Abstract
A 64-year-old Japanese man presented with dyspnea and shortness of breath during exertion. Chest computed tomography revealed bilateral pleural effusion. He was drowsy because of CO2 storage and died due to ventilatory impairment. His past medical history included a thymectomy and adjuvant radiotherapy with thymoma. He had undergone cardiac surgery and permanent pacemaker implantation. The autopsy examination revealed extensive bilateral pleural adhesions and diffuse visceral pleural thickening. An inspection of multiple lung sections failed to detect any asbestos body formation or mesothelioma. The patient's pleural effusion and diffuse pleural thickening may have exacerbated after cardiac surgery. In this case, the progression and pathophysiology of the pleural thickening could be traced by imaging and an autopsy, and we were able to estimate the factors that exacerbated the pleural thickening and ventilation impairment.
Keywords: Diffuse pleural thickening, Pleural effusion, Cardiac surgery
Introduction
Diffuse pleural thickening (DPT) refers to extensive fibrosis of the visceral pleura with frequent adhesions to the parietal pleura. It is usually the sequel to an exudative effusion and develops in patients with historical asbestos exposure [1]. Pleural thickening has been suggested in some patients with immunological disease, infection, hemothorax, specific medications, malignancy, post-coronary artery bypass grafting (CABG), and uremic pleurisy. Although in the present patient's case we initially considered radiation therapy, chemotherapy, and cardiac surgery as the causes of pleural thickening, it became apparent that his prior cardiac surgery might have been the most influential factor in the progression of his DPT. To the best of our knowledge, few reports have reported the clinical course of DPT. We describe our patient's clinical course and the DPT process from multiple viewpoints.
Case report
The patient was a 64-year-old Japanese male. Three years prior to this admission, he had undergone CABG and aortic valve replacement (AVR) and mitral valve replacement (MVR) for multivessel coronary artery disease, severe aortic regurgitation, and moderate mitral stenosis. Two years prior to this admission, a permanent pacemaker had been implanted for the patient's sick sinus syndrome. For approx. 1 year prior to this admission, the patient had experienced dyspnea and shortness of breath during exertion.
At the patient's admission to our emergency room, the following data were obtained: blood pressure, 92/64 mm Hg; pulse, regular at 88 beats/min; respiratory rate, 24/min; percutaneous oxygen saturation (SpO2), 96% on room air; and body temperature, 36.8°C. His heart sounds were mechanical, and there were crackles in both lower lung fields. The abdomen were flat and soft. The patient showed marked thinning due to undernutrition. However, no abnormalities suggestive of neuromuscular disease, collagen disease, or vascular disease were identified. He was a nonsmoker and was not sedentary. He had never been directly or indirectly exposed to asbestos.
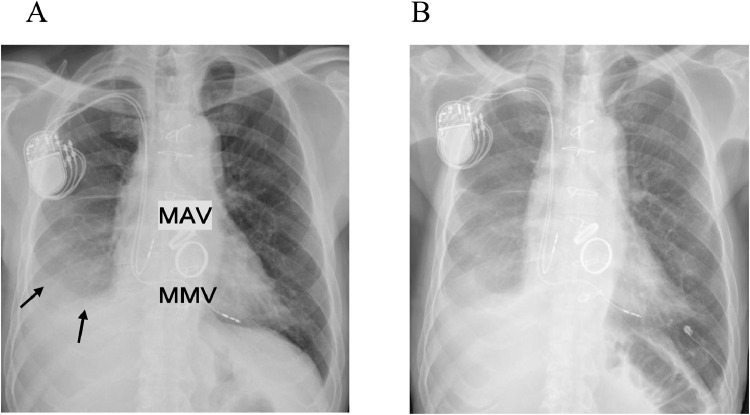
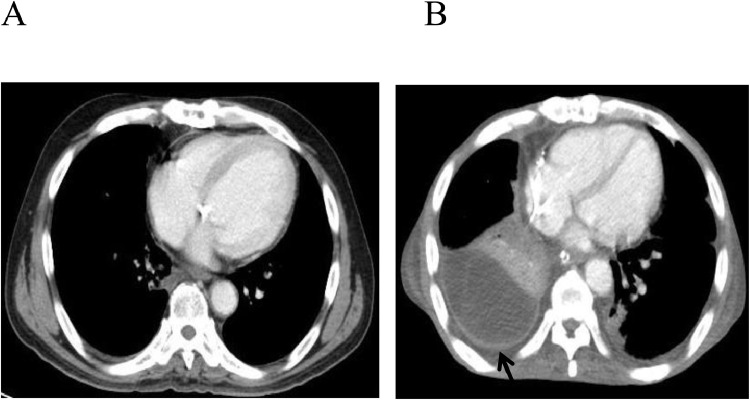
