Abstract
Introduction
Colistin is used to treat severe antibiotic-resistant Gram-negative infections (GNIs). With the rise of antibiotic resistance, colistin has been used increasingly as a ‘last-line’ therapy for multidrug-resistant GNIs. We evaluated the incidence of acute kidney injury (AKI) and mortality among patients receiving colistin or one of the new β-lactam/β-lactamase inhibitors (βL + βLI) (ceftazidime/avibactam, ceftolozane/tazobactam, or meropenem/vaborbactam).
Methods
This retrospective cohort study used data from the Premier Healthcare Database. The cohort included propensity score-matched adults with an inpatient stay between January 2016 and December 2018. Patients given both colistin and BL + BLI as treatment for ≥ 72 h were excluded. AKI was defined as acute renal failure or dialysis during hospitalization with antibiotic administration. Propensity score matching was used to control for selection bias and confounding. Logistic regression evaluated associations between treatment, AKI, and in-hospital mortality.
Results
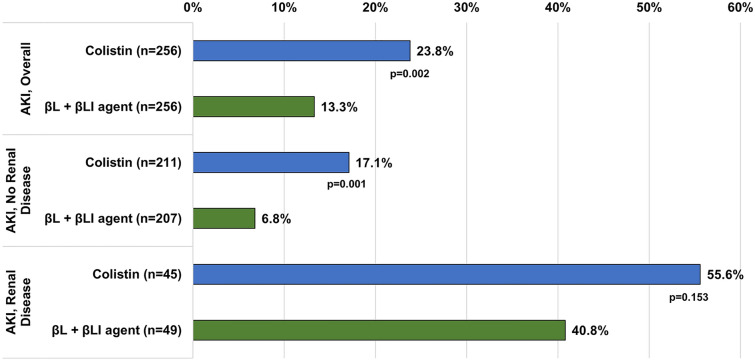
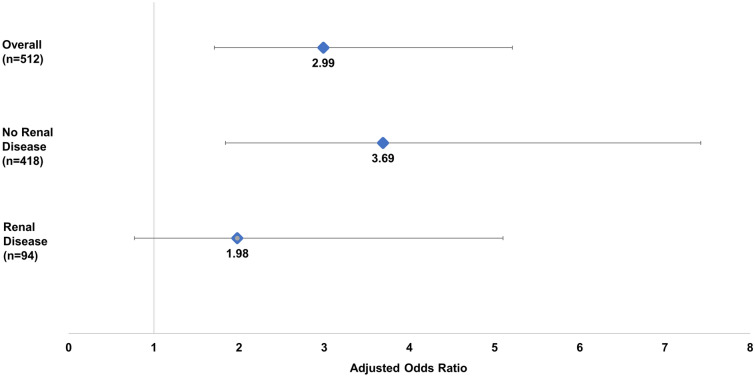
The total number of patients in the matched cohorts were 256 in each. Overall, 23.8% and 13.3% of patients receiving colistin or new βL + βLI agents, respectively, experienced AKI during hospitalization (p = 0.002); odds of AKI for colistin were 3.0 (95% CI 1.71, 5.21). Following propensity score-matching, patients without baseline renal disease experienced AKI during hospitalization to a higher degree in the colistin group compared to the βL + βLI group (17.1% vs. 6.8%); colistin use was associated with 3.7 times higher odds (95% CI 1.84, 7.42) of AKI compared to βL + βLI agents. The odds of mortality in patients on colistin developing AKI were more than three times that of patients receiving a BL + BLI agent who developed AKI. Among patients receiving colistin, incident AKI was associated with 6.1 times higher odds (95% CI 2.53, 14.71) of mortality.
Conclusions
Patients receiving colistin for GNIs had significantly higher odds of AKI and mortality than those receiving βL + βLI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00556-x.
