Abstract
In eukaryotes, DNA damage induced by ultraviolet light and other agents which distort the helix is removed by nucleotide excision repair (NER) in a fragment ∼25 to 30 nucleotides long. In humans, a deficiency in NER causes xeroderma pigmentosum (XP), characterized by extreme sensitivity to sunlight and a high incidence of skin cancers. Abasic (AP) sites are formed in DNA as a result of spontaneous base loss and from the action of DNA glycosylases involved in base excision repair. In Saccharomyces cerevisiae, AP sites are removed via the action of two class II AP endonucleases, Apn1 and Apn2. Here, we provide evidence for the involvement of NER in the removal of AP sites and show that NER competes with Apn1 and Apn2 in this repair process. Inactivation of NER in the apn1Δ or apn1Δ apn2Δ strain enhances sensitivity to the monofunctional alkylating agent methyl methanesulfonate and leads to further impairment in the cellular ability to remove AP sites. A deficiency in the repair of AP sites may contribute to the internal cancers and progressive neurodegeneration that occur in XP patients.
Abasic (AP) sites arise in DNA at a substantial rate by spontaneous hydrolysis of the N-glycosylic bond, and it has been estimated that as many as 104 purines are lost spontaneously in a human cell per day (27). AP sites are also formed in DNA as intermediates in base excision repair (BER), which removes damaged bases formed by oxidation and alkylation. The first step of BER involves the action of a DNA glycosylase which catalyzes the hydrolysis of the N-glycosylic bond linking the damaged base to the deoxyribose phosphate backbone. The ensuing AP site is recognized by a class II AP endonuclease which cleaves the phosphodiester backbone on the 5′ side of the AP site, leaving a 3′-hydroxyl group and a 5′-baseless deoxyribose 5′-phosphate residue. Removal of the deoxyribose 5′-phosphate residue, followed by DNA repair synthesis and ligation, completes the repair process (6, 39, 45).
Two class II AP endonucleases, Apn1 and Apn2, have been identified in the yeast Saccharomyces cerevisiae. Apn1 represents the major AP endonuclease activity in yeast, and it shares extensive homology with Escherichia coli endonuclease IV (31, 32). Apn2 is an homolog of E. coli exonuclease III and of human HAP1 (REF1) AP endonuclease (3, 23). Genetic and biochemical studies have indicated that Apn1 and Apn2 constitute alternate pathways for the removal of AP sites in yeast (23).
In contrast to BER, which removes damaged bases which do not perturb the helical structure of DNA, nucleotide excision repair (NER) removes DNA damages that cause significant distortion of the helix (36). For example, NER removes cyclobutane pyrimidine dimers and (6-4) photoproducts formed by ultraviolet light and is also involved in the removal of intrastrand and interstrand cross-links and bulky adducts formed in DNA upon treatment with a variety of chemical agents. NER is a highly conserved process among eukaryotes from yeast to humans (36). In S. cerevisiae, NER is accomplished via the concerted action of the following: Rad14, RPA, the Rad4-Rad23 complex, and the Rad7-Rad16 complex, all of which function in DNA damage recognition (5, 11, 12, 14–16, 22); TFIIH, which contains the Rad3 and Rad25 DNA helicases, essential for DNA unwinding (41); and the Rad1-Rad10 and Rad2 nucleases (18, 42, 43) which incise the damaged strand on the 5′ and 3′ sides of the lesion, respectively (2, 17). Both in yeast and humans, dual incision by the NER ensemble results in the release of an oligonucleotide fragment ∼25 to 30 nucleotides long (10, 28, 29).
In humans, a defect in NER results in xeroderma pigmentosum (XP). XP individuals are extremely sensitive to sunlight, and the frequencies of basal cell carcinoma, squamous cell carcinoma, and melanoma of the skin are increased 1,000-fold or more in these patients (24, 25). XP patients also manifest an increase in the incidence of cancers in sites not exposed to UV radiation, and there is a disproportionate increase in the frequency of malignant neoplasms of the brain and other parts of the central nervous system and extraglossal oral cavity in these individuals (24, 25). These observations have suggested the involvement of NER in the removal of non-UV-related DNA damage. Because AP sites are formed so frequently in mammalian cells, here we utilize the yeast system to examine the role of NER in the removal of AP sites in eukaryotes. Our studies indicate that Apn1, Apn2, and NER constitute alternate competing pathways for the removal of AP sites in yeast.
MATERIALS AND METHODS
Yeast strains and plasmids.
