Abstract
Background:
In the N-MOmentum trial, the risk of an adjudicated neuromyelitis optica spectrum disorder (NMOSD) attack was significantly reduced with inebilizumab compared with placebo.
Objective:
To demonstrate the robustness of this finding, using pre-specified sensitivity and subgroup analyses.
Methods:
N-MOmentum is a prospective, randomized, placebo-controlled, double-masked trial of inebilizumab, an anti-CD19 monoclonal B-cell-depleting antibody, in patients with NMOSD. Pre-planned and post hoc analyses were performed to evaluate the primary endpoint across a range of attack definitions and demographic groups, as well as key secondary endpoints.
Results:
In the N-MOmentum trial (ClinicalTrials.gov: NCT02200770), 174 participants received inebilizumab and 56 received placebo. Attack risk for inebilizumab versus placebo was consistently and significantly reduced, regardless of attack definition, type of attack, baseline disability, ethnicity, treatment history, or disease course (all with hazard ratios < 0.4 favoring inebilizumab, p < 0.05). Analyses of secondary endpoints showed similar trends.
Conclusion:
N-MOmentum demonstrated that inebilizumab provides a robust reduction in the risk of NMOSD attacks regardless of attack evaluation method, attack type, patient demographics, or previous therapy.
The N-MOmentum study is registered at ClinicalTrials.gov: NCT2200770.
Keywords: Attack risk, clinical trial, Devic’s disease, inebilizumab, neuromyelitis optica, neuromyelitis optica spectrum disorder, patient demographics, sensitivity analyses
Introduction
In observational studies, several risk factors are prognostic of disease outcomes in neuromyelitis optica spectrum disorder (NMOSD). Dietary or lifestyle factors associated with weight,1–4 delayed treatment from initial diagnosis or diagnostic delay,5,6 higher levels of disability or attack rates early in the disease course,1,7 and being of non-White ethnicity8–13 were linked to disease outcomes.
N-MOmentum is a prospective, randomized, placebo-controlled, double-masked trial of inebilizumab, an anti-CD19 monoclonal B-cell-depleting antibody, in patients with NMOSD. 14 In N-MOmentum, the risk of an adjudicated NMOSD attack was significantly reduced with inebilizumab compared with placebo (hazard ratio (HR), 0.272, (95% confidence interval (CI): 0.150–0.496); p < 0.0001). Although the N-MOmentum study recruited participants who were seropositive and seronegative for aquaporin 4 autoantibodies (AQP4-IgG), the majority of participants were AQP4-IgG seropositive, with only 17 participants (7.4%) who were AQP4-IgG seronegative. 14
Pre-planned sensitivity and subgroup analyses used to test the robustness of the primary endpoint in N-MOmentum (time to an adjudicated NMOSD attack) are presented. Data for key secondary endpoints are also presented.
Methods
Case selection and study population
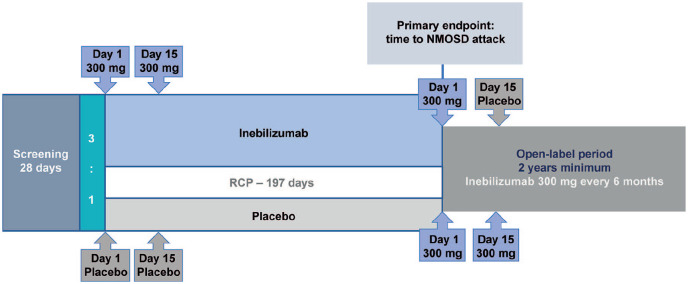
Eligible participants were 18 years or older with an Expanded Disability Status Scale (EDSS) score of 8.0 or less, with a documented history of one or more neuromyelitis optica acute attacks that required rescue therapy within the last year, or 2 or more such attacks within 2 years prior to screening AND either (a) positive serum anti-AQP4-IgG result at screening OR (b) negative serum anti-AQP4-IgG result at screening without evidence of brain lesion consistent with MS and also meeting clinical criteria for neuromyelitis optica. 15 AQP4-IgG seronegative subjects were reviewed by an independent eligibility committee for eligibility. Participants were randomly assigned (3:1) to receive inebilizumab 300 mg i.v. or placebo on days 1 and 15, with no other immune treatments allowed (Figure 1). 14
Figure 1.
N-MOmentum study design.
IDMC: Independent Data Monitoring Committee; NMOSD: neuromyelitis optica spectrum disorder; RCP: randomized controlled period.
N-MOmentum was a double-blind, placebo-controlled study at 99 medical centers in 25 countries, with a time-to-event design. End of RCP was defined as 67 NMOSD attacks, or when 252 participants had been randomized and had received study drug, whichever happened first. Enrollment was stopped early at 231 participants and 43 attacks owing to proven efficacy as determined by the IDMC. No background immunotherapy was permitted. The primary endpoint was the time to an NMOSD adjudicated attack within the RCP.
aParticipants eligible for the open-label period at the end of the RCP or after an adjudicated attack.
