Abstract
NOD-like receptor (NLR) family pyrin domain-containing-3 (NLRP3) inflammasome is implicated in inflammation-associated diseases such as multiple sclerosis, Parkinson’s disease, and stroke. Targeting the NLRP3 inflammasome is beneficial to these diseases, but few NLRP3 inflammasome-selective inhibitors are identified to date. Essential oils (EOs) are liquid mixtures of volatile and low molecular-weight organic compounds extracted from aromatic plants, which show various pharmacological activities, including antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory properties. In this study we screened active ingredients from essential oils, and identified 1,2,4-trimethoxybenzene (1,2,4-TTB) as a selective NLRP3 inflammasome inhibitor. We showed that 1,2,4-TTB (1 mM) markedly suppressed nigericin- or ATP-induced NLRP3 inflammasome activation, thus decreased caspase-1 activation and IL-1β secretion in immortalized murine bone marrow-derived macrophages (iBMDMs) and in primary mouse microglia. Moreover, 1,2,4-TTB specifically inhibited the activation of NLRP3 inflammasome without affecting absent in melanoma 2 (AIM2) inflammasome activation. We further demonstrated that 1,2,4-TTB inhibited oligomerization of the apoptosis-associated speck-like protein containing a CARD (ASC) and protein–protein interaction between NLRP3 and ASC, thus blocking NLRP3 inflammasome assembly in iBMDMs and in primary mouse macrophages. In mice with experimental autoimmune encephalomyelitis (EAE), administration of 1,2,4-TTB (200 mg · kg−1 · d−1, i.g. for 17 days) significantly ameliorated EAE progression and demyelination. In conclusion, our results demonstrate that 1,2,4-TTB is an NLRP3 inflammasome inhibitor and attenuates the clinical symptom and inflammation of EAE, suggesting that 1,2,4-TTB is a potential candidate compound for treating NLRP3 inflammasome-driven diseases, such as multiple sclerosis.
Keywords: essential oils; 1,2,4-Trimethoxybenzene; NLRP3 inflammasome; ASC; experimental autoimmune encephalomyelitis
Introduction
The NOD-like receptor (NLR) family pyrin domain-containing-3 (NLRP3) inflammasome is a multiple protein complex that is composed of NLRP3, the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC), and the cysteine protease caspase-1 (Casp-1) [1, 2]. After activation, NLRP3 binds to ASC and then recruits Casp-1 to form the inflammasome complex, which cleaves the proinflammatory cytokines pro-interleukin (IL)-1β and pro-IL-18, resulting in the maturation and secretion of IL-1β and IL-18 [3–5]. Thus, the NLRP3 inflammasome plays a crucial role in innate immunity and the inflammatory response. Emerging evidence has shown that aberrant NLRP3 inflammasome activation is implicated in several inflammation-associated diseases, including atherosclerosis, colitis, diabetes, Alzheimer’s disease, and multiple sclerosis [6–10]. Knockout of individual NLRP3 inflammasome components and NLRP3 inflammasome inhibitors have been demonstrated to inhibit inflammasome assembly or activity, thus ameliorating NLRP3-related diseases [11–15]. Although it has been suggested that targeting the NLRP3 inflammasome has multiple beneficial effects in the treatment of inflammation-associated diseases, currently available treatments for NLRP3-related diseases in the clinic are limited. The available therapeutic agents, including the recombinant IL-1 receptor antagonist anakinra, the neutralizing IL-1β antibody canakinumab and the soluble decoy IL-1 receptor rilonacept, target IL-1β but not the NLRP3 inflammasome itself and therefore might cause side effects related to the other biological functions of IL-1β [16, 17]. Recently, several NLRP3 inflammasome-specific inhibitors (e.g., MCC950, CY-09, and oridonin) with potent therapeutic effects in various animal models of inflammatory diseases have been identified [18–21]; however, their efficacy in the clinic remains unknown. Therefore, identifying and developing NLRP3 inflammasome-specific candidate inhibitors is urgently required for the treatment of NLRP3-related diseases.
Essential oils (EOs) are liquid mixtures of volatile and low molecular-weight organic compounds extracted from aromatic plants [22]. EOs have been used for medical and commercial purposes owing to their various biological activities, including antibacterial, antifungal, antiviral and antioxidant properties [23–26]. A growing number of studies have revealed the anti-inflammatory effects of EOs in different models of inflammation in vitro and in vivo [27–30]. However, the mechanism by which EOs exert their anti-inflammatory effects remains incompletely understood. As the components of EOs are complex, the active compound(s) that exhibit anti-inflammatory properties must be identified. In the present study, we first established a system to screen drugs targeting the NLRP3 inflammasome to identify the active compound(s) of different components of EOs that directly and specifically inhibit NLRP3 inflammasome activity. 1,2,4-Trimethoxybenzene (1,2,4-TTB), a major component of paulownia EO, was identified by unbiased drug screening as a selective NLRP3 inflammasome inhibitor. We found that 1,2,4-TTB decreased the aggregation of ASC and its interaction with NLRP3 and disrupted the assembly and activity of the NLRP3 inflammasome. Furthermore, 1,2,4-TTB treatment in vivo significantly prevented the development and progression of experimental autoimmune encephalomyelitis (EAE), a mouse model of chronic neuroinflammation disease.
Collectively, the current results revealed that 1,2,4-TTB selectively inhibited the activation of the NLRP3 inflammasome by disrupting the formation of NLRP3 oligomers. By blocking the activation of the NLRP3 inflammasome, 1,2,4-TTB attenuated the symptoms and pathogenesis of EAE, which suggests that 1,2,4-TTB could be a candidate treatment of NLRP3 inflammasome-related diseases, such as multiple sclerosis.
Materials and methods
Mice
C57BL/6 mice were purchased from SPF (Beijing) Biotechnology Co., Ltd. Conventional ASC knock mice were provided by Prof. Feng Shao (National Institute of Biological Sciences, Beijing, China) [31]. Four to five mice were housed per cage on a 12 h light/dark cycle and given unrestricted access to food and water. All experimental animal procedures were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Basic Medical Sciences.
Cell culture
Immortalized murine bone marrow-derived macrophages (iBMDMs) were maintained in Dulbecco’s modified Eagle’s medium (#11965-092, Life Technologies, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, #04-001-1A, Biological Industries, Beit Haemek, Israel) and 1% penicillin–streptomycin solution (#03-031-1B, Biological Industries) at 37 °C in a humidified atmosphere with 5% CO2.
Isolation and culture of primary macrophages and microglia
Primary peritoneal macrophages were isolated from adult wild-type and ASC KO mice. The mice were sacrificed by neck breaking method and immersed in 75% ethyl alcohol for 5 min. Macrophages were collected by washing the peritoneal cavity with 5 mL of ice-cold serum-free RPMI-1640 medium (#C11875500BT, Gibco, Shanghai, China). After centrifugation at 300 × g and 4 °C for 5 min, the cell pellet was resuspended in fresh RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 1% penicillin, and 1% streptomycin and seeded in culture plates for subsequent experiments.
Primary microglia from newborn mice were isolated and cultured as previously described [32].