Keywords: Colistin, β-lactam and β-lactamase inhibitor, Acute kidney injury, Mortality
Key Summary Points
| Why carry out this study? |
| Historically, colistin has been infrequently prescribed due to associated nephrotoxicity, but has recently been used more frequently for resistant pathogens, as it is the only generic antibiotic demonstrating reliable in vitro activity against some pathogens, such as A. baumannii. |
| Limited clinical experience of newer antibiotics to treat carbapenem-resistant Gram-negative infection (GNI) suggests a lower incidence of acute kidney injury (AKI). |
| The objectives of this study were to assess acute kidney injury and in-hospital mortality among patients receiving colistin or a new β-lactam/β-lactamase inhibitor to treat a severe Gram-negative infection in a large population-based dataset. |
| What was learned from the study? |
| A large retrospective cohort study of the Premier Healthcare Database revealed that patients receiving colistin to treat Gram-negative infections had higher odds of mortality and acute kidney injury than those receiving a β-lactam/β-lactamase inhibitor. |
| Utilizing newer, less toxic antibiotics to treat severe antibiotic-resistant Gram-negative infections could have significant implications for healthcare costs, morbidity, and mortality. |
Introduction
Colistin is a polymyxin class antibiotic originally introduced into clinical practice in the 1950s as one of the first antibiotics with significant efficacy for treating Gram-negative bacterial infections (GNIs) [1]. Due to reports of nephrotoxicity and neurotoxicity, historically, colistin was prescribed infrequently; thus, limited pharmacokinetic and pharmacodynamic data exist, and optimum dosing is ill-defined [2]. However, with the rise of antibiotic resistance, in particular carbapenem resistance (CR), colistin has been used increasingly as a ‘last-line’ therapy for multidrug-resistant (MDR) GNIs, including carbapenem-resistant Enterobacterales (CRE), Pseudomonas aeruginosa, and Acinetobacter baumannii (CRAB) [3]. MDR P. aeruginosa and CRAB, along with CRE, have, respectively, been identified by the CDC as serious and urgent threats [4].
The rise in colistin use renews concerns regarding the well-known potential for nephrotoxicity. Recent reports have described higher incidence of acute kidney injury (AKI) in patients treated with colistin, but most have small sample sizes, are not population-based, or study only one type of GNI [5, 6]. AKI occurs in approximately 25–50% of patients treated with colistin [7–10], but has been reported as high as 90% in certain patients [11]. Those with comorbid conditions, including existing kidney disease, have increased risk of AKI while receiving colistin [7, 12]. AKI post-colistin use often resolves [13], but increased risk of developing chronic kidney disease (CKD) after occurrence of AKI due to colistin use has been reported, with older age being a possible contributing factor [14].
The objectives of this study were to assess AKI and in-hospital mortality among patients hospitalized for severe GNIs who were receiving colistin or a new β-lactam/β-lactamase inhibitor (βL + βLI), stratified by the presence of baseline renal disease. The population-attributable risk of AKI due to colistin was used to estimate the potential amount of AKI that could be prevented by using a newer antibiotic in place of colistin.
Methods
Data Source
The Premier Healthcare Database (PHD) was used for this analysis, with data from January 2016 through December 2018. The PHD contains data from standard hospital discharge files, including patient demographics and disease states, insurance type, admission and discharge diagnoses, admission source and type, and discharge status. This database represents approximately 25% of US discharges from geographically diverse hospitals.
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Institutional Review Board approval was not required based on the decision tool from the US National Institution of Health assessing a human subject study (https://grants.nih.gov/policy/humansubjects/hs-decision.htm).
Study Design and Population
This is a retrospective cohort study which evaluated the incidence of AKI and in-hospital mortality among patients with GNIs treated with colistin compared to propensity score-matched patients treated with βL + βLI antibiotic agents.
Patients were included if they met all of the following criteria: (1) aged 18 + years with an inpatient stay between January 2016 and December 2018 (the ‘index’ period); (2) positive for a GNI; and (3) received at least 3 days of treatment with colistin (intravenous) or a βL + βLI antibiotic agent. The βL + βLI antibiotic agents included ceftazidime/avibactam, ceftolozane/tazobactam, or meropenem/vaborbactam. Imipenem/relabactam was not yet commercially available.
Patients were excluded for any of the following reasons: (1) evidence of cystic fibrosis or acute renal failure (ARF) present on admission by ICD-9/10 codes; (2) evidence of a dialysis procedure, defined using billing codes prior to the first dose of colistin or βL + βLI; and (3) received both colistin and a βL + βLI antibiotic during the inpatient stay for > 72 h (Table S1).
Patient and Hospital Characteristics
Patient demographics included age, sex, race, and payer type. The Charlson–Deyo Comorbidity Index (CCI) [15, 16] was calculated using ICD-9/10 codes. Prior exposure to other treatments that predispose to renal injury before the first dose of colistin or βL + βLI agents was also examined (Table S2). An intensive care unit (ICU) stay or ventilation during hospitalization prior to the first dose of colistin or βL + βLI agent, as well as sepsis present on admission or any inpatient hospitalization in the prior 30 days, was assessed. Hospital characteristics included geographic region, bed size, teaching status (yes/no), and location (urban/rural).