Chest radiography revealed pleural effusion in the patient's lower right and left lung fields, and there was no pulmonary congestion (Fig. 1A). Electrocardiography showed sinus rhythm and T-wave flattening. Chest computed tomography (CT) showed moderate right pleural effusion and mild left pleural effusion (Fig. 2B). A right thoracentesis was performed, but the fluid was not sufficiently drained. A left thoracentesis was performed; exudative pleural effusion with a total albumin level at 3.3 mg/dL, glucose at 114 mg/dL, and LDH at 210 IU/L was observed.
Fig. 1.
(A) Chest X-ray taken after the patient's cardiac surgery 30 years prior to this admission, showing density indicating right-sided and left-sided pleural effusion. (B) At this admission: chest X-ray showing the placement of a drainage tube (DT) in the left thoracic cavity. Arrows: pleural effusion. MAV: mechanical aortic valve, MMV: mechanical mitral valve.
Fig. 2.
(A) Chest CT from before the patient's earlier cardiac surgery. (B) CT revealing moderate pleural effusion and DPT in the posterior right lung after that cardiac surgery. Arrow: pleural thickening.
The pleural effusion was drained to 920 mL. No malignant cells were detected, and the adenosine deaminase (ADA) level was within the normal range. A chest CT examination revealed diffuse bilateral pleural thickening (Fig. 2B), and the retention of right pleural effusion after the patient's earlier cardiac surgery (3 years prior to this admission). In the CT examination of the patient conducted 3 years earlier, no pleural thickening or pleural effusion was detected (Fig. 2A). Respiratory function tests performed before the patient' prior cardiac surgery measured 62% vital capacity (VC%) and 82% forced expiratory volume in 1 s (FEV1.0%).
The left pleural effusion then increased, and on the 29th day of hospitalization, a drainage tube was placed in the left thoracic cavity (Fig. 1B). Between 100 and 300 mL of pleural effusion was drained per day. On the 68th day of hospitalization, the patient had impaired consciousness. A blood gas test revealed PCO2 at 76 mm Hg and narcosis due to CO2 storage. The patient's respiratory failure was exacerbated due to decreased lung compliance.
On the 72nd day of hospitalization, ascites increased, and chylous ascites were drained by ascites puncture. The patient died on the 90th day of hospitalization. His medical history revealed a maximal thymectomy operation and adjuvant radiotherapy with a diagnosis of thymoma 30 years ago.
The patient's cardiac function was maintained until his death, and considered the cause of death to be the progressive exacerbation of ventilation impairment. He had a bleeding tendency due to continued anticoagulant therapy, and no pleural biopsy was performed before birth.
The pathological dissection revealed diffuse pleural thickening (3 mm) in the lower right lung field plus partial thickening of the left pleura and pericardium and at the lower end of the tracheal branch and at the lower end of the diaphragm (Fig. 3). The replaced artificial mitral valve revealed perivalvular leakage in the septal anterior lesions. The inferior vena cava was observed to have exclusion stenosis at the site penetrating the diaphragm from the outside. The extensive bilateral diffuse thickening of the pleura showed increased fibrous tissue and elastic fibers (Fig. 4). A microscopic examination confirmed the presence of paucicellular hyaline collagen plaques. An inspection of multiple lung sections by light microscopy failed to detect any asbestos body formation or mesothelioma (Fig. 5).