Western blotting
Western blot analysis was performed as previously described [33]. Briefly, cells or tissues were lysed with RIPA lysis buffer comprising a cocktail of protease and phosphatase inhibitors. Total protein concentrations were measured using the BCA assay, and the proteins were separated by SDS-PAGE and then transferred to a polyvinylidene fluoride membrane (#ISEQ00010, Millipore, Darmstadt, Hessen, Germany). The membrane was blocked with 5% nonfat milk in Tris-buffered saline and incubated with primary antibodies overnight at 4 °C followed by horseradish peroxidase-conjugated secondary antibodies for protein detection. The primary antibodies used in the present study were anti-NLRP3 (#AG-20B-0014, AdipoGen, San Diego, CA, USA, 1:1000 for Western blotting, 2 μg/sample for immunoprecipitation, 1:200 for immunocytochemistry), anti-Casp-1 (#Ag-20B-0042, AdipoGen, 1:1000), anti-ASC (#67824, Cell Signaling Technology, Beverly, MA, USA, 1:1000 for Western blotting, 1:200 for immunochemistry), anti-IL-1β (#AF-401-NA; R&D Systems, Minneapolis, MN, USA, 1:1000), anti-MBP (#78896, Cell Signaling Technology, 1:1000), and anti-β-tubulin (#CW0098A, CWBiotech, Taizhou, JS, China, 1:2000).
Enzyme-linked immunosorbent assay (ELISA) for the detection of IL-1β
The culture medium was collected after cell stimulation and then centrifuged at 12,000 × g and 4 °C for 5 min. The levels of IL-1β (#432604, BioLegend, San Diego, CA, USA) in the supernatants were assayed using ELISA according to the manufacturer’s instructions.
ELISA for the detection of anti-MOG antibody
The MOG33–55 peptide was diluted in sodium bicarbonate buffer (0.1 M, pH 9.0) at 1000 ng/μL and added to a 96-well plate (NuncTM MaxiSorpTM ELISA plates, uncoated, Biolegend) at a volume of 100 μL/well. Then, the plate was incubated at 4 °C overnight and washed three times with PBS. After washing, the plate was blocked with blocking buffer (2% bovine serum albumin and 1× PBS) at 37 °C for 2 h. After blocking, the plate was washed three times with washing buffer (1× PBS and 0.05% Tween 20). Then, the serum was diluted 10 times with blocking buffer and added to the plate at a volume of 100 μL/well.
EAE induction and drug treatment
Six- to eight-week-old C57BL/6 female mice were used to establish the EAE model as previously described. Briefly, each mouse was subcutaneously immunized with 150 μg MOG35–55 peptide (synthesized by ChinaPeptides, Beijing, China) emulsified in complete Freund’s adjuvant (#F5881, Sigma-Aldrich, Shanghai, China) comprising 4 mg/mL heat-killed Mycobacterium tuberculosis (#231141, BD Bioscience, San Jose, CA, USA) and then intraperitoneally injected with 200 ng pertussis toxin (#516560, Sigma-Aldrich) on days 0 and 2. 1,2,4-TTB was diluted in sunflower oil and administered intragastrically (i.g.) at a dosage of 200 mg · kg−1 · d−1 from day 7 after MOG immunization. The same volume of sunflower oil was administered i.g. to the animals in the control group. Body weight and clinical scores were recorded every day post-immunization. Clinical scores were based on a five-point scale: 0, no disability; 1, flaccid tail; 2, single hind leg; 3, hind limb paralysis; 4, quadriplegia; and 5, death.
Immunochemistry
Mice were euthanized with tribromoethanol and then intracardially perfused with ice-cold saline. The lumbosacral spinal cords were fixed with 4% paraformaldehyde (PFA) for at least 48 h and processed for immunochemistry. Paraffin-embedded tissues were cut at a thickness of 5 μm, and the sections were stained with Luxol Fast Blue (#G3242, Solarbio Life Science, Beijing, China) or anti-MBP antibody for the evaluation of demyelination. In some experiments, 4% PFA-fixed tissues were cut at a thickness of 40 μm using a cryostat (#CM3050S, Leica, Buffalo Grove, IL, USA), processed for immunostaining with anti-ASC and anti-MBP antibodies, and subjected to confocal analysis.
Co-immunoprecipitation (Co-IP)
To investigate the effect of 1,2,4-TTB on the protein interaction between NLRP3 and ASC, iBMDMs (10 cm wells, ~107 cells) were primed with lipopolysaccharide (LPS) for 3 h. Then, the cells were pretreated with 1,2,4-TTB or vehicle for 30 min and stimulated with nigericin (Nig, #481990, Sigma-Aldrich) for 1 h. After two washes with ice-cold PBS, the cells were lysed with 1 mL IP buffer supplemented with cocktail protease inhibitor and processed for IP. Briefly, 2 μg anti-NLRP3 antibody was incubated with 40 μL Protein G beads (#10004D, Invitrogen, Shanghai, China) at 4 °C for 2 h with rotation. After three washes with IP buffer, the antibody-conjugated beads were added to the prepared cell lysates and incubated at 4 °C overnight with rotation. Afterwards, the beads were washed four times with IP buffer, eluted with 50 μL 1× SDS loading buffer, and subjected to immunoblotting analysis.
ASC oligomerization assay
Macrophages were exposed to 1 μg/mL LPS for 3.5 h, treated with 1,2,4-TTB for 0.5 h, and then stimulated with Nig for 45 min (for primary macrophages) or 1 h (for iBMDMs). The supernatant was removed, and the cells were rinsed in ice-cold PBS and then lysed with hypotonic lysis buffer (10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM PMSF, and 20 mM Tris; pH 7.5) with end-over-end rotation for 30 min at 4 °C. The lysates were centrifuged at 6000 × g for 8 min at 4 °C, and the supernatants contained the soluble components. The pellets were washed twice in 1 mL ice-cold PBS and resuspended in 500 µL CHAPS buffer (0.1% CHAPS, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM PMSF, and 20 mM Tris; pH 7.5). Disuccinimidyl suberate (DSS, #S1885, Sigma-Aldrich, 2 mM) was added to the resuspended pellets, which were incubated at 37 °C for 45 min with rotation. The samples were then centrifuged at 6000 × g for 15 min at 4 °C. The crosslinked pellets were resuspended in 60 µL sample buffer and boiled at 100 °C for 5 min. Then, oligomeric ASC and NLRP3 levels were analyzed by Western blotting.
Flow cytometry
The mice were sacrificed after being anesthetized, and the spleens were removed and ground into single cells with the plunger of a 2 mL syringe in cold PBS comprising 2% FBS. The cells were then filtered with a 70 μm strainer and harvested by centrifugation for 5 min at 250 × g and 4 °C. After being washed with ice-cold PBS, the cells were counted, and 2 × 106 cells from each sample were used for staining with PE-labeled anti-CD4 (#100408, BioLegend, 1:400), FITC-labeled anti-CD3 (#100204, BioLegend, 1:400), and APC-labeled anti-CD8a (#100712, BioLegend, 1:400) antibodies. After staining, the cells were washed with PBS and analyzed with a BD Aria III flow cytometer.
Real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the spinal cord using TRIzol reagent (#15596026, Invitrogen), and then cDNA was synthesized using a One-Step First-strand cDNA synthesis kit (#AT311-02, Transgen, Beijing, China). SYBR Green-based real-time qPCR (#A304, GenStar, Beijing, China) was performed to measure gene expression. The primer sequences used in the present study were listed in Supplementary Table S2.