The randomized controlled period (RCP) was 28 weeks or up to an adjudicated attack. Attacks were evaluated using predefined attack diagnosis criteria that were developed specifically for this study. 16 Study investigators and an independent adjudication committee (AC) composed of three members assessed attacks. 14 The primary endpoint was the time to an adjudicated attack; secondary endpoints included worsening from baseline in EDSS score at last visit, cumulative number of active magnetic resonance imaging (MRI) lesions (new gadolinium-enhancing T1 or new/enlarging T2), hospitalizations during the RCP, and change from baseline in low-contrast visual acuity binocular score. 14 Only attacks confirmed by an AC majority (at least 2/3) were used for the primary endpoint analysis; participants with events adjudicated as non-attacks continued in the RCP. The RCP ended if participants experienced an adjudicated attack, reached day 197, or were in the RCP when enrollment stopped. 14 Participants could then continue treatment in the open-label period for at least 1 year, during which they received inebilizumab 300 mg every 26 weeks to maintain B-cell depletion. 14 Eligibility criteria, settings and locations, sample size determination, and full details of interventions are available in the original publication of the N-MOmentum trial results, 14 and the full trial protocol is available from: https://ucsf.box.com/s/qn2uiij5lfxqj6h8ch2edkb2o9nzr9le.
Sensitivity and subgroup analyses
Sensitivity analyses were performed to assess whether the primary endpoint remained significant when accounting for various factors, and included: analysis of attacks only by unanimous AC decision, investigator-determined attacks, patient-reported symptoms, attacks including patients who discontinued prematurely, attacks categorized according to attack type, attacks adjusted for historical acute attacks and baseline EDSS score, and censoring of attacks during the first 15 days of the trial. These analyses were pre-planned and conducted for both the whole population and the AQP4-IgG seropositive population.
The primary endpoint was also analyzed across the following subgroups, where all characteristics are presumed to reflect disease severity or differential prognoses: disease duration (<5 years or ⩾5 years, from time of clinical diagnosis), time since last attack at enrollment (<26 weeks or ⩾26 weeks), number of previous attacks (<4 attacks or ⩾4 attacks), baseline EDSS score (<5 or ⩾5), use of prior immunosuppressive therapy (yes/no), White and non-White participants, Asian and non-Asian participants, underweight/healthy weight (<25 kg/m2) and overweight/obese participants (⩾25 kg/m2). The cutoff disease duration of ⩾ 5 years was chosen to reflect those with longer-term disease and the time since last attack of 26 weeks is representative of the length of the RCP. Originally, a cutoff of 2 previous attacks was chosen to reflect the inclusion criteria (⩾2 attacks in the previous 2 years). As the number of previous attacks experienced by participants was typically greater than this, a new cutoff of ⩾ 4 attacks was selected to be more representative of the population. A baseline EDSS score ⩾ 5 was selected as this represents the point where daily activities are impacted to a degree that requires special provisions. The same subgroup analyses were performed for attack-related outcomes, including the cumulative number of active (new gadolinium-enhancing T1 or new/enlarging T2) MRI lesions and the cumulative number of NMOSD-related inpatient hospitalizations seen in participants during the RCP.
Statistical analysis
All sensitivity and subgroup analyses of NMOSD attacks were analyzed by Cox proportional hazards regression with placebo as the reference group, and treatment and serotype as explanatory factors. Interaction tests for the subgroup analyses were also performed, where p > 0.05 indicates no statistically significant difference in treatment effect between subgroups. The rate ratio for the cumulative number of active MRI lesions from baseline and the cumulative number of NMOSD-related inpatient hospitalizations from baseline were assessed using negative binomial regression, with treatment and serostatus as explanatory variables. 14 Because of the low number of participants in many of the subgroups, the statistical power to detect significant differences in the secondary sensitivity analyses is limited.
Standard protocol approvals, registrations, and patient consents
Institutional review boards or ethics committees at each study site approved the protocol. All participants provided written informed consent. The study was conducted in accordance with the provisions of the International Conference on Harmonization Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The study is registered with ClinicalTrials.gov, number NCT02200770.
Data availability statement
Reasonable requests for anonymized data on defined study outcomes will be made available by request. Proposals should be directed to katze@vielabio.com. Requestors will be required to sign a data access agreement. Requests will be considered for up to 3 years after article publication.
Results
Participants
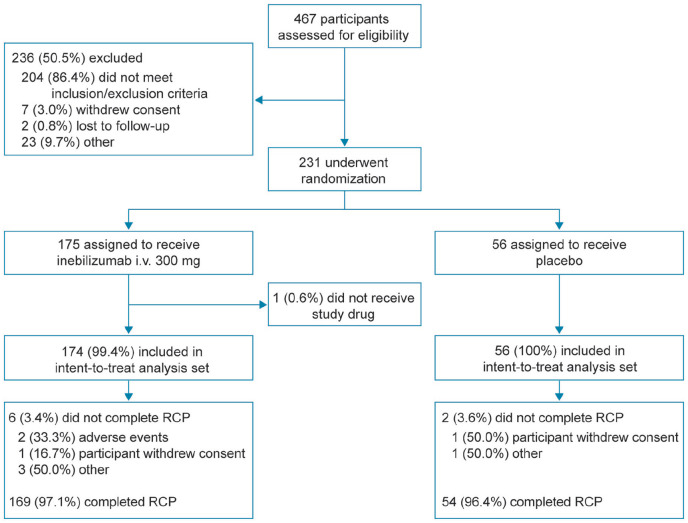
Of the 467 participants screened, 231 were randomized; 175 were assigned to inebilizumab (with one participant not dosed) and 56 to placebo (overall population) (Figure 2). In the subgroup of participants who were AQP4-IgG seropositive, 161 were randomized to inebilizumab and 52 to placebo. Baseline demographics and characteristics were generally similar between treatment groups in the overall population and in AQP4-IgG seropositive participants. 14
Figure 2.
