Abstract
Type 2 diabetes (T2D) impairs hypoxia-inducible factor (HIF)1α activation, a master transcription factor that drives cellular adaptation to hypoxia. Reduced activation of HIF1α contributes to the impaired post-ischemic remodeling observed following myocardial infarction in T2D. Molidustat is an HIF stabilizer currently undergoing clinical trials for the treatment of renal anemia associated with chronic kidney disease; however, it may provide a route to pharmacologically activate HIF1α in the T2D heart. In human cardiomyocytes, molidustat stabilized HIF1α and downstream HIF target genes, promoting anaerobic glucose metabolism. In hypoxia, insulin resistance blunted HIF1α activation and downstream signaling, but this was reversed by molidustat. In T2D rats, oral treatment with molidustat rescued the cardiac metabolic dysfunction caused by T2D, promoting glucose metabolism and mitochondrial function, while suppressing fatty acid oxidation and lipid accumulation. This resulted in beneficial effects on post-ischemic cardiac function, with the impaired contractile recovery in T2D heart reversed by molidustat treatment. In conclusion, pharmacological HIF1α stabilization can overcome the blunted hypoxic response induced by insulin resistance. In vivo this corrected the abnormal metabolic phenotype and impaired post-ischemic recovery of the diabetic heart. Therefore, molidustat may be an effective compound to further explore the clinical translatability of HIF1α activation in the diabetic heart.
Introduction
Hypoxia-inducible factor 1 (HIF1) is a heterodimeric transcription factor considered to be a master regulator of oxygen homeostasis, as it is key to the cellular and systemic adaptive responses to low oxygen availability (hypoxia) (1–3). HIF1 consists of a constitutively expressed and stable β subunit (HIF1β) and an α subunit whose regulation is oxygen dependent (HIF1α) (2). In normoxia, HIF1α is targeted for degradation by the proteasome via hydroxylation of proline residues, mediated by the oxygen-dependent prolyl hydroxylase domain (PHD) family of enzymes (4). When oxygen delivery is compromised, as occurs during myocardial ischemia, the PHDs are inhibited and HIF1α escapes hydroxylation, allowing it to migrate to the nucleus and induce transcription of HIF-target genes. HIF regulates many hundreds of different genes involved in metabolism, cell growth, angiogenesis, and erythropoiesis, allowing the tissue/organism to survive during hypoxia (5). Optimal HIF activation is essential for the heart to maintain its contractile function following myocardial infarction (MI) and pressure-overload hypertrophy (6–8). Recognition of HIF’s central role in physiology and medicine was recently demonstrated by the Nobel Prize award in 2019 to William Kaelin Jr., Sir Peter Ratcliffe, and Gregg Semenza.
A key element of HIF activation is the promotion of glycolysis and suppression of fatty acid oxidation (9–14), reprogramming cardiac substrate metabolism (15). Interestingly, this HIF-mediated metabolic shift is in the opposite direction to that caused by diabetes, in which glucose metabolism is decreased and fatty acid oxidation is increased (16,17). Thus, from a metabolic perspective, HIF and diabetes have opposing effects on cardiac metabolism (15). In type 2 diabetes (T2D), the shift toward fatty acid metabolism is proportional to the severity of insulin resistance (18,19). In addition, this abnormal metabolism is associated with abnormal cardiac function in T2D (20). Thus, activating HIF in diabetes may provide a mechanism to shift metabolism back toward greater glucose use and suppress fatty acid metabolism, resulting in improved cardiac function (21).
We have previously shown that in diabetes HIF1α stabilization is blunted, and downstream activation of the adaptive hypoxic response is reduced in insulin-resistant cardiomyocytes (22). This reduction in HIF activation is mediated by the increased fatty acids present within the myocardium. In hypoxia, fatty acids suppress the accumulation of succinate and fumarate, which are needed for optimal HIF stabilization via inhibition of the PHD enzymes (23). This results in diabetic hearts being less able to tolerate hypoxia and less able to adapt in the longer term (15). The clinical consequence for people with T2D is a more rapid progression into heart failure following MI (24), as the decreased collateral vessel development present post-MI in patients with diabetes is due to decreased activation of HIF-dependent processes (25,26). Thus, strategies that pharmacologically activate HIF may correct the blunted HIF activation caused by increased fatty acids in T2D.
Cardiovascular disease is the leading cause of mortality in diabetes, and even with optimal managed risk factors (glucose, blood pressure, cholesterol), people with T2D still have a 21% increased risk of cardiovascular disease (27). Therefore, there is an unmet need for new therapies for the diabetic heart. Molidustat (BAY85-3934) is an orally bioavailable PHD inhibitor that successfully stabilizes HIF-1α and is undergoing phase III clinical trials to treat anemia in patients with chronic kidney disease (28–30). In cancer cells, molidustat stabilizes HIF1α and induces downstream targets involved in angiogenesis and erythropoiesis (31,32). We therefore questioned whether molidustat could be repurposed for use in the heart to overcome the blunted HIF activation and correct the abnormal cardiac metabolism induced by diabetes. Using both human cardiomyocytes and animal models we demonstrate here that molidustat can improve cardiac metabolism and HIF signaling in diabetes, resulting in improved recovery of the heart post-ischemia.
Research Design and Methods
Human Induced Pluripotent Stem Cell–Derived Cardiomyocyte Differentiation and Maturation
Healthy human induced pluripotent stem cell (hiPSC) lines IMR90 and M180 were cultured in mTeSR1 medium on Matrigel-coated plates and were dissociated using ReLeSR. The cells were then transferred onto Reduced Growth Factor (RF)-Matrigel–coated plates for differentiation into hiPSC-derived cardiomyocytes (hiPSC-CM). Differentiation was initiated on day 0 using differentiation medium (RPMI medium [Gibco] supplemented with 1% B27 minus insulin (Gibco) and 6 μmol/L CHIR99201 (Tocris Bioscience) for 2 days. On day 3, the differentiation medium was supplemented with 2.5 μmol/L Wnt-C59 (Tocris Bioscience). The glucose-depletion method was performed on days 11 and 13 by changing of medium to no-glucose RPMI with 1% B27 minus insulin (33). hiPSC-CM maturation was initiated from day 16 with replating cells onto RF-Matrigel–coated plates and culturing in DMEM containing 5 mmol/L glucose and supplemented with 0.4 mmol/L oleic acid conjugated to BSA, for 1 week (34). Results from the IMR90 line are presented in Figs. 1 and 2, with key findings confirmed in the M180 line presented in Supplementary Fig. 1.