Statistical analysis
All data are expressed as the mean ± standard error of the mean of three independent experiments. All statistical analyses were performed using GraphPad Prism software (version 8.0). The significance of differences was assessed by unpaired Student’s t test or one-way or two-way analysis of variance followed by Tukey’s multiple comparisons test. Differences were considered significant at P < 0.05.
Results
Screening of the active compounds of EOs that target the NLRP3 inflammasome
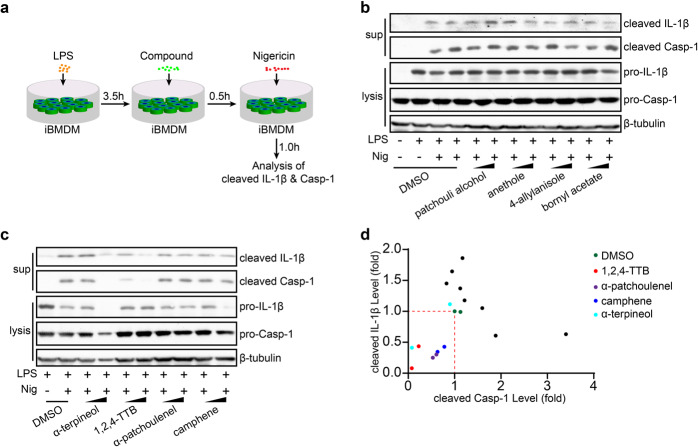
To identify the ingredients of EOs that exert anti-inflammatory effects, we established a potential bioactive compound library composed of the major components of patchouli EO, camphor EO, eucalyptus EO and paulownia flower EO. Then, the effects of these compounds on the activation of the NLRP3 inflammasome were tested by measuring Casp-1 activation and IL-1β secretion. We first determined whether the EO compounds affected the secretion of IL-1β in primary macrophages. Among the 22 EO compounds (Supplementary Table S1), we found that eight significantly suppressed the secretion of IL-1β (Supplementary Fig. S1a). For more stringent screening, iBMDMs were primed with lipopolysaccharide for 3.5 h and then pretreated with individual active compounds of EOs for 0.5 h before being stimulated with nigericin (Nig) (Fig. 1a). The results showed that four of the components, including 1,2,4-TTB (a major component of paulownia EO), α-patchoulenel (a major components of patchouli EO), α-terpineol and camphene (the components of pine EO), exerted potent inhibitory effects on both Casp-1 cleavage and IL-1β secretion in a dose-dependent manner (Fig. 1b–d). Further confirmatory experiments showed that 1,2,4-TTB (the structure of which is shown in Fig. 2a) exhibited the most significant inhibitory effect on NLRP3 inflammasome activation (Fig. 2b). In addition, high doses of α-patchoulenel, α-terpineol, and camphene caused cytotoxicity (Supplementary Fig. S1b–d). Therefore, 1,2,4-TTB is a candidate inhibitor of the NLRP3 inflammasome.
Fig. 1. Screening of NLRP3 inflammasome-targeting natural compounds derived from essential oils.
a The working model of the screening system for NLRP3 inflammasome-targeting natural compounds. b–d iBMDMs pretreated with LPS were then treated with natural compounds (two doses of each compound, 0.5 and 1 mM) from essential oils (EOs) and nigericin (Nig) for 1 h, and the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved caspase-1 (Casp-1) by Western blotting (b, c). The gray values were analyzed by ImageJ software (d).
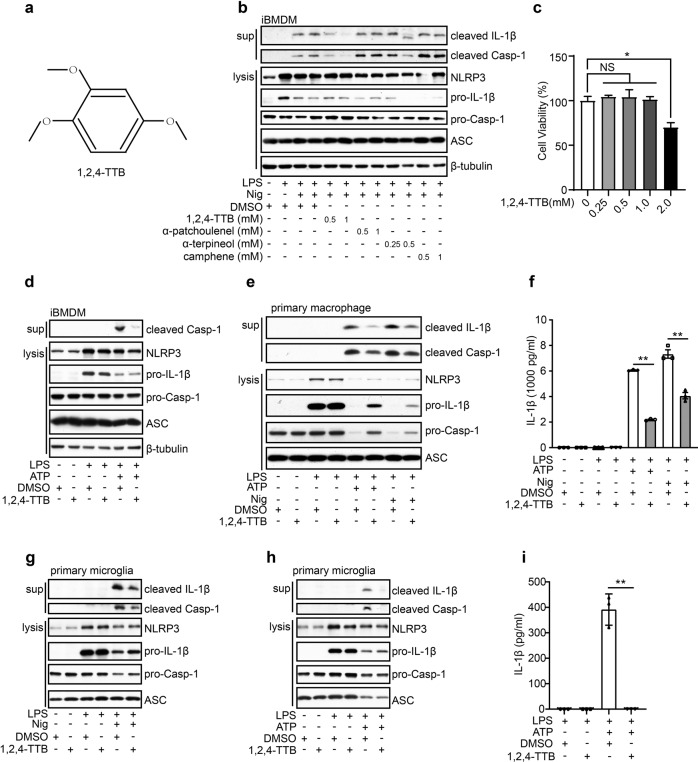
Fig. 2. 1,2,4-TTB inhibits the activation of the NLRP3 inflammasome in vitro.
a Structure of 1,2,4-TTB. b iBMDMs pretreated with LPS for 3.5 h were treated with 1,2,4-TTB, α-patchoulenel, α-terpineol or camphene for 0.5 h and then treated with Nig for another 1 h. The cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting. c iBMDMs were treated with various doses of 1,2,4-TTB for 24 h, and cell viability was assessed by the CellTiter-GLO luminescent assay. d iBMDMs pretreated with LPS were then treated with 1,2,4-TTB and ATP for 1 h, and the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting. e, f Primary macrophages pretreated with LPS were treated with 1,2,4-TTB and Nig or ATP for 45 min, and the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting (e) and ELISA (f). g Primary microglia pretreated with LPS were treated with 1,2,4-TTB and Nig, and then the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting. h, i Primary microglia pretreated with LPS were treated with 1,2,4-TTB and ATP for 45 min, and then the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting (h) and ELISA (i). (* indicates P < 0.05, ** indicates P < 0.01 by One-way or Two-way ANOVA).
1,2,4-TTB selectively inhibits the activation of the NLRP3 inflammasome
To further investigate the pharmacological activity of 1,2,4-TTB, we first examined its toxic effects on iBMDMs. Analysis of cell viability showed that 1,2,4-TTB did not have an obvious toxic effect on cells when the concentration was below 1 mM (Fig. 2c). At 1 mM, 1,2,4-TTB exerted the most significant inhibitory effect on NLRP3 inflammasome activation induced by Nig (Fig. 2b); thus, this dose was used in subsequent experiments. The NLRP3 inflammasome can also be activated by stimuli other than Nig, such as ATP. Therefore, we tested whether 1,2,4-TTB was effective in inhibiting NLRP3 inflammasome activation induced by ATP. ATP-induced Casp-1 activation and IL-1β secretion were significantly suppressed by 1,2,4-TTB in both iBMDMs and primary macrophages (Fig. 2d–f), indicating that 1,2,4-TTB might be a broad-spectrum inhibitor of the NLRP3 inflammasome. We also examined the effect of 1,2,4-TTB on microglia, central nervous system-resident immune cells that mediate neuroinflammation in neurological diseases. Consistent with the results in macrophages, Casp-1 activation and IL-1β maturation were markedly inhibited by 1,2,4-TTB treatment when NLRP3 inflammasome activation was induced in primary microglia by Nig or ATP (Fig. 2g–i). 1,2,4-TTB treatment did not affect the expression of pro-caspase-1 and pro-IL-1β in the cell lysates, suggesting that 1,2,4-TTB might specifically inhibit the activation but not the priming of the NLRP3 inflammasome.