CONSORT flow diagram.
CONSORT: Consolidated Standards of Reporting Trials; i.v.: intravenous; RCP: randomized controlled period.
No notable differences in previous therapies for NMOSD were observed between treatment groups, with almost all participants receiving some form of prior medication or therapy for NMOSD (inebilizumab: 172/174 (98.9%); placebo: 55/56 (98.2%)). Similar numbers of participants had previously received intravenous immunoglobulin (inebilizumab: 8/174 (5.4%); placebo: 3/56 (4.6%)) or immunosuppressive therapies (inebilizumab: 82/174 (47.1%); placebo: 26/56 (46.4%)) prior to enrollment, such as azathioprine (inebilizumab: 65/174 (37.4%); placebo: 22/56 (39.3%)), mycophenolate (inebilizumab: 27/174 (21.8%); placebo: 7/56 (12.5%)) or rituximab (inebilizumab: 13/174 (7.5%); placebo: 4/56 (7.1%)).
Sensitivity and subgroup analyses of time to adjudicated attack
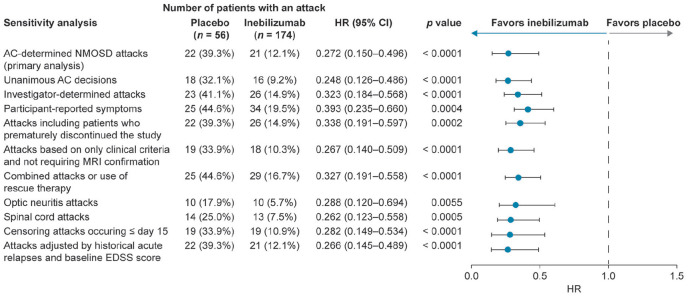
According to majority AC decision, 21 of the 174 participants (12.1%) who received inebilizumab and 22 of the 56 (39.3%) who received placebo experienced attacks. The risk reduction for inebilizumab versus placebo was 72.8% (HR, 0.272 (95% CI: 0.150–0.496); p < 0.0001). 14 The reduction in the risk of attack with inebilizumab versus placebo was significant in all sensitivity and subgroup analyses (Figure 3). The HR for all sensitivity and subgroup analyses was ⩽ 0.4 in the inebilizumab group compared with in the placebo group.
Figure 3.
Sensitivity analyses for primary endpoint (time to adjudicated attack; overall population).
AC: adjudication committee; CI: confidence interval; EDSS: Expanded Disability Status Scale; HR: hazard ratio; NMOSD: neuromyelitis optica spectrum disorder.
Based on Cox regression method, with placebo as the reference group.
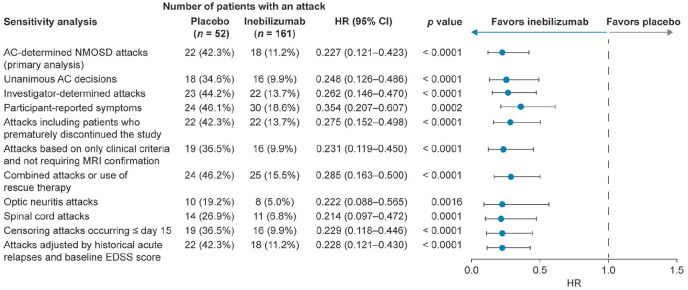
Risk of attack was reduced in the inebilizumab group compared with the placebo group when attacks were limited to unanimous AC decisions, investigator-determined attacks, or when participants reported attack symptoms. A reduction in attack risk was also seen for participants who prematurely discontinued the study, and for attacks based solely on clinical attack criteria. When the definition of attacks was broadened to include events that required clinical intervention (such as intravenous corticosteroids, intravenous immunoglobulin, and/or plasma exchange), but may not have fully met the attack criteria, the risk of attacks was still reduced in the inebilizumab group. Risk of attacks in the inebilizumab group compared with the placebo group was reduced for optic neuritis attacks and for spinal cord attacks. Brainstem attacks (two in the placebo group and none in the inebilizumab group) occurred too infrequently for statistical analysis. Attack risk was lower in the inebilizumab group than in the placebo group when attacks that occurred on or before day 15 were censored (two in the inebilizumab group and three in the placebo group). When adjusted by the number of historical NMOSD attacks and baseline EDSS score, the reduction in attack risk was maintained in the inebilizumab group. Reduction in the risk of attack with inebilizumab was observed in all sensitivity and subgroup analyses in the AQP4-IgG seropositive population (HR ⩽ 0.4; p ⩽ 0.0016) (Figure 4), which is not unexpected based on the small proportion of participants who were AQP4-IgG seronegative. Only 17/230 study participants (7.4%) were AQP4-IgG seronegative, and therefore could not be analyzed statistically as an independent subgroup. Details and post hoc analyses of the AQP4-IgG seronegative subgroup are the subject of a separate manuscript.
Figure 4.
Sensitivity analyses for primary endpoint (time to adjudicated attack; AQP4-IgG seropositive population).