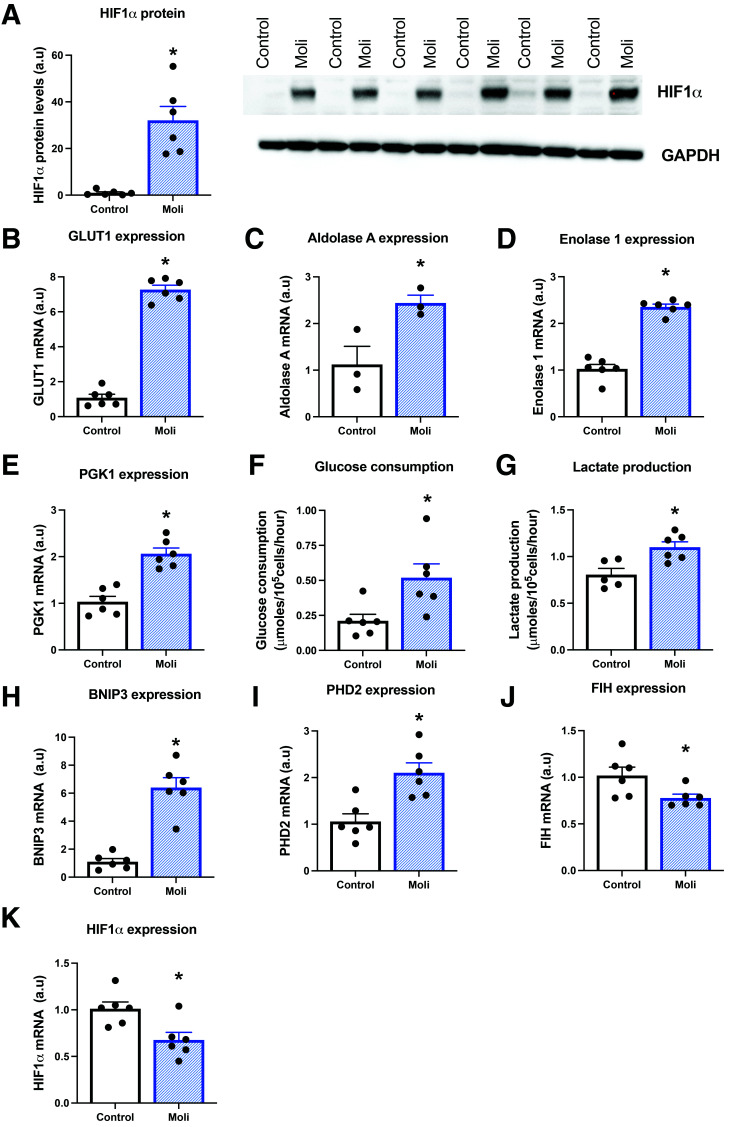
Figure 1.
Molidustat increases HIF signaling in human cardiomyocytes. Hypoxia-inducible factor (HIF)1α protein levels in control and molidustat-treated (Moli) IMR90 hiPSC-CM (A). Expression of HIF1α target genes in control and molidustat-treated cardiomyocytes, including GLUT1, aldolase A, enolase 1, phosphoglycerate kinase 1 (PGK1), bcl2/adenovirus E1B 19kDa–interacting protein 3 (BNIP3), prolyl hydroxylase domain (PHD)2, factor inhibiting HIF (FIH), and HIF1α mRNA (B–E and H–K). F and G: Glucose consumption by and lactate release from control and molidustat-treated cardiomyocytes. *P < 0.05 vs. control. a.u, arbitrary units.
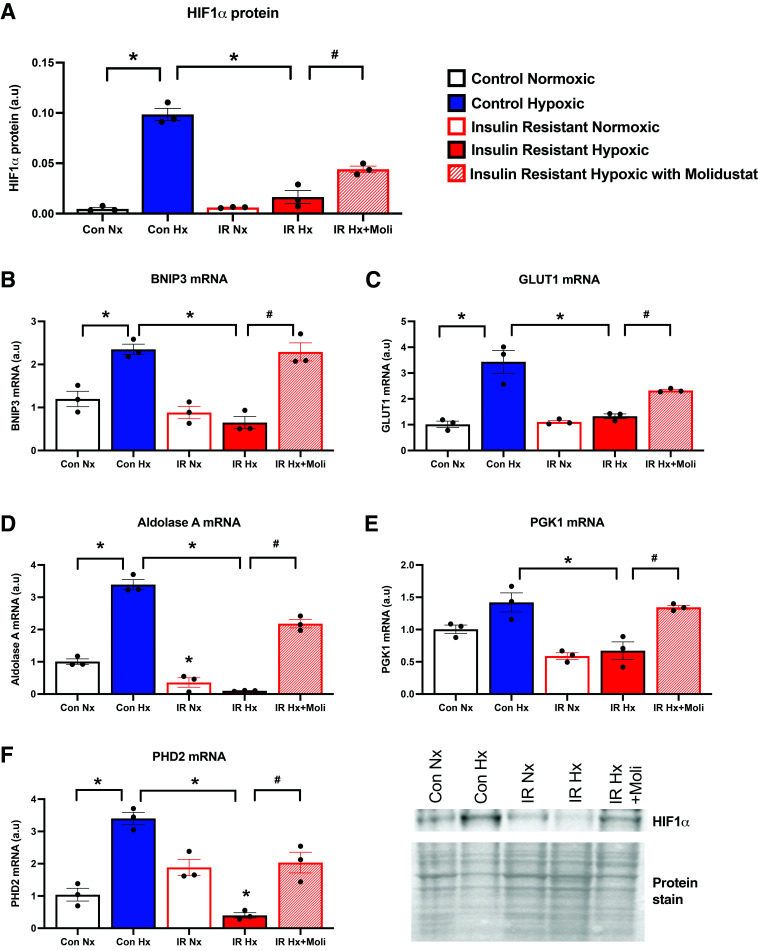
Figure 2.
Molidustat overrides the impaired HIF signaling caused by insulin resistance. Hypoxia-inducible factor (HIF)1α protein levels in control (Con) and insulin-resistant (IR) human cardiomyocytes, cultured in a state of normoxia (Nx) or hypoxia (Hx) for 16 h. Molidustat (Moli) was added to insulin-resistant cells just prior to entry into hypoxic chamber (A). Expression of HIF1α target genes under the respective conditions, including bcl2/adenovirus E1B 19kDa–interacting protein 3 (BNIP3), GLUT1, aldolase A, phosphoglycerate kinase 1 (PGK1), and prolyl hydroxylase domain (PHD)2 (B–F). *P < 0.05 vs. respective control group; #P < 0.05 vs. respective insulin-resistant group. a.u, arbitrary units.
Insulin Resistance in hiPSC-CM
Insulin resistance was induced by culturing mature hiPSC-CM in glucose-free insulin resistance media comprising DMEM without glucose, 0.3 mmol/L palmitic acid:BSA (bound 6:1), 50 nmol/L insulin, and 2 mmol/L nonessential amino acids for 3 days. On day 4, the media was switched to insulin resistance media as described above but also containing 12 mmol/L glucose for a further 3 days.