All yeast strains used in this study were derived from EMY74.7 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52). Deletion mutations were generated in yeast using the gene replacement method (34) and the URA3 gene blaster (1). The plasmids used for constructing a genomic deletion mutation of APN1 and APN2, pPM750 and pPM838, respectively, have been described previously (23). Genomic deletions of the RAD2, RAD4, and RAD14 genes were generated by using plasmids pR2.35, pDG38, and pR14.4, respectively.
MMS sensitivity and mutagenesis.
For determining sensitivity to methyl methanesulfonate (MMS) and for measuring the rate of MMS-induced forward mutations at the CAN1 locus, cells were grown overnight in YPD (yeast extract-peptone-dextrose) medium, sonicated to disperse clumps, washed, and resuspended in 0.05 M KPO4 buffer, pH 7.0. Appropriate dilutions of 0.5 ml MMS at two times the desired final concentration were added to 0.5-ml suspensions of cells adjusted to 3 × 108 cells per ml and incubated with vigorous shaking at 30°C for 20 min. The reaction was terminated by the addition of 1 ml of 10% sodium thiosulfate. Appropriate dilutions of cells were plated on YPD medium for viability determinations and on synthetic complete medium lacking arginine but containing canavanine for determining the frequency of can1r mutations. Plates were incubated at 30°C and counted after 3 and 4 or 5 days for viability and mutagenesis determinations, respectively.
Alkaline sucrose gradients.
[rho0] derivatives lacking mitochondrial DNA were obtained by ethidium bromide mutagenesis, resulting in the following isogenic strains used in these experiments: YRP276 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 [rho0]), YRP210 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 apn1Δ [rho0]), YR14-30 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 rad14Δ [rho0]), YR14-29 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 apn1Δ rad14Δ [rho0]), YRP292 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 apn1Δ apn2Δ [rho0]), and YR14-39 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52 apn1Δ apn2Δ rad14Δ [rho0]). Growth of strains, conditions for treatment with MMS, preparation of spheroplasts, alkaline sucrose gradient sedimentation procedures, and processing of samples were as previously described (23, 44).
RESULTS
Inactivation of NER enhances the sensitivity of apnΔ strains to alkylation damage.
The various NER proteins in yeast are part of tightly associated multiprotein complexes that can be purified intact and which have been named nucleotide excision repair factors (NEFs). NEF1 consists of the damage recognition protein Rad14 and the Rad1-Rad10 endonuclease (13), NEF2 is comprised of the Rad4 and Rad23 proteins (10), and NEF3 contains the Rad2 endonuclease and TFIIH (19). To examine the role of NER in the repair of AP sites, we constructed genomic deletion mutations of RAD14, RAD4, and RAD2, each of which encodes one component of these NEFs, and tested the sensitivity of these mutant strains, singly and in combination with the apn1Δ and apn2Δ mutations, to the alkylating agent MMS. MMS alkylates, in particular, adenine at the N3 position, forming 3-methyl adenine (3MeA), and guanine at the N7 position, forming 7-methyl guanine (7MeG). An N-methyl purine DNA glycosylase removes these and a variety of other alkylated bases (4, 35). The resulting AP sites can then be removed by the APN1- or APN2-encoded endonucleases or by the action of the NER ensemble.
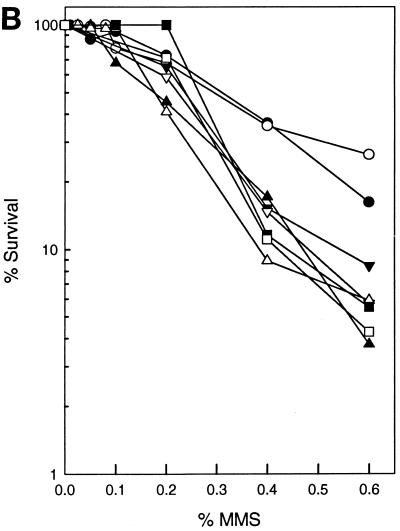
As shown in Fig. 1A, deletion of any of the NER genes confers a modest level of sensitivity to MMS that is intermediate between that of the wild type and the apn1Δ strain. However, yeast strains carrying a deletion of the APN1 gene and a deletion of any of the NER genes exhibit a synergistic increase in MMS sensitivity, suggesting that Apn1 and NER compete for the repair of AP sites. By contrast, deletion of APN2 alone has no effect on MMS sensitivity and deletion of APN2 in NER-defective mutants also does not cause a further increase in MMS sensitivity (Fig. 1B). This suggests that at these MMS concentrations, in the absence of both Apn2 and NER, Apn1 alone can remove most of the AP sites.