AC: adjudication committee; AQP4-IgG: aquaporin-4 immunoglobulin G; CI: confidence interval; EDSS: Expanded Disability Status Scale; HR: hazard ratio; NMOSD: neuromyelitis optica spectrum disorder.
Based on Cox regression method, with placebo as the reference group.
Efficacy among demographic groups
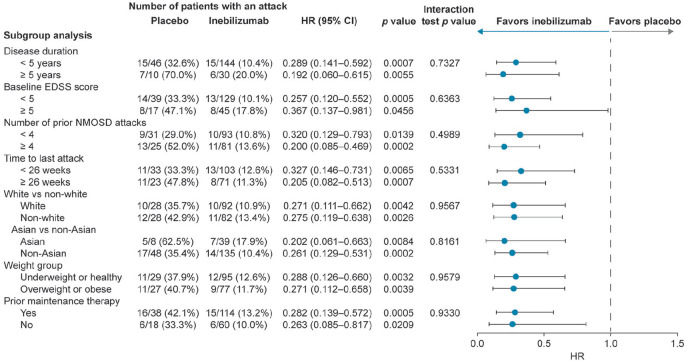
The time to adjudicated attack was analyzed across a variety of demographic subgroups to investigate whether inebilizumab is equally effective in patients with different ethnicity, weight, disease course, and treatment history. The HR of inebilizumab treatment versus placebo was < 0.4 for all demographic subgroups (all p < 0.05). Interaction tests were p > 0.05 in all cases (Figure 5). Risk of attack was reduced in participants receiving inebilizumab regardless of disease duration, attack occurrence within 6 months of study entry, or attack number before screening. Inebilizumab efficacy was not influenced by baseline EDSS score, race, weight, or prior immunosuppressive maintenance therapy.
Figure 5.
Primary endpoint by demographic and baseline characteristic subgroup (time to adjudicated attack; overall population).
CI: confidence interval; EDSS: Expanded Disability Status Scale; HR: hazard ratio; NMOSD: neuromyelitis optica spectrum disorder.
Based on Cox regression method, with placebo as the reference group.
Subgroup analyses of secondary endpoints
Subgroup analyses were conducted for the attack-related secondary endpoints of the cumulative numbers of active MRI lesions (Table 1) and NMOSD-related inpatient hospitalizations during the RCP (Table 2). MRI scans were performed at baseline, during attacks, and at the end of the RCP. Therefore, cumulative new lesions would be assessed in participants either at the time of an adjudicated attack during the RCP or at day 197. Rate ratio point estimates favored inebilizumab in all cases. Inebilizumab treatment appeared to be associated with significantly fewer new central nervous system (CNS) MRI lesions in participants with baseline EDSS scores less than 5, four or more attacks before study entry, a disease duration of less than 5 years, with a time to last attack before enrollment of at least 26 weeks, or who were White, non-Asian, overweight/obese or had previously received prior immunosuppressive maintenance therapy. Significant effects were not identified in the other corresponding subgroups, namely in participants with a disease duration of equal to or more than 5 years, baseline EDSS score of equal to or more than 5, fewer than 4 previous NMOSD attacks, time to attack before enrollment of less than 26 weeks, or who were non-White, Asian, underweight or healthy weight, or had previously not received immunosuppressive maintenance therapy.
Table 1.
Subgroup analyses of key secondary endpoints: cumulative number of active MRI lesions, randomized controlled period.
| Subgroup | Placebo (N = 56) | Inebilizumab (N = 174) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Patients, n/N | Lesions, mean (SD) | Patients, n/N | Lesions, mean (SD) | RR (95% CI) | p value | Interaction test p value | |
| Disease duration < 5 years | 25/46 | 2.3 (1.2) | 64/144 | 1.6 (1.0) | 0.555 (0.363–0.849) | 0.0066 | 0.8462 |
| Disease duration ⩾ 5 years | 7/10 | 2.3 (1.7) | 15/30 | 1.9 (1.1) | 0.603 (0.259–1.406) | 0.2419 | |
| Baseline EDSS score < 5 | 22/39 | 2.5 (1.4) | 59/129 | 1.6 (1.0) | 0.538 (0.341–0.848) | 0.0076 | 0.6901 |
| Baseline EDSS score ⩾ 5 | 10/17 | 2.0 (1.1) | 20/45 | 1.7 (0.9) | 0.643 (0.319–1.295) | 0.2163 | |
| Prior NMOSD attacks < 4 | 16/31 | 1.9 (1.1) | 39/93 | 1.6 (1.0) | 0.650 (0.372–1.139) | 0.1322 | 0.4503 |
| Prior NMOSD attacks ⩾ 4 | 16/25 | 2.8 (1.3) | 40/81 | 1.7 (1.0) | 0.484 (0.288–0.813) | 0.0061 | |
| Time to last attack < 26w | 17/33 | 2.3 (1.3) | 48/103 | 1.6 (0.9) | 0.645 (0.378–1.062) | 0.0844 | 0.4460 |
| Time to last attack ⩾ 26w | 15/23 | 2.3 (1.3) | 31/71 | 1.7 (1.2) | 0.476 (0.265–0.855) | 0.0130 | |
| White | 15/28 | 2.3 (1.3) | 37/92 | 1.3 (0.5) | 0.428 (0.254–0.722) | 0.0015 | 0.1887 |
| Non-White | 17/28 | 2.4 (1.3) | 42/82 | 1.9 (1.3) | 0.709 (0.416–1.209) | 0.2065 | |
| Asian | 6/8 | 1.7 (0.8) | 21/39 | 2.0 (1.1) | 0.886 (0.385–2.043) | 0.7772 | 0.2638 |
| Non-Asian | 26/48 | 2.5 (1.3) | 58/135 | 1.5 (1.0) | 0.489 (0.318–0.752) | 0.0011 | |
| Underweight/healthy | 15/29 | 2.5 (1.4) | 39/95 | 1.9 (1.3) | 0.584 (0.316–1.080) | 0.0864 | 0.8479 |
| Overweight/obese | 17/27 | 2.1 (1.2) | 38/77 | 1.4 (0.6) | 0.543 (0.344–0.859) | 0.0090 | |
| Prior maintenance therapy, yes | 23/38 | 2.3 (1.3) | 56/114 | 1.7 (1.0) | 0.609 (0.393–0.944) | 0.0265 | 0.5903 |
| Prior maintenance therapy, no | 9/18 | 2.4 (1.3) | 23/60 | 1.5 (1.1) | 0.493 (0.236–1.030) | 0.5930 | |
CI: confidence interval; EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; NMOSD: neuromyelitis optica spectrum disorder; RR: rate ratio; SD: standard deviation; w: weeks.