Rat Model of T2D
All animal experiments conformed to the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act, 1986, and were approved by a local ethics committee (University of Oxford, U.K.). T2D was induced as previously described (35,36), generating an early-stage model of the disease presenting with mild hyperglycemia, hyperinsulinemia, and hyperlipidemia. Briefly, male Wistar rats (starting body weight ∼300 g; Envigo) were fed a high-fat diet (cat. no. 829197, 60% calories from fats; Special Diet Services) for 5 weeks, and on day 14 they received a single low-dose injection of streptozotocin (25 mg/kg body wt i.p. in citrate buffer, pH 4) in the fasted state. Control rats were fed a standard chow diet for 5 weeks.
Molidustat Administration
Human Cardiomyocytes
Mature hiPSC-CMs were cultured in the presence or absence of 50 μmol/L molidustat (cat. no. ADV465750852, Merck, and R16009, Advanced ChemBlocks) for 16 h. A subgroup of control and insulin-resistant hiPSC-CMs was exposed to hypoxia (2% O2, 5% CO2) for 16 h, with molidustat added just prior to the insulin-resistant cells entering the hypoxic incubator. Cell media was collected and assayed spectrophotometrically for glucose and lactate concentrations, with measurement of the change in absorbance following incubation with glucose assay reagent (Merck) for the former or lactate dehydrogenase and NAD for the latter. Glucose consumption rates and lactate production rates were normalized to cell number.
T2D Rats
Molidustat was given orally (5 mg/kg body wt/day) to T2D and control rats, dissolved in sugar-free jelly (to ensure the precise dose was administered, yet avoiding the daily stress of gavaging). Molidustat was given daily for the final 5 days of the 5-week protocol. Untreated rats were given an equivalent amount of drug-free jelly. Rats were terminally anesthetized in the fed state 24 h after the final dose of molidustat, using an injection of pentobarbital sodium (0.7 mL of 200 mg/mL i.p. Euthatal). Blood was collected for measurement of hematocrit (HemoCue 201+ System meter), and glucose concentrations were measured from a sample of blood with an Accu-chek Aviva meter following heart removal. Epididymal fat pads were collected and weighed for assessment of adiposity. Hearts were perfused for measurement of function and metabolic flux, used for mitochondrial isolation, or freeze clamped in liquid nitrogen for subsequent molecular biology analyses.
Isolated Heart Perfusion
Hearts were isolated and arrested in ice-cold Krebs-Henseleit buffer, rapidly cannulated via the aorta, and then perfused in retrograde Langendorff mode according to our published protocol (35,37). Hearts contracted against a constant afterload pressure of 100 mmHg (to represent the arterial blood pressure [38]). A fluid-filled polyvinyl chloride balloon connected to a pressure transducer was inserted into the left ventricle and inflated to give an end-diastolic pressure of 4–8 mmHg. This balloon allowed measurement of intraventricular pressure during systole and diastole, and the subsequent calculation of heart rate (systolic pressure peaks per minute). Left ventricular developed pressure was calculated as peak systolic pressure minus end-diastolic pressure for each contraction cycle and expressed as mmHg. Rate pressure product was calculated as the developed pressure multiplied by the heart rate, and expressed as mmHg/minute. Hearts were perfused with recirculating Krebs-Henseleit buffer containing 11 mmol/L glucose, 0.4 mmol/L palmitate (bound to BSA), and 3 units/L insulin, and gassed with 95% O2 and 5% CO2 at 37°C. Hearts were perfused for 20 mins (baseline pre-ischemia), followed by 30 mins of low-flow ischemia (0.3 mL/min/gram wet weight [gww]) and then 25 min of reperfusion.
3H Perfusion and Metabolic Rates
For measurement of glycolytic rates, buffer was supplemented with 0.2 µCi/mL of [5-3H]glucose. For measurement of palmitate oxidation rates, buffer was supplemented with 0.2 µCi/mL [9,10-3H]palmitate. Buffer aliquots were collected at 4-min intervals throughout the baseline perfusion, and 3H metabolic rates were calculated according to published protocols (15). Lactate efflux rates were calculated spectrophotometrically using these timed aliquots, following addition of lactate dehydrogenase and NAD.
Tissue Analyses
Cardiac and hepatic triglyceride concentrations were measured spectrophotometrically using a Randox triglyceride assay kit following Folch extraction. Glycogen content was measured with use of amyloglucosidase to convert glycogen to glucose, which was subsequently quantified spectrophotometrically with glucose assay reagents (Merck). Medium-chain acyl-CoA dehydrogenase (MCAD) activity was measured spectrophotometrically according to a previously published protocol (37).
Mitochondrial Isolation and Respiration
Mitochondria were isolated as previously described and respired with pyruvate (10 mmol/L) and malate (5 mmol/L) as carbohydrate substrates (39). Respiration was measured under ADP-stimulated (0.2 mmol/L) state 3 conditions and maximally stimulated with the uncoupling agent carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) (20 μmol/L).
Western Blotting
Tissue and cells were lysed in ice-cold lysis buffer (22), a protein assay was used to calculate protein concentration, and 10 μg cell protein or 25 μg tissue protein was loaded onto SDS-PAGE gels and separated by electrophoresis. For measurement of sarcolemmal transporters, separation of the sarcolemmal membrane fraction was carried out using density centrifugation according to the established protocol of Luiken et al. (40). Primary antibodies to HIF1α (10006421; Cayman Chemical), lactate dehydrogenase (LDH) (Ab47010; Abcam), GLUT1 (ab652; Abcam), GLUT4 (a kind gift from Geoff Holman, University of Bath, U.K.), uncoupling protein 3 (UCP3) (Ab180643; Abcam), glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (60004-1-Ig; Proteintech), pyruvate dehydrogenase kinase 1 (PDK1) (3062; Cell Signaling Technology), and fatty acid translocase (FAT/CD36, monoclonal antibody MO25; a gift from Narendra Tandon, Otsuka Maryland Medicinal Laboratories, Rockville, MD) were used, in combination with the relevant secondary antibodies (Abcam). Even protein loading and transfer were confirmed by ponceau S total protein staining as a housekeeping loading control for tissue and normalized to GAPDH for molidustat-treated cells. Bands were quantified using LI-COR C-DiGit chemiluminescent detection system (LI-COR), Chemidoc XRS+ imaging system (Bio-Rad) and Image Studio Software version 5.2.5.
