (2.5 points), and SF-36 domain (5 points) scores.74,75
A similar impact on fatigue has been described with belimumab SC. 28
What is the effectiveness of belimumab in real-world medical practice?
The impact of belimumab in the real-world has also been explored, and as of 2020, post-marketing experience with belimumab is estimated at 108,477 patient-years. Data from the evaluation Of use of Belimumab in clinical practice SEttings (OBSErve) program, which included 830 patients from 6 countries (Argentina, Canada, Germany, Spain, Switzerland, and USA), support the real-world effectiveness and steroid-sparing ability of belimumab.76–81 Across all countries, after 6 months of belimumab treatment, 48% of patients with SLE experienced a ≥50% physician-assessed improvement in their condition, with 13% achieving a ≥80% improvement, indicating near-normalisation of their condition (equal to ≥80% improvement). Of those patients with high disease activity at enrolment (defined either as SLEDAI-2K/SELENA-SLEDAI score ≥10, or high anti-dsDNA or C3 and/or C4 below lower limit of normal), 96% experienced an improvement in their overall condition with 12–14% achieving near-normalisation. Among the 80% of patients who were taking oral glucocorticoids at enrolment, 78% were able to reduce or discontinue steroid use after 6 months of belimumab treatment, and of those who were still using steroids, >50% achieved doses ≤7.5 mg/day. 82 Ethnicity did not appear to affect magnitude of improvement in clinical response, transitions toward milder disease or patterns of steroid use, with similar levels of improvement observed among white and Black African ancestry patients up to 24 months of follow-up. 77 In the subgroup of patients of Black African ancestry in OBSErve US who continued to receive belimumab over 24 months (n = 69/123), substantial reductions in disease activity and severity, and oral corticosteroid use were observed. 83
The benefits of belimumab on disease activity and steroid sparing were also demonstrated in a prospective multicentre, observational study of 91 consecutive patients with SLE in Greece who received belimumab for treatment of active disease despite ≥1 previous conventional immunosuppressant. Belimumab significantly decreased average SLEDAI-2K, PGA and daily prednisone dose over time, with >20% of patients able to discontinue corticosteroid use from as early as 3 months post-initiation, and >40% achieving a lupus low disease activity state (LLDAS) by 9–12 months post-initiation. 65
The Belimumab in Real-Life Setting Study (BeRLiSS) conducted in Italy reported improvement in disease activity score (DAS)-28 in patients with SLE and musculoskeletal involvement at baseline, and in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) activity score in patients with SLE and cutaneous manifestations (N = 466), who were followed for up to 60 months (median: 18 months). In this study, initiation of IV belimumab treatment early in the disease (≤2 years of disease duration) was associated with an increased likelihood of SRI-4 response compared with initiation after a longer disease duration. Disease remission and low disease activity were likely to persist over time, as demonstrated by low disease activity being achieved by 56%, 72%, 75%, 79% and 76% of patients at 6, 12, 24, 36 and 48 months of follow-up, respectively, and remission by approximately half of the patients throughout follow-up. 84
A retrospective US insurance claims database analysis of 1879 patients with SLE showed a significant decrease in inpatient costs in the 6 months after IV belimumab initiation, while outpatient costs were higher during the same period. Notably, after stratification by mean oral prednisone-equivalent daily dose category (≤7.5, >7.5 to ≤15, and >15 mg/day), a significantly higher proportion of patients were in the ≤7.5 mg/day dose category in the post-index period compared with the pre-index period. 85 In a separate retrospective claims database analysis of patients with SLE (N = 49,413 eligible for analysis), those initiating belimumab during follow-up (n = 1710) significantly decreased their oral corticosteroid use and average daily dose in the 6 months after initiation compared with the 6 months prior to initiation. 86
Belimumab has changed the SLE treatment paradigm
Several key milestones in the history of belimumab have contributed to a greater understanding of SLE and clinical study design:
Belimumab has an unrivalled body of evidence across its clinical development program, which includes four successful pivotal clinical studies in patients with SLE26–28,30 and a study in patients with LN; 34
Belimumab has been successful in multiple studies of diverse patient population, including paediatric patients, patients in North East Asia, and in patients of black African ancestry characterised by high disease activity;7,30,40
Refinements in SLE clinical study design have been driven by the development of the SRI-4 composite endpoint and its implementation in several clinical studies of belimumab. 87
Conducting belimumab studies in diverse patient populations has enabled greater insight into the barriers to recruitment and highlighted effective strategies that can be used to engage and motivate patients, their families, HCPs and local advocacy groups to participate and advocate for clinical studies. Such engagement is critical for traditionally under-represented ethnic and racial populations in clinical studies; notably in SLE, this includes patients of Black African ancestry. Enrolment of this racial group in the Phase 2 and 3 belimumab studies were not reflective of the racial distribution in the general US population with SLE, resulting in incomplete data on the appropriate treatment and management of this important patient group, who have a disproportionately high unmet need. In the EMBRACE study, various strategies to improve recruitment were implemented, such as national awareness campaigns, Sponsor website with a study screener and 24-hour call centre, US site locator and provision of study educational materials.