Table 2.
Subgroup analyses of key secondary endpoints: cumulative number of NMOSD-related inpatient hospitalizations, randomized controlled period.
| Subgroup a | Placebo (N = 56) | Inebilizumab (N = 174) | ||
|---|---|---|---|---|
| Patients, n/N (%) | Hospitalizations, mean (SD) | Patients, n/N (%) | Hospitalizations, mean (SD) | |
| Disease duration < 5 years | 6/46 (13.0%) | 1.5 (0.8) | 6/144 (4.2%) | 1.0 (0.0) |
| Disease duration ⩾ 5 years | 2/10 (20.0%) | 1.0 (0.0) | 4/30 (13.3%) | 1.0 (0.0) |
| Baseline EDSS score < 5 | 5/39 (12.8%) | 1.6 (0.9) | 6/129 (4.7%) | 1.0 (0.0) |
| Baseline EDSS score ⩾ 5 | 3/17 (17.6%) | 1.0 (0.0) | 4/45 (8.9%) | 1.0 (0.0) |
| Prior NMOSD attacks < 4 | 5/31 (16.1%) | 1.6 (0.9) | 2/93 (2.2%) | 1.0 (0.0) |
| Prior NMOSD attacks ⩾ 4 | 3/25 (12.0%) | 1.0 (0.0) | 8/81 (9.9%) | 1.0 (0.0) |
| Time to last attack < 26w | 1/33 (3.0%) | 1.0 (0.0) | 7/103 (6.8%) | 1.0 (0.0) |
| Time to last attack ⩾ 26w | 7/23 (30.4%) | 1.4 (0.8) | 3/71 (4.2%) | 1.0 (0.0) |
| White | 3/28 (10.7%) | 1.3 (0.6) | 6/92 (6.5%) | 1.0 (0.0) |
| Non-White | 5/28 (17.9%) | 1.4 (0.9) | 4/82 (4.9%) | 1.0 (0.0) |
| Asian | 1/8 (12.5%) | 1.0 (0.0) | 2/39 (5.1%) | 1.0 (0.0) |
| Non-Asian | 7/48 (14.6%) | 1.4 (0.8) | 8/135 (5.9%) | 1.0 (0.0) |
| Underweight/heathy | 2/29 (6.9%) | 1.0 (0.0) | 7/95 (7.4%) | 1.0 (0.0) |
| Overweight/obese | 6/27 (22.2%) | 1.5 (0.8) | 3/77 (3.9%) | 1.0 (0.0) |
| Prior maintenance therapy, yes | 6/38 (15.8%) | 1.5 (0.8) | 6/114 (5.3%) | 1.0 (0.0) |
| Prior maintenance therapy, no | 2/18 (11.1%) | 1.0 (0.0) | 4/60 (6.7%) | 1.0 (0.0) |
NMOSD: neuromyelitis optica spectrum disorder; SD: standard deviation; w: weeks; EDSS: Expanded Disability Status Scale.
The numbers of cases were too low to generate meaningful rate ratios or statistical analyses for these outcomes.
This lack of significance may possibly be related to smaller numbers of participants in these subgroups. Indeed, interaction tests detected no significant differences in treatment effect between the dichotomized groups (Table 1, all p > 0.05). The overall number of participants who had NMOSD-related inpatient hospitalizations was low in both groups (inebilizumab, n = 10; placebo, n = 8). As such, meaningful point estimates of rate ratios and p values could not be calculated, but the proportion of patients who required inpatient hospitalization was generally lower with inebilizumab (Table 2). Disability endpoint analyses are presented in a separate manuscript.