To test this hypothesis, we treated iBMDMs with 1,2,4-TTB 0.5 h before LPS stimulation and then performed qPCR and Western blotting analysis. Consistently, 1,2,4-TTB treatment failed to alter the expression levels of pro-IL-1β, pro-caspase-1, and NLRP3 (Supplementary Fig. S2a–d), demonstrating that 1,2,4-TTB did not suppress LPS-induced priming of the NLRP3 inflammasome. Together, these results show that 1,2,4-TTB selectively inhibited NLRP3 inflammasome activation.
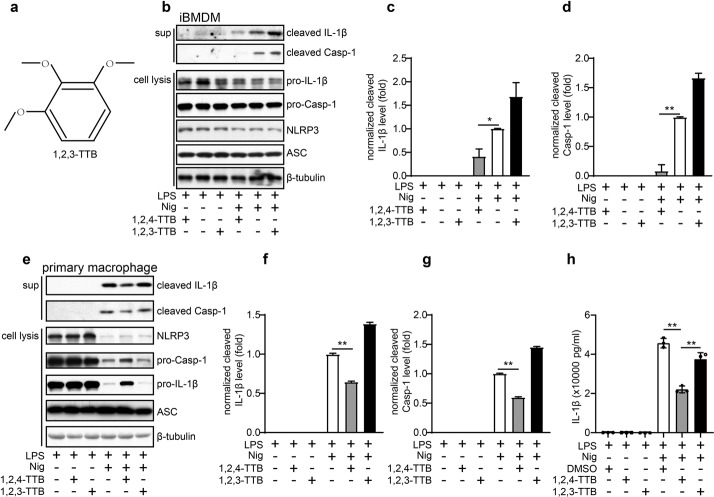
1,2,3-TTB fails to inhibit activation of the NLRP3 inflammasome
To explore whether 1,2,3-TTB (Fig. 3a), an isomer of 1,2,4-TTB, also exhibits anti-NLRP3 inflammasome activity, we performed similar experiments. As expected, we found that 1,2,4-TTB significantly inhibited Nig-induced NLRP3 inflammasome activation in both iBMDMs and primary macrophages, as indicated by decreased Casp-1 cleavage and IL-1β secretion (Fig. 3b–g). In contrast, 1,2,3-TTB failed to alter the activation of Casp-1 and maturation of IL-1β (Fig. 3b–g); therefore, 1,2,3-TTB could not inhibit NLRP3 inflammasome activation. Similarly, ELISA analysis showed that the levels of IL-1β were markedly reduced by 1,2,4-TTB treatment; however, no obvious difference was observed between the 1,2,3-TTB and vehicle (DMSO) groups (Fig. 3h). Together, these data suggest that the structure-dependent activity of 1,2,4-TTB might be required for NLRP3 inflammasome inhibition.
Fig. 3. The effect of TTB on NLRP3 inflammasome activation is structure dependent.
a Structure of 1,2,3-TTB. b, c iBMDMs pretreated with LPS were treated with 1,2,4-TTB/Nig or 1,2,3-TTB/Nig for 1 h, and the cells and supernatants were harvested for analysis of cleaved IL-1β and cleaved Casp-1 expression by Western blotting (b). The gray values of the cleaved IL-1β (c) and cleaved Casp-1 (d) bands were analyzed with ImageJ. e–g Primary macrophages pretreated with LPS were treated with 1,2,4-TTB/Nig or 1,2,3-TTB/Nig for 45 min, and the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting (e). The gray values of the cleaved IL-1β (f) and cleaved Casp-1 (g) bands were analyzed with ImageJ. h Primary macrophages pretreated with LPS were treated with 1,2,4-TTB/Nig or 1,2,3-TTB/Nig for 45 min, and the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β by ELISA. (* indicates P < 0.05, ** indicates P < 0.01 by one-way ANOVA).
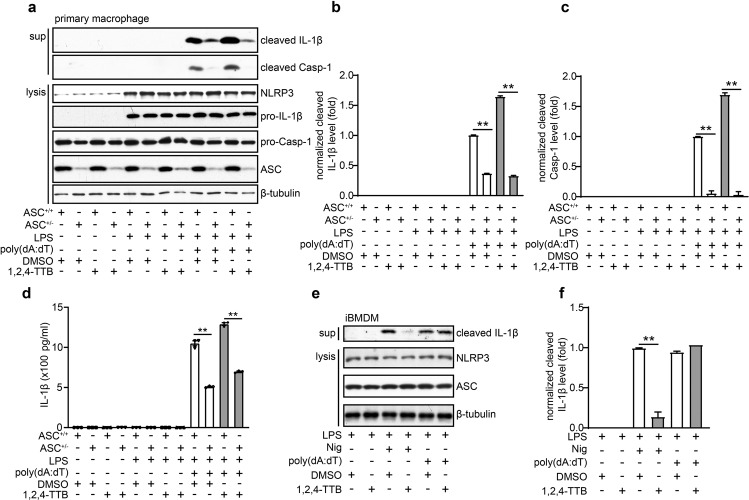
1,2,4-TTB does not inhibit activation of the AIM2 inflammasome
Next, we investigated whether 1,2,4-TTB could inhibit the activation of the absent in melanoma 2 (AIM2) inflammasome induced by the dsDNA analog poly(dA:dT) in primary peritoneal macrophages and iBMDMs. Western blotting (Fig. 4a–c) and ELISA (Fig. 4d) indicated that 1,2,4-TTB did not affect the LPS- and poly(dA:dT)-induced cleavage and release of IL-1β in primary peritoneal macrophages. Consistently, 1,2,4-TTB failed to alter the release of IL-1β induced by LPS and poly(dA:dT) in iBMDMs (Fig. 4e, f). Therefore, 1,2,4-TTB did not inhibit the activation of the AIM2 inflammasome. After activation, both NLRP3 and AIM2 bind to ASC and recruit Casp-1 for inflammasome assembly. ASC heterozygote knockout (ASC+/−) macrophages, which were used as positive controls, displayed significantly decreased caspase-1 activation and IL-1β maturation (Fig. 4a–d). Collectively, these results demonstrate that 1,2,4-TTB does not inhibit AIM2 inflammasome activation.
Fig. 4. 1,2,4-TTB had no significant effect on the activation of the AIM2 inflammasome.
a–c Primary macrophages pretreated with LPS were treated with 1,2,4-TTB/Nig for 45 min or 1,2,4-TTB/poly(dA:dT) for 2 h. Then, the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β and cleaved Casp-1 by Western blotting (a), and the gray values of the cleaved IL-1β (b) and cleaved Casp-1 (c) bands were analyzed with ImageJ. d Primary macrophages pretreated with LPS were treated with 1,2,4-TTB/Nig for 45 min or 1,2,4-TTB/poly(dA:dT) for 2 h, and then the supernatants were harvested for analysis of the expression of cleaved IL-1β by ELISA. e, f iBMDMs pretreated with LPS were then treated with 1,2,4-TTB/Nig for 1 h or 1,2,4-TTB/poly(dA:dT) for 2 h. Then, the cells and supernatants were harvested for analysis of the expression of cleaved IL-1β by Western blotting (e), and the gray values of the cleaved IL-1β (f) bands were analyzed with ImageJ. (* indicates P < 0.05, ** indicates P < 0.01 by two-way ANOVA).