FIG. 1.
Effect of inactivation of NER genes in apnΔ strains upon MMS sensitivity. (A) MMS sensitivity of various apn1Δ radΔ yeast strains. Cells grown overnight in YPD medium were treated with MMS at the concentrations indicated for a 20-min period. Appropriate dilutions were spread onto YPD plates. Each curve represents the average of two or more experiments for each strain. Symbols: ●, EMY74.7 (wild type); ○, YRP190 (apn1Δ); ■, EMY75 (rad2Δ); □, YR4-1 (rad4Δ); ▵, YR14-21 (rad14Δ); ▾, YR2-46 (apn1Δ rad2Δ); ▿, YR4-21 (apn1Δ rad4Δ); ▴, YR14-25 (apn1Δ rad14Δ). (B) MMS sensitivity of various apn2Δ radΔ yeast strains. Conditions were as described above for panel A. Symbols: ●, EMY74.7 (wild type); ○, YRP263 (apn2Δ); ■, EMY75 (rad2Δ); □, YR4-1 (rad4Δ); ▵, YR14-21 (rad14Δ); ▾, YR2-55 (apn2Δ rad2Δ); ▿, YR4-31 (apn2Δ rad4Δ); ▴, YR14-34 (apn2Δ rad14Δ). (C) MMS sensitivity of various apn1Δ apn2Δ radΔ strains. Conditions were as described above for panel A. Symbols: ●, EMY74.7 (wild type); ○, YRP269 (apn1Δ apn2Δ); ■, EMY75 (rad2Δ); □, YR4-1 (rad4Δ); ▵, YR14-21 (rad14Δ); ▾, YR2-53 (apn1Δ apn2Δ rad2Δ); ▿, YR4-29 (apn1Δ apn2Δ rad4Δ); ▴, YR14-32 (apn1Δ apn2Δ rad14Δ). The survival curves for rad2Δ, rad4Δ, and rad14Δ strains are indistinguishable.
We next examined the MMS sensitivity of rad2Δ, rad4Δ, or rad14Δ mutations in combination with the apn1Δ apn2Δ mutations. As shown in Fig. 1C, simultaneous deletion of APN1 and APN2 causes a large increase in MMS sensitivity, and introduction of the rad2Δ, rad4Δ, or rad14Δ mutation into the apn1Δ apn2Δ strain causes a further enhancement in MMS sensitivity. These observations suggest that in the absence of Apn1, yeast cells depend heavily upon Apn2 and NER for repairing AP sites.
Inactivation of NER enhances MMS-induced mutagenesis in apnΔ strains.
Since AP sites are noncoding, they present a block to the DNA replicational machinery. Replication through such sites could occur by translesion synthesis by the REV3-REV7-encoded DNA polymerase ζ (23), or the gap left opposite the AP site may be filled in by a recombinational mechanism or by a “copy choice” type of DNA synthesis in which the undamaged sister duplex is used as the template for copying the missing information (20). Previously, we have shown that AP sites are highly mutagenic, and consequently, the frequency of MMS-induced mutations is greatly elevated in the apn1Δ apn2Δ strain over that in the wild-type strain (23).
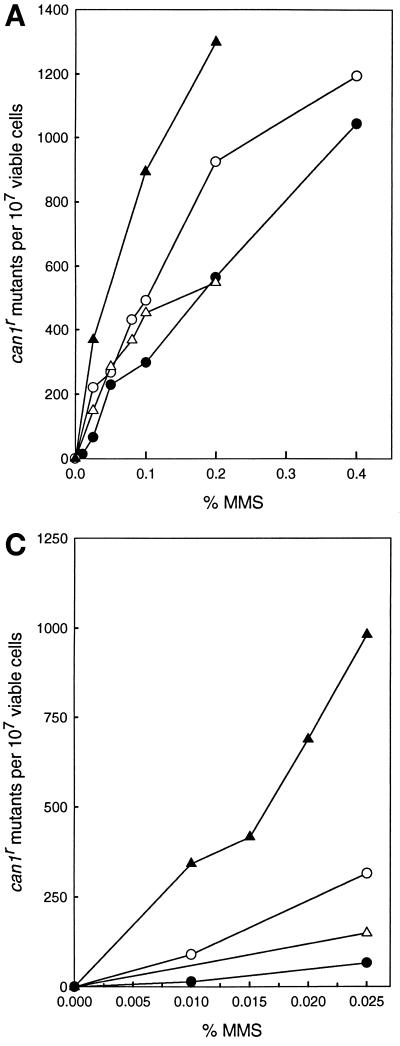
To further evaluate the role of NER in the removal of AP sites, we examined the effect of incorporation of the rad14Δ mutation into the apnΔ strains on the frequency of MMS-induced CAN1s to can1r mutations. As shown in Fig. 2A, the frequency of MMS-induced can1r mutations was higher in the apn1Δ rad14Δ double mutant than in the apn1Δ or rad14Δ single mutant. The incorporation of the apn2Δ mutation into the rad14Δ strain, however, did not confer any increase in MMS-induced can1r mutagenesis (Fig. 2B), whereas the frequency of MMS-induced can1r mutations increased sharply in the apn1Δ apn2Δ rad14Δ strain over that in the apn1Δ apn2Δ strain (Fig. 2C). For instance, whereas treatment with 0.01% MMS produced 90 can1r mutants per 107 viable cells in the apn1Δ apn2Δ strain, this treatment produced 340 can1r mutants per 107 viable cells in the apn1Δ apn2Δ rad14Δ strain.
