Abstract
Background:
The Oxford Big Data Institute, multiple sclerosis (MS) physicians and Novartis aim to address unresolved questions in MS with a novel comprehensive clinical trial data set.
Objective:
The objective of this study is to describe the Novartis–Oxford MS (NO.MS) data set and to explore the relationships between age, disease activity and disease worsening across MS phenotypes.
Methods:
We report key characteristics of NO.MS. We modelled MS lesion formation, relapse frequency, brain volume change and disability worsening cross-sectionally, as a function of patients’ baseline age, using phase III study data (≈8000 patients).
Results:
NO.MS contains data of ≈35,000 patients (>200,000 brain images from ≈10,000 patients), with >10 years follow-up. (1) Focal disease activity is highest in paediatric patients and decreases with age, (2) brain volume loss is similar across age and phenotypes and (3) the youngest patients have the lowest likelihood (<25%) of disability worsening over 2 years while risk is higher (25%–75%) in older, disabled or progressive MS patients. Young patients benefit most from treatment.
Conclusion:
NO.MS will illuminate questions related to MS characterisation, progression and prognosis. Age modulates relapse frequency and, thus, the phenotypic presentation of MS. Disease worsening across all phenotypes is mediated by age and appears to some extent be independent from new focal inflammatory activity.
Keywords: Multiple sclerosis, phenotypes, disease progression, magnetic resonance imaging, age
Introduction
Multiple sclerosis (MS) is described by clinical phenotypes (relapsing–remitting (RRMS), primary and secondary progressive (PPMS and SPMS)), disease activity (clinical relapses or imaging parameters) and the presence or absence of progression. 1 However, there is increasing evidence of a disease continuum rather than distinct disease entities, with a heterogeneous endophenotype. 2 Even within clinical phenotypes, the MS course and disease activity are highly variable from one patient to another. 3 Despite considerable progress over the last 30 years, factors affecting MS disease evolution and disease activity, improving prognosis4,5 or help optimising treatments and disease management 6 warrant further elucidation.
Advanced analytics and machine learning approaches have provided relevant new insights in- and outside of medicine and their utility needs to be explored in large MS data sets. There have only been a few attempts,7–12 not surprising given the practical complexities of data access and the curating required.
A collaboration between Novartis, the Oxford Big Data Institute (BDI), and MS physicians aims for better disease characterisation and identification of prognostic factors, using advanced analytical approaches applied on the novel NO.MS data set, composed of 34 Novartis MS clinical trials.
This work describes the key features of the NO.MS data set and explores the contribution of patients’ age at baseline to disease activity, disease worsening and brain volume changes across the MS spectrum.
Methods
NO.MS comprises clinical trials from 2003 to January 2020, approved by institutional review boards or ethics committees and conducted following the principles of the Declaration of Helsinki and Good Clinical Practice. Trial protocols prospectively defined the objectives, eligibility, endpoints, assessments and statistical analysis. Individual study results have been previously published. Data have been de-identified in a risk-based approach as reported elsewhere. 13 In brief, identifiers (including facial features on scans) were either removed, generalised or modified to minimise the risk of re-identification.
We describe patients’ baseline characteristics, visualise the availability of cross-sectional and longitudinal data and display baseline distributions of key variables. Data of all phase III trials in NO.MS were used to model the relationship between age and disease activity (magnetic resonance imaging (MRI) lesions and confirmed MS relapses) or worsening (brain volume change and likelihood of 3 months confirmed disability worsening (3mCDW) based on expanded disability status scale (EDSS)) across MS phenotypes: (1) The cumulative number of gadolinium-enhancing (Gd+) lesions over 1 year after baseline was analysed in a negative binomial model with sex and the presence of a prior treatment as binary factors, age as a continuous covariate, treatment as a factor and treatment by age interaction, with the number of evaluable scans as the offset. (2) Relapses were analysed in a negative binomial model using the same covariates and factors and the time in study as the offset variable. Results from the negative binomial models, fitted separately for each phenotype, are reported with means and 95% confidence intervals (CIs) as a function of age, for all patients ‘total’ and patients receiving ‘placebo’ cohorts. (3) Brain volume change (reported as annualised rate of brain volume change (ARBVC) was calculated based on percentage of brain volume loss observed for up to 2 years from baseline, as measured by the trial-specific MRI reading centres. (4) The probability of 3mCDW (defined as +1.5 points EDSS change if baseline EDSS = 0, +1 point with baseline EDSS = 1–5.0, and +0.5 with EDSS > 5.0 at baseline, confirmed after 3 months based on another EDSS assessment) was estimated based on the data within each phenotype using a survival model with exponential distribution and sex and the presence of a prior treatment as binary factors, age as continuous covariate, treatment, and baseline EDSS as factors (EDSS factorised as levels ‘<2’, ‘2–5’ and ‘⩾5.5’) and binary pairwise interactions (age-by-treatment, age-by-EDSS category and treatment-by-EDSS). The probability of a 3mCDW within 2 years is presented graphically as a function of age and MS phenotype, with 95% prediction interval, for total and placebo cohorts. To assess the impact of the time of data acquisition and treatment eras, supplementary analyses for relapse frequency, Gd+ T1 lesions, brain volume change and disability worsening were conducted comparing more recent data (‘decade 2’: 2010 until 2020) versus older data (‘decade 1’: <2010)
Results
NO.MS includes data from 34 MS clinical studies (phases II–IV) conducted worldwide since 2003 by Novartis (Supplementary Table S1) which enrolled ≈35,000 patients, the majority (>31,000 patients) with RRMS. Five phase II and nine phase III randomised, blinded clinical trials enrolled >9500 patients, of which >2300 patients received placebo. Open label, single arm or observational studies enrolled ≈25,000 patients, the vast majority diagnosed with RRMS. Table 1 illustrates data availability, by variable and MS phenotype, at baseline and longitudinally. Smaller data sets, mainly based on phase III trials, come from SPMS (N = 1873) and PPMS patients (N = 986), respectively. Relapse data are available for all patients and the entire disability range (from EDSS = 0 – no disability to EDSS = 10 – death due to MS) is covered by longitudinal data from ≈22,000 patients. Approximately 10,000 patients contributed >200,000 brain scans in Digital Imaging and Communications in Medicine (DICOM) format, allowing for a new, harmonised voxel-wise analysis of all MRI scans across studies using artificial intelligence (AI). The typical MRI imaging results provided by respective MRI evaluating centres per study are also available. Longitudinal blood neurofilament light chain (NfL) concentrations of over 4400 patients across all phenotypes allow for new insights into neuronal injury and loss. Patient-reported outcome (PRO) and cognitive measures cover further clinically relevant domains.