RNA Isolation and Quantitative PCR
RNA was extracted from cells and tissue with a RNeasy mini kit (QIAGEN), with cDNA conversion carried out with a high-capacity RNA-to-cDNA kit (Applied Biosystems) using a SensoQuest Labcycler (Geneflow). Quantitative PCR amplification was performed with Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) with 15 ng/well of cDNA, using a StepOnePlus Real-Time PCR System machine (Applied Biosystems). Relative gene expression was calculated with the 2−ΔΔCt method, normalized to the housekeeper gene (SDHA for hearts, UBC for cells). Primer sequences are located in Supplementary Table 1.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Statistics
Results are presented as means ± SEM and considered significant at P values of <0.05, analyzed with GraphPad Prism version 8. Data sets containing two groups were analyzed with a two-tailed parametric unpaired t test with Welch correction (Fig. 1). Data sets containing multiple groups were analyzed with one-way ANOVA, with Tukey post hoc correction for multiple comparisons (Fig. 2). Data sets with two groups (control vs. diabetic) and two variables (vehicle vs. molidustat) were analyzed with two-way ANOVA, with Sidak post hoc correction for multiple comparisons (Figs. 3–6).
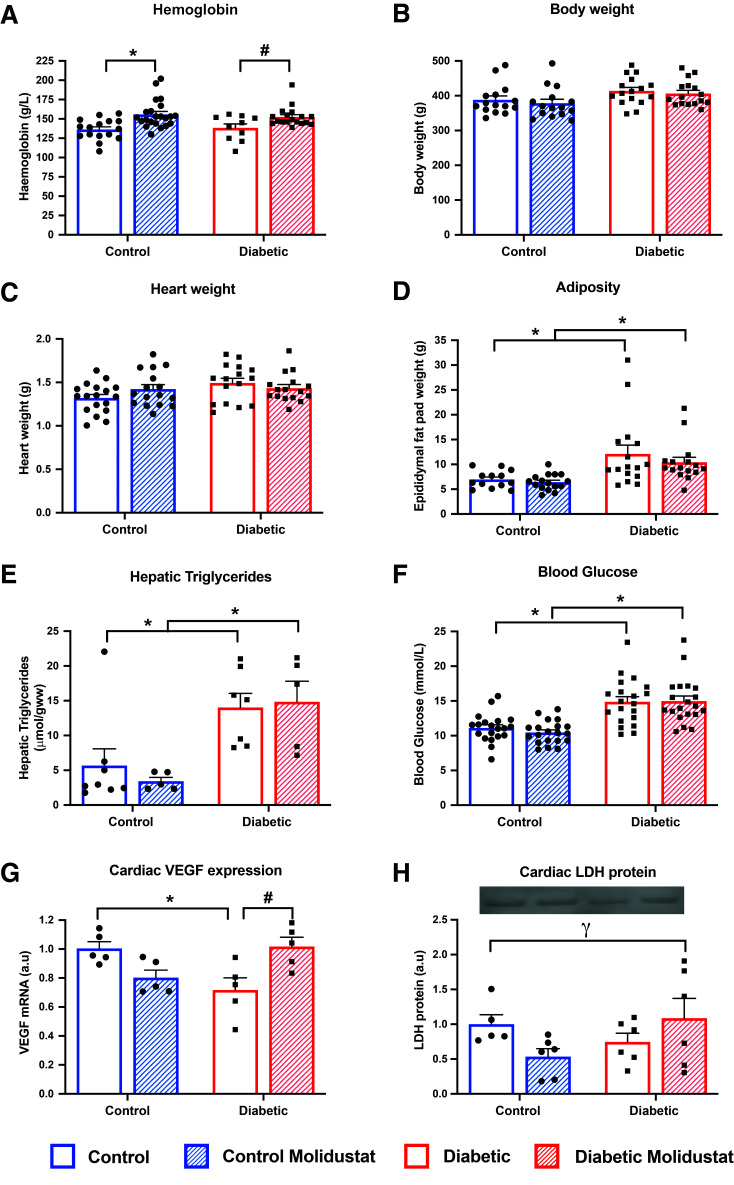
Figure 3.
Molidustat increases systemic HIF targets in T2D. Hemoglobin content, body weight, heart weight, and epididymal fat pad weight in control and diabetic rats, with and without molidustat treatment (A–D). Hepatic triglycerides and fed blood glucose concentrations in control and diabetic rats, with and without molidustat treatment (E and F). Cardiac VEGF expression and lactate dehydrogenase (LDH) protein levels in control and diabetic rats, with and without molidustat treatment (G and H). *P < 0.05 vs. respective control group; #P < 0.05 vs. diabetic untreated; γP < 0.05 significant interaction between disease and drug treatment. a.u, arbitrary units.
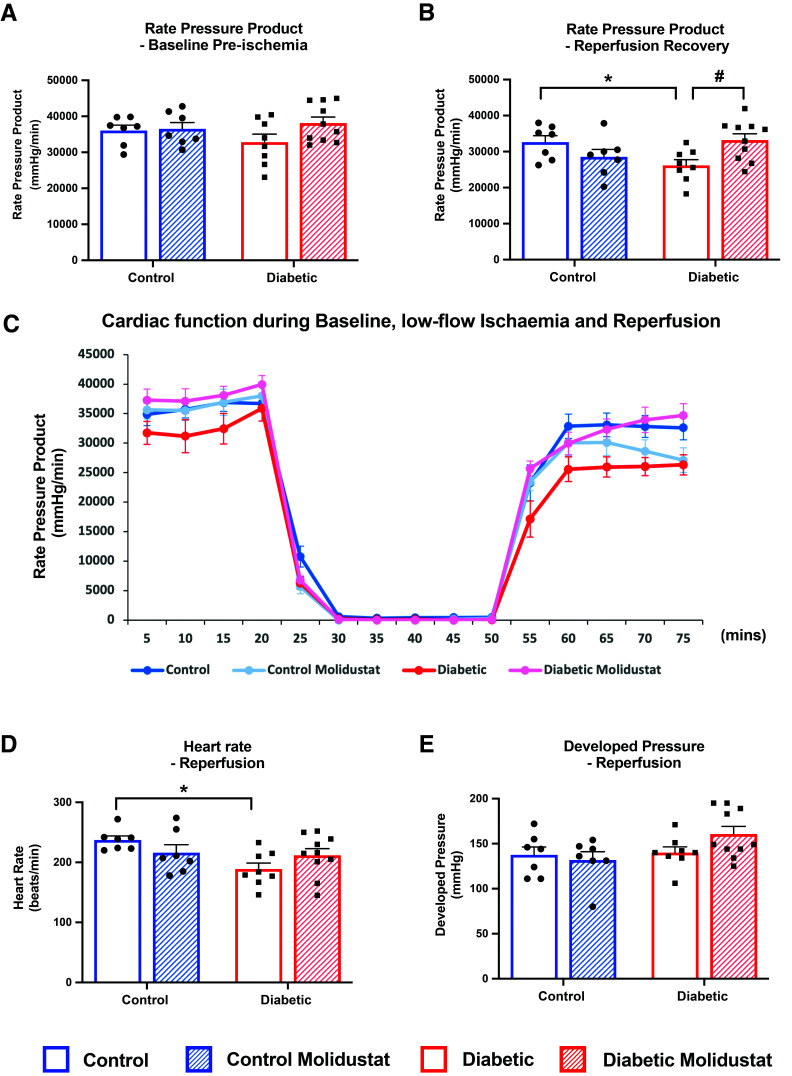
Figure 6.