FIG. 2.
MMS-induced mutations at the CAN1 locus. Cells grown overnight in YPD medium were treated with MMS at the concentrations indicated for a 20-min period. Appropriate dilutions were spread onto YPD plates for viability determinations and onto synthetic complete medium lacking arginine and containing canavanine for the determination of CAN1s to can1r mutagenesis. Each curve represents the average of three or more experiments for each strain. (A) Enhanced can1r mutagenesis in the apn1Δ rad14Δ strain. Symbols: ●, EMY74.7 (wild type); ○, YRP190 (apn1Δ); ▵, YR14-21 (rad14Δ); ▴, YR14-25 (apn1Δ rad14Δ). (B) can1r mutagenesis in the apn2Δ rad14Δ strain. Symbols: ●, EMY74.7 (wild type); ○, YRP263 (apn2Δ); ▵, YR14-21 (rad14Δ); ▴, YR14-34 (apn2Δ rad14Δ). (C) Enhanced can1r mutagenesis in the apn1Δ apn2Δ rad14Δ strain. Symbols: ●, EMY74.7 (wild type); ▵, YR14-21 (rad14Δ); ○, YRP269 (apn1Δ apn2Δ); ▴, YR14-32 (apn1Δ apn2Δ rad14Δ).
Defective removal of AP sites in apn1Δ rad14Δ and apn1Δ apn2Δ rad14Δ mutant strains.
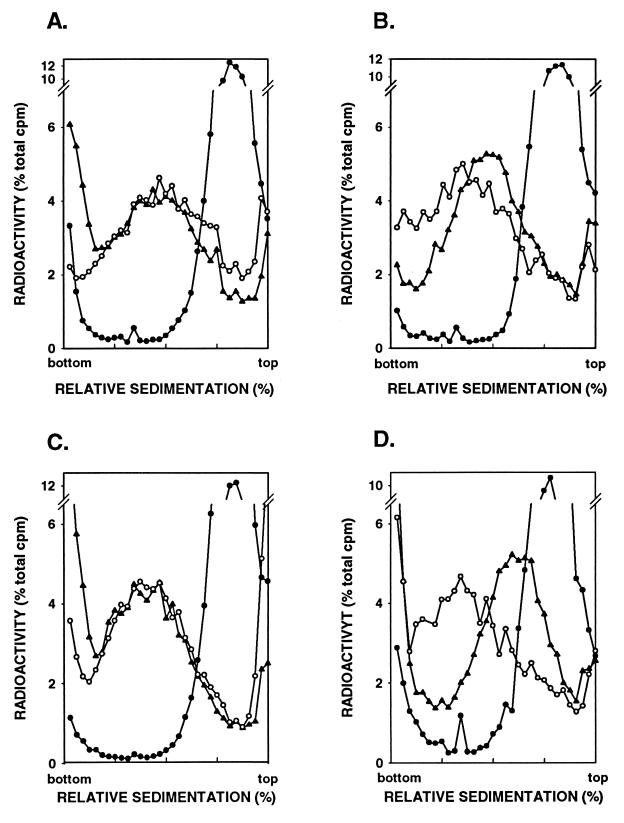
The enhancement in MMS sensitivity and in MMS-induced mutagenesis seen upon inactivation of NER in the apn1Δ and apn1Δ apn2Δ strains is consistent with a role of NER in the removal of AP sites. To directly assess this, we examined the removal of AP sites in MMS-treated wild type, apn1Δ, rad14Δ, and apn1Δ rad14Δ strains and in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains by sedimentation of DNA in alkaline sucrose gradients. Since NaOH hydrolyzes the phosphodiester bond near each AP site, the presence of AP sites can be evaluated by the size reduction in DNA upon sedimentation in alkaline sucrose gradients. Yeast cells were treated with 0.1% MMS for 20 min, and the sedimentation profile of DNA was examined immediately following this treatment or after a 2-h incubation period to allow time for DNA repair to occur. As has been shown previously (23) and reproduced here for comparison (Fig. 3A and B), incubation of MMS-treated wild-type cells for 2 h restores normal-sized DNA, indicating that all the AP sites have been repaired in this period (Fig. 3A). The rate of removal of AP sites is lower in the apn1Δ strain, as DNA is not restored to normal size after the 2-h incubation period (Fig. 3B), whereas the rad14Δ strain displays proficient repair of AP sites (Fig. 3C). The rate of removal of AP sites, however, is reduced further in the apn1Δ rad14Δ strain (Fig. 3D) than in the apn1Δ strain (Fig. 3B).