Effective strategies included developing detailed educational materials for patients and their families on what to expect during the study, promoting community awareness about the importance of study participation, site participation in community activities, and enhancing referral programs at the local level, with outreach to local advocacy groups and trusted HCPs being pivotal to this process. This process highlighted that multiple, concurrent, ongoing approaches were necessary for successful enrolment and retention of patients who self-identified as being of black race in the EMBRACE study. In addition, it was necessary to address logistical barriers, such as the provision of door to door transportation to clinical study sites. These lessons learnt from EMBRACE are being used to improve the recruitment pathway and the importance of community collaboration in future clinical studies, not only in SLE, but in other therapeutic areas.
The increased understanding of belimumab gained through this comprehensive clinical development program has supported a shift in the SLE treatment paradigm away from reactive disease management towards a more proactive and holistic approach. A decline in glucocorticoid use has been associated with belimumab treatment; this is particularly important given the lack of supporting data and issues relating to long-term glucocorticoid treatment, 88 and steroid-sparing approaches continue to be encouraged among both clinicians and patients. Overall, the steroid-sparing effects of belimumab, in addition to the prevention of flares and reduced disease activity, have resulted in a reduction of organ damage accumulation and support the concept of belimumab as a disease–modifying treatment.
Belimumab’s current position in the SLE treatment paradigm
Current clinical guideline recommendations regarding belimumab are presented in Table 2. The EULAR 2019 guidelines recommend belimumab as add-on therapy for patients with an inadequate response to ST that includes combinations of hydroxychloroquine and glucocorticoids, with or without immunosuppressive agents. 93 Add-on belimumab should be considered in patients with persistently active or flaring extrarenal disease inadequately controlled on first-line therapies and an inability to taper glucocorticoid daily dose to acceptable levels (i.e. maximum 7.5 mg/day). 93 Similarly, the Pan-American League of Associations of Rheumatology (PANLAR) recommends belimumab for musculoskeletal, cutaneous and cardiac manifestations after ST. 92 Belimumab may also be beneficial early in the management of SLE as a disease-modifying agent in order to limit damage accumulation related to recurrent flares and corticosteroid use. 95
Table 2.
Guideline recommendations for the use of belimumab in SLE.
| Guideline | Recommendation |
|---|---|
| American College of Rheumatology (ACR; 1999, 2012)89,90 | SLE guidelines (1999): Belimumab not mentionedLupus nephritis guidelines (2012): Mentions FDA approval of belimumab for use in seropositive patients with active SLE who have active disease in spite of prior therapies. Belimumab had not been studied for use in lupus nephritis at the time the guidelines were published |
| Asian Lupus Nephritis Network (ALNN; 2014) 91 | Belimumab not mentioned |
| Pan-American League of Associations of Rheumatology (PANLAR; 2018) 92 | Musculoskeletal manifestations• If disease remains active after ST, add either methotrexate or leflunomide or belimumab or abatacept over other immunosuppressants (quality of evidence: low to moderate; strength of recommendation: weak)Cutaneous manifestations• If disease remains active after ST, add methotrexate, azathioprine, mycophenolate mofetil, cyclosporine A, cyclophosphamide or belimumab over other immunosuppressants (quality of evidence: low to moderate; strength of recommendation: weak)Cardiac manifestations• Use ST plus colchicine over ST plus NSAIDs or belimumab |
| European League Against Rheumatism (EULAR; 2019) 93 | In patients with inadequate response to ST (combinations of hydroxychloroquine and glucocorticoids, with or without immunosuppressive agents), defined as residual disease activity not allowing tapering of glucocorticoids and/or frequent relapses, add-on treatment with belimumab should be considered (level of evidence: 1a/A)Add-on belimumab should be considered in persistently active or flaring extrarenal disease• Belimumab should be considered in extrarenal disease with inadequate control (ongoing disease activity or frequent flares) on first-line treatments (typically including combination of hydroxychloroquine and prednisone with or without immunosuppressive agents), and inability to taper glucocorticoid daily dose to acceptable levels (i.e. maximum 7.5 mg/day)Patients with persistent disease may benefit from belimumab; higher likelihood of response associated with high disease activity (e.g. SLEDAI score >10), prednisone dose >7.5 mg/day and serological activity (low C3/C4, high anti-dsDNA titres), with cutaneous, musculoskeletal and serological manifestations responding the most |
| Asia‐Pacific League of Associations for Rheumatology (APLAR; 2021) 94 | Belimumab may be considered for active SLE manifestations that are refractory to standard therapies |
anti-dsDNA: anti-double stranded DNA; FDA: Food and Drug Administration; NSAID: non-steroidal anti-inflammatory drug; SELENA-SLEDAI: Safety of Estrogens in Lupus Erythematosus National Assessment–SLE Disease Activity Index; SLE: systemic lupus erythematosus; ST: standard therapy.