Identification of Gram-Negative Infections, Pathogens and Treatments
All GNIs were confirmed by a positive culture from laboratory pathogen data or identification through pathogen susceptibility data. Information regarding infection site (grouped as respiratory, urinary, or bloodstream sources), susceptibility to carbapenems, and the type of pathogen [Enterobacterales, non-glucose fermenter, or other Gram-negative (GN) bacteria] were noted. The index date was the date of the first culture sample with a GN, CR pathogen. If a patient did not have any CR cultures identified, then the first carbapenem-sensitive GN culture became the reference culture and its associated index date.
Patients were classified as receiving colistin or one of the new βL + βLI antibiotic agents active against GNIs, including ceftazidime-avibactam, ceftolozane-tazobactam, or meropenem-vaborbactam.
Acute Kidney Injury, Acute Renal Failure, and In-Hospital Mortality Outcomes
The incidence of ARF during treatment with colistin or βL + βLI was defined using ICD-10 codes (Table S1). Incident AKI was defined as a dialysis procedure or ARF with or without a dialysis procedure (Table 1). In-hospital mortality was assessed during the index hospitalization.
Table 1.
Cohort attrition stratified by treatment regimen
| Colistina | βL + βLI agent | |
|---|---|---|
| n = 6811 | n = 7952 | |
| Patients ≥ 18 years of age | 6505 (95.5%) | 7891 (99.2%) |
| Inpatient hospitalization | 6434 (94.5%) | 7654 (96.3%) |
| Non-cystic fibrosis patients | 4499 (66.1%) | 7235 (91.0%) |
| Patients receiving ≥ 3 days of treatment | 3320 (48.7%) | 5713 (71.8%) |
| Patients w/any microbiological results | 734 (10.8%) | 1520 (19.1%) |
| Patients w/confirmed Gram negative pathogen | 649 (9.5%) | 1267 (15.9%) |
| Patients w/no evidence of ARF POA or dialysis prior to 1st dose | 319 (4.7%) | 657 (8.3%) |
| Propensity score matched 1:1 | 256 (3.8%) | 256 (3.2%) |
ARF acute renal failure, POA present on admission
aStarting with patients receiving ≥ 1 day of treatment
Statistical Analysis
Risk factors were identified a priori to assess differences in demographic and clinical characteristics between the colistin and βL + βLI treatment groups, and to identify potential confounders and matching variables. A propensity score-matching approach was used to account for variables which may confound the relationship between treatments (colistin and βL + βLI agents) and AKI.
Propensity scores were generated by logistic regression with treatment (colistin vs. any of the βL + βLI agents) as the dependent variable. The independent variables in the model are shown in Table 2. The matching algorithm used a caliper of 0.2 times the standard deviation of the logit of the propensity score to produce a 1:1 match, and the absolute standardized difference was used to compare characteristics between the colistin and βL + βLI treatment groups post-match. In-hospital mortality was assessed during the index hospitalization and defined as death occurring in the hospital any time after initiating antibiotic therapy of interest. SAS v.9.3 (Cary, NC, USA) was used for all statistical analyses.
Table 2.
Patient, infection, and hospital characteristics pre- and post-propensity score matches
| Unmatched sample | 1:1 PSM sample | SDa | |||
|---|---|---|---|---|---|
| Colistin | βL + βLI agent | Colistin | βL + βLI agent | ||
| n = 319 | n = 657 | n = 256 | n = 256 | ||
| Patient | |||||
| Age, mean ± SD | 58.7 ± 17.4 | 61.2 ± 15.6 | 59.7 ± 16.8 | 59.2 ± 15.2 | 3% |
| Sex | |||||
| Female | 136 (43%) | 283 (43%) | 102 (40%) | 106 (41%) | 3% |
| Male | 183 (57%) | 374 (57%) | 154 (60%) | 150 (59%) | 3% |
| Race | |||||
| White | 254 (80%) | 462 (70%) | 198 (77%) | 194 (76%) | 4% |
| Non-White | 65 (20%) | 195 (30%) | 58 (23%) | 62 (24%) | 4% |
| Payer type | |||||
| Commercial | 32 (10%) | 92 (14%) | 28 (11%) | 20 (8%) | 11% |
| Medicaid | 55 (17%) | 112 (17%) | 45 (18%) | 49 (19%) | 4% |