1,2,4-TTB blocks NLRP3 inflammasome assembly
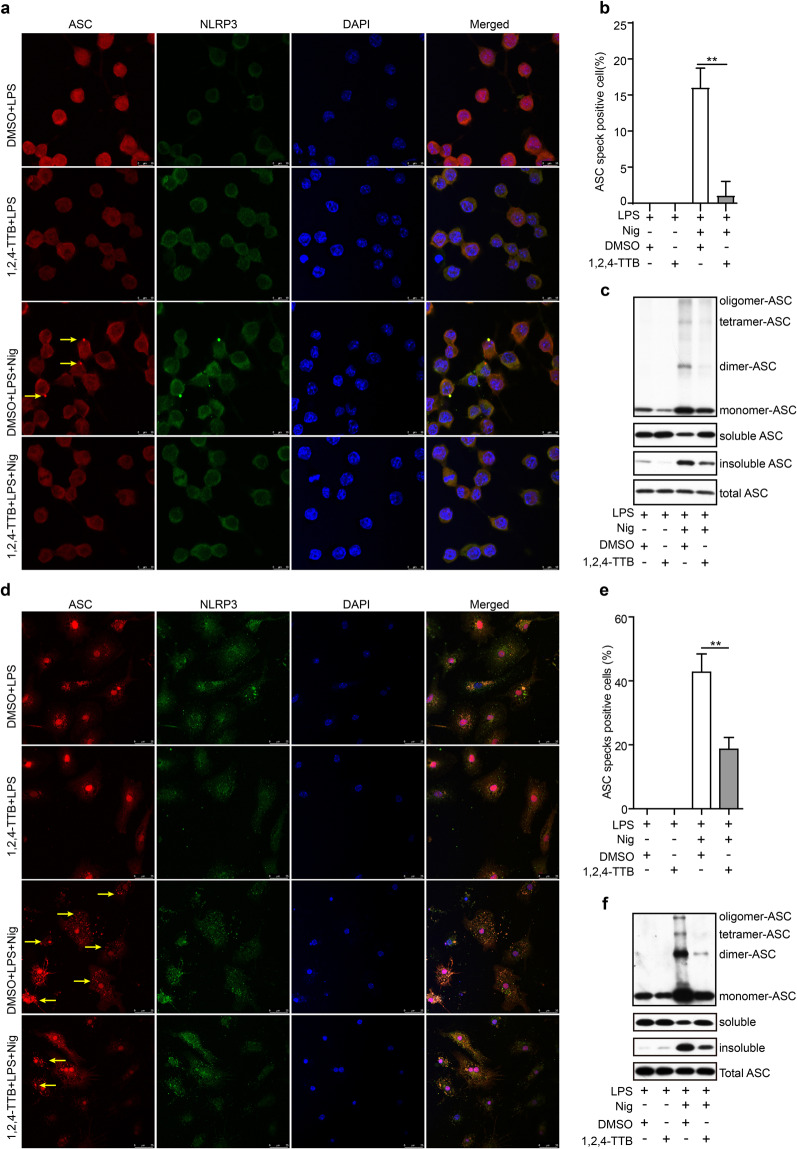
To clarify how 1,2,4-TTB specifically inhibits NLRP3 inflammasome activation, we tested the effect of 1,2,4-TTB on NLRP3 inflammasome assembly, which is required for NLRP3 inflammasome activation. Because 1,2,4-TTB did not alter the expression of ASC in cell lysates (Fig. 4a), we next examined whether it impaired NLRP3-dependent ASC oligomer formation. Immunofluorescence revealed that ASC was condensed into specks after Nig-induced NLRP3 activation in iBMDMs; however, the number of these specks was markedly decreased upon 1,2,4-TTB treatment (Fig. 5a, b). The DSS crosslinking experiment showed that the formation of ASC monomers, dimers, tetramers and higher-order complexes was significantly attenuated by 1,2,4-TTB (Fig. 5c). Correspondingly, while 1,2,4-TTB treatment decreased the level of insoluble ASC (the activated form), it increased the level of soluble ASC (the inactivated form) without obviously changing total ASC protein expression (Fig. 5c), indicating that 1,2,4-TTB inhibits ASC oligomerization during NLRP3 inflammasome activation. In addition, the inhibitory effect of 1,2,4-TTB on speck formation was confirmed in primary microglia (Fig. 5d, e). Consistently, the amount of ASC dimers, tetramers and oligomers formed as a result of NLRP3 activation was reduced in 1,2,4-TTB-treated microglia (Fig. 5f).
Fig. 5. Effect of 1,2,4-TTB on the formation of ASC specks.
a, b iBMDMs pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h. Then, the cells were fixed and stained with a rabbit anti-ASC antibody and mouse anti-NLRP3 antibody (a), and the number of cells containing ASC specks (yellow arrows) was analyzed (b). c iBMDMs pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h, and then the cells were harvested and crosslinked with disuccinimidyl suberate (DSS) for analysis of the oligomerization of ASC. d, e Primary microglia pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h, fixed and stained with a rabbit anti-ASC antibody and mouse anti-NLRP3 antibody (d), and the number of cells containing ASC specks (yellow arrows) was analyzed (e). f Primary microglia pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h, and then the cells were harvested and crosslinked with DSS for analysis of the expression of oligomeric ASC. (* indicates P < 0.05, ** indicates P < 0.01 by Student’s t test).
We next sought to investigate whether 1,2,4-TTB impaired the protein interaction between NLRP3 and ASC. The results of the Co-IP assay showed that the NLRP3-ASC interaction was enhanced upon Nig stimulation but that this change was blocked by 1,2,4-TTB treatment (Fig. 6a, b), suggesting that 1,2,4-TTB might disrupt the binding of ASC to NLRP3 after inflammasome activation. A subsequent study showed that NLRP3 oligomers formed after NLRP3 activation and that this phenomenon was also suppressed by 1,2,4-TTB (Fig. 6c–f). Together, these data demonstrate that 1,2,4-TTB inhibits NLRP3 inflammasome activation by preventing NLRP3 inflammasome assembly in the initial stage.
Fig. 6. 1,2,4-TTB inhibits the protein interactions of NLRP3.
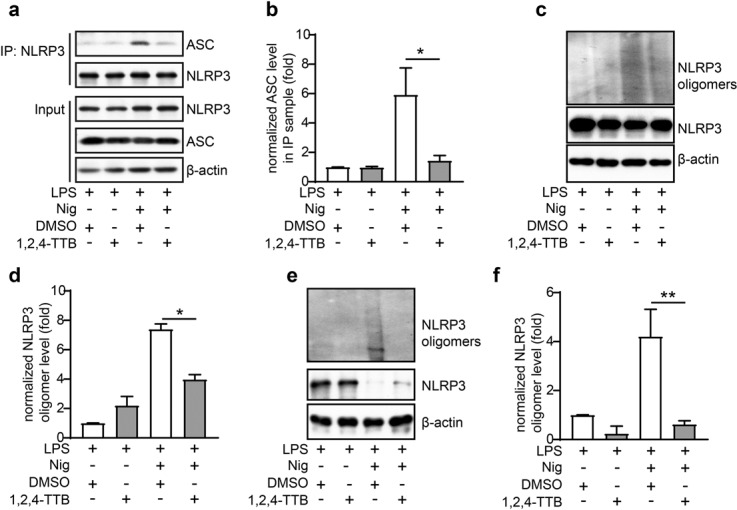
a, b iBMDMs pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h. Then, the cells were harvested, and NLRP3 was immunoprecipitated with an anti-NLRP3 antibody. The protein levels of NLRP3 and ASC were analyzed by Western blotting (a), and the gray values of the ASC bands in the IP samples were analyzed by ImageJ (b). c–f iBMDMs (c, d) and primary macrophages (e, f) pretreated with LPS were treated with 1,2,4-TTB and Nig for 1 h, and then the cells were harvested and crosslinked with DSS for analysis of the oligomerization of NLRP3. The gray values of the NLRP3 oligomer bands were analyzed by ImageJ. (* indicates P < 0.05, ** indicates P < 0.01 by Student’s t test).
