Abstract
Background
Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer that has a poor prognosis in patients with advanced disease. Avelumab [anti-programmed death-ligand 1 (PD-L1)] became the first approved treatment for patients with metastatic MCC (mMCC), based on efficacy and safety data observed in the JAVELIN Merkel 200 trial. We report long-term overall survival (OS) data after >5 years of follow-up from the cohort of patients with mMCC whose disease had progressed after one or more prior lines of chemotherapy.
Patients and methods
In Part A of the single-arm, open-label, phase II JAVELIN Merkel 200 trial, patients with mMCC that had progressed following one or more prior lines of chemotherapy received avelumab 10 mg/kg by intravenous infusion every 2 weeks until confirmed disease progression, unacceptable toxicity, or withdrawal. In this analysis, long-term OS was analyzed.
Results
In total, 88 patients were treated with avelumab. At data cut-off (25 September 2020), median follow-up was 65.1 months (range 60.8-74.1 months). One patient (1.1%) remained on treatment, and an additional patient (1.1%) had reinitiated avelumab after previously discontinuing treatment. Median OS was 12.6 months [95% confidence interval (CI) 7.5-17.1 months], with a 5-year OS rate of 26% (95% CI 17% to 36%). In patients with PD-L1+ versus PD-L1− tumors, median OS was 12.9 months (95% CI 8.7-29.6 months) versus 7.3 months (95% CI 3.4-14.0 months), and the 5-year OS rate was 28% (95% CI 17% to 40%) versus 19% (95% CI 5% to 40%), respectively (HR 0.67; 95% CI 0.36-1.25).
Conclusion
Avelumab monotherapy resulted in meaningful long-term OS in patients with mMCC whose disease had progressed following chemotherapy. These results further support the role of avelumab as a standard of care for patients with mMCC.
Key words: Merkel cell carcinoma, avelumab, immunotherapy, overall survival
Highlights
-
•
Avelumab led to meaningful long-term OS in patients with previously treated mMCC.
-
•
After >5 years of follow-up, median OS was 12.6 months and the 5-year OS rate was 26%.
-
•
Longer OS was observed in patients with PD-L1+ versus PD-L1– tumors.
-
•
The survival benefit with avelumab greatly exceeds that seen in retrospective analyses of second-line or later chemotherapy.
-
•
These results further support the role of avelumab as a standard of care for patients with mMCC.
Introduction
Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer, and patients with distant metastatic MCC (mMCC) have a poor prognosis,1 with an historical 5-year overall survival (OS) rate from diagnosis of ∼14%.2 Although mMCC is considered sensitive to chemotherapy, duration of response tends to be limited.3, 4, 5 Historically, in patients with chemotherapy-refractory mMCC, the 1-year OS rate following second-line or later chemotherapy treatment was 0%.4,5
Avelumab is an anti-programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor that targets the PD-L1/programmed death 1 (PD-1) pathway. In 2017, avelumab became the first approved treatment for patients with mMCC on the basis of results from the phase II JAVELIN Merkel 200 trial, which investigated avelumab monotherapy in patients with mMCC in two cohorts: as second-line or later treatment in patients who had disease progression after one or more lines of chemotherapy (Part A) and as first-line treatment (Part B).6,7 In Part A (N = 88), after ≥3 years of follow-up, the objective response rate (ORR) was 33.0%, including a complete response in 11.4% of patients.8 Median OS after ≥44 months of follow-up was 12.6 months, and the 42-month OS rate was 31%.8 A numerically higher ORR and longer median OS were seen in patients with PD-L1+ (n = 57) versus PD-L1− (n = 16) tumors {ORR 36.8% [95% confidence interval (CI) 24.4% to 50.7%] versus 18.8% (95% CI 4.0% to 45.6%); median OS, 12.9 months (95% CI 8.7-29.6 months) versus 7.3 months (95% CI 3.4-14.0 months), respectively}.8 Here, we report 5-year OS data from Part A of the JAVELIN Merkel 200 trial.