| Medicare | 222 (70%) | 434 (66%) | 174 (68%) | 178 (70%) | 3% |
| Other | 10 (3%) | 19 (3%) | 9 (4%) | 9 (4%) | 0% |
| CCI, mean ± SD | 2.68 ± 2.22 | 2.75 ± 2.29 | 2.63 ± 2.14 | 2.76 ± 2.08 | 6% |
| CCI categories | |||||
| Renal disease (CCI) | 56 (18%) | 140 (21%) | 45 (18%) | 49 (19%) | 4% |
| Diabetes w/complications (CCI) | 86 (27%) | 182 (28%) | 68 (27%) | 70 (27%) | 2% |
| Diabetes w/o complications (CCI) | 34 (11%) | 106 (16%) | 30 (12%) | 32 (13%) | 2% |
| Chronic pulmonary disease (CCI) | 153 (48%) | 215 (33%) | 115 (45%) | 118 (46%) | 2% |
| Hospitalization in prior 30 days | 97 (30%) | 206 (31%) | 75 (29%) | 85 (33%) | 8% |
| ICU prior to 1st dose | 164 (51%) | 283 (43%) | 133 (52%) | 139 (54%) | 5% |
| Exposure ARF drugs prior to 1st dose | 271 (85%) | 564 (86%) | 218 (85%) | 224 (88%) | 7% |
| ARF in Prior 3 months | 42 (13%) | 116 (18%) | 36 (14%) | 35 (14%) | 1% |
| Ventilator prior to 1st dose | 169 (53%) | 222 (34%) | 129 (50%) | 132 (52%) | 2% |
| Sepsis present on admission | 172 (54%) | 333 (51%) | 140 (55%) | 140 (55%) | 0% |
| Index infection | |||||
| Infection site | |||||
| Blood | 20 (6%) | 59 (9%) | 16 (6%) | 15 (6%) | 2% |
| IAI | 1 (0.3%) | 9 (1%) | 0 (0%) | 0 (0%) | – |
| Other | 18 (6%) | 64 (10%) | 17 (7%) | 16 (6%) | 2% |
| Respiratory | 207 (65%) | 195 (30%) | 152 (59%) | 152 (59%) | 0% |
| Skin | 21 (7%) | 120 (18%) | 20 (8%) | 17 (7%) | 5% |
| Urine | 52 (16%) | 210 (32%) | 51 (20%) | 56 (22%) | 5% |
| Pathogen | |||||
| Enterbacterales | 82 (26%) | 257 (39%) | 72 (28%) | 80 (31%) | 7% |
| Non-fermenter | 236 (74%) | 389 (59%) | 183 (71%) | 175 (68%) | 7% |
| Other G-bacteria | 1 (0.3%) | 11 (2%) | 1 (0.4%) | 1 (0.4%) | 0% |
| Carbapenem susceptibility | |||||
| Not susceptible | 225 (71%) | 489 (74%) | 184 (72%) | 179 (70%) | 4% |
| Not tested | 18 (6%) | 44 (7%) | 14 (5%) | 20 (8%) | 9% |
| Susceptible | 76 (24%) | 124 (19%) | 58 (23%) | 57 (22%) | 1% |
| Hospital | |||||
| Bed size | |||||
| < 300 | 107 (34%) | 171 (26%) | 86 (34%) | 86 (34%) | 0% |
| 300–499 | 97 (30%) | 149 (23%) | 73 (29%) | 74 (29%) | 1% |
| 500+ | 115 (36%) | 337 (51%) | 97 (38%) | 96 (38%) | 1% |
| Teaching facility | 156 (49%) | 406 (62%) | 128 (50%) | 130 (51%) | 2% |
| Region | |||||
| Midwest | 99 (31%) | 245 (37%) | 86 (34%) | 93 (36%) | 6% |
| Northeast | 29 (9%) | 45 (7%) | 21 (8%) | 25 (10%) | 5% |
| South | 174 (55%) | 325 (49%) | 133 (52%) | 126 (49%) | 5% |
| West | 17 (5%) | 42 (6%) | 16 (6%) | 12 (5%) | 7% |
| Location | |||||
| Rural | 65 (20%) | 48 (7%) | 40 (16%) | 37 (14%) | 3% |
| Urban | 254 (80%) | 609 (93%) | 216 (84%) | 219 (86%) | 3% |
ARF acute renal failure, CCI Charlson Comorbidity Index, IAI intra-abdominal infection, ICU intensive care unit, SD standard deviation
aAbsolute standardized difference for 1:1 propensity score-matched sample
The rates of AKI and in-hospital mortality post-initiation of treatment were compared between patients receiving colistin or βL + βLI after matching. P-values were calculated for differences in the rates of AKI overall and by baseline renal disease status using the Chi-square test. The rates of AKI and in-hospital mortality post-treatment initiation were compared between propensity score-matched treatment groups using logistic regression models to calculate adjusted odds ratios (ORs) while controlling for covariates. These analyses were additionally stratified by baseline renal disease, defined by the individual component of the CCI [15, 16]. Associations of AKI with in-hospital mortality were also examined after stratifying by treatment (colistin vs. βL + βLI agent). Since interaction was observed between treatment group and AKI, logistic regression models included joint effects. The population attributable risk percentage (PAR%) of ARF due to colistin use was estimated using national projected utilization data from the Premier database and the risk difference of AKI incidence. We used Levin’s formula to calculate the PAR%.