Fig. 3.
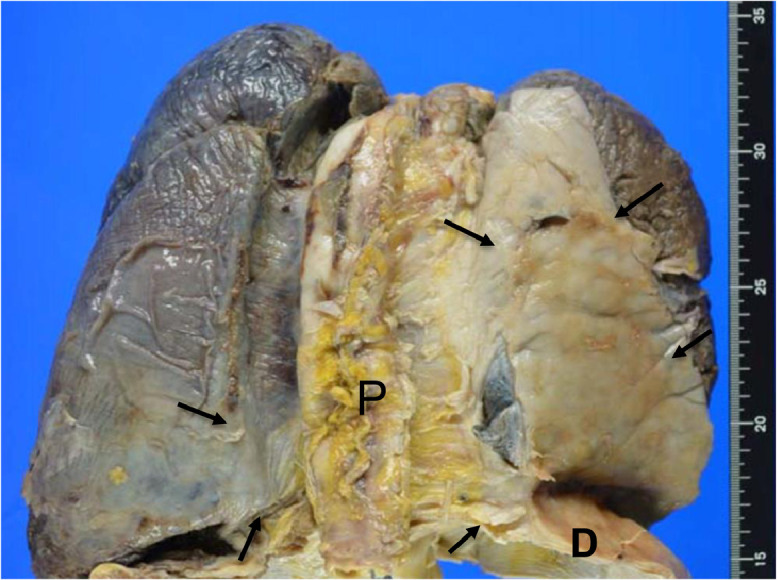
Autopsy findings of the pleura. Gross findings revealed severe thickening of the right pleura, which adhered to the diaphragm and pericardium. Arrows: thickened pleura. D: diaphragm P: pericardium.
Fig. 4.
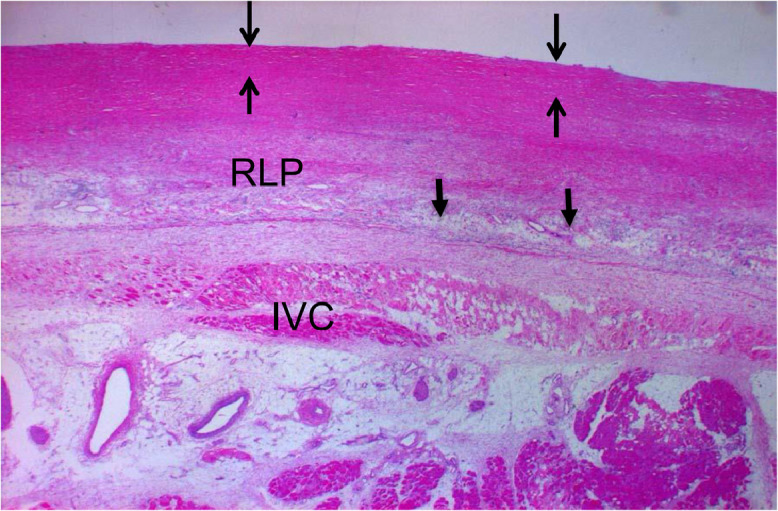
Microscopic findings of the right parietal pleura. Victoria blue staining shows DPT in the right parietal pleura (>3 mm). Arrows: thickened pleura. 1.25 × magnification.
Fig. 5.