Methods
Study design and patients
The design of the phase II, single-arm, open-label JAVELIN Merkel 200 trial (NCT02155647) has been reported previously.6,8 Briefly, in Part A, eligible patients were aged ≥18 years and had measurable (per RECIST 1.1) and histologically confirmed stage IV MCC that had progressed following one or more prior lines of chemotherapy. Other inclusion criteria included having an Eastern Cooperative Oncology Group performance status of 0 or 1; an estimated life expectancy of ≥3 months; and adequate hematologic, hepatic, and renal function. Patients were excluded if they had received previous therapy with immune checkpoint inhibitors, concurrent anticancer treatment, or systemic treatment with corticosteroids; or had human immunodeficiency virus (HIV), immunosuppression, previous solid-organ transplantation, hematologic malignancies, or clinically significant comorbidities. Patients received avelumab 10 mg/kg by 1-h intravenous infusion every 2 weeks until confirmed disease progression, unacceptable toxicity, or withdrawal. Patients received mandatory premedication with antihistamine and acetaminophen, according to local standards, 30-60 min prior to every infusion.
Outcomes and statistical analysis
In this analysis, long-term OS (a secondary endpoint) was assessed; updated data for other efficacy endpoints, including the primary endpoint of best overall response per independent review committee and additional secondary endpoints of duration of response and progression-free survival, were not obtained. Outcomes from the primary analysis have been reported previously.6 OS was analyzed using the Kaplan–Meier method, and 95% CIs for the median were calculated using the Brookmeyer–Crowley method. PD-L1 expression in tumor cells was measured in formalin-fixed, paraffin-embedded tumor samples using the PD-L1 73-10 immunohistochemistry assay (Dako). PD-L1 positivity was defined as PD-L1 expression in ≥1% of tumor cells.
Results
Patient disposition
A total of 88 patients were enrolled and treated with avelumab (Table 1). The baseline characteristics of these patients have been reported previously and are listed in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100290.8 All patients had received one or more prior lines of systemic anticancer treatment, and 36 patients (40.9%) had received two or more prior lines of therapy.8 After >5 years of follow-up (data cut-off, 25 September 2020), median follow-up was 65.1 months (range 60.8-74.1 months). At data cut-off, one patient (1.1%) remained on treatment; this patient's first dose was in September 2014 and the patient initially had a partial response that deepened to a complete response and was ongoing at data cut-off (duration of response, 64.9 months). An additional patient (1.1%) had reinitiated avelumab after previously discontinuing treatment. This patient's first dose was in June 2015 and this patient had a complete response in September 2015 and subsequently discontinued avelumab in July 2016 due to ongoing complete response. After disease progression was confirmed in November 2019 (duration of response, 47.5 months), this patient reinitiated avelumab in December 2019 and is currently still on treatment (last visit to receive avelumab, 10 September 2021). After reinitiating avelumab treatment, the best response was reported as a partial response.
Table 1.
Patient disposition (N = 88)
| Patient disposition | n (%) |
|---|---|
| Received one or more doses of study treatment | 88 (100.0) |
| Treatment ongoing | 1 (1.1) |
| Off treatment | 87 (98.9) |
| Reason for discontinuation of treatment | 87 (98.9) |
| Adverse event | 11 (12.5) |
| Lost to follow-up | 1 (1.1) |
| Protocol noncompliance | 1 (1.1) |
| Death | 10 (11.4) |
| Disease progression | 45 (51.1) |
| Withdrawal of consent | 9 (10.2) |
| Other | 10 (11.4) |
| Discontinued treatment but still in follow-up | 19 (21.6) |
| Reinitiated treatment with avelumab | 1 (1.1) |
| Discontinued from the trial | 68 (77.3) |
| Lost to follow-up | 3 (3.4) |
| Death | 58 (65.9) |
| Withdrawal of consent | 7 (8.0) |
Reasons for discontinuing treatment were disease progression [n = 45 (51.1%)], adverse event [AE; n = 11 (12.5%)], death [n = 10 (11.4%)], withdrawal of consent [n = 9 (10.2%)], and other reasons [n = 10 (11.4%), including complete response for ≥6 months on treatment (per protocol) in five patients (5.7%) and switch to commercial avelumab for patient convenience in two (2.3%); Table 1]. Of those who had discontinued treatment, 19 patients (21.6%) remained in follow-up at data cut-off.