Results
Attrition and Pathogen Characteristics
After exclusion criteria were applied, 319 (4.7%) patients were eligible for propensity score matching in the colistin group, and a total of 657 (8.3%) for βL + βLI agents (Table 1). Patient counts were consistently higher for the βL + βLI group following application of each restriction, particularly after CF patients were excluded (66.1% vs. 91.0%). No patients receiving meropenem-vaborbactam were part of the matched sample (Table 1).
In the propensity matched sample (n = 256 in each treatment group), for both the colistin- and βL + βLI treated groups, the distribution between CRE and non-fermenter pathogens was similar, 28% vs. 31% and 71% vs. 68% respectively (Table 2). The most frequently treated pathogen was Pseudomonas aeruginosa (n = 96, 37.5% vs. n = 157, 61.3%). The actual distribution of specific pathogens is shown in Table S3.
Patient and Hospital Characteristics
Table 2 displays baseline characteristics for the colistin and βL + βLI treatment groups, and pre- and post-propensity score matching. Overall, the matched cohorts were similar for patient, infection site and hospital characteristics.
The most common comorbid conditions were chronic pulmonary disease (45% vs. 46%) and diabetes with and without complications (39% vs. 40%). Baseline renal disease was present in 18% of the colistin and 19% of the βL + βLI group.
Associations of Treatments with AKI
Overall, AKI had developed in 23.8% of the colistin and 13.3% of the βL + βLI group (Fig. 1). Following post-propensity score matching, patients without baseline renal disease (n = 418) experienced AKI during hospitalization to a higher degree in the colistin group compared to the βL + βLI group (17.1% vs. 6.8%). Among patients with baseline renal disease, there was no statistically significant difference between the groups.
Fig. 1.
Rates of AKI by treatment group and baseline renal disease status among propensity score-matched sample
After additional adjustment with logistic regression, among the propensity score-matched sample, patients receiving colistin had approximately threefold higher odds (OR = 3.0; 95% CI 1.71, 5.21) of AKI versus βL + βLI agents (Fig. 2). Baseline renal disease (OR = 5.1; 95% CI 2.70, 9.62) was also a significant independent risk factor for AKI (data not shown). Stratified analysis revealed that, among patients without existing renal disease (n = 418), colistin use was associated with 3.7 higher odds of AKI versus βL + βLI agents (95% CI 1.84, 7.42) (Fig. 2). In contrast, among those with existing renal disease (n = 94), odds of AKI were not statistically different between the colistin and βL + βLI agent treatment groups (OR = 2.0; 95% CI 0.77. 5.10).
Fig. 2.
Adjusted odds of AKI for colistin versus βL + βLI agents, stratified by baseline renal disease, among propensity score-matched sample
Associations of Treatments with In-hospital Mortality
In-hospital mortality was higher in the colistin group compared to the βL + βLI group (15.2% vs. 10.5%). While adjusted odds of inpatient mortality for colistin versus βL + βLI treatment was not statistically significant, occurrence of AKI during the inpatient visit was associated with almost four-fold higher odds of mortality compared to those without AKI (OR = 3.8; 95% CI 2.00, 7.27) (Table 3). The joint risk of mortality among patients receiving colistin and having AKI was more than 3 times that of patients receiving new agents with AKI (OR = 3.23, 95% CI 1.09, 9.52) (Table 4). Similarly, adjusted odds of mortality for patients with AKI treated with colistin was six times that of colistin patients without AKI (OR = 6.13; 95% CI 2.53, 14.71). Conversely, among patients on new agents, no association was observed between AKI and mortality.
Table 3.
Adjusted mortality risk for colistin versus new agents among propensity score-matched sample, without accounting for joint effects of treatment and AKI
| Final stepwise model: treatment and AKI forced into modela | OR estimate | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| Ventilated at ANY point | 13.80 | 5.02 | 37.88 | < 0.0001 |
| Age | 1.03 | 1.01 | 1.06 | 0.006 |
| Mild liver disease | 2.65 | 0.96 | 7.33 | 0.060 |
| Malignancy | 12.53 | 5.04 | 31.15 | < 0.0001 |
| Treatment colistin vs. new agents | 1.36 | 0.74 | 2.51 | 0.318 |
| AKI yes vs. no | 3.81 | 2.00 | 7.27 | < 0.0001 |
AKI acute kidney injury
aStepwise algorithm eliminating covariates with p value > 0.15 except for treatment and AKI
Table 4.