Molidustat improves post-ischemic recovery in T2D. Cardiac function was measured as rate pressure product (the multiple of heart rate and developed pressure) in control and diabetic rats, with and without molidustat treatment. Hearts were perfused at baseline (A) and subjected to 30 min of low-flow ischemia followed by 25 min of reperfusion (B–E). Average rate pressure product traces are shown from the time course of the perfusion experiment (C). *P < 0.05 vs. control; #P < 0.05 vs. diabetic untreated.
Results
Molidustat Stabilizes HIF1α Signaling and Downstream Metabolic Adaptation in Human Cardiomyocytes
Firstly we set out to determine whether molidustat could regulate HIF-dependent pathways in hiPSC-CM. In control cardiomyocytes, HIF1α protein was barely detectable; however, following 16 h exposure to molidustat there was robust 30-fold stabilization of HIF1α protein (Fig. 1A). Molidustat increased the expression of multiple genes involved in glycolysis, increasing glucose transporter GLUT1 sevenfold, and increasing by more than twofold the expression of aldolase A, enolase 1, and phosphoglycerate kinase (PGK)1 (Fig. 1B–E). This resulted in a twofold increase in glucose metabolism and a 36% increase in lactate release in molidustat-treated human cardiomyocytes (Fig. 1F and G). Other downstream targets of HIF1α also changed accordingly, with molidustat increasing the expression of the mitophagy gene BNIP3 sixfold and prolyl hydroxylase domain 2 (PHD2) twofold (Fig. 1H and I). Molidustat decreased HIF1α mRNA by 33%, demonstrating a feedback loop from HIF1α protein stabilization (Fig. 1K). These key findings in the IMR90 hiPSC-CM line were confirmed in the M180 hiPSC-CM line (Supplementary Fig. 1).
Molidustat Overrides the Blunted HIF Signaling Caused by Insulin Resistance
Exposure of control cardiomyocytes to hypoxia stabilized HIF1α protein and induced transcription of downstream HIF target genes BNIP3, GLUT1, aldolase A, and PHD2 (Fig. 2 and Supplementary Fig. 2A). In contrast, when insulin-resistant human cardiomyocytes were exposed to the same hypoxic challenge, stabilization of HIF1α protein and induction of downstream gene targets were all suppressed, demonstrating a blunted adaptive response to hypoxia in insulin resistance (Fig. 2). Addition of molidustat to insulin-resistant cells just prior to hypoxia improved hypoxic signaling. Molidustat increased HIF1α protein (Fig. 2A) and induced transcription of the HIF target genes (BNIP3, GLUT1, aldolase A, PGK1, and PHD2 [Fig. 2B–F]), overriding the inhibitory effect of insulin resistance on hypoxic adaptation. Thus, molidustat provides a pharmacological approach to restore cardiac HIF signaling and hypoxic adaptation in insulin resistance.
Molidustat Increases Systemic HIF Targets in the T2D Rat
We next administered molidustat orally to control and T2D rats. Molidustat upregulated hemoglobin content, by 14% and 10%, respectively, in control and diabetic rats (Fig. 3A), but did not affect body weight or heart weight in any group (Fig. 3B and C). Molidustat did not correct the adiposity or hepatic triglycerides, which remained elevated in our diabetic rats; thus, molidustat did not correct the systemic diabetic phenotype (Fig. 3D and E). Similarly, molidustat did not correct the hyperglycemia in the diabetic rats (Fig. 3F and Supplementary Fig. 3A).
In agreement with published literature (31), the HIF1α protein did not remain elevated once the drug was metabolized from the circulation (Supplementary Fig. 2B), but the downstream genes and products remained elevated 24 h post–final dose. The HIF target gene, VEGF, was decreased by 29% in T2D hearts (Fig. 3G). In vivo treatment with molidustat increased VEGF mRNA by 42% in the diabetic hearts, back to that found in control hearts (Fig. 3G). Similarly, LDH, another cardiac HIF target, showed a significant interaction between diabetes and molidustat (Fig. 3H).
Molidustat Improves Cardiac Glucose Metabolism in Diabetes
Diabetes impairs normal cardiac metabolism, forcing the heart to metabolize less glucose and more fatty acid. Given that molidustat promoted glucose metabolism in the hiPSC-CM, we asked whether pharmacological HIF1α activation may provide a route to correct cardiac metabolism in vivo in T2D. T2D hearts had impaired glycolytic rates, lactate efflux rates, and mitochondrial pyruvate oxidation (Fig. 4A, B, G, and H). In vivo treatment with molidustat increased cardiac glycolytic rates by 77% in T2D rats (Fig. 4A). Similarly, lactate efflux rates were increased by 70% following molidustat treatment of T2D rats (Fig. 4B). We investigated whether changes in glucose transporters were associated with the increased glycolysis and lactate production with molidustat treatment. Changes in total GLUT1 protein in the heart did not reach significance (Fig. 4C); however, when we specifically looked at GLUT1 at the sarcolemma we found a significant increase with molidustat treatment (Fig. 4E). This contrasted with GLUT4, which was decreased in the diabetic heart and was not upregulated with molidustat treatment (Fig. 4D and F); thus, the increase in glucose uptake was driven by GLUT1 and not GLUT4. The improvement in glycolysis was accompanied by improvements in mitochondrial pyruvate oxidation. Mitochondria from diabetic hearts had impaired respiration when oxidizing pyruvate, but when they were treated with molidustat this resulted in a 34% increase in maximal respiration (Fig. 4G and H). In contrast to glucose catabolism, cardiac glycogen content was not modified by molidustat (Supplementary Fig. 3B).
Figure 4.
Molidustat increases cardiac glucose metabolism in T2D. Cardiac glycolytic rates and lactate efflux rates in control and diabetic rats, with and without molidustat treatment (A and B). GLUT1 and GLUT4 protein levels in control and diabetic rats, with and without molidustat treatment (C and D). Sarcolemmal GLUT1 and GLUT4 protein levels in control and diabetic rats, with and without molidustat treatment (E and F). State 3 ADP-stimulated and maximally FCCP-stimulated pyruvate respiration rates in isolated mitochondria from control and diabetic rats, with and without molidustat treatment (G and H). *P < 0.05 vs. control, #P < 0.05 vs. diabetic untreated, †P < 0.05 significant effect of molidustat treatment at two-way ANOVA. a.u, arbitrary units.