Overall survival
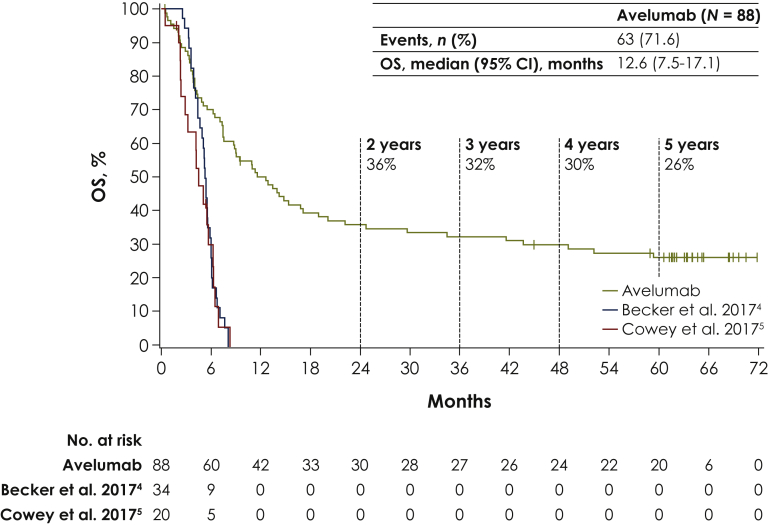
Median OS was 12.6 months (95% CI 7.5-17.1 months; Figure 1). OS rates at 3, 4, and 5 years were 32% (95% CI 23% to 42%), 30% (95% CI 20% to 40%), and 26% (95% CI 17% to 36%), respectively. For illustrative purposes, Kaplan–Meier estimates of OS from retrospective analyses of second-line or later chemotherapy in patients with mMCC are also depicted in Figure 1.
Figure 1.
Overall survival (OS) for avelumab in comparison with retrospective analyses of second-line or later chemotherapy in patients with metastatic Merkel cell carcinoma.
CI, confidence interval.
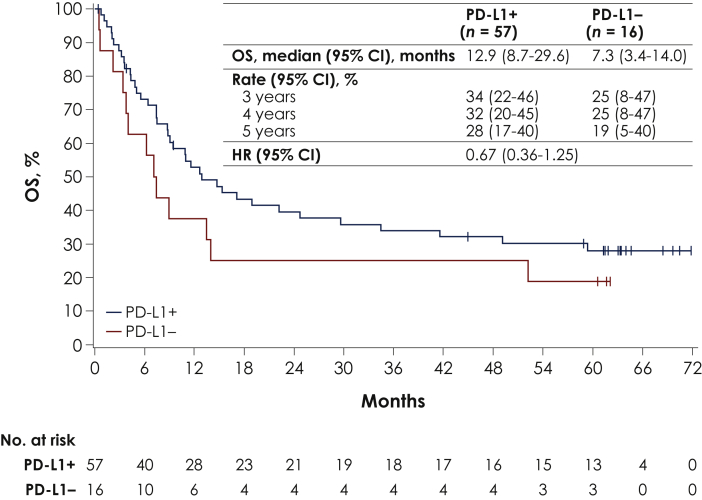
Median OS in patients with PD-L1+ (n = 57) or PD-L1− (n = 16) tumors was 12.9 months (95% CI 8.7-29.6 months) and 7.3 months (95% CI 3.4-14.0 months), respectively [HR 0.67 (95% CI 0.36-1.25)]. OS rates at 4 and 5 years in patients with PD-L1+ tumors were 32% (95% CI 20% to 45%) and 28% (95% CI 17% to 40%), and in patients with PD-L1− tumors were 25% (95% CI 8% to 47%) and 19% (95% CI 5% to 40%), respectively (Figure 2).
Figure 2.
Overall survival (OS) in subgroups defined by programmed death-ligand 1 (PD-L1) status.
CI, confidence interval.
At data cut-off, 63 patients (71.6%) had died. The most common cause of death was disease progression [n = 49 (55.7%)]; other causes were unknown reason [n = 9 (10.2%)], AE not related to study treatment [n = 3 (3.4%)], and other reason [n = 2 (2.3%)]. No deaths due to treatment-related AEs were reported.
Subsequent treatment
In total, 26 patients (29.5%) received subsequent anticancer therapy. The most common subsequent therapies after trial discontinuation were avelumab (n = 4; 4.5%), carboplatin + etoposide (n = 4; 4.5%), and pembrolizumab (n = 4; 4.5%; Table 2).
Table 2.