Risk of mortality among propensity score-matched sample, colistin vs. new agents by AKI status
| Final stepwise model—joint effect of AKI and treatment typea | OR estimate | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| Ventilated at ANY point | 15.87 | 5.69 | 44.23 | < 0.0001 |
| Age | 1.03 | 1.01 | 1.06 | 0.0056 |
| Rheumatologic disease | 4.81 | 0.67 | 34.78 | 0.1196 |
| Mild liver disease | 3.01 | 1.08 | 8.44 | 0.0358 |
| Malignancy | 12.95 | 5.16 | 32.49 | < 0.0001 |
| New agent AKI vs. new agent no AKI | 1.67 | 0.60 | 4.67 | 0.3312 |
| Colistin no AKI vs. new agent no AKI | 0.88 | 0.41 | 1.90 | 0.7415 |
| Colistin AKI vs. new agent no AKI | 5.37 | 2.31 | 12.49 | < 0.0001 |
| Colistin AKI vs. new agent AKIb | 3.23 | 1.09 | 9.52 | 0.0347 |
| Colistin AKI vs. colistin no AKIb | 6.13 | 2.53 | 14.71 | < 0.0001 |
AKI acute kidney injury
aStepwise algorithm eliminating covariates with p value > 0.15 except for treatment and AKI
bNot a model coefficient
Population Attributable Risk of Colistin in AKI
Based on the calculated PAR%, we estimated that, at the end of 2018, 28.6% of AKI in the population treated with either colistin or a βL + βLI agent for a GNI could have been avoided by not using colistin. We estimated the proportion of use in 2018 of colistin to be 23% of the total of both colistin and βL + βLI agents used for GNIs and used the OR of 3.0 of the risk of AKI for colistin versus βL + βLI agents as an approximation of the relative risk. This assumes that colistin and βL + βLI agents were used only to treat CR GNIs. If the βL + βLI group was used more empirically with some infections being found to be Gram-positives or pathogens less likely to be treated with colistin, then the true proportion of colistin use would be greater. This would make our estimate a lower bound estimate.
Discussion
The use of colistin has increased in recent years along with the incidence of CR GNIs [2], renewing concerns over colistin-associated nephrotoxicity. In this retrospective cohort study, 512 patients with GNIs were matched on baseline characteristics. There was an increased risk of AKI in patients treated with colistin (23.8%) versus newer βL + βLI antibiotics (13.3%) that may have equal or better coverage for CR GNIs. These findings corroborate prior studies supporting a link between colistin use and incident AKI [9, 13]. A recent meta-analysis identified 5 randomized controlled trials comparing nephrotoxicity of colistin to minimally nephrotoxic antibiotics. The authors estimated a colistin-associated nephrotoxicity rate of 36%, and 140% increased risk, compared to βL-based regimens [17]. We estimated that the percentage of AKI attributable to colistin use in 2018 was 25–29%, suggesting that roughly 1 in 4 incident cases of AKI may be prevented by utilizing newer, less toxic antibiotics. To our knowledge, no previous studies have reported the PAR% for AKI due to colistin. This finding may have significant implications for healthcare costs, and morbidity and mortality associated with colistin use.
Elevated risk of AKI was observed among patients without pre-existing renal disease who were treated with colistin compared to those treated with βL + βLI. Among patients with renal disease at baseline, 56% of colistin- and 41% of βL + βLI-treated patients had subsequent AKI, although this difference was not significant, possibly due to the relatively small sample size of patients with renal disease. Several studies have documented significantly higher risk of AKI among patients with existing renal disease or chronic kidney disease [7, 18]. To our knowledge, colistin-associated AKI has not been studied specifically among those without existing renal disease. It is possible that clinicians who prescribe colistin to patients who have pre-existing renal disease may take additional precautions to prevent incident AKI.
The current study demonstrates that, among those who develop AKI, patients treated with colistin have threefold greater adjusted odds of mortality compared to βL + βLI. This aligns with previous studies that reported colistin use to treat GNIs was associated with an increased risk of mortality. A meta-analysis estimated the odds of 30-day mortality at 1.5 times higher for patients receiving colistin versus βL + βLI antibiotics [13], a result not inconsistent with the point estimate of 1.36 in this study, without taking into consideration the joint effect of AKI and treatment. Moreover, a prospective observational study of 99 patients receiving colistin and 38 patients receiving ceftazidime-avibactam found that patients receiving colistin had a significantly higher rate of 30-day in-hospital mortality (32% vs. 9%), although this was limited to treatment of CRE [19].