The role of belimumab beyond SLE
Besides SLE, belimumab has also been investigated in several other diseases and conditions (Table 3), including primary Sjögren’s Syndrome, 96 myasthenia gravis, 97 primary membranous nephropathy, 98 renal transplantation, 99 and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis.100,101 Additionally, GlaxoSmithKline has supported or is currently supporting a number of belimumab investigator-initiated studies that extend beyond rheumatology and SLE; including in emphysema (NCT03244059), immune thrombocytopenia, 102 myositis, systemic sclerosis, 103 immune-mediated kidney diseases, among others.
Table 3.
Studies of belimumab in indications other than systemic lupus erythematosus.
| Study name or indication | Description and findings |
|---|---|
| BELISS 96 | • Prospective, open-label, Phase 2 study evaluating the use of belimumab in patients with autoantibody-seropositive primary Sjögren’s Syndrome and either early disease (duration <5 years), salivary gland enlargement, current systemic manifestation or increased biomarkers of B-cell activation • Significant improvements in disease activity, fatigue, mucosal dryness and markers of B-cell activation were reported up to 52 weeks, with no effect on oral or ocular dryness |
| MG 97 | • Randomised study in patients with generalised MG who remained symptomatic despite standard of care • Did not meet its primary endpoint (mean change in Quantitative MG score from baseline at Week 24), and there were no significant differences between belimumab and placebo groups in any secondary endpoints, including the MG Composite and MG–Activity of Daily Living scores |
| PMN 98 | • Prospective, open-label, experimental medicine study of belimumab monotherapy for up to 2 years in patients with PMN and persistent nephrotic-range proteinuria (N = 11) • Significant decreases in proteinuria and PLA2R-Ab observed at Weeks 28 and 104 • Nine patients achieved partial (n = 8) or complete (n = 1) remission • Patients with abnormal albumin and/or cholesterol at baseline had normal/near normal levels by last follow-up |
| Renal transplantation 99 | • Double-blind, randomised, placebo-controlled, Phase 2 study of belimumab in addition to standard-of-care immunosuppression (basiliximab, mycophenolate mofetil, tacrolimus, and prednisolone) in adult kidney transplant recipients • No major increased risk of infection observed • Co-primary endpoint was not met (reduction in naive B cells from baseline to Week 24) |
| AAV 100 | • Multicentre, randomised, double-blind, placebo-controlled study in patients with ANCA-associated vasculitis • Belimumab as adjunctive therapy to maintain remission, administered with azathioprine and low-dose glucocorticoids, did not reduce the risk of relapse • The study was truncated due to difficulties in enrolment, so was not powered to support definitive conclusions |
| COMBIVAS 101 | • Randomised, double blind, placebo-controlled study to evaluate the mechanistic effect of belimumab combined with rituximab in patients with active proteinase 3-AAV • Study is ongoing |
Note: All studies were/are supported by GlaxoSmithKline, and GlaxoSmithKline was a sponsor of the AAV study.
AAV: ANCA-associated vasculitis; ANCA: antineutrophil cytoplasmic antibody; MG: myasthenia gravis; PLA2R-Ab: anti-phospholipase A2 receptor autoantibodies; PMN: primary membranous nephropathy; SLE: systemic lupus erythematosus.
What does the future hold for belimumab and patients?
Recent and ongoing studies of belimumab will provide valuable data to address remaining areas of unmet need in SLE and LN. A key aspect of belimumab’s evolution in the SLE treatment paradigm is its use in combination therapy regimens. Several studies have evaluated or are evaluating the use of belimumab in sequential treatment with a cycle of rituximab.