1,2,4-TTB ameliorates EAE severity
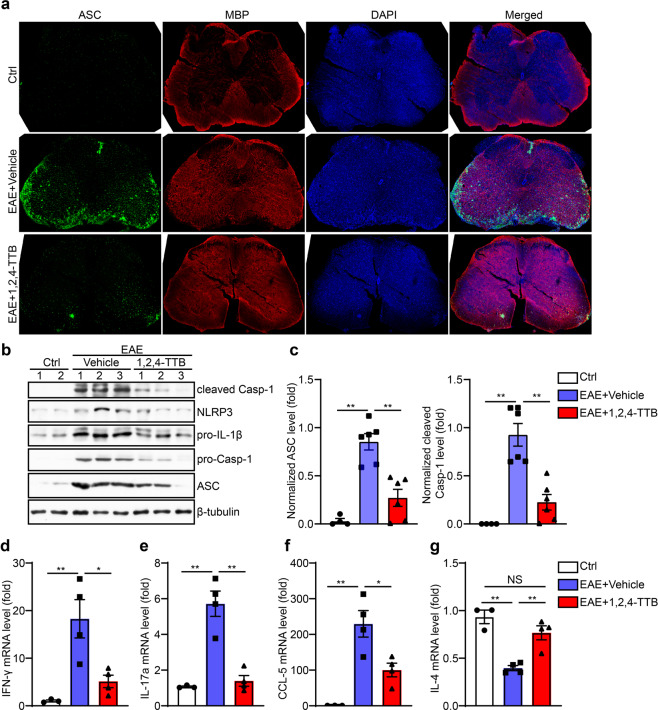
We next evaluated the effect of 1,2,4-TTB in vivo. The EAE mouse model is a well-established model of human multiple sclerosis, an autoimmune disease associated with demyelination, reactive gliosis, and chronic neuroinflammation. Recent evidence has demonstrated that NLRP3 inflammasome activation occurs during multiple sclerosis and is required for EAE development [10, 34]. Therefore, we tested the possibility therapeutic effect of 1,2,4-TTB on EAE. The mice received i.g. administration of 1,2,4-TTB at a dosage of 200 mg · kg−1 · d−1 starting from day 7 after MOG immunization (Fig. 7a). The mice treated with 1,2,4-TTB displayed lower morbidity and less severe EAE than the mice treated with the vehicle (Fig. 7b). Accordingly, reduced body weight loss was observed in 1,2,4-TTB-treated mice compared to control mice (Fig. 7c). 1,2,4-TTB also attenuated disease pathology, as indicated by a smaller MBP-negative area (Fig. 7d) and smaller demyelination area (Fig. 7e) measured by immunofluorescence and Luxol Fast Blue staining, respectively.
Fig. 7. 1,2,4-TTB ameliorates the clinical symptoms of EAE model mice.
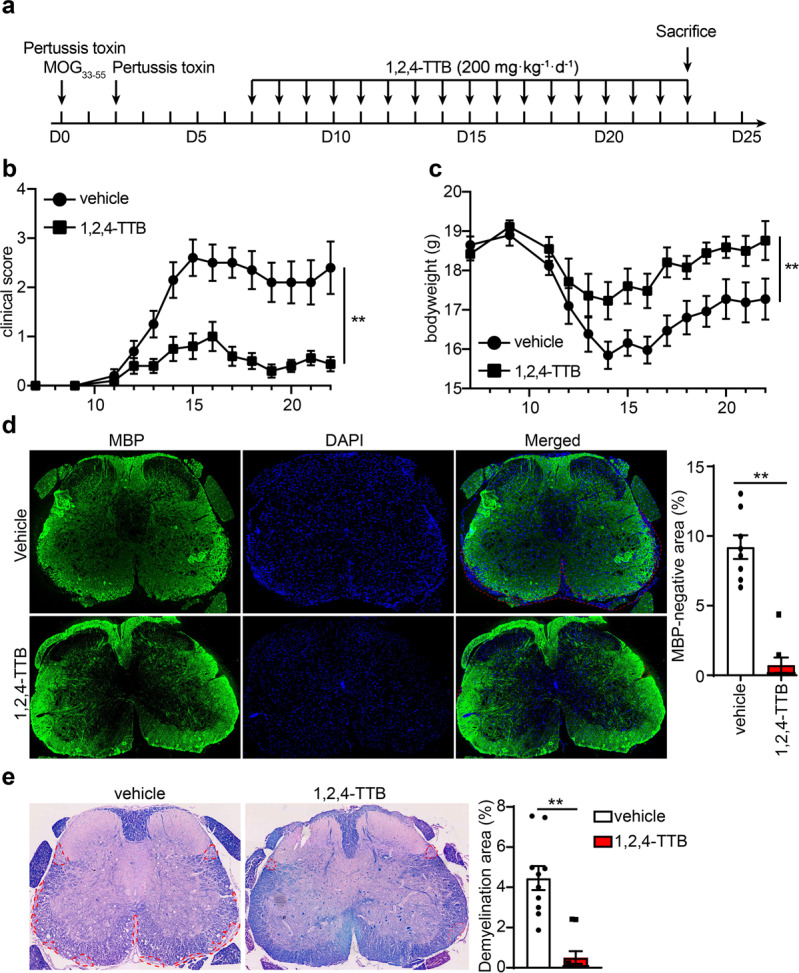
a Schedule of the EAE model establishment and administration of 1,2,4-TTB. b, c Clinical scores and body weights of EAE model mice administered 1,2,4-TTB or vehicle. d, e Spinal cord slices from EAE model mice administered 1,2,4-TTB or vehicle were stained with an anti-MBP antibody (d), and the percentage of the demyelination area (e) was analyzed by ImageJ. (* indicates P < 0.05, ** indicates P < 0.01 by Student’s t test or one-way ANOVA).
To investigate whether 1,2,4-TTB ameliorated EAE by suppressing the adaptive immune response, we counted T cells and measured the serum anti-MOG antibody level. First, we analyzed T cell subpopulations when the mice were killed on day 23. FACS analysis of the spleen revealed that 1,2,4-TTB did not alter the percentage of CD4+ cells (Supplementary Fig. S3a). The 1,2,4-TTB-treated mice displayed a slightly increase in the number of CD8+ cells (Supplementary Fig. S3a), suggesting that 1,2,4-TTB did not limit the proliferation of T cells in the spleen. Similarly, no obvious alterations in MOG antibody levels in the serum were observed between the vehicle and 1,2,4-TTB groups (Supplementary Fig. S3b), indicating that 1,2,4-TTB did not affect B-cell activation or MOG antibody secretion. Together, these in vivo results show that 1,2,4-TTB attenuates EAE severity without affecting peripheral adaptive immune responses.