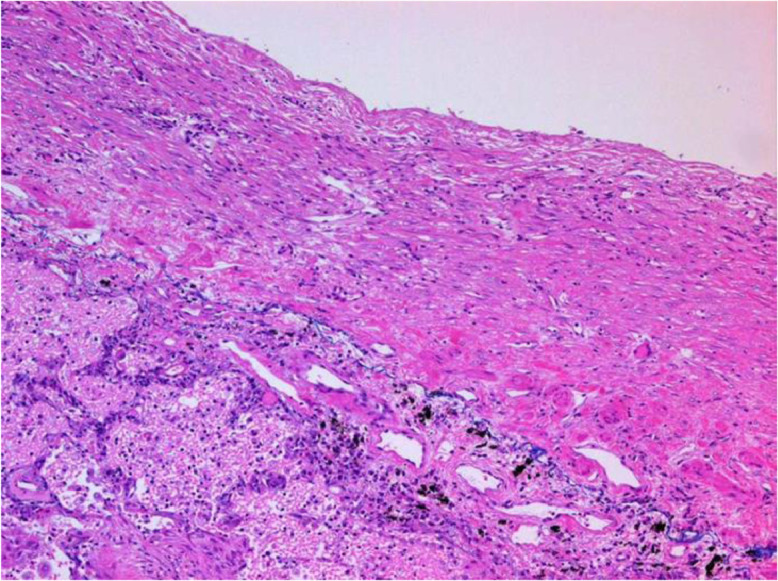
Microscopic findings of the left lower pleura. Victoria blue staining shows paucicellar hyaline collagen plaques, fibrous tissues, and elastic fibers. 12.5 × magnification.
Discussion
In this 64-year-old male with prior cardiac procedures (CABG, AVR, MVR, and permanent pacemaker) and a thymectomy, we speculated that the cause of the rapid progression of restrictive ventilation impairment was the patient's difficulty in sputum production as a result of malnutrition and pulmonary infection. Respiratory muscle exhaustion may have also been a progression factor. Chylous ascites were removed via ascites puncture. We believe that the patient's inferior vena cava stenosis due to external exclusion caused increased venous pressure, which led to the chylous ascites.
There are many reports of asbestos as a cause of diffuse pleural thickening [2], but there are also other reports of pneumonia, empyema, tuberculosis, post-CABG status, radiation, and drugs being responsible for DPT [3]. Evison et al. reported that pleural effusions caused by heart failure can trigger the development of DPT [4]. Our patient's case did not involve heart failure. Huggins et al. explained the pathogenesis of DPT as the extension of the parenchymal fibrotic process to the visceral and parietal surfaces, which involves a complex interaction of inflammatory cells and cytokines with the pleural cavity [5]. Our patient had a history of thymoma removal, chemotherapy, and radiation therapy for thymoma 30 years ago, but the details of the radiation dose and irradiation site were not available. The possibility that the irradiation contributed to the causes and exacerbating factors of this patient's DPT cannot be ruled out.
Shrinkage and pleural changes due to radiation-induced lung disease were present in 82% of the cases examined by Veiga et al. [6] A recurrence of thymoma after 11 years, presenting as DPT, was described by Koksal et al. [7] In our patient's case, there was no thymoma in the pathological findings. Three years prior to the patient's admission to our ER, he had undergone CABG, AVR, and MVR, and we speculate that this cardiac surgery may has been a factor in promoting his DPT. The pleura was clearly thickened after the cardiac surgery, as shown by the chest CT scan at that time (Fig. 2B). The respiratory function tests performed before that cardiac surgery revealed restrictive ventilation impairment. The patient may thus have had potential ventilation impairment before the cardiac surgery; the dates of the imaging were not provided in the images [8]. The patient's radiation therapy and chemotherapy after his thymoma surgery also involved the pleura, and the patient's DPT and ventilation impairment might have been further exacerbated by the cardiac surgery.