SynBioSe (NCT02284984) is a Phase 2, open-label, single-arm, proof-of-concept study in patients with severe, refractory SLE (N = 16) treated with rituximab and sequential belimumab. After 2 years of follow-up, sequential treatment led to long-lasting and specific reductions of several anti-nuclear autoantibodies, prevented full repopulation of B cells, and enabled patients to discontinue use of mycophenolate mofetil.104,105 Belimumab in combination therapy for the treatment of patients with severe SLE is being investigated in the Phase 2 SynBioSe-2 study (NCT03747159). CALIBRATE, a multicentre safety, mechanistic and preliminary efficacy study in 43 patients with refractory LN did not find a significant increase in the incidence of adverse events with rituximab, cyclophosphamide and glucocorticoids then belimumab, versus without belimumab. The sequential therapy diminished the maturation of transitional to naïve B cells and increased autoreactive B cell negative selection, although no additional benefit from the sequential treatment could be demonstrated. 106 BEAT-LUPUS (ISRCTN: 4,78,73,003 EudraCT number: 2015-005543-14) is an investigator-sponsored study evaluating the safety and efficacy of belimumab after rituximab treatment in patients with active SLE resistant to ST. 107 The GlaxoSmithKline-sponsored BLISS-BELIEVE study (NCT03312907) is an ongoing Phase 3 study assessing the use of belimumab with a cycle of rituximab in adult patients with SLE, with the rationale of controlling rising BLyS levels due to rituximab-mediated depletion of B cells; results are expected in the second half of 2021. 108
Real-world studies are evaluating IV and SC belimumab use in clinical practice in Japan (NCT03370263), and belimumab safety and effectiveness in patients with active, autoantibody-positive SLE over 5 years of follow-up (SABLE; NCT01729455). A non-randomised Phase 3 study (NCT02119156) has evaluated treatment holidays and the rebound phenomenon following belimumab treatment in patients with stable, low SLE disease activity. 109 Additional ongoing studies include an aggregated analysis of pooled data from older belimumab-treated patients with SLE and an ongoing surveillance program of belimumab use in pregnancy.
Summary: where is belimumab today?
Since initial approval, belimumab has established its position as a disease-modifying treatment for SLE. Robust evidence from randomised clinical studies, and observational and real-world studies, has demonstrated its tolerability and efficacy in reducing disease activity and SLE flares, enabling patients to taper glucocorticoid use, and limiting damage accumulation caused by the disease. Over the past 10 years, belimumab’s efficacy and safety has been consistently demonstrated for patients with SLE, and findings from its development program have contributed much information about SLE, as well as informed SLE clinical study design. Further clinical studies are ongoing to evaluate the effectiveness of belimumab in various indications beyond SLE, and post-marketing and registry studies are gathering additional valuable data on key areas such as pregnancy outcomes after belimumab exposure, and co-administration of belimumab with other biologics and treatments for SLE.
Acknowledgements
The authors thank the patients and their families, and the investigators, clinical practitioners, and staff who participated in protocols involving belimumab over the past 10 years. The authors would also like to thank Herbert Struemper for his contribution on the rationale for the dosing schedules used in the study designs. Medical writing support was provided by Casmira Brazaitis, PhD, of Fishawack Indicia Ltd, part of Fishawack Health.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RAL, TGR, MK, NLF, AJL, BR, SWB, KG, AVM, and DAR are employees of GlaxoSmithKline and hold stocks and shares in the company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by GlaxoSmithKline.
References
- 1.Friebus-Kardash J, Branco L, Ribi C, et al. Immune complexes containing serum B-cell activating factor and immunoglobulin G correlate with disease activity in systemic lupus erythematosus. Nephrol Dial Transplant 2018; 33: 54–64. [DOI] [PubMed] [Google Scholar]
- 2.Touma Z, Gladman DD, Tulloch-Reid D, et al. Burden of autoantibodies and association with disease activity and damage in systemic lupus erythematosus. Clin Exp Rheumatol 2010; 28: 525–531. [PubMed] [Google Scholar]
- 3.Livingston B, Bonner A, Pope J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus 2011; 20: 1345–1355. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon GS, McGwin G, Jr, Petri M, et al.; PROFILE Study Group. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 2002; 11: 95–101. [DOI] [PubMed] [Google Scholar]
- 5.Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford ) 2016; 55: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM, et al. Characterization of patients with lupus nephritis included in a large cohort from the Spanish society of rheumatology registry of patients with systemic lupus erythematosus (RELESSER). Medicine (Baltimore) 2016; 95: e2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner HI, Abud-Mendoza C, Viola DO, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis 2020; 79: 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui-Yuen JS, Reddy A, Taylor J, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol 2015; 42: 2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancro MP, D'Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009; 119: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys 2009; 53: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amissah-Arthur MB, Gordon C. Contemporary treatment of systemic lupus erythematosus: an update for clinicians. Ther Adv Chronic Dis 2010; 1: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. Lupus therapies continue to evolve. USA: Author, 2017.