Discussion
In N-MOmentum, inebilizumab consistently provided a statistically significant reduction in the risk of attacks compared with placebo, regardless of who evaluated or reported attacks, whether decisions were based on clinical criteria alone, and when considering anatomical attack locations separately. These results suggest that the result for the primary endpoint was robust and insensitive to these potential confounders; indeed, the study was designed to maximize clinically informative data by using independent AQP4-IgG seronegative patient eligibility-, attack adjudication-, and data monitoring-committees,14,16 and establishing predefined attack criteria. 16 However, it is also important to note that a robust treatment effect was observed, even according to metrics that were more in line with the general clinical setting (investigator-determined attacks or patient-reported symptoms, attacks adjudicated by attack criteria solely using clinical data). As such, while the methods employed in N-MOmentum may help to inform future trial design in NMOSD, more importantly, they provide meaningful information to support clinicians in making treatment decisions.
Attacks in NMOSD tend to cluster, 17 and therefore attacks are more likely to occur in patients beginning a new program of treatment if they have had frequent recent attacks. It is important to note that inebilizumab was effective regardless of whether participants had relapses within 6 months of enrollment, showing treatment benefit regardless of whether participants had recent attacks.
Inebilizumab also provided a statistically significant reduction in the risk of attacks compared with placebo across a broad spectrum of baseline characteristics and demographics, demonstrating efficacy regardless of ethnicity, body habitus, previous treatment, or disease duration. Subgroup analyses of cumulative numbers of new CNS MRI lesions supported the efficacy of inebilizumab in the same subgroups, and the proportion of participants who required NMOSD-related hospitalizations was generally lower with inebilizumab. Given that these secondary endpoints are related to attacks (new MRI lesions typically occur during attacks and NMOSD-related hospitalizations occur with severe attacks), the subgroup analyses for these secondary endpoints bolster the sensitivity and subgroup analyses conducted on the primary endpoint.
Although carefully designed to make interpretation of results relatively straightforward, the N-MOmentum study had several limitations. 7 Of relevance to the sensitivity and subgroup analyses reported here, these include: the small number of AQP4-IgG seronegative participants; the exclusion of patients with several comorbidities or laboratory abnormalities for participant safety (which may reduce the extent to which the trial participants are representative of the general NMOSD population); and the relatively small size of the study population, which, nevertheless, constitutes the largest prospective randomized study to be conducted in NMOSD. Criteria for the pre-planned subgroup analyses could have been chosen to be more representative of the actual cohort recruited. In the N-MOmentum trial, the Bonferroni-based chain procedure was used to control the overall type 1 error rate for the primary and key secondary endpoints. 14 This rigorous correction for multiple comparisons was not performed for the analyses presented here. Nonetheless, that all of the sensitivity analyses of the primary endpoint were pre-planned, lends some confidence to the results.
The relatively small size of the study was reflected in the small size of the subgroups analyzed for secondary endpoints. Even where these subgroups were relatively large (e.g. non-White participants or healthy/underweight participants, Table 1), further division by treatment group reduced the number of subjects available for analysis. It should be noted that rate ratio point estimates and 95% CIs, which are demonstrative of the actual size of treatment effect, favored inebilizumab in all cases. While the results presented here are promising, and may help inform treatment decisions by clinicians, further studies designed to investigate the effect of baseline or demographic characteristics on inebilizumab treatment efficacy are needed, including in patient populations at risk of more severe disease.
The results of the N-MOmentum study demonstrate that inebilizumab provides a robust reduction in the risk of attacks in patients with NMOSD, regardless of attack evaluation methods, attack types, patient demographics, and previous therapies. These findings provide important information for clinicians who are making treatment decisions for patients with NMOSD.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_1352458521988926 for Sensitivity analysis of the primary endpoint from the N-MOmentum study of inebilizumab in NMOSD by Bruce AC Cree, Jeffrey L Bennett, Ho Jin Kim, Brian G Weinshenker, Sean J Pittock, Dean Wingerchuk, Kazuo Fujihara, Friedemann Paul, Gary R Cutter, Romain Marignier, Ari J Green, Orhan Aktas, Hans-Peter Hartung, Ian M Williams, Jorn Drappa, Dewei She, Daniel Cimbora, William Rees, John N Ratchford and Eliezer Katz in Multiple Sclerosis Journal
Acknowledgments
Medical writing and editorial support was provided by Oxford PharmaGenesis.