Duneesha et al. reported reductions of the vital capacity and total lung capacity in DPT patients, without cost-phrenic angle obliterance [9]. Asbestos-induced respiratory function and pulmonary lesions require >10 years to develop [8], and heart disease and mediastinal disease can be treated with cardiac surgery or radiation therapy [10], which may contribute to the progression of pleural disease. Gary et al. reported that persistent post-CABG effusion can occur, and thatinflammatory changes are replaced by fibrosis [11]. Our patient's pleural abnormalities that were not detected by imaging may have been caused by his radiation therapy and chemotherapy [12], which may have worsened the DPT after cardiac his surgery. Several factors may thus have contributed to this patient's DPT.
Clinicians' familiarity with the present case might be useful for elucidating the etiology and pathology of DPT, ventilation impairment, and intractable pleural effusion.
Ethical approval
Our institution does not require ethical approval for reporting individual cases or case series,
Patient consent
Written informed consent was obtained from a legally authorized representation for anonymized patient information to be published in this article.
Footnotes
Acknowledgments: The author(s) received no financial support for research, authorship, and/or publication of this article. We thank Dr. Iwao Nakazato for the pathology image.
Competing Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.JeeBurn V, Stenton SC. The presentation and natural history of asbestos-induced diffuse pleural thickening. Occup Med. 2012;62:266–268. doi: 10.1093/occmed/kqs028. [DOI] [PubMed] [Google Scholar]
- 2.Greillier L, Astoul P. Mesothelioma and asbestos-related pleural diseases. Respiration. 2008;76:1–15. doi: 10.1159/000127577. [DOI] [PubMed] [Google Scholar]
- 3.Miles SE, Sandrini A, Johonson AR, Yates DH. Clinical consequences of asbestos-related diffuse pleural thickening: a review. J Occup Med Toxicol. 2008;3:20. doi: 10.1186/1745-6673-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evison M, Barbet P. Diffuse pleural thickening following heart failure-related pleural effusion in an asbestos exposed patient. Int J Occup Environ Health. 2015;21:169–171. doi: 10.1179/2049396714Y.0000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huggins JT, Sahn SA. Causes and management of pleural fibrosis. Respirology. 2004;9:441–447. doi: 10.1111/j.1440-1843.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Veiga C, Landau D, McClelland JR, Ledeman JA, Hawkes D, Janes SM. Long-term radiological features of radiation-induced lung damage. Radiother Oncl. 2018;126(2):300–306. doi: 10.1016/j.radonc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Köksal D, Bayiz H, Gülgösteren M, Basay N, Mutluay N, Boyagi E. Recurrence of thymoma after 11 years presenting as diffuse pleural thickening. Tuberk Toraks. 2012;60:62–65. doi: 10.5578/tt.1993. [DOI] [PubMed] [Google Scholar]
- 8.Corris PA, Best JJK, Gibson GJ. Effects of diffuse pleural thickening on respiratory mechanics. Eur Respir J. 1988;1:248–252. PMID: 3384077. [PubMed] [Google Scholar]
- 9.Duneeha DF, Anthony E, Louise S, Jason v, Michael D, Nick AM. The physiological consequences of different distributions of diffuse pleural thickening on CT imaging. Br J Radiol. 2017;90:1–7. doi: 10.1259/bjr.20170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmi RR, Chandrasekharan G. Pulmonary injury associated with radiation therapy-Assessments, complications, and therapeutic targets. Biomed Pharmacother. 2017;89:1092–1104. doi: 10.1016/j.biopha.2017.02.106. [DOI] [PubMed] [Google Scholar]
- 11.Lee YCG, McDonald EC, Nesbitt LC, Marceio ACV, Ely KA, Light RW. Symptomatic persistent post-coronary bypass graft pleural effusions requiring operative treatment. Chest. 2001;119:795–800. doi: 10.1378/chest.119.3.795. [DOI] [PubMed] [Google Scholar]
- 12.Antony VB. Drug-induced pleural disease. Clin Chest Med. 1998;19(2):331–340. doi: 10.1016/s0272-5231(05)70080-0. [DOI] [PubMed] [Google Scholar]