- 13.Sprague RG, Power MH. Observations on the physiologic effects of cortisone and ACTH in man. Arch Intern Med (Chic) 1950; 85: 199–258. [DOI] [PubMed] [Google Scholar]
- 14.Basta F, Fasola F, Triantafyllias K, Schwarting A. Systemic lupus erythematosus (SLE) therapy: the old and the new. Rheumatol Ther 2020; 7: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. FDA approves Benlysta to treat lupus. USA: Author, 2011.
- 16.Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS-lupus connection. Nat Biotechnol 2012; 30: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001; 44: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166: 6–10. [DOI] [PubMed] [Google Scholar]
- 19.Furie R, Stohl W, Ginzler EM, et al.; Belimumab Study Group. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther 2008; 10: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace DJ, Stohl W, Furie RA, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum 2009; 61: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999; 8: 685–691. [DOI] [PubMed] [Google Scholar]
- 22.Hui-Yuen JS, Li XQ, Askanase AD. Belimumab in systemic lupus erythematosus: a perspective review. Ther Adv Musculoskelet Dis 2015; 7: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hruskova Z, Tesar V. Lessons learned from the failure of several recent trials with biologic treatment in systemic lupus erythematosus. Expert Opin Biol Ther 2018; 18: 989–996. [DOI] [PubMed] [Google Scholar]
- 24.Furie RA, Petri MA, Wallace DJ, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum 2009; 61: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furie R, Wang L, Drappa J, Illei G. OP0044 systemic lupus erythematosus (SLE) responder index [SRI(4)] response is associated with global benefit in patients with moderate to severe SLE. Ann Rheum Dis 2016; 75: 70. [Google Scholar]
- 26.Furie R, Petri M, Zamani O, et al.; BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 28.Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017; 69: 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yapa SW, Roth D, Gordon D, Struemper H. Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus phase 3 program. Lupus 2016; 25: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang F, Bae SC, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis 2018; 77: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Zhang W, Leng X, et al.; CSTAR co-authors. Chinese SLE treatment and research group (CSTAR) registry: I. Major clinical characteristics of Chinese patients with systemic lupus erythematosus. Lupus 2013; 22: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 32.Dooley MA, Houssiau F, Aranow C, et al.; BLISS-52 and -76 Study Groups. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013; 22: 63–72. [DOI] [PubMed] [Google Scholar]
- 33.Sciascia S, Radin M, Yazdany J, et al. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev 2017; 16: 287–293. [DOI] [PubMed] [Google Scholar]
- 34.Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020; 383: 1117–1128. [DOI] [PubMed] [Google Scholar]
- 35.Struemper H. Case examples of using quantitative pharmacology in developing therapeutic proteins in systemic lupus erythematosus – belimumab. In: Honghui Z and Diane RM (eds) Quantitative pharmacology and individualized therapy strategies in development of therapeutic proteins for immune‐mediated inflammatory diseases 2019, pp.389–400.
- 36.Dall'Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus nephritis cohort. Arthritis Rheumatol 2015; 67: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 37.Tamirou F, Lauwerys BR, Dall'Era M, et al.; on behalf of the MAINTAIN Nephritis Trial investigators. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN nephritis trial. Lupus Sci Med 2015; 2: e000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the joint European league against rheumatism and European renal Association-European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020; 79: 713–723. [DOI] [PubMed] [Google Scholar]
- 39.GlaxoSmithKline. Highlights of prescribing information: BENLYSTA (belimumab) for injection. 2021.