Table 1.
Heat map showing availability of variables by MS phenotype expressed in number of patients (variables available cross-sectionally at baseline and variables available longitudinally).
| Colour scale | ||||||
|---|---|---|---|---|---|---|
| 0% | <5% | 5% – 25% | 25% – 50% | 50% – 75% | 75% – 100% | |
| Variables available at baseline | ||||||
| Total | POMS | RRMS | SPMS | PPMS | ||
| 34,957 | 235 | 31,863 | 1873 | 986 | ||
| Demography | Age | 34,928 (99.9%) | 235 (100%) | 31,834 (99.9%) | 1873 (100%) | 986 (100%) |
| Sex | 34,955 (100%) | 235 (100%) | 31,861 (100%) | 1873 (100%) | 986 (100%) | |
| Race | 29,693 (84.9%) | 233 (99.1%) | 26,614 (83.5%) | 1860 (99.3%) | 986 (100%) | |
| BMI | 20,701 (59.2%) | 219 (93.2%) | 17,772 (55.8%) | 1737 (92.7%) | 973 (98.7%) | |
| MS Disease History | Treatment naϊve status | 26,373 (75.4%) | 226 (96.2%) | 23,338 (73.2%) | 1832 (97.8%) | 977 (99.1%) |
| Duration since first symptoms | 32,362 (92.6%) | 227 (96.6%) | 29,325 (92%) | 1826 (97.5%) | 984 (99.8%) | |
| Number of relapses in last 1 year | 31,464 (90%) | 227 (96.6%) | 29,397 (92.3%) | 1827 (97.5%) | 983 (99.7%) | |
| Number of relapses in last 2 year | 30,809 (88.1%) | 227 (96.6%) | 28,744 (90.2%) | 1825 (97.4%) | 983 (99.7%) | |
| EDSS | Total score | 30,316 (86.7%) | 232 (98.7%) | 27,256 (85.5%) | 1852 (98.9%) | 976 (99%) |
| Functional scores | 13,474 (38.5%) | 216 (91.9%) | 10,505 (33%) | 1784 (95.2%) | 969 (98.3%) | |
| Ambulation score | 10,115 (28.9%) | 215 (91.5%) | 7146 (22.4%) | 1784 (95.2%) | 970 (98.4%) | |
| MSFC | Timed 25-foot walking test | 8915 (25.5%) | 2 (0.9%) | 6166 (19.4%) | 1777 (94.9%) | 970 (98.4%) |
| Nine-hole peg test | 8836 (25.3%) | 2 (0.9%) | 6152 (19.3%) | 1732 (92.5%) | 950 (96.3%) | |
| PASAT | 6958 (19.9%) | 1 (0.4%) | 4342 (13.6%) | 1651 (88.1%) | 964 (97.8%) | |
| MRI | Sum. results from central reader | 13,220 (37.8%) | 216 (91.9%) | 10,297 (32.3%) | 1738 (92.8%) | 969 (98.3%) |
| Scans available for re-analysis | 6744 (19.3%) | 1 (0.4%) | 4264 (13.4%) | 1719 (91.8%) | 760 (77.1%) | |
| Other | SDMT | 2491 (7.1%) | 211 (89.8%) | 648 (2%) | 1632 (87.1%) | 0 (0%) |
| NfL | 2601 (7.4%) | 0 (0%) | 840 (2.6%) | 1414 (75.5%) | 347 (35.2%) | |
| EQ-5D | 6428 (18.4%) | 1 (0.4%) | 3849 (12.1%) | 1628 (86.9%) | 950 (96.3%) | |
| MFIS | 1947 (5.6%) | 0 (0%) | 1947 (6.1%) | 0 (0%) | 0 (0%) | |
| MSIS-29 | 5934 (17%) | 0 (0%) | 4180 (13.1%) | 1748 (93.3%) | 6 (0.6%) | |
| PRO | MSWS-12 | 2415 (6.9%) | 0 (0%) | 0 (0%) | 1623 (86.7%) | 792 (80.3%) |
| Relapses | 34,957 (100%) | 235 (100%) | 31,863 (100%) | 1873 (100%) | 986 (100%) | |
| EDSS | Total score | 22,286 (63.8%) | 227 (96.6%) | 19,279 (60.5%) | 1815 (96.9%) | 965 (97.9%) |
| Variables available longitudinally | ||||||
| Functional scores | 12,712 (36.4%) | 217 (92.3%) | 9782 (30.7%) | 1756 (93.8%) | 957 (97.1%) | |
| Ambulation score | 9983 (28.6%) | 215 (91.5%) | 7053 (22.1%) | 1758 (93.9%) | 957 (97.1%) | |
| MSFC | Timed 25-foot walking test | 8988 (25.7%) | 2 (0.9%) | 6296 (19.8%) | 1736 (92.7%) | 954 (96.8%) |
| Nine-hole peg test | 8867 (25.4%) | 2 (0.9%) | 6214 (19.5%) | 1713 (91.5%) | 938 (95.1%) | |
| PASAT | 6993 (20%) | 1 (0.4%) | 4432 (13.9%) | 1608 (85.9%) | 952 (96.6%) | |
| MRI | Sum. results from central reader | 11,556 (33.1%) | 211 (89.8%) | 8849 (27.8%) | 1632 (87.1%) | 864 (87.6%) |
| Scans available for re-analysis | 6355 (18.2%) | 1 (0.4%) | 4067 (12.8%) | 1619 (86.4%) | 668 (67.7%) | |
| Other | SDMT | 4268 (12.2%) | 209 (88.9%) | 2364 (7.4%) | 1695 (90.5%) | 0 (0%) |
| NfL | 4417 (12.6%) | 0 (0%) | 2531 (7.9%) | 1549 (82.7%) | 337 (34.2%) | |
| PRO | EQ-5D | 8898 (25.5%) | 1 (0.4%) | 6248 (19.6%) | 1704 (91%) | 945 (95.8%) |
| MFIS | 1876 (5.4%) | 0 (0%) | 1876 (5.9%) | 0 (0%) | 0 (0%) | |
| MSIS | 5394 (15.4%) | 0 (0%) | 3674 (11.5%) | 1714 (91.5%) | 6 (0.6%) | |
| MSWS | 2394 (6.8%) | 0 (0%) | 0 (0%) | 1584 (84.6%) | 810 (82.2%) | |
The percentage of missing data relates to the overall NO.MS database; Note that different types of studies collected data for different sets of endpoints as pre-specified in the respective study protocols, i.e. missing information may not be equally distributed throughout NO.MS, rather dependent on the type of study.BMI: body mass index; EDSS: expanded disability status scale; EQ-5D: EuroQol 5-dimension; MFIS: modified fatigue impact scale; MRI: magnetic resonance imaging; MS: multiple sclerosis; MSFC: multiple sclerosis functional composite; MSIS-29: multiple sclerosis impact scale 29-item; MSWS-12: multiple sclerosis walking scale 12-item; NfL: neurofilament light; PASAT: paced auditory serial addition test; POMS: paediatric-onset MS; PPMS: primary progressive MS; PRO: patient-reported outcome; RRMS: relapsing–remitting MS; SDMT: symbol digit modalities test; SPMS: secondary progressive MS; WPAI: work productivity and activity impairment; Sum. results from central reader: common MRI parameters read by central MRI readers: number of Gd+ lesions, T2 lesion volume and normalised brain volume; scans available for re-analysis (raw scans were obtained in DICOM format, after defacing and anonymising the scans and they are now available for re-analysis in NIFTI format).