1,2,4-TTB inhibits NLRP3 inflammasome activation in vivo
To address how 1,2,4-TTB ameliorated EAE, we next explored whether 1,2,4-TTB could inhibit NLRP3 inflammasome activation in vivo. We first performed immunofluorescence analysis of spinal sections. As expected, the NLRP3 inflammasome was aberrantly activated in the EAE group, as reflected by elevated ASC expression and aggregation in the EAE group compared to the control group (naive mice) (Fig. 8a). In contrast, 1,2,4-TTB-treated mice showed much lower ASC expression and aggregation than mice treated with the vehicle (Fig. 8a), suggesting that 1,2,4-TTB inhibits NLRP3 inflammasome activation in EAE. Correspondingly, Western blot analysis of the spinal cord showed that the expression of components of the NLRP3 inflammasome complex, including NLRP3, ASC and cleaved Casp-1, was significantly downregulated in the mice treated with 1,2,4-TTB (Fig. 8b, c). These data again demonstrate that 1,2,4-TTB inhibits NLRP3 inflammasome activation in vivo. In addition, we found that 1,2,4-TTB markedly reduced the mRNA levels of IFN-γ and IL-17a, indicating that 1,2,4-TTB suppressed pathogenic T cell activation in local areas (Fig. 8d, e). Accordingly, while the expression of chemokine CCL-5 was decreased, the expression of the anti-inflammatory factor IL-4 was upregulated by 1,2,4-TTB treatment (Fig. 8f, g). Taken together, these results demonstrate that 1,2,4-TTB ameliorates neuroinflammation by inhibiting NLRP3 inflammasome activation, thus preventing EAE progression (Fig. 9).
Fig. 8. 1,2,4-TTB inhibits activation of the NLRP3 inflammasome in vivo.
a Spinal cord slices from EAE model mice administered 1,2,4-TTB or vehicle were stained with an anti-ASC antibody to analyze the expression of ASC. b–d The expression of cleaved Casp-1, NLRP3, pro-IL-1β, pro-Casp-1, ASC, and β-actin was analyzed by Western blotting (b), and the gray values of the ASC (c) and cleaved Casp-1 (d) bands were analyzed by ImageJ. e–h RNA was extracted from the spinal cords of EAE model mice administered 1,2,4-TTB or vehicle, and the mRNA levels of IFN-γ (e), IL-17a (f), CCL-5 (g), and IL-4 (h) were analyzed by real-time PCR. (* indicates P < 0.05, ** indicates P < 0.01 by one-way ANOVA).
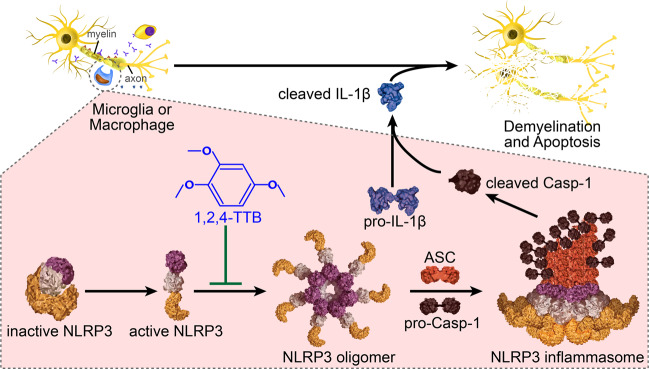
Fig. 9. Schematic diagram of our study.
Inhibition of NLRP3 oligomer formation by 1,2,4-TTB results in decreased assembly of the NLRP3 inflammasome. Because NLRP3 inflammasome formation is reduced under 1,2,4-TTB treatment, the production of cleaved Casp-1 and cleaved IL-1β induced by LPS and Nig stimulation is decreased as well. By inhibiting the formation of the NLRP3 inflammasome, 1,2,4-TTB attenuates neuroinflammation and demyelination in EAE.
Discussion
The NLRP3 inflammasome is one of the major inflammasomes and has been indicated to promote the development of many diseases. Inhibition of the NLRP3 inflammasome can be achieved by targeting the priming, boosting, and posttranslational modification of NLRP3 components [35–37]. However, agents that target only NLRP3 selectively block the activation of the NLRP3 inflammasome and have certain advantages over biologic inhibitors of NF-κB and IL-1β. Here, we identified 1,2,4-TTB, a natural component of EOs that inhibits the activation of the NLRP3 inflammasome without affecting the activation of the AIM2 inflammasome.
EOs are secondary metabolites that are mainly derived from aromatic plants and have potential application value in the medical and commercial fields [38]. EOs of patchouli, paulownia, rosemary, lavender, pine, and clove exert potent anti-inflammatory effects [23, 24, 38–40]. However, the mechanisms involved in the anti-inflammatory properties of EOs remain unknown. As natural products with multiple biological activities, EOs are promising sources for the development of novel therapeutic molecules to treat human diseases [41, 42]. In the current study, we identified 1,2,4-TTB, a major component of paulownia EO, as a selective inhibitor of the NLRP3 inflammasome. Although 1,2,3-TTB is an isomer of and is very similar to 1,2,4-TTB, it fails to inhibit the activation of the NLRP3 inflammasome, indicating that inhibition of the NLRP3 inflammasome by 1,2,4-TTB is structure dependent. To optimize the inhibitory effect of 1,2,4-TTB, its structure-dependent biological activity should be explored in future work.
Basic and clinical studies have demonstrated that abnormal activation of the NLRP3 inflammasome is implicated in several human inflammatory diseases, such as atherosclerosis, colitis, diabetes, Alzheimer’s disease, multiple sclerosis, and post-traumatic stress disorder [6–11]. MCC950, the first compound identified to specifically inhibits the NLRP3 inflammasome, has been demonstrated to have a strong beneficial effect on mouse models of several of these diseases [18]. Moreover, recent studies have identified several compounds that can directly inhibit NLRP3 inflammasome activation and effectively attenuate inflammation-associated diseases in animal models [19, 20, 43]. Therefore, the NLRP3 inflammasome is a diagnostic biomarker and an attractive therapeutic target for these diseases [34, 44, 45]. However, there are currently no available agents that directly and specifically target the NLRP3 inflammasome in the clinic.
In the present study, we report that 1,2,4-TTB is a novel inhibitor that selectively blocks NLRP3 inflammasome activation and is thus an optimal choice for the treatment of NLRP3-driven diseases. Our data indicated that 1,2,4-TTB selectively inhibited NLRP3 inflammasome activation without altering the status of the AIM2 inflammasome. Mechanistically, 1,2,4-TTB blocked NLRP3 inflammasome assembly by suppressing NLRP3 oligomer formation, the NLRP3-ASC interaction, and NLRP3-dependent ASC oligomerization. Importantly, 1,2,4-TTB treatment in vivo significantly ameliorated EAE severity and pathological progression. Consistent with previous studies, our data suggest that the NLRP3 inflammasome is a potential therapeutic target for inflammation-associated diseases. However, for future development of 1,2,4-TTB and its application in clinical settings, more optimization, preclinical trials, and safety testing of the compound are required.