FIG. 3.
Alkaline sucrose gradient analysis of DNA from cells treated with 0.1% MMS for 20 min. Strains YRP276 (wild type) (A), YRP210 (apn1Δ) (B), YR14-30 (rad14Δ) (C), and YR14-29 (apn1Δ rad14Δ) (D) were tested. Symbols, ○, untreated cells; ●, cells treated with MMS for 20 min; ▴, cells treated with MMS for 20 min and then given a 2-h repair period.
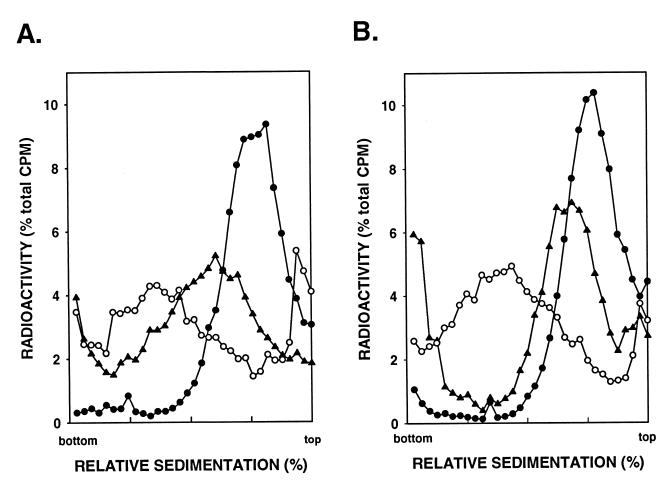
Because removal of the AP sites is severely impaired in the apn1Δ apn2Δ double mutant (23), to examine the effect of inactivation of NER in this strain, we treated the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ cells with 0.04% MMS and examined the size of DNA in cells after a 2-h incubation period. Even at this low MMS concentration, the repair of AP sites is considerably reduced in the apn1Δ apn2Δ strain (Fig. 4A), and this repair defect is further exacerbated in the apn1Δ apn2Δ rad14Δ strain (Fig. 4B). Interestingly, this triple mutant still repairs some of the AP sites (Fig. 4B), suggesting that yeast cells harbor yet another AP endonuclease activity.
FIG. 4.
Alkaline sucrose gradient analysis of DNA from cells treated with 0.04% MMS for 20 min. Strains YRP292 (apn1Δ apn2Δ) (A) and YR14-39 (apn1Δ apn2Δ rad14Δ) (B) were tested. Symbols, ○, untreated cells; ●, cells treated with MMS for 20 min; ▴, cells treated with MMS for 20 min and then given a 2-h repair period.
DISCUSSION
The results presented here provide evidence for the involvement of NER in the removal of AP sites in vivo in yeast, and they strongly suggest a similar role of NER in humans. Deletion of any of the yeast NER genes, RAD2, RAD4, or RAD14, increases the sensitivity of the apn1Δ and apn1Δ apn2Δ strains to MMS. Consistent with this, the removal of AP sites is reduced in the apn1Δ rad14Δ double mutant compared to that in the apn1Δ or rad14Δ mutant strain, and the ability to repair AP sites is further impaired in the apn1Δ apn2Δ rad14Δ strain over that in the apn1Δ apn2Δ or apn1Δ rad14Δ strain. From these observations, we conclude that Apn1, Apn2, and NER constitute alternate competing pathways for the repair of AP sites.