Table 2 summarises patient demographics and disease characteristics at baseline, which were representative of the respective MS phenotypes. Patients diagnosed with RRMS, compared to patients with progressive MS (PMS; includes SPMS and PPMS) were younger (RRMS: 39 years vs. PMS: 48 years), less disabled (RRMS: EDSS = 2.7 vs. PMS > 4.5) and had more relapses in the year prior to study entry (RRMS: 1.3 vs. SPMS ⩽ 0.3 and no previous relapses in PPMS). Subclinical disease activity as reflected in the proportion of patients with Gd+ lesions at baseline was highest in RRMS (38%), followed by SPMS (23%) and lowest in PPMS (13%). The data set covers both newly diagnosed as well as patients with long-lasting MS.
Table 2.
Demography and baseline characteristics of NO.MS cohort.
| Baseline characteristics | All patients | Cohort of patients by indication | ||
|---|---|---|---|---|
| Total (N = 34,957) | RRMS (N = 32,098) | SPMS (N = 1873) | PPMS (N = 986) | |
| Treatment naïve n (n/N′, %) |
N′ = 26,373 (75.4%) 5445 (20.6%) |
N′ = 23,564 (73.4%) 4282 (18.2%) |
N′ = 1832 (97.8%) 397 (21.7%) |
N′ = 977 (99.1%) 766 (78.4%) |
| Age (years) |
N′ = 34,928
(99.9%) 40.0 ± 10.6 |
N′ = 32,069
(99.9%) 39.3 ± 10.5 |
N′ = 1873
(100%) 47.6 ± 8.2 |
N′ = 986
(100%) 48.5 ± 8.5 |
| Sex | N′ = 34,955 (99.9%) | N′ = 32’096 (99.99%) | N′ = 1873 (100%) | N′ = 986 (100%) |
| Female, n (%) | 24,465 (70.0 %) | 22,865 (71.2 %) | 1123 (60 %) | 477 (48.8 %) |
| Male, n (%) | 10,490 (30.0 %) | 9231 (28.8 %) | 750 (40 %) | 509 (51.6 %) |
| MS since first symptoms (years) | N′ = 32,362 (92.6%) | N′ = 29,552 (92.1%) | N′ = 1826 (97.5%) | N′ = 984 (99.8%) |
| 0 to <2 | 4213 (13%) | 4201 (14.2%) | 5 (0.3%) | 7 (0.7%) |
| 2 to <5 | 6454 (19.9%) | 5968 (20.2%) | 99 (5.4%) | 387 (39.3%) |
| 5 to <10 | 8779 (27.1%) | 7891 (26.7%) | 334 (18.3%) | 554 (56.3%) |
| 10 to <30 | 12,210 (37.7%) | 10,909 (36.9%) | 1265 (69.3%) | 36 (3.7%) |
| 30 to <50 | 702 (2.2%) | 579 (2%) | 123 (6.7%) | 0 (0%) |
| 50 to <70 | 3 (0%) | 3 (0%) | 0 (0%) | 0 (0%) |
| 70 to <90 | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| EDSS |
N′ = 30,316
(86.7%) 2.9 ± 1.7 |
N′ = 27,488
(85.6%) 2.7 ± 1.6 |
N′ = 1852
(98.9%) 5.4 ± 1.1 |
N′ = 976
(99%) 4.6 ± 1.0 |
| Number of relapses last year |
N′ = 31,464
(90.0%) 1.2 ± 1.1 |
N′ = 29,624
(92.3%) 1.3 ± 1.1 |
N′ = 1827
(97.5%) 0.3 ± 0.6 |
N′ = 983
(99.7%) 0 ± 0.1 |
| Number of relapses last 2 years |
N′ = 30,809
(88.1%) 1.9 ± 1.7 |
N′ = 28,971
(90.3%) 2 ± 1.7 |
N′ = 1825
(97.4%) 0.8 ± 1.2 |
N′ = 983
(99.7%) 0 ± 0.2 |
| Patients with Gd+ T1 lesions, n (%) |
N′ = 12,926 (37.0%) 4399 (34.0 %) |
N′ = 10,227 (31.9%) 3884 (38.0 %) |
N′ = 1732 (92.5%) 391 (22.6 %) |
N′ = 967 (98.1%) 124 (12.8 %) |
| Number of Gd+ T1 lesions |
N′ = 12,926
(37.0%) 1.2 ± 3.5 |
N′ = 10,227
(31.9%) 1.3 ± 3.6 |
N′ = 1732
(92.5%) 0.9 ± 3.5 |
N′ = 967
(98.1%) 0.3 ± 1.0 |
| T2 lesion volume (mm3) |
N′ = 8883
(25.4%) 9963 ± 12,490 |
N′ = 6178
(19.2%) 8375 ± 10,764 |
N′ = 1738
(92.8%) 15,716 ± 16,227 |
N′ = 967
(98.1%) 9764 ± 12,014 |
| Normalised brain volume (cm3) |
N′ = 8735
(25.0%) 1473 ± 106 |
N′ = 6064
(18.9%) 1485 ± 110 |
N′ = 1708
(91.2%) 1421 ± 87 |
N′ = 963
(97.7%) 1492 ± 86 |
Gd+ T1: gadolinium-enhancing T1; PPMS: primary pogressive multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; SD: standard deviation; SPMS: secondary progressive multiple sclerosis.
Data are presented as mean ± SD, unless otherwise stated.