Molidustat Decreases Cardiac Fatty Acid Metabolism in Diabetes
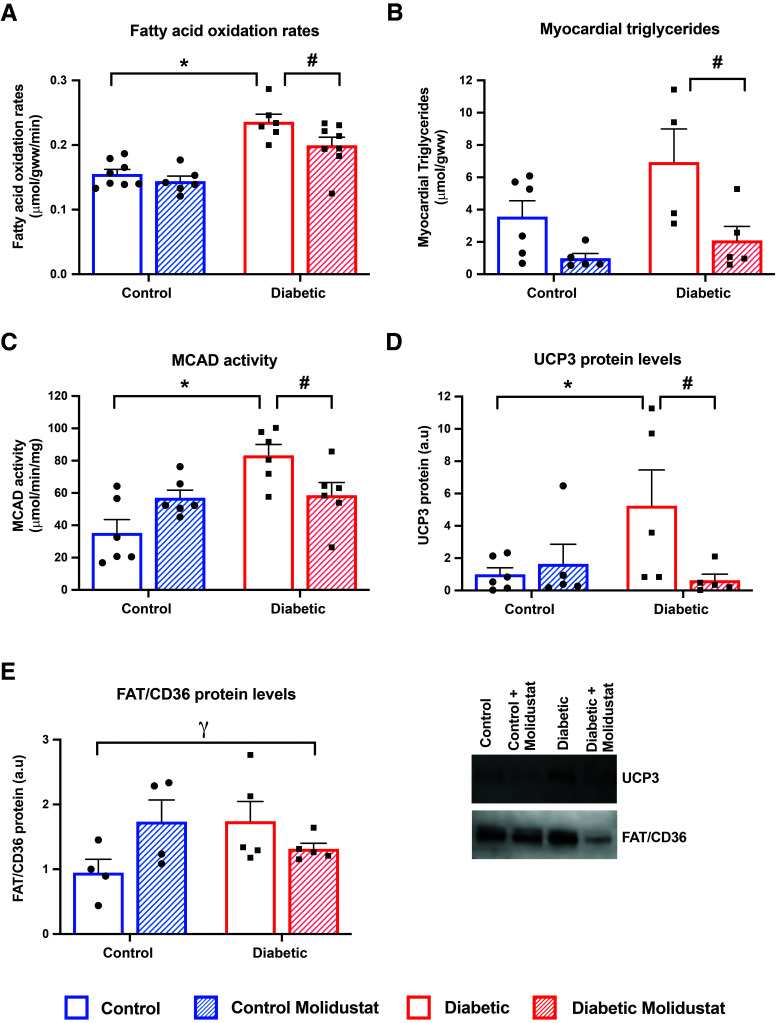
T2D hearts had elevated rates of fatty acid oxidation, concentrations of myocardial triglycerides, and activity/expression of lipid metabolism proteins (Fig. 5A–E). In T2D hearts, treatment with molidustat decreased fatty acid oxidation rates by 15%, compared with untreated T2D rats (Fig. 5A). Myocardial triglyceride concentrations were significantly decreased by 70% with molidustat treatment in T2D (Fig. 5B). Thus, both aspects of fatty acid metabolism, oxidation and esterification, were downregulated in the heart by in vivo molidustat treatment. PPARα is the predominant regulator of fatty acid metabolism in the heart and has been shown to be under the control of HIF; therefore, we measured activity and protein expression of several PPARα targets. MCAD activity was increased twofold in T2D hearts compared with control hearts, but molidustat treatment decreased MCAD activity by 30% in T2D (Fig. 5C). Uncoupling protein 3 (UCP3) protein levels were increased fivefold in T2D hearts compared with control hearts, but molidustat treatment decreased UCP3 levels by 88% in T2D (Fig. 5D). There was a significant interaction term for fatty acid translocase (FAT/CD36) protein levels between diabetes and molidustat treatment (Fig. 5E), decreasing in T2D hearts treated with molidustat, which mirrored the changes in the other PPARα-responsive proteins.
Figure 5.
Molidustat decreases cardiac fatty acid metabolism in T2D. Cardiac fatty acid oxidation rates in control and diabetic rats, with and without molidustat treatment (A). Myocardial triglyceride concentrations, MCAD activity, uncoupling protein 3 (UCP3) protein, and fatty acid translocase (FAT/CD36) protein within the myocardium of control and diabetic rats, with and without molidustat treatment (B–E). *P < 0.05 vs. control; #P < 0.05 vs. diabetic untreated; γP < 0.05 significant interaction between disease and drug treatment. a.u, arbitrary units.
Molidustat Improves Post-Ischemic Function in Diabetes
Molidustat treatment had no effect on baseline cardiac function, with no change in rate pressure product (Fig. 6A), heart rate, or developed pressure (Supplementary Fig. 3C and D) under normal aerobic perfusion conditions. Hearts were then challenged with 30 min of low-flow ischemia, followed by 25 min of reperfusion. T2D hearts had significantly decreased post-ischemic recovery compared with control hearts, as shown by a 20% decrease in rate pressure product during reperfusion (Fig. 6B). Molidustat treatment significantly increased reperfusion recovery by 27% in T2D rats, back to control levels (Fig. 6B and C). Molidustat caused small increases in heart rate and developed pressure during reperfusion, which culminated in the significant improvement in rate pressure product in T2D rats (Fig. 6D and E). Thus, molidustat restored blunted HIF stabilization and downstream signaling in both human cardiomyocytes and rodent hearts, to improve cardiac substrate metabolism and post-ischemic functional recovery in T2D.
Discussion
The diabetic heart has impaired HIF1α stabilization, which subsequently blunts critical metabolic adaptations to hypoxia, contributing to decreased cardiac function following myocardial ischemia. Molidustat, a HIF stabilizer developed for the treatment of anemia associated with chronic kidney disease, provides a potential pharmacological route to promote HIF signaling in the diabetic heart, thereby improving metabolism and post-ischemic function. Here we show for the first time that molidustat can stabilize HIF1α in hiPSC-CM, and can overcome the inhibitory effect of insulin resistance on hypoxic signaling. In a rodent model of T2D we further show that molidustat can correct cardiac metabolism by improving glucose metabolism and suppressing fatty acid metabolism. This metabolic remodeling results in improved cardiac recovery following ischemia in T2D. Thus, repurposing molidustat may be beneficial for metabolism and function in the diabetic heart.