Because AP sites are noncoding, they represent a block to the normal DNA replication machinery. The simultaneous inactivation of the APN1 and APN2 genes results in a steep rise in the frequency of mutations induced in cells upon treatment with MMS. Since MMS-induced mutations are not recovered in the apn1Δ apn2Δ rev3Δ or apn1Δ apn2Δ rev7Δ strains, the REV3-REV7-encoded DNA polymerase ζ functions in the mutagenic bypass of AP sites (23). Here, we show that the frequency of MMS-induced can1r mutations is higher in the apn1Δ rad14Δ strain than in the apn1Δ or rad14Δ strain, and mutation frequency is further elevated in the apn1Δ apn2Δ rad14Δ strain over that in the apn1Δ apn2Δ strain. Thus, inactivation of NER in the apn1Δ or apn1Δ apn2Δ strain causes a pronounced decrease in cellular ability to remove AP sites, resulting in enhanced mutagenesis.
In vitro studies with human cell extracts have indicated that a large variety of lesions—AP sites, N6-methyladenine, O6-methylguanine, DNA mismatches, 8-oxoguanine, and thymine glycol—can be removed by the human NER system (21, 33). Our studies provide evidence for the involvement of NER in the removal of AP sites in eukaryotic cells, and they indicate that in vivo, NER competes with Apn1 and Apn2 for the removal of AP sites. The frequency of spontaneous GC-to-TA mutations is increased in the yeast ogg1Δ rad14Δ double mutant over that in either single mutant, suggesting a role for NER in the removal of 8-oxoguanine as well (38).
In addition to the large increase in the incidence of skin cancer, XP patients experience an increase in the occurrence of cancers in sites not exposed to UV radiation. The frequency of cancers of the brain and other regions of the central nervous system is elevated about 50-fold, and there is a >100-fold increase in the frequency of cancers of the oral cavity (excluding tongue and lip) in XP patients (24, 25). The role of NER in the removal of DNA damage from internal organs is also supported from studies on XPA−/− mice, where the frequency of spontaneous mutations in liver as well as the incidence of hepatocellular adenomas increases as these mice age (7, 9). The high frequency of spontaneous depurinations in mammalian cells may impose an even more significant role for NER in the repair of AP sites in humans than in yeast, and elevated mutability resulting from a deficiency in the removal of AP sites may contribute to the increase in the incidence of internal cancers in XP.
XP patients also suffer from progressive neurological abnormalities. Of the seven XP genes XPA through XPG, XPB and XPD encode the two DNA helicases present in transcription factor TFIIH (8, 37, 40), and the XPG gene product is tightly associated with TFIIH (29). Mutations in XPB, XPD, and XPG can also result in Cockayne's syndrome (26), a disease characterized by growth retardation and by progressive neurological dysfunction and mental retardation (30). Even though the neurological problems in these patients may result from a deficiency in some aspect of transcription, XPA patients also exhibit early onset of severe neurological abnormalities (26), and there is no evidence for the involvement of XPA in any function other than NER. Because of the high rate of metabolic activity, neurons may experience considerable damage to their DNA from reactive oxygen species, and AP sites resulting from the action of DNA glycosylases on damaged bases may be repaired less efficiently in the absence of NER. Thus, a deficiency in the repair of AP sites may also contribute to the progressive neurological dysfunction in XP.