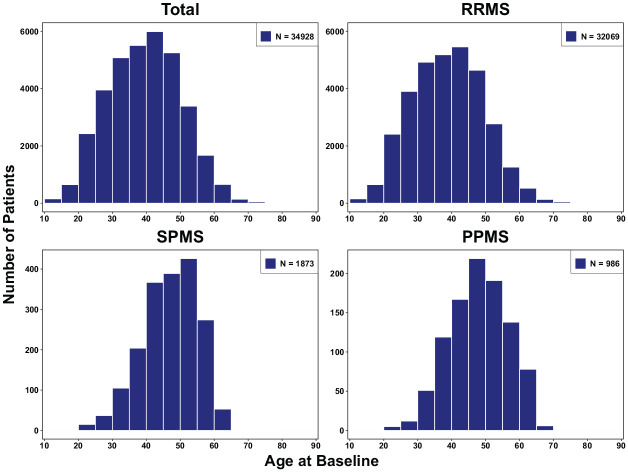
Figure 1 presents the baseline age distribution ranging from paediatric (>10 years) to elderly MS patients (aged >70 years). Paediatric-onset MS (POMS) data are available only in RRMS, since MS in young patients is almost exclusively of the relapsing–remitting phenotype. SPMS and PPMS patients were on average 8–9 years older than RRMS patients.
Figure 1.
Age distribution at baseline by MS phenotype.
MS: multiple sclerosis; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
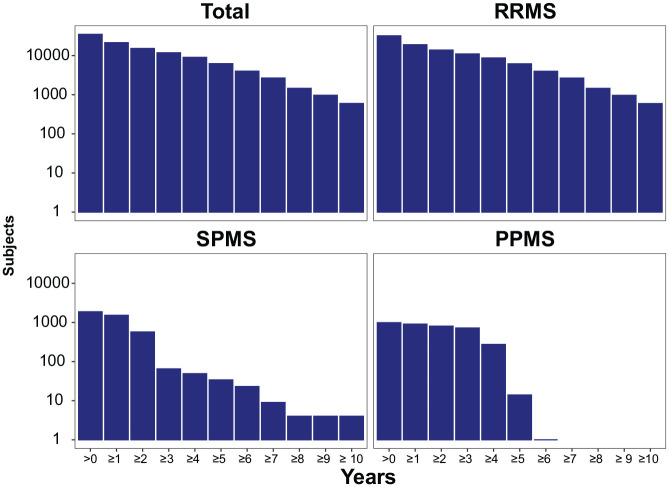
Figure 2 shows the follow-up times by phenotype. Data for ⩾2, up to 5 and 10 years are available for ≈15,000, ≈6200 and ≈600 patients, respectively. Follow-up data for >2 years are available for ≈570 SPMS, and ≈800 PPMS patients, and more is currently being collected in the ongoing extension study in SPMS patients.
Figure 2.
Number of subjects with specified follow-up times by MS phenotype.
MS: multiple sclerosis; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
Overall, the NO.MS data set covers ≈88,800 patient-years of follow-up. Placebo data are available for all phenotypes, which allow studying the disease in the absence of disease-modifying therapy (DMT). Long-term data come primarily from fingolimod-treated patients (≈61,500 treatment years, highest exposure), but substantial data are also available for other DMTs from observational trials and control arms. The exposure, expressed in number of patients-years, by treatment and MS phenotype is shown in Supplementary Figure S1.
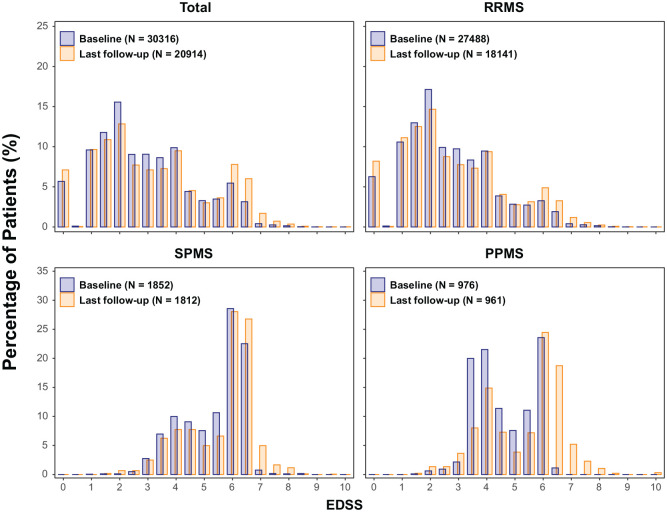
Baseline EDSS distributions represent ambulatory patients (EDSS 0–6.5) in line with inclusion criteria of contributing trials (Figure 3). Worsening in EDSS scores was observed in all phenotypes (most prominently in PMS); at the last follow-up, scores covered the full EDSS range, providing an invaluable data source to study worsening, progression and prognostic factors. Some decreases in EDSS were also seen, reflecting either recovery or EDSS scale properties.
Figure 3.
EDSS distributions at baseline and last measurement by MS phenotype.
EDSS: expanded disability status scale; MS: multiple sclerosis; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
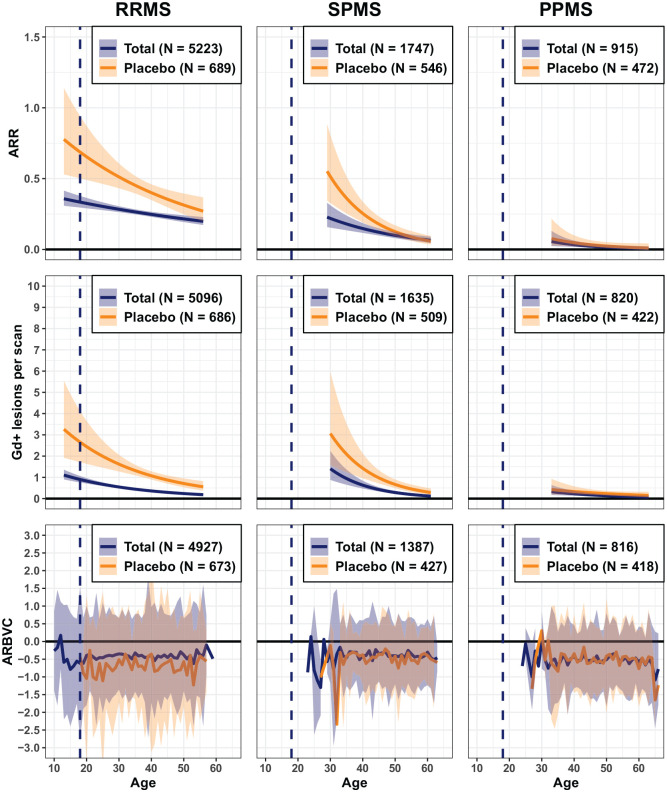
The relationship between age, focal disease activity (annualised relapse rate (ARR) and Gd+ T1 lesions/scan) and brain volume change across phenotypes was investigated and described separately for total (all patients enrolled in respective phase III trials) and placebo cohorts (Figure 4). Clinical relapses and MRI lesions occur with highest frequency in the youngest (i.e. POMS) patients and gradually decrease with older age, also in patients receiving placebo. The qualitative interpretation of results remained similar, irrespective of different data acquisition/treatment eras (Supplementary Figures S2 and S3), although the absolute number of Gd+ T1 lesions was lower in the first compared to the second decade, possibly related to changes in MRI scan quality and MRI methodology. Interestingly, there is a similar pattern of higher disease activity at younger ages in SPMS (and to some extent in PPMS patients). The impact of treatment on focal inflammatory activity is higher in younger patients. Baseline characteristics of the small number of young SPMS and PPMS patients below the age of 30 or 25 years, respectively, are presented in (Supplementary Table S2). Young SPMS patients had pronounced disability and high T2 lesion loads, suggesting an active and aggressive course with early transition to SPMS, and/or long-lasting disease, many with POMS. At baseline, young PPMS patients (<30 years) were significantly disabled and had a considerable T2 lesion volume (mean >10 cm3), despite a short-reported disease duration and a low frequency of new lesion formation on trial. This suggests a history of clinically silent focal inflammation, well before the first clinical symptoms became apparent.