Suppression of HIF Signaling by Diabetes
We previously showed that insulin resistance suppresses HIF1α stabilization and downstream hypoxic adaptation in rodent cardiomyocytes (22). This is due to elevated fatty acids in diabetes suppressing myocardial succinate concentrations, as succinate and fumarate inhibit the PHD enzymes necessary to promote HIF stabilization (22). Here we demonstrate impaired HIF stabilization and hypoxic signaling in human insulin-resistant cardiomyocytes, demonstrating translation to the human disease state. We have shown in the heart, in contrast to fibroblasts and retinal cells (41,42), that the driver for HIF dysfunction is specifically the hyperlipidemia—rather than the hyperinsulinemic or hyperglycemic state in diabetes (22). As highlighted by Catrina and Zheng (43), this divergence between tissues is likely due to the differential effects of diabetes on glycolysis in these tissues, with the T2D heart presenting with decreased glucose metabolism and decreased intracellular glucose concentrations (18,35).
Impaired adaptation to hypoxia has profound effects in cardiac disease, as genetic models of HIF1α deletion exhibited impaired function during pressure overload hypertrophy (8), whereas HIF1α overexpression resulted in improved post-MI function and reduced infarct size (6,7). Determining that insulin resistance blunts HIF activation provides a mechanism to explain decreased VEGF and collateral vessel development seen in patients with diabetes (25,26), with the former identified as a seminal event in the development of diabetic cardiomyopathy (44). However, given the far-reaching effects of the HIF transcription factor, regulating many hundreds of different genes, the effects of impaired HIF stabilization in response to hypoxia would be far-reaching, affecting metabolism, cell growth and death, oxygen homeostasis, iron homeostasis, and vasomotor regulation. Thus, impaired HIF signaling in diabetes would impair the ability of the heart to adapt following MI and would result in accelerated progression into heart failure. In agreement, Stone et al. (24) reported that patients with diabetes have a 72% increase in the progression to heart failure 3 months after MI.
Molidustat Can Restore HIF Signaling in Human Cardiomyocytes
Molidustat (BAY85-3934) is an orally bioavailable triazole-based PHD inhibitor that is undergoing phase III clinical trials to treat anemia in patients with chronic kidney disease (28,29,31). Molidustat confers various benefits over other clinical PHD inhibitors in development, as it has a higher stability upon binding, increased potency, and greater selectivity for PHD enzymes (30,32). Despite its development to target the kidney, we show here that molidustat can robustly stabilize HIF1α in human cardiomyocytes and induce downstream signaling across several HIF targets in the heart. Furthermore, molidustat can overcome the inhibition of HIF signaling induced by insulin resistance, thus providing a mechanism to correct the hypoxic signaling defects associated with diabetes. Therefore, molidustat is an effective compound to further explore the clinical translatability of HIF stabilization in the diabetic heart.
Molidustat Reprograms Substrate Metabolism in Diabetes
Cardiac metabolism is dysregulated in T2D, with decreased dependence on glucose and increased dependence on fatty acid metabolism. This metabolic shift toward greater fat use is associated with contractile dysfunction and impaired recovery post-ischemia (20,35). Here we show that molidustat treatment restores the diabetes-induced suppression of glucose metabolism in the heart. HIF1α upregulates enzymes and proteins involved in glucose uptake, glycolysis, and lactate production, which resulted in an increase in both glycolytic flux within the heart and lactate efflux from the heart in the molidustat-treated diabetic animals. Concomitantly, molidustat decreased fatty acid oxidation and triglyceride accumulation within the myocardium in diabetic rats, reducing the dependency on fatty acids for ATP generation in diabetes. PPARα, the master transcription factor controlling fatty acid metabolism, has been shown to be downregulated in response to HIF stabilization (12,13), decreasing the transcription of PPARα target genes in hypoxia. The benefit of targeting PPARα in diabetes is that it corrects both fatty acid oxidation and the lipotoxicity associated with lipid overload within the cytosol, which are hallmarks of the pathophysiology of the diabetic heart. Molidustat also restored the impaired mitochondrial pyruvate oxidation observed in diabetic hearts, which was initially counterintuitive given that in cells HIF1α increases pyruvate dehydrogenase kinase (PDK)1, the suppressor of pyruvate dehydrogenase (PDH) (45). However, we showed previously that PDK1 is not regulated by hypoxia in the heart (46) (Supplementary Fig. 3D). Therefore, the increased pyruvate oxidation is most likely due to the correction of fatty acid metabolism, removing the allosteric inhibition on PDH activity.
Several preclinical metabolic therapies have been proposed to treat the diabetic heart, specifically targeting glucose or fatty acid metabolic pathways. The reciprocal relationship between these two substrate pathways was described by Randle et al. (47), whereby as metabolism of one fuel is increased the other is decreased due to intracellular cross talk between pathways. Targeting HIF as an approach to correct metabolism has an additional advantage over these alternative metabolic therapies, as it transcriptionally regulates both glucose and fatty acid metabolism simultaneously, directly upregulating glucose and downregulating fatty acid metabolism. Thus, molidustat-dependent HIF stabilization has the added advantage as a metabolic regulator by directly changing both metabolic pathways, in addition to the endogenous Randle cycle, which will further operate between pathways.
Molidustat Improves Recovery Post-Ischemia
Here we have shown that molidustat treatment shows functional benefit for the diabetic heart in the setting of ischemia by improving recovery at reperfusion. We postulate that improved glycolytic flux in the diabetic heart from molidustat treatment primes the heart to be able to tolerate ischemia to a greater extent, due to its ability to generate ATP under oxygen-restricted conditions. However, given the far-reaching targets for HIF and the integrated nature of metabolism, it is likely that the benefit is the culmination of multiple pathways. We previously showed a similar functional improvement in diabetes using the nonspecific HIF stabilizer dimethyloxallyl glycine, corroborating our findings here with a more selective and clinically relevant compound (22). The work presented here highlights the potential to repurpose molidustat as a cardiac therapy for patients with diabetes. Given that cardiovascular disease is the leading cause of mortality in individuals with diabetes, and that even with optimal managed risk factors (glucose, blood pressure, cholesterol) people with T2D still have a 21% increased risk of cardiovascular disease (27), there is an unmet need for treatments for the heart in diabetes.
Molidustat did not modify systemic markers of diabetes in our early-stage T2D model, as animals remained hyperglycemic, with increased adiposity and elevated hepatic triglycerides (as a marker for fatty liver disease). However, it did raise blood hematocrit in the diabetic animals, in line with the drug’s development as a treatment for anemia. Though anemia is not a facet of our early-stage model of T2D, people with diabetes commonly have anemia associated with diabetic kidney disease—estimated at one in four patients with diabetes also being anemic (48). Thus, while this study was designed to assess molidustat for the treatment of the diabetic heart, there is potential that this compound could serve as a pan-complication therapy for diabetes.