Timing of AKI in colistin-treated patients may play a role in mortality risk, with early-stage AKI (within 7 days of first colistin dose) conferring more than fourfold higher odds of mortality compared to later-stage AKI [20]. In our study, patients receiving colistin who developed AKI had approximately 6 times the odds of mortality compared to those without AKI, while odds of mortality among patients treated with βL + βLI were not significantly higher for those with AKI versus no AKI. Prior research has demonstrated that, among patients on colistin with AKI, those whose AKI resolved had better survival compared to those whose AKI did not (88% vs. 53%, p = 0.001) [21]. The current study is consistent with previous findings, with higher mortality in the colistin treatment group compared to the βL + βLI treatment group, and, among patients on colistin, greater odds of mortality associated with incident AKI. Patients prescribed colistin in the context of AKI may have more unidentified adverse characteristics, leading their physicians to use colistin despite their AKI. Additional research is indicated to address this question.
Our study’s findings should be interpreted in the context of its strengths and limitations. Strengths include that these data are real-world from a large US hospital discharge database. Some prior studies were not population-based, only included one or two GN pathogens, or did not adjust for confounders. We controlled for selection bias using propensity-score techniques to match treatment groups on measured variables, as well as multiple logistic regression. The current study was agnostic in terms of pathogen, although propensity score matching controlled for Enterobacterales versus non-fermenters. More patients with Acinetobacter infection were treated with colistin than new agents. As Acinetobacter spp. are known to be associated with higher mortality than other GN pathogens [22], this was included as an indicator variable in our mortality regression model and did not alter the results.
Limitations include that this analysis utilizes an administrative dataset with an observational retrospective approach with the possibility of selection bias and misclassification. If a patient was admitted with high risk for AKI not noted in the index hospitalization, there may have been a higher probability of being treated with a βL + βLI antibiotic. Similarly, not every nephrotoxic agent used prior to treatment would necessarily be identified, but any bias would be in the direction of the βL + βLI antibiotics; clinicians normally refrain from using additional nephrotoxic agents such as colistin. This would result in a conservative bias away from an association of colistin with AKI or mortality. We also could not time the diagnosis of AKI during the hospitalization. Some incident AKI may have occurred prior to treatment but after admission, resulting in misclassification. This would also result in a bias away from a colistin association with AKI. Severity could be a potential confounder. We do not have an index of severity in Premier data; rather, we used a surrogate measure, the presence of mechanical ventilation, that may not be as granular as a severity score. Laboratory results, if available, would have improved the classification of a new AKI diagnosis. However, others have shown the validity of using administrative codes for classifying incident AKI in an observational setting [23, 24]. Higher doses of colistin have been associated with increased risk of AKI [25], but we were unable to evaluate dosing differences.
Conclusions
Patients receiving colistin for GNIs had significantly higher odds of developing AKI compared to those on βL + βLI antibiotics, including patients without baseline renal disease. Among patients on colistin, there were significantly higher odds of mortality for those who developed AKI versus those who did not, which was not observed among βL + βLI-treated patients. Further research is warranted on the nephrotoxicity of colistin in real-world settings. Our findings suggest that almost 1 in 4 incident cases of AKI may be prevented by utilizing more recently developed, less toxic antibiotics. The implications for healthcare costs, morbidity, and mortality also merit future investigations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work and the Rapid Service Fee were supported by Shionogi, Inc, Florham Park, NJ, USA. Employees of the study sponsor were involved in the study design, as well as collection, analysis, interpretation of the data, and in critically revising the manuscript for important intellectual content. No funding to the authors was provided for the preparation of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval to publish this version. All authors read and approved the final manuscript.
Author Contributions
Casey Doremus and Stephen W Marcella conceived and designed the study, performed the analysis, contributed to data interpretation, and critically revised the manuscript for intellectual content. Roger M Echols and Bin Cai critically revised the manuscript.
Medical Writing/Editorial Assistance
Medical writing support was provided by Eve Hunter-Featherstone and Jenifer Wogen at Genesis Research. Funding for this service was provided by Shionogi Inc.
Prior Presentation
A subset of the findings in this study were presented as a poster at the virtual European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in 2020.
Disclosures
Casey Doremus, Stephen W Marcella, and Bin Cai are employees of Shionogi, Inc., Florham Park, NJ USA, and Roger M Echols is a consultant for Shionogi, Inc.
Compliance with Ethics Guidelines
This study was conducted using an anonymous, publicly available secondary dataset that meets the US HIPAA requirement. Ethics approval and consent to participate are not applicable for the current study per US 45 Code of Federal Regulations Part 46, human participant protection regulations, and Protections of Human Subjects.