Figure 4.
Disease activity and brain volume loss in relation to age by MS phenotype (total and placebo cohorts).
Vertical dashed line represents patients with POMS. Data are presented as mean and 95% confidence interval; ARR: annualised relapse rate; ARBVC: annualised rate of brain volume change; Gd+ T1: gadolinium-enhancing T1; MS: multiple sclerosis; POMS: paediatric-onset MS; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
Brain volume loss was assessed over 2 years from baseline and is expressed as the average rate in brain volume change per year (ARBVC) with 95% prediction interval. The plotting range (x-axis) covers the age range where >30 patients were available. The impact of the time of data acquisition was investigated by splitting the data into two data acquisition periods (see Supplementary Figure S2 for relapse rates, Figure S3 for Gd+ T1 lesions and Figure S4 for brain volume change).
A high rate of total brain volume loss was observed across all phenotypes and was already present in POMS. Prediction intervals were broad, reflecting high variability, especially in the very young. However, beyond an age of 35 years, brain volume change was remarkably similar across disease phenotypes with generally ⩾0.5% loss over 2 years. The difference in rate of brain volume loss between the placebo cohort and the total cohort (which included both placebo and active-treated patients) was mostly apparent in RRMS patients (Figure 4 and Supplementary Figure S4).
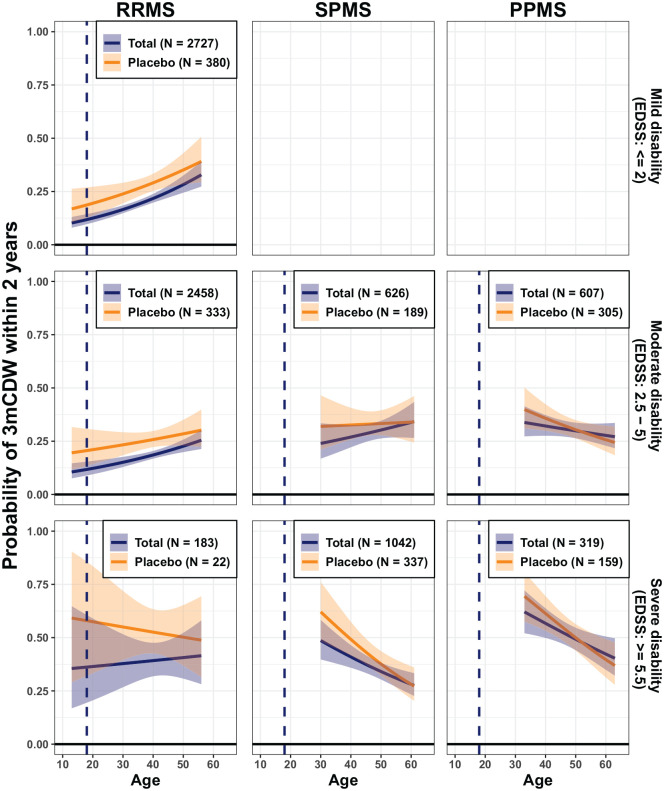
The probability of 3mCDW to occur within 2 years from baseline as a function of age, baseline disability and MS phenotype is illustrated in Figure 5 (and Supplementary Figure S5; by period of data acquisition in Supplementary Figure S6). This probability was lowest (10%–15%) in the youngest RRMS patients with no disability at baseline (EDSS = 0) and increased with higher age and/or level of disability. In patients older than 40 years, the probability of 3mCDW was surprisingly similar and reliably higher than 25%, irrespective of phenotype.
Figure 5.
Probability of 3 months confirmed disability worsening (3mCDW) within 2 years from baseline in relation to age by MS phenotype, for different baseline EDSS categories (total and placebo cohorts).
EDSS: expanded disability status scale; MS: multiple sclerosis; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
Data are presented as mean and 95% prediction interval. Empty plots indicate less than 20 disability events available for this category. The plotting range (x-axis) covers the age range where >30 patients were available. The probability of 3-month EDSS-confirmed disability as a more granular function of age and disability is provided in the Supplementary Figure S5. The impact of the time of data acquisition was investigated by splitting the data into two data acquisition periods (Supplementary Figure S6).
Discussion
MS is complex and heterogeneous, with highly variable individual disease courses.1–3 Particularly, the development of irreversible disability, including the contribution of focal inflammation, needs further elucidation.14,15 Analyses of large, homogeneous high quality data sets across all phenotypes could help to revisit current disease characterisations, which are clinically useful but less so for individual prognosis of disease course or treatment response. Applying big data analytics might help explain heterogeneity and improve individual prognosis.
NO.MS
Large patient-level data sets from clinical trials hold great promise for understanding complex diseases and ultimately may guide ways to improve individual prognosis. The Oxford BDI brings expertise in data analysis in epidemiology, genomics, imaging and computer science,16,17 which allowed combining heterogeneous data sets, conducting image analysis with unified methods across the studies and extracting novel insights with advanced analytical methods.
NO.MS is currently the largest, most comprehensive clinical trial data set across MS phenotypes and uniquely set to characterise ambulatory MS. It includes both randomised controlled and observational trials, thus allowing both identification of causal relationships and translation into settings closer to clinical practice. We summarise the strengths and limitations of NO.MS for future analyses in Table 3.