Subsequent treatment (N = 88)
| Subsequent treatment | n (%) |
|---|---|
| Received subsequent therapy | 26 (29.5) |
| Avelumab | 4 (4.5) |
| Carboplatin + etoposide | 4 (4.5) |
| Pembrolizumab | 4 (4.5) |
| Everolimus | 3 (3.4) |
| Nivolumab | 3 (3.4) |
| Pazopanib | 3 (3.4) |
| Capecitabine | 2 (2.3) |
| Cyclophosphamide + doxorubicin + vincristine | 2 (2.3) |
| Paclitaxel | 2 (2.3) |
| Pegylated liposomal doxorubicin hydrochloride | 2 (2.3) |
| Temozolomide | 2 (2.3) |
| Topotecan | 2 (2.3) |
| Amrubicin | 1 (1.1) |
| Carboplatin | 1 (1.1) |
| Carboplatin + paclitaxel | 1 (1.1) |
| Cisplatin | 1 (1.1) |
| Combinations of antineoplastic agents | 1 (1.1) |
| Cyclophosphamide | 1 (1.1) |
| Ipilimumab + nivolumab | 1 (1.1) |
| Octreotide | 1 (1.1) |
| Sunitinib | 1 (1.1) |
| Somatostatin | 1 (1.1) |
| Other therapeutic product | 1 (1.1) |
Discussion
To our knowledge, this is the longest follow-up reported to date for a cohort of patients with mMCC treated with an immune checkpoint inhibitor. These updated data show that avelumab monotherapy resulted in meaningful long-term OS in patients with mMCC whose disease had progressed following chemotherapy, with a 5-year OS rate of 26%. Although responses to avelumab occurred irrespective of PD-L1 status,8 longer OS was observed in patients with PD-L1+ versus PD-L1− tumors, including median OS of 12.9 versus 7.3 months and 5-year OS rates of 28% versus 19%, respectively, consistent with trends seen in earlier analyses.8 However, the OS observed in both subgroups greatly exceeded that seen in retrospective analyses of second-line or later chemotherapy in patients with mMCC, which reported a 1-year OS rate of 0%,4,5 supporting the conclusion that avelumab can provide a meaningful OS benefit irrespective of tumor PD-L1 status.
After >5 years of follow-up, OS data were mature, with death having occurred in 71.6% of patients. No treatment-related AEs led to death. In the majority of patients, the cause of death was disease progression. In patients who received subsequent therapy, the most commonly received treatments were chemotherapy or other immune checkpoint inhibitors (including patients who switched to commercial avelumab).
The study does have limitations, including its single-arm, phase II design, and the small sample size; however, MCC is a rare disease, which makes large randomized clinical trials unlikely to be feasible. In addition, the study was not designed or powered to show statistical differences in biomarker-defined subgroups. Lastly, the long-term analyses reported here are limited to OS, which was a secondary endpoint.
In conclusion, this 5-year follow-up analysis shows the benefit of avelumab treatment on OS in patients with mMCC who had disease progression after one or more lines of chemotherapy and further supports the role of avelumab as a standard of care for patients with mMCC.
Acknowledgements
The authors thank the patients and their families, investigators, co-investigators, and study teams at each of the participating centers.
Funding
This trial was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945), Germany, as part of an alliance between Merck, Germany and Pfizer, United States. Medical writing support was provided by Felicia Barklund of ClinicalThinking and funded by Merck, Germany and Pfizer, United States (no grant number).
Disclosure
SPD has served in a consulting or advisory role for Adaptimmune, Amgen, GlaxoSmithKline, Immune Design, Immunocore, Incyte, Merck, and Nektar; has received travel and accommodations expenses from Adaptimmune, Merck, and Nektar; and has received institutional research funding from Amgen, Bristol Myers Squibb, Deciphera, Incyte, Merck, MSD, and Nektar. SB has served in a consulting or advisory role for Bristol Myers Squibb, Exicure, Genentech/Roche, Merck, and Sanofi/Regeneron (self and institution); has received travel and accommodations expenses from NantWorks and Sanofi/Regeneron; has received honoraria from Bristol Myers Squibb, Genentech/Roche, Merck, and Sanofi/Regeneron; and has received institutional research funding from Bristol Myers Squibb, Exicure, Immune Design, Merck, MSD, NantWorks, Nektar, Novartis, OncoSec, and Incyte. ASB has served in a consulting or advisory role for Bayer, Deciphera, and Merck; and their immediate family member has provided expert testimony for GlaxoSmithKline. OH has served in a consulting or advisory role for Aduro Biotech, Akeso Biopharma, Amgen, BeiGene, BioAtla, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Janssen, MSD, NextCure, Novartis, Pfizer, Regeneron, Roche, Sanofi, Seattle Genetics, Tempus, and Zelluna; has provided speaker services for Bristol Myers Squibb, Novartis, Sanofi/Regeneron, and Pfizer; has received honoraria from Bristol Myers Squibb, Pfizer, Novartis, and Sanofi/Regeneron; and has received institutional research funding from Aduro Biotech, Akeso Biopharma, Amgen, Arcus Biosciences, BioAtla, Bristol Myers Squibb, CytomX Therapeutics, Exelixis, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance Biotherapeutics, Merck, MSD, Moderna Therapeutics, NextCure, Novartis, Pfizer, Regeneron, Roche, Sanofi, Seattle Genetics, Torque, and Zelluna. JMM has served in a consulting or advisory role for Bristol Myers Squibb, MSD, Sanofi/Regeneron, and Seattle Genetics; has received travel and accommodations expenses from Array BioPharma, Bristol Myers Squibb, Merck, and MSD; has stock and other ownership interests with Pfizer; has received honoraria from Merck and Pfizer; and has received institutional research funding from Amgen, AstraZeneca, Bristol Myers Squibb, Incyte, MacroGenics, MSD, Novartis, and Regeneron. PT has served in a consulting or advisory role for Bristol Myers Squibb, Merck, Novartis, Pierre Fabre, Roche, and Sanofi; has received travel and accommodations expenses from Bristol Myers Squibb and Pierre Fabre; and has received honoraria from Bristol Myers Squibb, CureVac, Merck, MSD, Novartis, and Roche. CL has served in a consulting or advisory role for Amgen, Bristol Myers Squibb, Merck, MSD, Novartis, Pierre Fabre, Roche, and Sanofi; has provided speaker services for Amgen, Bristol Myers Squibb, Novartis, MSD, and Roche; has received travel and accommodations expenses from Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, and Sanofi; reports other relationships with Avantis Medical Systems; has received honoraria from Amgen, Bristol Myers Squibb, Incyte, MSD, Novartis, Pfizer, Pierre Fabre, and Roche; and has received institutional research funding from Bristol Myers Squibb and Roche. KDL has served in a consulting or advisory role for Array BioPharma, Iovance Biotherapeutics, Merck, Regeneron, Roche, and Sanofi; has received travel and accommodations expenses from Alkermes, Merck, Neon Therapeutics, Regeneron, and Roche/Genentech; has received honoraria from Array BioPharma and Iovance Biotherapeutics; has received institutional research funding from Alkermes, Array BioPharma, Bristol Myers Squibb, Incyte, Iovance Biotherapeutics, Kartos Therapeutics, Merck, Nektar, Neon Therapeutics, OncoSec, Regeneron, Roche/Genentech, and Ultimovacs; and has uncompensated relationships with Regeneron and Roche/Genentech. MM has served in a consulting or advisory role for Novartis and Pfizer and has received travel and accommodations expenses from Novartis. HX is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. GG is an employee of Merck Healthcare KGaA, Darmstadt, Germany. PTN has served in a consulting or advisory role for 4SC, Merck, MSD, Pfizer, and Sanofi/Regeneron; received travel and accommodations expenses from MSD and Sanofi/Regeneron; has a patent pending for high-affinity T-cell receptors that target the Merkel polyomavirus; and has received research funding from Bristol Myers Squibb and Merck. All other authors have declared no conflicts of interest.
Data sharing
For all new products or new indications approved in both the European Union and the United States after 1 January 2014, Merck will share patient-level and study-level data after deidentification, as well as redacted study protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researcher’s request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company’s data sharing portal. More information can be found at https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. Where Merck has a co-research, co-development, or co-marketing/co-promotion agreement or where the product has been out-licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Ethics approval and consent to participate
The trial was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines. The protocol was approved by the independent ethics committee or institutional review board at each participating center, and all patients provided written informed consent before enrollment.
Supplementary data
References
- 1.NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. v1. 2021. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf [Google Scholar]
- 2.Harms K.L., Healy M.A., Nghiem P. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer J.G., Blom A., Doumani R. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5:2294–2301. doi: 10.1002/cam4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker J.C., Lorenz E., Ugurel S. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget. 2017;8:79731–79741. doi: 10.18632/oncotarget.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowey C.L., Mahnke L., Espirito J., Helwig C., Oksen D., Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13:1699–1710. doi: 10.2217/fon-2017-0187. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman H.L., Russell J., Hamid O. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Angelo S.P., Lebbé C., Mortier L. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer. 2021;9:e002646. doi: 10.1136/jitc-2021-002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo S.P., Bhatia S., Brohl A.S. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer. 2020;8:e000674. doi: 10.1136/jitc-2020-000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.