Collectively, our results demonstrated that 1,2,4-TTB specifically inhibited the activation of the NLRP3 inflammasome by suppressing inflammasome assembly and displayed marked beneficial effects in EAE (Fig. 9). Given the role of the NLRP3 inflammasome in the development of Parkinson’s disease, gout and multiple sclerosis, 1,2,4-TTB or its derivatives could be potential pharmaceutical agents for NLRP3-driven inflammatory diseases.
Supplementary information
Acknowledgements
We sincerely thank Prof. Feng Shao (National Institute of Biological Sciences, Beijing, China) for providing the ASC knockout mice. This work was supported by the National Natural Science Foundation of China (81701187 to YJL), the Open Project of Modern Preparation of TCM, Ministry of Education, Jiangxi University of Traditional Chinese Medicine (TCM-201914 to YJL), and the Open Project of NHC Key Laboratory of Birth Defects Research, Prevention and Treatment (KF2019001 to YJL).
Author contributions
YJL conceived, designed, and performed the experiments with the assistance of RYP and XXK; HYZ, ZCW, and LD performed the virtual screening and established a library of the bioactive compounds of EOs; CY provided technical support on confocal imaging. YJL, RYP, XXK, ZQY, JBC, and YC analyzed the data; and RYP and YJL wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Rui-yuan Pan, Xiang-xi Kong
Change history
4/13/2021
A Correction to this paper has been published: 10.1038/s41401-021-00670-z
Contributor Information
Hai-yan Zhang, Email: haiyansl@163.com.
Ya-jin Liao, Email: lyajin@muc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00613-8.
References
- 1.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–59. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding Z, Wang X, Liu S, Zhou S, Kore RA, Mu S, et al. NLRP3 inflammasome via IL-1beta regulates PCSK9 secretion. Theranostics. 2020;10:7100–10. doi: 10.7150/thno.45939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617–29. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 5.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–9. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 8.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay W, Shinohara ML. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Brain Pathol. 2017;27:213–9. doi: 10.1111/bpa.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y, Li S, Lu Y, Li X, Liao Y, Peng Z, et al. Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J Neuroinflammation. 2020;17:205. doi: 10.1186/s12974-020-01842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019;137:599–617. doi: 10.1007/s00401-018-01957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci USA. 2018;115:E6065–74. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy. 2019;15:1860–81. doi: 10.1080/15548627.2019.1596481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32:468–78. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–84. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–38. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Varadarajan S, Munoz-Planillo R, Burberry A, Nakamura Y, Nunez G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289:1142–50. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant RM, Dinh L, Argo S, Shah M. The essentials of essential oils. Adv Pediatr. 2019;66:111–22. doi: 10.1016/j.yapd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Silva GL, Luft C, Lunardelli A, Amaral RH, Melo DA, Donadio MV, et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An da Acad Bras Cienc. 2015;87:1397–408. doi: 10.1590/0001-3765201520150056. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Parker TL. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm Biol. 2017;55:1619–22. doi: 10.1080/13880209.2017.1314513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lavor EM, Fernandes AWC, de Andrade Teles RB, Leal A, de Oliveira Junior RG, Gama ESM, et al. Essential oils and their major compounds in the treatment of chronic inflammation: a review of antioxidant potential in preclinical studies and molecular mechanisms. Oxid Med Cell Longev. 2018;2018:6468593. doi: 10.1155/2018/6468593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen L, Laird K, Wilson PB. Structure-activity modelling of essential oils, their components, and key molecular parameters and descriptors. Mol Cell Probes. 2018;38:25–30. doi: 10.1016/j.mcp.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Guo H, Cheng BC, Su T, Fu XQ, Li T, et al. Dingchuan tang essential oil inhibits the production of inflammatory mediators via suppressing the IRAK/NF-kappaB, IRAK/AP-1, and TBK1/IRF3 pathways in lipopolysaccharide-stimulated RAW264.7 cells. Drug Des Dev Ther. 2018;12:2731–48. doi: 10.2147/DDDT.S160645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho CL, Li LH, Weng YC, Hua KF, Ju TC. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-kappaB pathways. BMC Complement Med Ther. 2020;20:200. doi: 10.1186/s12906-020-02999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Li Q, Fang M, Ma Y, Liu N, Yan X, et al. The kidney injury induced by short-term PM2.5 exposure and the prophylactic treatment of essential oils in BALB/c Mice. Oxid Med Cell Longev. 2018;2018:9098627. doi: 10.1155/2018/9098627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao Z, Xu F, Wen T, Wang F, Sang W, Zeng N. Protective effects of essential oils from Rimulus cinnamon on endotoxin poisoning mice. Biomed Pharmacother. 2018;101:304–10. doi: 10.1016/j.biopha.2018.02.092. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–41. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 32.Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M, et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10:9644–62. doi: 10.7150/thno.47651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan RY, Ma J, Wu HT, Liu QS, Qin XY, Cheng Y. Neuroprotective effects of a Coeloglossum viride var. Bracteatum extract in vitro and in vivo. Sci Rep. 2017;7:9209. doi: 10.1038/s41598-017-08957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra S, Costa C, Eixarch H, Keller CW, Amman L, Martinez-Banaclocha H, et al. NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain. 2020;143:1414–30. doi: 10.1093/brain/awaa084. [DOI] [PubMed] [Google Scholar]
- 35.Kong X, Liao Y, Zhou L, Zhang Y, Cheng J, Yuan Z, et al. Hematopoietic cell kinase (HCK) is essential for NLRP3 inflammasome activation and lipopolysaccharide-induced inflammatory response in vivo. Front Pharmacol. 2020;11:581011. doi: 10.3389/fphar.2020.581011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–97. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Stutz A, Kolbe CC, Stahl R, Horvath GL, Franklin BS, van Ray O, et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med. 2017;214:1725–36. doi: 10.1084/jem.20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod. 2014;62:250–64. [Google Scholar]
- 39.Leong W, Huang G, Khan I, Xia W, Li Y, Liu Y, et al. Patchouli essential oil and its derived compounds revealed prebiotic-like effects in C57BL/6J mice. Front Pharmacol. 2019;10:1229. doi: 10.3389/fphar.2019.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basholli-Salihu M, Schuster R, Hajdari A, Mulla D, Viernstein H, Mustafa B, et al. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm Biol. 2017;55:1553–60. doi: 10.1080/13880209.2017.1309555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang ZJ, Heinbockel T. Essential oils and their constituents targeting the GABAergic system and sodium channels as treatment of neurological diseases. Molecules. 2018;23:1061. doi: 10.3390/molecules23051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsey JT, Shropshire BC, Nagy TR, Chambers KD, Li Y, Korach KS. Essential oils and health. Yale J Biol Med. 2020;93:291–305. [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Fernandez A, Skouras DB, Dinarello CA, Lopez-Vales R. OLT1177 (Dapansutrile), a selective NLRP3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front Immunol. 2019;10:2578. doi: 10.3389/fimmu.2019.02578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusco R, Siracusa R, Genovese T, Cuzzocrea S, Di Paola R. Focus on the role of NLRP3 inflammasome in diseases. Int J Mol Sci. 2020;21:4223. doi: 10.3390/ijms21124223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirzada RH, Javaid N, Choi S. The roles of the NLRP3 inflammasome in neurodegenerative and metabolic diseases and in relevant advanced therapeutic interventions. Genes (Basel) 2020;11:131. doi: 10.3390/genes11020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.