This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Analysis of commercially available de-identified secondary data sources is considered exempt from the requirements for “human subjects research” in the US. Since this study utilized only de-identified data and did not involve collection, use or transmittal of individually identifiable data, Institutional Review Board (IRB) approval was not required.
Data Availability
The data that support the findings of this study are available from Premier Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Premier Inc.
References
- 1.Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30(2):557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim LM, Ly N, Anderson D, et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30(12):1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global priority list of antibiotic resistant bacteria to guide research, discovery and development of new antibiotics. 2017.
- 4.US Department of Health and Human Services Center for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2019.
- 5.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7(4):439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motsch J, Murta-de-Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs. colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–1808. doi: 10.1093/cid/ciz530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durante-Mangoni E, Andini R, Signoriello S, et al. Acute kidney injury during colistin therapy: a prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clin Microbiol Infect. 2016;22(12):984–989. doi: 10.1016/j.cmi.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Hassan MM, Gaifer Z, Al-Zakwani IS. Incidence and risk factors of nephrotoxicity in patients on colistimethate sodium. Int J Clin Pharm. 2018;40(2):444–449. doi: 10.1007/s11096-018-0607-y. [DOI] [PubMed] [Google Scholar]
- 9.Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis. 2019;94(1):41–49. doi: 10.1016/j.diagmicrobio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing acute kidney injury reports among antibiotics: a pharmacovigilance study of the FDA adverse event reporting system (FAERS) Drug Saf. 2020;43(1):17–22. doi: 10.1007/s40264-019-00873-8. [DOI] [PubMed] [Google Scholar]
- 11.Thammathiwat T, Tiranathanagul K, Srisawat N, Susantitaphong P, Praditpornsilpa K, Eiam-Ong S. Clinical and subclinical acute kidney injury in multidrug-resistant septic patients treated with colistimethate sodium: Incidence and clinical outcomes. Nephrology (Carlton) 2020;25(1):32–39. doi: 10.1111/nep.13663. [DOI] [PubMed] [Google Scholar]
- 12.Gunay E, Kaya S, Baysal B, Yuksel E, Arac E. Evaluation of prognosis and nephrotoxicity in patients treated with colistin in intensive care unit. Ren Fail. 2020;42(1):704–709. doi: 10.1080/0886022X.2020.1795878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien HT, Lin YC, Sheu CC, Hsieh KP, Chang JS. Is colistin-associated acute kidney injury clinically important in adults? A systematic review and meta-analysis. Int J Antimicrob Agents. 2020;55(3):105889. doi: 10.1016/j.ijantimicag.2020.105889. [DOI] [PubMed] [Google Scholar]
- 14.Meraz-Munoz A, Gomez-Ruiz I, Correa-Rotter R, Ramirez-Sandoval JC. Chronic kidney disease after acute kidney injury associated with intravenous colistin use in survivors of severe infections: a comparative cohort study. J Crit Care. 2018;44:244–248. doi: 10.1016/j.jcrc.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Eljaaly K, Bidell MR, Gandhi RG, et al. Colistin nephrotoxicity: meta-analysis of randomized controlled trials. Open Forum Infect Dis 2021;8(2). [DOI] [PMC free article] [PubMed]
- 18.Min KL, Son ES, Kim JS, Kim SH, Jung SM, Chang MJ. Risk factors of colistin safety according to administration routes: intravenous and aerosolized colistin. PLoS ONE. 2018;13(11):e0207588. doi: 10.1371/journal.pone.0207588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko H, Jeon M, Choo E, et al. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin Pract. 2011;117(3):c284–c288. doi: 10.1159/000320746. [DOI] [PubMed] [Google Scholar]
- 21.Miano TA, Lautenbach E, Wilson FP, Guo W, Borovskiy Y, Hennessy S. Attributable risk and time course of colistin-associated acute kidney injury. Clin J Am Soc Nephrol. 2018;13(4):542–550. doi: 10.2215/CJN.06980717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leão ACQ, Menezes PR, Oliveira MS, Levin AS. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect Dis 2016;16(1). [DOI] [PMC free article] [PubMed]
- 23.Patel UD, Hardy NC, Smith DH, et al. Validation of acute kidney injury cases in the mini-sentinel distributed database. Mini-Sentinel, 2013.
- 24.Waikar SS, Wald R, Chertow GM, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 25.Almutairy R, Aljrarri W, Noor A, et al. Impact of colistin dosing on the incidence of nephrotoxicity in a tertiary care hospital in Saudi Arabia. Antibiotics. 2020;9(8):485. doi: 10.3390/antibiotics9080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Premier Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Premier Inc.