- 40.D'Cruz D, Maksimowicz-McKinnon K, Oates J, et al. Efficacy and safety of belimumab in patients of black race with systemic lupus erythematosus: results from the EMBRACE study. Lupus Sci Med 2019; 6: A149–A50. [Google Scholar]
- 41.Sheikh SZ, Scheinberg MA, Wei JC-C, et al. Mortality and adverse events of special interest with intravenous belimumab for adults with active, autoantibody-positive systemic lupus erythematosus (BASE): a multicentre, double-blind, randomised, placebo-controlled, phase 4 trial. Lancet Rheumatol 2021; 3: e122–e130. [DOI] [PubMed] [Google Scholar]
- 42.Bruce IN, Urowitz M, van Vollenhoven R, et al. Long-term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus 2016; 25: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furie RA, Wallace DJ, Aranow C, et al. Long-Term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase III parent study in the United States. Arthritis Rheumatol 2018; 70: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginzler EM, Wallace DJ, Merrill JT, et al.; The LBSL02/99 Study Group. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheumatol 2014; 41: 300–309. [DOI] [PubMed] [Google Scholar]
- 45.Merrill JT, Ginzler EM, Wallace DJ, et al.; LBSL02/99 Study Group. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum 2012; 64: 3364–3373. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Bass D, Chu M, Egginton S, Ji B, Roth D. Organ system improvements in Japanese patients with systemic lupus erythematosus treated with belimumab: a subgroup analysis from a phase 3 randomized placebo-controlled trial. Mod Rheumatol 2020; 30: 313–320. [DOI] [PubMed] [Google Scholar]
- 47.van Vollenhoven RF, Navarra SV, Levy RA, et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a phase III study extension. Rheumatology (Oxford) 2020; 59: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace DJ, Ginzler EM, Merrill JT, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol 2019; 71: 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Zheng J, Bass DL, et al. Long-term, open-label extension study of safety and efficacy of belimumab in patients with systemic lupus erythematosus in China. Int J Rheum Dis 2020; 23: 330–331. [Google Scholar]
- 50.Strand V, Berry P, Lin X, Asukai Y, Punwaney R, Ramachandran S. Long-term impact of belimumab on Health-Related quality of life and fatigue in patients with systemic lupus erythematosus: Six years of treatment. Arthritis Care Res 2019; 71: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace DJ, Navarra S, Petri MA, et al.; BLISS-52 and -76, and LBSL02 Study Groups. Safety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosus. Lupus 2013; 22: 144–154. [DOI] [PubMed] [Google Scholar]
- 52.Bernatsky S, Boivin JF, Joseph L, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum 2005; 52: 1481–1490. [DOI] [PubMed] [Google Scholar]
- 53.Leblanc-Trudeau C, Masetto A, Bocti C. Progressive multifocal leukoencephalopathy associated with belimumab in a patient with systemic lupus erythematosus. J Rheumatol 2015; 42: 551–552. [DOI] [PubMed] [Google Scholar]
- 54.Fredericks CA, Kvam KA, Bear J, Crabtree G, Josephson SA. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus 2014; 23: 711–713. [DOI] [PubMed] [Google Scholar]
- 55.Nino A, Bass D, Eriksson G, et al. Efficacy and safety of intravenous belimumab in children with systemic lupus erythematosus: an across-trial comparison with the adult belimumab studies [abstract 2864]. Arthritis Rheumatol 2019; 71. [Google Scholar]
- 56.GlaxoSmithKline. Intravenous Benlysta is the first biologic treatment to be approved for children with lupus in Europe. Brentford: Author, 2019.
- 57.Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology (Oxford) 2012; 51: 491–498. [DOI] [PubMed] [Google Scholar]
- 58.Segura BT, Bernstein BS, McDonnell T, et al. Damage accrual and mortality over long-term follow-up in 300 patients with systemic lupus erythematosus in a multi-ethnic British cohort. Rheumatology (Oxford) 2020; 59: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urowitz MB, Gladman DD, Ibanez D, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2012; 64: 132–137. [DOI] [PubMed] [Google Scholar]
- 60.Urowitz MB, Gladman DD, Ibanez D, et al. Effect of disease activity on organ damage progression in systemic lupus erythematosus: University of Toronto lupus clinic cohort. J Rheumatol 2021; 48: 67–73. [DOI] [PubMed] [Google Scholar]
- 61.Ghazali WSW, Daud SMM, Mohammad N, Wong KK. Slicc damage index score in systemic lupus erythematosus patients and its associated factors. Medicine (Baltimore ) 2018; 97: e12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker-Merok A, Nossent HC. Damage accumulation in systemic lupus erythematosus and its relation to disease activity and mortality. J Rheumatol 2006; 33: 1570–1577. [PubMed] [Google Scholar]