In conclusion, pharmacological HIF1α stabilization with molidustat corrects the blunted HIF response to hypoxia caused by insulin resistance in both human and rodent cardiomyocytes. In vivo this corrects the abnormal metabolic phenotype of the diabetic heart, resulting in improved post-ischemic contractile recovery.
Article Information
Acknowledgments. The authors thank Matthew Kerr (University of Oxford), Noelia Diaz Morales (University of Valencia), David Hauton (University of Oxford), and Javier Gilbert Jaramillo (University of Oxford) for technical assistance.
Funding. This work was funded by grants from the British Heart Foundation (FS/17/58/33072, FS/14/65/31292, and FS/19/61/34900) and the Rosetrees Trust (PGS19-2/10121).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.d.L.S.F., U.P., K.M.J.H.D., C.N.M.A., M.C.-G., E.M., and L.C.H. researched data. K.M.J.H.D., C.N.M.A., and L.C.H. wrote the manuscript. D.J.T., C.A.C., and L.C.H. contributed to discussion and edited the manuscript. L.C.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
M.d.L.S.F. and U.P. contributed equally.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16539774.
References
- 1. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 1995;270:1230–1237 [DOI] [PubMed] [Google Scholar]
- 2. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995;92:5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998;12:149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001;107:43–54 [DOI] [PubMed] [Google Scholar]
- 5. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 2004;5:343–354 [DOI] [PubMed] [Google Scholar]
- 6. Jatho A, Zieseniss A, Brechtel-Curth K, et al. Precisely tuned inhibition of HIF prolyl hydroxylases is key for cardioprotection after ischemia. Circ Res 2021;128:1208–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kido M, Du L, Sullivan CC, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 2005;46:2116–2124 [DOI] [PubMed] [Google Scholar]
- 8. Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007;446:444–448 [DOI] [PubMed] [Google Scholar]
- 9. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 1994;269:23757–23763 [PubMed] [Google Scholar]
- 10. Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem 1995;270:29083–29089 [DOI] [PubMed] [Google Scholar]
- 11. Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem 1995;270:21021–21027 [DOI] [PubMed] [Google Scholar]
- 12. Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem 2001;276:27605–27612 [DOI] [PubMed] [Google Scholar]
- 13. Belanger AJ, Luo Z, Vincent KA, et al. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun 2007;364:567–572 [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Ma Z, Zhao C, et al. HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol Lett 2014;226:117–123 [DOI] [PubMed] [Google Scholar]
- 15. Mansor LS, Mehta K, Aksentijevic D, et al. Increased oxidative metabolism following hypoxia in the type 2 diabetic heart, despite normal hypoxia signalling and metabolic adaptation. J Physiol 2016;594:307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hafstad AD, Solevåg GH, Severson DL, Larsen TS, Aasum E. Perfused hearts from type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol 2006;290:H1763–H1769 [DOI] [PubMed] [Google Scholar]
- 17. Mazumder PK, O’Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366–2374 [DOI] [PubMed] [Google Scholar]
- 18. McGill JB, Peterson LR, Herrero P, et al. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol 2011;18:421–429; quiz 432–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–2196 [DOI] [PubMed] [Google Scholar]
- 20. Le Page LM, Rider OJ, Lewis AJ, et al. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 2015;64:2735–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol 2011;50:598–605 [DOI] [PubMed] [Google Scholar]
- 22. Dodd MS, Sousa Fialho MDL, Montes Aparicio CN, et al. Fatty acids prevent hypoxia-inducible factor-1α signaling through decreased succinate in diabetes. JACC Basic Transl Sci 2018;3:485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Timm KN, Perera C, Ball V, et al. Early detection of doxorubicin-induced cardiotoxicity in rats by its cardiac metabolic signature assessed with hyperpolarized MRI. Commun Biol 2020;3:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone PH, Muller JE, Hartwell T, et al.; The MILIS Study Group . The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J Am Coll Cardiol 1989;14:49–57 [DOI] [PubMed] [Google Scholar]
- 25. Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 2002;105:373–379 [DOI] [PubMed] [Google Scholar]
- 26. Marfella R, Esposito K, Nappo F, et al. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes 2004;53:2383–2391 [DOI] [PubMed] [Google Scholar]
- 27. Wright AK, Suarez-Ortegon MF, Read SH, et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings. Circulation 2020;142:1925–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Böttcher M, Lentini S, Arens ER, et al. First-in-man-proof of concept study with molidustat: a novel selective oral HIF-prolyl hydroxylase inhibitor for the treatment of renal anaemia. Br J Clin Pharmacol 2018;84:1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol 2019;14:28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beck H, Jeske M, Thede K, et al. Discovery of molidustat (BAY 85-3934): a small-molecule oral HIF-prolyl hydroxylase (HIF-PH) inhibitor for the treatment of renal anemia. ChemMedChem 2018;13:988–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One 2014;9:e111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeh TL, Leissing TM, Abboud MI, et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci (Camb) 2017;8:7651–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013;12:127–137 [DOI] [PubMed] [Google Scholar]
- 34. Lopez CA, Al-Siddiqi HHAA, Purnama U, et al. Physiological and pharmacological stimulation for in vitro maturation of substrate metabolism in human induced pluripotent stem cell-derived cardiomyocytes. Sci Rep 2021;11:7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mansor LS, Sousa Fialho MDL, Yea G, et al. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc Res 2017;113:737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mansor LS, Gonzalez ER, Cole MA, et al. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc Diabetol 2013;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heather LC, Cole MA, Lygate CA, et al. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 2006;72:430–437 [DOI] [PubMed] [Google Scholar]
- 38. Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 2000;41:613–627 [DOI] [PubMed] [Google Scholar]
- 39. Heather LC, Cole MA, Tan JJ, et al. Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res Cardiol 2012;107:268. [DOI] [PubMed] [Google Scholar]
- 40. Luiken JJ, Koonen DP, Willems J, et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 2002;51:3113–3119 [DOI] [PubMed] [Google Scholar]
- 41. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 2008;105:19426–19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bento CF, Fernandes R, Ramalho J, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One 2010;5:e15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Catrina SB, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021;64:709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoon YS, Uchida S, Masuo O, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 2005;111:2073–2085 [DOI] [PubMed] [Google Scholar]
- 45. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006;3:177–185 [DOI] [PubMed] [Google Scholar]
- 46. Le Page LM, Rider OJ, Lewis AJ, et al. Assessing the effect of hypoxia on cardiac metabolism using hyperpolarized 13 C magnetic resonance spectroscopy. NMR Biomed 2019;32:e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 48. Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 2003;26:1164–1169 [DOI] [PubMed] [Google Scholar]