Table 3.
NO.MS: strengths and limitations of the data set for future analyses.
| Strengths | Limitations |
|---|---|
| 1. Data set - Rich data set from prospectively acquired clinical and imaging trials - All MS phenotypes and POMS included - High quality assessments and data (study protocols, harmonised assessments and data curation) - Broad age- and disability ranges - Placebo data (all phenotypes) - Randomised-controlled trials as well as observational trials - Standardised assessments of relapses and disability (EDSS, including functional scores) by trained physicians - Definitions of outcomes relatively standardised or differences understood (since all trials conducted by a single sponsor), enabling data harmonisation or selection for analysis - MRI scans (defaced) available in NIFTI format for unified image analyses - Additional valuable data on measures such as cognition, PROs and biomarker |
1. Data set - Selection bias: Patients represent selected populations based on the eligibility criteria of study protocols and may be non-representative of routine clinical practice (including selective DMT use) - Studies conducted by single sponsor - Limited biological and genetic characterisation - Study populations may change over time (e.g. to less activity) |
| 2. Follow-up duration - Long (up to 15 years) follow-up - Patient-level longitudinal high quality clinical data, including regular standardised neurological assessments - Includes RRMS patients who transitioned to SPMS while on trial, allowing to study the onset of progressive disease - Patient-level longitudinal MRI scans (defaced) available in NIFTI format to support re-analysis of MRI scans and linkable to the de-identified clinical data |
2. Follow-up duration - Variable longitudinal follow-up - Informative censoring is a possibility in some cases - Limited follow-up in PMS cohorts (additional long-term data are being collected in SPMS) |
| 3. Data analysis - Longitudinal, harmonised, robust and scalable voxel-wise analysis of MRI scans across studies is ongoing to extract new features - Applicable for advanced analytical approaches including supervised and unsupervised machine learning on top of conventional approaches |
3. Data analysis - Challenging as MRI scans are heterogeneous from multicentre trials over almost 20 years (scanner/software, sites and resolution) |
DMT: disease modifying therapy; EDSS: expanded disability status scale; MRI: magnetic resonance imaging; MS: multiple sclerosis; PMS: progressive MS; POMS: paediatric-onset MS; PRO: patient-reported outcomes; SPMS: secondary progressive MS.
Long follow-up (>2–15 years) of many patients and data under placebo treatment are valuable to study disability worsening or to find prognostic markers. MRI analyses performed per study are available; however, heterogeneity across studies limits the utility. 18 We are currently re-analysing >200,000 DICOM images from ≈10,000 patients to obtain a voxel-wise lesion segmentation and to quantify global and regional brain atrophy with a consistent methodology across studies in our future analyses. This new longitudinal MRI data set, together with the clinical data, will reduce heterogeneity and allow to detect associations or causal relationships of imaging and clinical measures.
Despite the heterogeneous patient population recruited in these clinical trials, NO.MS is still limited to the eligible trial populations and biological and genetic characterisation is sparse. Selection bias in clinical trial populations, the fact that NO.MS data are from clinical trials of one sponsor, and the more limited number of DMTs studied compared to those used in clinical practice may also impact the broad generalisability of future findings. This may require replications in independent, large and diverse MS data sets.
Previous attempts to compile and analyse pooled MS clinical trial data sets (Supplementary Table S3) include the Sylvia Lawry Centre for Multiple Sclerosis Research, which published on clinical outcomes, disease evolution and prognosis 19 and the Multiple Sclerosis Outcome Assessments Consortium (MSOAC) to develop better disability progression measures. 20 Recently, the International Progressive MS Alliance (IPMSA) collated industry sponsored trial data from >14,000 patients and used machine learning/AI to predict progression, accelerate clinical trials and improve individual treatment decisions in patients with PMS.7,8,12 Several academic centres follow prospectively medium sized, well characterised observational patient cohorts, which have importantly contributed to our understanding of MS (Supplementary Table S3). They recently formed a consortium (SUMMIT) pooling data of ⩾3000 patients to study factors related to MS progression. 21 Registries, collecting observational data from clinical practice, have also formed data networks (Supplementary Table S3) addressing questions around therapeutic effectiveness, 22 comparative safety or outcome and prognosis, among others.22,23 They differ from controlled trial data sets regarding completeness (including MRI), 24 standardisation, homogeneity, quality and biases due to lack of randomisation. Meta-analyses combine information across published studies; however, lack of individual patient data, publication bias and selective outcome reporting bias are limitations. 25 In comparison, NO.MS seems well suited to further characterise MS, study disease worsening and identify prognostic markers (or signatures) across MS phenotypes. However, given that studies included in NO.MS measured heterogeneous sets of endpoints, the selection of an adequate study subset may be required to address a specific scientific question.
Characterisation of MS phenotypes across the age span
As a first application of NO.MS, we explored the relationship between age and (1) focal disease activity, (2) subclinical worsening, as measured by brain volume change and (3) 3mCDW in all phase III studies across phenotypes. By modulating the occurrence and frequency of demyelinating events, age is a key contributor of how the disease is experienced by patients and seen by physicians. Our results confirm that disease activity is highest in patients with POMS26–28 and decreases with ageing across phenotypes, possibly related to immunosenescence. 29 This is in line with previous findings in the London Ontario, the British Columbia MS and the CLIMB cohorts.26,30,31 The prevailing disease activity in POMS as compared to adult patients may be due to intrinsically higher inflammatory disease rather than poorer treatment response, as suggested by others. 28 The difference in disease activity between the ‘placebo ’ and the ‘total’ cohorts (consisting of all placebo and DMT-treated patients in the phase III studies) shrank with increasing age, as focal disease activity decreases over time, also in untreated patients. Based on our descriptive analysis, we conclude that age is an important factor in the presentation of MS patients, as young age is strongly associated with high levels of focal inflammatory disease activity and relapse frequency. Inversely, in the placebo cohort, there were fewer relapses in older patients. The difference between untreated placebo patients and the ‘total’ cohort in brain lesions and relapse activity was highest in the youngest patients. These results were consistent with the phase III results from randomised controlled clinical trials 27 and a meta-analysis; 32 however, the descriptive nature of our analyses and the composite ‘total’ cohort do not allow to draw conclusions about the relative effectiveness of DMTs across the age range studied. The relevance of age in modulating the clinical disease course can be used to inform future MS clinical trials. 33
The brain volume change of approximately 0.5% over 2 years was strikingly similar in adult patients across phenotypes, and relatively independent of age, only RRMS patients receiving placebo fared worse, consistent with other reports. 34 Brain volume change was most pronounced in adolescent patients, where focal disease activity was highest overall. Acute inflammation may be involved in initiating the pathological processes relevant for irreversible tissue loss which then continue rather independently likely based on mechanisms other than focal inflammation. Very similar atrophy rates across age ranges and MS phenotypes have also been reported previously for a much smaller data set. 35 A large recent study found significantly different age-dependent rates of lateral ventricle and brain volume changes. 36 Longer follow-up times and MRI cohorts including patients with an age up to 80 years may explain the different results. Finally, variability was high, reflecting both biological and methodological variations, but overall, brain tissue loss seems to be more similar than different between MS phenotypes.