Footnotes
Declaration of Conflicting Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.A.C. Cree reports personal fees for consulting from Akili, Alexion, Autobahn, EMD Serono, Novartis, Sanofi, TG Therapeutics, and Therini. J.L. Bennett reports payment for study design/consultation from MedImmune/Viela Bio; personal fees from AbbVie, Alexion, Chugai, Clene Nanomedicine, Genentech, Genzyme, Mitsubishi-Tanabe, Reistone Bio, and Roche; grants and personal fees from EMD Serono and Novartis; grants from the Guthy–Jackson Charitable Foundation, Mallinckrodt, and the National Institutes of Health; and has a patent for Aquaporumab issued. H.J. Kim has received a grant from the National Research Foundation of Korea; consultancy/speaker fees or research support from Alexion, Aprilbio, Celltrion, Eisai, HanAll BioPharma, MDimune, Merck Serono, Novartis, Sanofi Genzyme, Teva-Handok, and Viela Bio; serves on a steering committee for MedImmune/Viela Bio and is a coeditor for the Multiple Sclerosis Journal and an associate editor for the Journal of Clinical Neurology. B.G. Weinshenker receives payments for serving as chair of attack adjudication committees for clinical trials in NMOSD for Alexion, MedImmune, and Viela Bio; has consulted with Chugai, Genentech, Roche Pharmaceuticals, and Mitsubishi Tanabe Pharma regarding clinical trial design for NMOSD; and has a patent for NMO-IgG for diagnosis of neuromyelitis optica, with royalties paid by Hospices Civils de Lyon, MVZ Labor PD Dr. Volkmann und Kollegen GbR, Oxford University, and RSR. S.J. Pittock reports grants, personal fees, and non-financial support from Alexion Pharmaceuticals, Inc.; grants from Autoimmune Encephalitis Alliance, Grifols; grants, personal fees, non-financial support, and other from MedImmune and Viela Bio; consulting support from Astellas; personal fees for consulting services from UCB; and has a patent # 9,891,219 (Application#12-573942) “Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive.” D. Wingerchuk reports personal fees from Arcus Medica, Biogen, Celgene, Genentech, MedImmune, Novartis, Reistone Biopharma, TG Therapeutics, and Third Rock Ventures; research support paid to the Mayo Clinic by Alexion and Terumo BCT; and serves on a clinical trial adjudication committee for MedImmune and Viela Bio. K. Fujihara serves on scientific advisory boards for Alexion, Bayer Schering, Biogen Idec, Chugai, MedImmune, Merck Serono, Mitsubishi Tanabe Pharma, Nihon Pharmaceutical, Novartis, Ono, and Viela Bio; has received funding for travel and speaker honoraria from Asahi Kasei Medical, Astellas, Bayer Schering, Biogen Idec, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Mitsubishi Tanabe Pharma, Nihon Pharmaceutical, Novartis, and Takeda; and research support from Asahi Kasei Medical, Bayer Schering, Biogen Idec, Chemo-Sero-Therapeutic Research Institute, Chugai, Genzyme Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Welfare and Labor of Japan, Mitsubishi Tanabe Pharma, Nihon Pharmaceutical, Ono, Teijin, and Teva. G.R. Cutter has received personal fees for participation on Data and Safety Monitoring Boards from AstraZeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Orphazyme, Sanofi-Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, Viela Bio Inc., Vivus, NHLBI (Protocol Review Committee) and NICHD (OPRU oversight committee); personal fees for consulting or advisory board partici-pation from Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche and TG Therapeutics. Dr Cutter is employed by the University of Alabama at Birmingham and is President of Pythagoras, Inc., a private consulting company based in Birmingham, AL, USA. F. Paul has received research support, speaker fees, and travel reimbursement from Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, and Teva; is supported by the German Competence Network for Multiple Sclerosis and the German Research Council (DFG Exc 257); has received travel reimbursement from the Guthy–Jackson Charitable Foundation; and serves on the steering committee of the OCTIMS study sponsored by Novartis. R. Marignier serves on scientific advisory boards for MedImmune and Viela Bio; and has received funding for travel and fees from Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva, and Viela Bio. A.J. Green reports grants from the Conrad N. Hilton Foundation and the Tom Sherak MS Hope Foundation; other financial relationships (for activities as expert witness, associate editor, advisory board/steering committee participation, and endpoint adjudication) with Bionure, Inception Sciences, JAMA Neurology, MedImmune/Viela Bio, Mylan, Synthon, and Trims Pharma; and personal fees from and other financial relationships with Pipeline Therapeutics. O. Aktas reports grants from the German Ministry of Education and Research (BMBF) and the German Research Foundation (DFG); grants and personal fees from Bayer HealthCare, Biogen, Genzyme, Novartis, Teva, and Viela Bio; and personal fees from Almirall, MedImmune, Merck Serono, and Roche. H.-P. Hartung has received fees for consulting, speaking, and serving on steering committees from Bayer HealthCare, Biogen Idec, Celgene Receptos, CSL Behring, GeNeuro, Genzyme, MedDay, MedImmune, Merck Serono, Novartis, Roche, Sanofi, TG Therapeutics, and Viela Bio with approval by the Rector of Heinrich Heine University Düsseldorf. I.M. Williams is an employee of Oxford PharmaGenesis. J. Drappa, D. She, D. Cimbora, W. Rees, J.N. Ratchford, and E. Katz are employees of Viela Bio.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The N-MOmentum trial was funded by MedImmune and Viela Bio (Gaithersburg, MD, USA). Viela Bio supported the development of this manuscript, provided data analyses according to the direction of the authors, and paid for medical writing support provided by Oxford PharmaGenesis.
ORCID iDs: Bruce AC Cree  https://orcid.org/0000-0001-7689-2533
https://orcid.org/0000-0001-7689-2533
Ho Jin Kim  https://orcid.org/0000-0002-8672-8419
https://orcid.org/0000-0002-8672-8419
Sean J Pittock  https://orcid.org/0000-0002-8387-6491
https://orcid.org/0000-0002-8387-6491
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bruce AC Cree, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA, USA.
Jeffrey L Bennett, University of Colorado School of Medicine, Anschutz Medical Campus, University of Colorado, Aurora, CO, USA.
Ho Jin Kim, Research Institute and Hospital of National Cancer Center, Goyang, South Korea.
Brian G Weinshenker, Mayo Clinic, Rochester, MN, USA.