- 63.Urowitz MB, Ohsfeldt RL, Wielage RC, Kelton KA, Asukai Y, Ramachandran S. Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Ann Rheum Dis 2019; 78: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urowitz MB, Ohsfeldt RL, Wielage RC, et al. Comparative analysis of long-term organ damage in patients with systemic lupus erythematosus using belimumab versus standard therapy: a post hoc longitudinal study. Lupus Sci Med 2020; 7: e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fanouriakis A, Adamichou C, Koutsoviti S, et al. Low disease activity-irrespective of serologic status at baseline-associated with reduction of corticosteroid dose and number of flares in patients with systemic lupus erythematosus treated with belimumab: a real-life observational study. Semin Arthritis Rheum 2018; 48: 467–474. [DOI] [PubMed] [Google Scholar]
- 66.van Vollenhoven RF, Petri M, Wallace DJ, et al. Cumulative corticosteroid dose over fifty-two weeks in patients with systemic lupus erythematosus: pooled analyses from the phase III belimumab trials. Arthritis Rheumatol 2016; 68: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kent T, Davidson A, Newman D, Buck G, D'Cruz D. Burden of illness in systemic lupus erythematosus: results from a UK patient and carer online survey. Lupus 2017; 26: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 68.Zonana-Nacach A, Roseman JM, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. LUMINA study group. LUpus in MInority populations: NAture vs nurture. Lupus 2000; 9: 101–109. [DOI] [PubMed] [Google Scholar]
- 69.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus 2006; 15: 633–643. [DOI] [PubMed] [Google Scholar]
- 70.Basta F, Margiotta DPE, Vadacca M, et al. Is fatigue a cause of work disability in systemic lupus erythematosus? Results from a systematic literature review. Eur Rev Med Pharmacol Sci 2018; 22: 4589–4597. [DOI] [PubMed] [Google Scholar]
- 71.Bruce IN, Mak VC, Hallett DC, Gladman DD, Urowitz MB. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis 1999; 58: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golder V, Ooi JJY, Antony AS, et al. Discordance of patient and physician health status concerns in systemic lupus erythematosus. Lupus 2018; 27: 501–506. [DOI] [PubMed] [Google Scholar]
- 73.Strand V, Levy RA, Cervera R, et al.; for the BLISS-52 and -76 Study Groups. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis 2014; 73: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bangert E, Wakani L, Merchant M, Strand V, Touma Z. Impact of belimumab on patient-reported outcomes in systemic lupus erythematosus: review of clinical studies. Patient Relat Outcome Meas 2019; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furie R, Petri MA, Strand V, et al.; the BLISS-52 and BLISS-76 Study Groups. Clinical, laboratory and health-related quality of life correlates of systemic lupus erythematosus responder index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med 2014; 1: e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babini A, Cappuccio AM, Caprarulo C, et al. Evaluation of belimumab treatment in patients with systemic lupus erythematosus in a clinical practice setting: results from a 24-month OBSErve study in Argentina. Lupus 2020; 29: 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins CE, Dall'Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med 2016; 3: e000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortés J, Andreu JL, Calvo J, García-Aparicio AM, Coronell CG, Díaz-Cerezo S. Evaluation of use of belimumab in clinical practice settings (observe study) in Spain: health resource utilization and labour absenteeism. Value Health 2014; 17: A534. [DOI] [PubMed] [Google Scholar]
- 79.Schwarting A, Schroeder JO, Alexander T, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther 2016; 3: 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Touma Z, Sayani A, Pineau CA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada study. Rheumatol Int 2017; 37: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Kempis J, Duetsch S, Reuschling N, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly 2019; 149: w20022. [DOI] [PubMed] [Google Scholar]
- 82.Collins CE, Cortes-Hernandez J, Garcia MA, et al. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumatol Ther 2020; 7: 949–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins C, Kerr G, Von Feldt J, et al. 24-Month outcomes associated with belimumab in black/African-American patients with systemic lupus erythematosus in a clinical practice setting in the United States. Arthritis Rheumatol 2019; 71. [Google Scholar]
- 84.Gatto M, Saccon F, Zen M, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a Real-Life setting. Arthritis Rheumatol 2020; 72: 1314–1324. [DOI] [PubMed] [Google Scholar]
- 85.Bell CF, Priest J, Stott-Miller M, et al. Real-world treatment patterns, healthcare resource utilisation and costs in patients with systemic lupus erythematosus treated with belimumab: a retrospective analysis of claims data in the USA. Lupus Sci Med 2020; 7: e000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Birt JA, Wu J, Griffing K, et al. Corticosteroid dosing and opioid use are high in patients with SLE and remain elevated after belimumab initiation: a retrospective claims database analysis. Lupus Sci Med 2020; 7: e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Runkel L, Stacey J. Lupus clinical development: will belimumab's approval catalyse a new paradigm for SLE drug development? Expert Opin Biol Ther 2014; 14: 491–501. [DOI] [PubMed] [Google Scholar]