We also investigated the probability of 3mCDW in relation to age and disease phenotype. The risk of a disability worsening was lowest (10%–15%) in the youngest, least disabled RRMS patients, probably due to higher reserve capacity or plasticity, 37 and increased with older age. Across phenotypes, the differences in the age- and baseline disability-adjusted risk of progression were less pronounced than may have been expected, which is in line with findings from the Swedish MS Registry. 38 However, pronounced inflammatory activity and early brain volume loss is likely the harbinger of disability progression later, since the risk of clinical worsening is increasing with decreasing brain volume, 39 and treatment of early MS with efficacious medication is likely beneficial for long-term outcomes. 40 Some patients diagnosed with SPMS were young (e.g. ⩽25 or ⩽30 years of age) when enrolled. Their baseline characteristics are presented in Supplementary Table S2, showing significant disability and relatively high T2 lesion volumes. Many had a POMS, suggesting an active and aggressive course with early transition to SPMS. The negative trajectories in the high EDSS range in both young SPMS and PPMS phenotypes may be representative for patients with an early onset of progressive disease, or could be caused by a selection bias in the clinical trial setting. The overall higher and rather constant risk of progression (>25% over 2 years) across phenotypes with longer disease durations (reflected by either higher age or pre-existing disability) is in line with the notion of an increasingly compromised CNS with diminishing compensatory abilities. 37 Clinical trials usually report the risk of 3mCDW by treatment arm as a ‘population average’ on the basis of a time-to-event analysis. Our study reveals that depending on the patient’s age and level of disability at baseline, the risk of 3mCDW can be substantially lower or higher than what is reported in clinical trials. Brain volume loss and disability worsening differed between the ‘placebo’ and the ‘total’ cohorts most visibly in young adult and paediatric patients, presumably related to DMT use, in line with the results from phase III randomised controlled trials.
Conclusion
NO.MS is a novel comprehensive clinical trial data set across all MS phenotypes, observed over nearly two decades, that is well suited to further characterise MS, study individual patient trajectories and identify prognostic markers (or signatures) using advanced analytical methods. A first application of NO.MS suggests that age is a key factor modulating relapse frequency and focal MRI activity and thus the phenotypic presentation of MS. However, brain volume loss and disability worsening occur throughout the disease at similar rates except in the youngest patients with least disease burden, suggesting that worsening, once started, occurs to some extent independent from new focal inflammatory disease.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_1352458520988637 for Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): Age is a key contributor to presentation by Frank Dahlke, Douglas L Arnold, Piet Aarden, Habib Ganjgahi, Dieter A Häring, Jelena Čuklina, Thomas E Nichols, Stephen Gardiner, Robert Bermel and Heinz Wiendl in Multiple Sclerosis Journal
Acknowledgments
The authors acknowledge the physicians and patients from all these trials for their valuable contribution towards advancement of our knowledge in MS. The authors acknowledge the work of Ann-Marie Mallon and Luis Santos in coordinating and leading the BDI data wrangling team. They also thank Uma Kundu (Medical Communications, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for assistance with literature search, formatting, referencing, preparation of tables and figures as per journal guidelines and incorporating the authors’ revisions and finalising the draft for submission, all under the direction of the authors. All authors edited the manuscript for intellectual content, provided guidance during manuscript development and approved the final version submitted for publication.
Footnotes
Data sharing statement: The data from NO.MS cohort are currently only available within the collaboration, due to privacy requirements, derived from the original signed informed consent forms and the risk-based anonymization, which takes IT security and access considerations into account. Anonymised clinical data from the individual studies are available on reasonable request provided that it is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed through ClinicalStudyDataRequest.com.
Declaration of Conflicting Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: F.D., P.A., D.A.H. and J.Č. are employees of Novartis. D.L.A. had received grants and personal fees from Novartis, during the conduct of the study; personal fees from Acorda; grants and personal fees from Biogen; personal fees from Celgene; personal fees from Roche; personal fees from Frequency Therapeutics; personal fees from GeNeuro; personal fees from MedImmune; personal fees from Merck-Serono; personal fees from Sanofi-Aventis; grants from Immunotec, outside the submitted work; and Equity interest in NeuroRx Research. H.G., T.E.N. and S.G. have nothing to disclose. R.B. reports grants, personal fees and non-financial support from Novartis, during the conduct of the study; grants, personal fees and non-financial support from Biogen, personal fees and non-financial support from Sanofi/Genzyme; grants, personal fees and non-financial support from Genentech/Roche; and personal fees from VielaBio, outside the submitted work. H.W. reports grants, personal fees and non-financial support from Novartis, during the conduct of the study; grants and personal fees from Bayer Healthcare; grants, personal fees and non-financial support from Bayer Vital GmbH; personal fees and non-financial support from Bayer Schering AG; grants, personal fees and non-financial support from Biogen; grants, personal fees and non-financial support from Sanofi Genzyme; personal fees and non-financial support from Sanofi-Aventis; grants from Sanofi US; grants, personal fees and non-financial support from Merck-Serono; personal fees and non-financial support from EMD Serono; personal fees and non-financial support from CSL Behring; personal fees and non-financial support from Fresenius Medical Care; personal fees and non-financial support from Omniamed; personal fees from Roche; and grants from Teva, outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Frank Dahlke, Novartis Pharma AG, Basel, Switzerland.
Douglas L Arnold, Brain Imaging Centre, Montreal Neurological Institute and Hospital, McGill University, Montréal, QC, Canada.
Piet Aarden, Novartis Pharma AG, Basel, Switzerland.
Habib Ganjgahi, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Dieter A Häring, Novartis Pharma AG, Basel, Switzerland.
Jelena Čuklina, Novartis Pharma AG, Basel, Switzerland.
Thomas E Nichols, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Stephen Gardiner, MRC Harwell Institute, Oxfordshire, UK.
Robert Bermel, Department of Neurology, Mellen MS Center, Cleveland Clinic, Cleveland, OH, USA.
Heinz Wiendl, Department of Neurology, University Hospital Münster, Münster, Germany.