Sean J Pittock, Mayo Clinic, Rochester, MN, USA.
Dean Wingerchuk, Mayo Clinic, Scottsdale, AZ, USA.
Kazuo Fujihara, Department of Multiple Sclerosis Therapeutics, Fukushima Medical University, Fukushima, Japan/Multiple Sclerosis and Neuromyelitis Optica Center, Southern Tohoku Research Institute for Neuroscience, Koriyama, Japan.
Friedemann Paul, Experimental and Clinical Research Center, Max Delbrück Center for Molecular Medicine, Berlin, Germany/Charité–Universitätsmedizin Berlin, Berlin, Germany.
Gary R Cutter, University of Alabama at Birmingham, Birmingham, AL, USA.
Romain Marignier, Service de Neurologie, Sclérose en Plaques, Pathologies de la Myéline et Neuroinflammation, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Lyon, France.
Ari J Green, Department of Neurology and Department of Ophthalmology, UCSF Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA, USA.
Orhan Aktas, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Hans-Peter Hartung, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Ian M Williams, Oxford PharmaGenesis Ltd, Oxford, UK.
Jorn Drappa, Viela Bio, Gaithersburg, MD, USA.
Dewei She, Viela Bio, Gaithersburg, MD, USA.
Daniel Cimbora, Viela Bio, Gaithersburg, MD, USA.
William Rees, Viela Bio, Gaithersburg, MD, USA.
John N Ratchford, Viela Bio, Gaithersburg, MD, USA.
Eliezer Katz, Viela Bio, Gaithersburg, MD, USA.
References
- 1. Wu K, Wen L, Duan R, et al. Triglyceride level is an independent risk factor in first-attacked neuromyelitis optica spectrum disorders patients. Front Neurol 2019; 10: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi M, Seok JM, Kim YS, et al. Association between body mass index and clinical outcome in neuromyelitis optica spectrum disorder. ECTRIMS Online Library 2016; 146828: P988. [Google Scholar]
- 3. Baek SH, Kim JS, Jang MJ, et al. Low body mass index can be associated with the risk and poor outcomes of neuromyelitis optica with aquaporin-4 immunoglobulin G in women. J Neurol Neurosurg Psychiatry 2018; 89(11): 1228–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X, Fan R, Peng F, et al. Blood pressure and body fat percent in women with NMOSD. Brain Behav 2019; 9(9): e01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mealy MA, Mossburg SE, Kim SH, et al. Long-term disability in neuromyelitis optica spectrum disorder with a history of myelitis is associated with age at onset, delay in diagnosis/preventive treatment, MRI lesion length and presence of symptomatic brain lesions. Mult Scler Relat Disord 2019; 28: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee A, Ng J, Coleman J, et al. Outcomes from acute attacks of neuromyelitis optica spectrum disorder correlate with severity of attack, age and delay to treatment. Mult Scler Relat Disord 2019; 28: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asgari N, Skejoe HP, Lillevang ST, et al. Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): A population-based, descriptive study. BMC Neurol 2013; 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sepulveda M, Armangue T, Sola-Valls N, et al. Neuromyelitis optica spectrum disorders: Comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm 2016; 3(3): e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mealy MA, Kessler RA, Rimler Z, et al. Mortality in neuromyelitis optica is strongly associated with African ancestry. Neurol Neuroimmunol Neuroinflamm 2018; 5(4): e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SH, Mealy MA, Levy M, et al. Racial differences in neuromyelitis optica spectrum disorder. Neurology 2018; 91: e2089–e2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandit L, Asgari N, Apiwattanakul M, et al. Demographic and clinical features of neuromyelitis optica: A review. Mult Scler 2015; 21(7): 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimbrough DJ, Mealy MA, Simpson A, et al. Predictors of recurrence following an initial episode of transverse myelitis. Neurol Neuroimmunol Neuroinflamm 2014; 1(1): e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amezcua L, Cook L, Yeaman M, et al. Black race is an independent risk factor for disability in neuromyelitis optica spectrum disorder. ECTRIMS Online Library 2018; 228162: P1784. [Google Scholar]
- 14. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 15. Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006; 66: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 16. Cree BA, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica-Ethical and design considerations. Mult Scler 2016; 22(7): 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akaishi T, Nakashima I, Takahashi T, et al. Neuromyelitis optica spectrum disorders with unevenly clustered attack occurrence. Neurol Neuroimmunol Neuroinflamm 2020; 7(1): e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_1352458521988926 for Sensitivity analysis of the primary endpoint from the N-MOmentum study of inebilizumab in NMOSD by Bruce AC Cree, Jeffrey L Bennett, Ho Jin Kim, Brian G Weinshenker, Sean J Pittock, Dean Wingerchuk, Kazuo Fujihara, Friedemann Paul, Gary R Cutter, Romain Marignier, Ari J Green, Orhan Aktas, Hans-Peter Hartung, Ian M Williams, Jorn Drappa, Dewei She, Daniel Cimbora, William Rees, John N Ratchford and Eliezer Katz in Multiple Sclerosis Journal
Data Availability Statement
Reasonable requests for anonymized data on defined study outcomes will be made available by request. Proposals should be directed to katze@vielabio.com. Requestors will be required to sign a data access agreement. Requests will be considered for up to 3 years after article publication.