- 88.Fanouriakis A, Bertsias G. Changing paradigms in the treatment of systemic lupus erythematosus. Lupus Sci Med 2019; 6: e000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guidelines for referral and management of systemic lupus erythematosus in adults. American college of rheumatology ad hoc committee on systemic lupus erythematosus guidelines. Arthritis Rheum 1999; 42: 1785–1796. [DOI] [PubMed] [Google Scholar]
- 90.Hahn BH, McMahon MA, Wilkinson A, et al.; American College of Rheumatology. American college of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mok CC, Yap DY, Navarra SV, et al.; Asian Lupus Nephritis Network (ALNN). Overview of lupus nephritis management guidelines and perspective from Asia. Nephrology (Carlton) 2014; 19: 11–20. [DOI] [PubMed] [Google Scholar]
- 92.Pons-Estel BA, Bonfa E, Soriano ER, et al.; Grupo Latino Americano de Estudio del Lupus (GLADEL) and Pan-American League of Associations of Rheumatology (PANLAR). First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American group for the study of lupus (GLADEL, grupo Latino americano de estudio del lupus)-Pan-American league of associations of rheumatology (PANLAR). Ann Rheum Dis 2018; 77: 1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 94.Mok CC, Hamijoyo L, Kasitanon N, et al. The Asia-Pacific league of associations for rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. Epub ahead of print 2021. DOI: 10.1016/S2665-9913(21)00009-6. [DOI] [PubMed] [Google Scholar]
- 95.Lutalo PM, D'Cruz DP. Update on belimumab for the management of systemic lupus erythematosus. Expert Opin Biol Ther 2014; 14: 1701–1708. [DOI] [PubMed] [Google Scholar]
- 96.De Vita S, Quartuccio L, Seror R, et al. Efficacy and safety of belimumab given for 12 months in primary Sjogren's syndrome: the BELISS open-label phase II study. Rheumatology (Oxford) 2015; 54: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 97.Hewett K, Sanders DB, Grove RA, et al.; BEL115123 Study Group. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology 2018; 90: e1425–e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barrett C, Willcocks LC, Jones RB, et al. Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy. Nephrol Dial Transplant 2020; 35: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banham GD, Flint SM, Torpey N, et al. Belimumab in kidney transplantation: an experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 2018; 391: 2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jayne D, Blockmans D, Luqmani R, et al.; BREVAS Study Collaborators. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled study. Arthritis Rheumatol 2019; 71: 952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McClure M, Gopaluni S, Wason J, et al. 322. A randomised, double-blind, controlled, mechanistic study of rituximab and belimumab combination therapy in PR3 ANCA-associated vasculitis (COMBIVAS): study protocol. Rheumatology 2019; 58: ii144. [Google Scholar]
- 102.Mahevas M, Azzaoui I, Crickx E, et al. Efficacy, safety and immunological profile of combining rituximab with belimumab for adults with persistent or chronic immune thrombocytopenia: results from a prospective phase 2b trial. Haematologica. Epub ahead of print 13 August 2020. DOI: 10.3324/haematol.2020.259481. [DOI] [PMC free article] [PubMed]
- 103.Gordon JK, Martyanov V, Franks JM, et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol 2018; 70: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kraaij T, Arends EJ, van Dam, et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant 2020: 1–10. [DOI] [PMC free article] [PubMed]
- 105.Kraaij T, Kamerling SWA, de Rooij ENM, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun 2018; 91: 45–54. [DOI] [PubMed] [Google Scholar]
- 106.Atisha-Fregoso Y, Malkiel S, Harris KM, et al. CALIBRATE: a phase 2 randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol 2021; 73: 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muller P, Chowdhury K, Gordon C, Ehrenstein MR, Dore CJ. Safety and efficacy of belimumab after B cell depletion therapy in systemic LUPUS erythematosus (BEAT-LUPUS) trial: statistical analysis plan. Trials 2020; 21: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teng YKO, Bruce IN, Diamond B, et al. Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open 2019; 9: e025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bae S-C, Dimelow R, Ji B, et al. Results of the open-label, non-randomized 52-Week study to evaluate treatment holidays and rebound phenomenon after treatment with belimumab in patients with SLE. Arthritis & Rheumatology 2019; 71: 2544. abstract [Google Scholar]