ACKNOWLEDGMENT
This work was supported by grant CA41261 from the NCI, National Institutes of Health.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted genes. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell A J, Bardwell L, Tomkinson A E, Friedberg E C. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994;265:2082–2085. doi: 10.1126/science.8091230. [DOI] [PubMed] [Google Scholar]
- 3.Bennett R A O. The Saccharomyces cerevisiae ETH1 gene, an inducible homolog of exonuclease III that provides resistance to DNA-damaging agents and limits spontaneous mutagenesis. Mol Cell Biol. 1999;19:1800–1809. doi: 10.1128/mcb.19.3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjoras M, Klungland A, Johansen R F, Seeberg E. Purification and properties of the alkylation repair DNA glycosylase encoded MAG gene from Saccharomyces cerevisiae. Biochemistry. 1995;34:4577–4582. doi: 10.1021/bi00014a010. [DOI] [PubMed] [Google Scholar]
- 5.Burns J L, Guzder S N, Sung P, Prakash S, Prakash L. An affinity of human replication protein A for ultraviolet-damaged DNA: implications for damage recognition in nucleotide excision repair. J Biol Chem. 1996;271:11607–11610. doi: 10.1074/jbc.271.20.11607. [DOI] [PubMed] [Google Scholar]
- 6.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 7.de Vries A, van Oostrom C T M, Dortant P M, Beems R B, van Kreijl C F, Capel P J A, van Steeg H. Spontaneous liver tumors and benzo[a]pyrene-induced lymphomas in XPA-deficient mice. Mol Carcinog. 1997;19:46–53. [PubMed] [Google Scholar]
- 8.Drapkin R, Reardon J T, Ansari A, Huang J C, Zawel L, Ahn K, Sancar A, Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994;368:769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- 9.Giese H, Dolle M E T, Hezel A, van Steeg H, Vijg J. Accelerated accumulation of somatic mutations in mice deficient in the nucleotide excision repair gene XPA. Oncogene. 1999;18:1257–1260. doi: 10.1038/sj.onc.1202404. [DOI] [PubMed] [Google Scholar]
- 10.Guzder S N, Habraken Y, Sung P, Prakash L, Prakash S. Reconstitution of yeast nucleotide excision repair with purified Rad proteins, replication protein A, and transcription factor TFIIH. J Biol Chem. 1995;270:12973–12976. doi: 10.1074/jbc.270.22.12973. [DOI] [PubMed] [Google Scholar]
- 11.Guzder S N, Sung P, Prakash L, Prakash S. Affinity of yeast nucleotide excision repair factor 2, consisting of the Rad4 and Rad23 proteins, for ultraviolet damaged DNA. J Biol Chem. 1998;273:31541–31546. doi: 10.1074/jbc.273.47.31541. [DOI] [PubMed] [Google Scholar]
- 12.Guzder S N, Sung P, Prakash L, Prakash S. The DNA-dependent ATPase activity of yeast nucleotide excision repair factor 4 and its role in DNA damage recognition. J Biol Chem. 1998;273:6292–6296. doi: 10.1074/jbc.273.11.6292. [DOI] [PubMed] [Google Scholar]
- 13.Guzder S N, Sung P, Prakash L, Prakash S. Nucleotide excision repair in yeast is mediated by sequential assembly of repair factors and not by a pre-assembled repairosome. J Biol Chem. 1996;271:8903–8910. doi: 10.1074/jbc.271.15.8903. [DOI] [PubMed] [Google Scholar]
- 14.Guzder S N, Sung P, Prakash L, Prakash S. Synergistic interaction between yeast nucleotide excision repair factors NEF2 and NEF4 in the binding of ultraviolet-damaged DNA. J Biol Chem. 1999;274:24257–24262. doi: 10.1074/jbc.274.34.24257. [DOI] [PubMed] [Google Scholar]
- 15.Guzder S N, Sung P, Prakash L, Prakash S. Yeast DNA repair gene RAD14 encodes a zinc metalloprotein with affinity for ultraviolet damaged DNA. Proc Natl Acad Sci USA. 1993;90:5433–5437. doi: 10.1073/pnas.90.12.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzder S N, Sung P, Prakash L, Prakash S. Yeast Rad7-Rad16 complex specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J Biol Chem. 1997;272:21665–21668. doi: 10.1074/jbc.272.35.21665. [DOI] [PubMed] [Google Scholar]
- 17.Habraken Y, Sung P, Prakash L, Prakash S. Structure-specific nuclease activity in yeast nucleotide excision repair protein Rad2. J Biol Chem. 1995;270:30194–30198. doi: 10.1074/jbc.270.50.30194. [DOI] [PubMed] [Google Scholar]
- 18.Habraken Y, Sung P, Prakash L, Prakash S. Yeast excision repair gene RAD2 encodes a single-stranded DNA endonuclease. Nature. 1993;366:365–368. doi: 10.1038/366365a0. [DOI] [PubMed] [Google Scholar]
- 19.Habraken Y, Sung P, Prakash S, Prakash L. Transcription factor TFIIH and DNA endonuclease Rad2 constitute yeast nucleotide excision repair factor 3: implications for nucleotide excision repair and Cockayne syndrome. Proc Natl Acad Sci USA. 1996;93:10718–10722. doi: 10.1073/pnas.93.20.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins N P, Kato K, Strauss B. A model for replication repair in mammalian cells. J Mol Biol. 1976;101:417–425. doi: 10.1016/0022-2836(76)90156-x. [DOI] [PubMed] [Google Scholar]