References
- 1. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83(3): 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Confavreux C, Vukusic S. Natural history of multiple sclerosis: A unifying concept. Brain 2006; 129(Pt. 3): 606–616. [DOI] [PubMed] [Google Scholar]
- 3. Scalfari A, Neuhaus A, Daumer M, et al. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol 2013; 70(2): 214–222. [DOI] [PubMed] [Google Scholar]
- 4. Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: A systematic review. Arch Neurol 2006; 63(12): 1686–1691. [DOI] [PubMed] [Google Scholar]
- 5. Brown FS, Glasmacher SA, Kearns PKA, et al. Systematic review of prediction models in relapsing remitting multiple sclerosis. PLoS ONE 2020; 15(5): e0233575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: An evolving treatment paradigm. Curr Opin Neurol 2019; 32(3): 365–377. [DOI] [PubMed] [Google Scholar]
- 7. Doyle A, Elliott C, Karimaghaloo Z, et al. Lesion detection, segmentation and prediction in multiple sclerosis clinical trials. Cham: Springer International Publishing, 2018. [Google Scholar]
- 8. Eshaghi A, Young A, Wijertane P, et al. Defining multiple sclerosis phenotypes using MRI, 2020, https://www.medrxiv.org/content/10.1101/19011080v2
- 9. Walsh J, Smith A, Pouliot Y, et al. Modeling subject-level disease progression for multiple sclerosis clinical trials with machine learning. Neurology 2020; 94(15 Suppl.): 2232. [Google Scholar]
- 10. Zhao Y, Healy BC, Rotstein D, et al. Exploration of machine learning techniques in predicting multiple sclerosis disease course. PLoS ONE 2017; 12(4): e0174866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iaffaldano P, Butzkueven H, Hillert J, et al. Big multiple sclerosis data network: Data sharing among five large MS registries (P738), http://www.ofsep.org/ECTRIMS/2017/P738_BIG%20MS%20DATA_poster1.pdf (accessed 15 August 2020).
- 12. Tousignant A, Lemaître P, Precup D, et al. Prediction of disease progression in multiple sclerosis patients using deep learning analysis of MRI data. In: Cardoso MJ, Aasa F, Ben G, et al. (eds) Proceedings of the 2nd international conference on medical imaging with deep learning, 2019, pp.483–492, http://proceedings.mlr.press/v102/tousignant19a.html
- 13. Ann-Marie Mallon DAH, Dahlke F, Aarden P, et al. Advancing data science in drug development through an innovative computational framework for data sharing and statistical analysis. BMC Medical Res. Methodol. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018; 391(10127): 1263–1273. [DOI] [PubMed] [Google Scholar]
- 15. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77(9): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudlow C, Gallacher J, Allen N, et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12(3): e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 2016; 19(11): 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schach S, Scholz M, Wolinsky JS, et al. Pooled historical MRI data as a basis for research in multiple sclerosis: A statistical evaluation. Mult Scler 2007; 13(4): 509–516. [DOI] [PubMed] [Google Scholar]
- 19.http://www.slcmsr.net/en/about/start.html (accessed on 15 August 2020).
- 20. LaRocca NG, Hudson LD, Rudick R, et al. The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability. Mult Scler 2018; 24(11): 1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bove R, Chitnis T, Cree B, et al. SUMMIT (Serially Unified Multicenter Multiple Sclerosis Investigation): Creating a repository of deeply phenotyped contemporary multiple sclerosis cohorts. Multi Scler 2017; 24: 1352458517726657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis – Insights from real-world observational studies. Nat Rev Neurol 2017; 13(2): 105–118. [DOI] [PubMed] [Google Scholar]
- 23. Cohen JA, Trojano M, Mowry EM, et al. Leveraging real-world data to investigate multiple sclerosis disease behavior, prognosis, and treatment. Mult Scler 2020; 26(1): 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalincik T, Kuhle J, Pucci E, et al. Data quality evaluation for observational multiple sclerosis registries. Mult Scler 2017; 23(5): 647–655. [DOI] [PubMed] [Google Scholar]
- 25. Goodman S, Dickersin K. Metabias: A challenge for comparative effectiveness research. Ann Intern Med 2011; 155(1): 61–62. [DOI] [PubMed] [Google Scholar]
- 26. Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord 2014; 3(2): 186–193. [DOI] [PubMed] [Google Scholar]
- 27. Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: A post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin 2018; 4(2): 2055217318778610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Wyl V, Décard BF, Benkert P, et al. Influence of age at disease onset on future relapses and disability progression in patients with multiple sclerosis on immunomodulatory treatment. Eur J Neurol 2020; 27(6): 1066–1075. [DOI] [PubMed] [Google Scholar]
- 29. Bolton C, Smith PA. The influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE). Ageing Res Rev 2018; 41: 64–81. [DOI] [PubMed] [Google Scholar]
- 30. Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79(12): 1368–1374. [DOI] [PubMed] [Google Scholar]
- 31. Scalfari A, Lederer C, Daumer M, et al. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler 2016; 22(13): 1750–1758. [DOI] [PubMed] [Google Scholar]
- 32. Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 2017; 8: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidli H, Haring DA, Thomas M, et al. Beyond randomized clinical trials: Use of external controls. Clin Pharmacol Therapeut 2020; 107(4): 806–816. [DOI] [PubMed] [Google Scholar]
- 34. De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010; 74(23): 1868–1876. [DOI] [PubMed] [Google Scholar]
- 35. De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87(1): 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghione E, Bergsland N, Dwyer MG, et al. Aging and brain atrophy in multiple sclerosis. J Neuroimaging 2019; 29(4): 527–535. [DOI] [PubMed] [Google Scholar]
- 37. Rocca MA, Absinta M, Moiola L, et al. Functional and structural connectivity of the motor network in pediatric and adult-onset relapsing-remitting multiple sclerosis. Radiology 2010; 254(2): 541–550. [DOI] [PubMed] [Google Scholar]
- 38. McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology 2019; 92(24): e2764–e2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sormani MP, Kappos L, Radue E-W, et al. Defining brain volume cutoffs to identify clinically relevant atrophy in RRMS. Mult Scler 2017; 23(5): 656–664. [DOI] [PubMed] [Google Scholar]
- 40. He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol 2020; 19(4): 307–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_1352458520988637 for Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): Age is a key contributor to presentation by Frank Dahlke, Douglas L Arnold, Piet Aarden, Habib Ganjgahi, Dieter A Häring, Jelena Čuklina, Thomas E Nichols, Stephen Gardiner, Robert Bermel and Heinz Wiendl in Multiple Sclerosis Journal