- 21.Huang J-C, Hsu D S, Kazantsev A, Sancar A. Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc Natl Acad Sci USA. 1994;91:12213–12217. doi: 10.1073/pnas.91.25.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen L E T, Verhage R A, Brouwer J. Preferential binding of yeast Rad4-Rad23 complex to damaged DNA. J Biol Chem. 1998;273:33111–33114. doi: 10.1074/jbc.273.50.33111. [DOI] [PubMed] [Google Scholar]
- 23.Johnson R E, Torres-Ramos C A, Izumi T, Mitra S, Prakash S, Prakash L. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 1998;12:3137–3143. doi: 10.1101/gad.12.19.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraemer K H, Lee L L, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 25.Kraemer K H, Lee M-M, Andrews A D, Lambert W C. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 26.Kraemer K H, Parris C N, Gozukara E M, Levy D D, Adelberg S, Seidman M M. Human DNA repair-deficient diseases: clinical disorders and molecular defects. In: Bohr V A, Wasserman K, Kraemer K H, editors. DNA repair mechanisms. Vol. 35. Copenhagen, Denmark: Munksgaard; 1993. pp. 15–26. [Google Scholar]
- 27.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3617. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 28.Mu D, Hsu D S, Sancar A. Reaction mechanism of human DNA repair excision nuclease. J Biol Chem. 1996;271:8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- 29.Mu D, Park C-H, Matsunaga T, Hsu D S, Reardon J T, Sancar A. Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem. 1995;270:2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- 30.Nance M A, Berry S A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- 31.Popoff S C, Spira A I, Johnson A W, Demple B. Yeast structural gene (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc Natl Acad Sci USA. 1990;87:4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramotar D, Popoff S C, Gralla E B, Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3′-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991;11:4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reardon J T, Bessho T, Kung H C, Bolton P H, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci USA. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 35.Roy R, Brooks C, Mitra S. Purification and biochemical characterization of recombinant N-methylpurine-DNA glycosylase of the mouse. Biochemistry. 1994;33:15131–15140. doi: 10.1021/bi00254a024. [DOI] [PubMed] [Google Scholar]
- 36.Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffer L, Moncollin V, Roy R, Staub A, Mezzina M, Sarasin A, Weeda G, Hoeijmakers J H J, Egly J M. The ERCC2/DNA repair protein is associated with the class II BTF2/TFIIH transcription factor. EMBO J. 1994;13:2388–2392. doi: 10.1002/j.1460-2075.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott A D, Neishabury M, Jones D H, Reed S H, Boiteux S, Waters R. Spontaneous mutation, oxidative DNA damage, and the roles of base and nucleotide excision repair in the yeast Saccharomyces. Yeast. 1999;15:205–218. doi: 10.1002/(SICI)1097-0061(199902)15:3<205::AID-YEA361>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Seeberg E, Eide L, Bjorås M. The base excision repair pathway. Trends Biochem Sci. 1995;20:391–396. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 40.Sung P, Bailly V, Weber C, Thompson L H, Prakash L, Prakash S. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993;365:852–855. doi: 10.1038/365852a0. [DOI] [PubMed] [Google Scholar]
- 41.Sung P, Guzder S N, Prakash L, Prakash S. Reconstitution of TFIIH and requirement of its DNA helicase subunits, Rad3 and Rad25, in the incision step of nucleotide excision repair. J Biol Chem. 1996;271:10821–10826. doi: 10.1074/jbc.271.18.10821. [DOI] [PubMed] [Google Scholar]
- 42.Sung P, Reynolds P, Prakash L, Prakash S. Purification and characterization of the Saccharomyces cerevisiae RAD1/RAD10 endonuclease. J Biol Chem. 1993;268:26391–26399. [PubMed] [Google Scholar]
- 43.Tomkinson A E, Bardwell A J, Bardwell L, Tappe N J, Friedberg E C. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature. 1993;362:860–862. doi: 10.1038/362860a0. [DOI] [PubMed] [Google Scholar]
- 44.Torres-Ramos C A, Yoder B L, Burgers P M J, Prakash S, Prakash L. Requirement of proliferating cell nuclear antigen in RAD6-dependent postreplicational DNA repair. Proc Natl Acad Sci USA. 1996;93:9676–9681. doi: 10.1073/pnas.93.18.9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace S. Oxidative stress defenses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. Oxidative damage to DNA and its repair; pp. 49–90. [Google Scholar]