Keywords: mitotic drive, preferential fertilization, recombination, chromosomal knobs, genomic conflict, engineered minichromosomes
Abstract
The supernumerary B chromosome of maize is dispensable, containing no vital genes, and thus is variable in number and presence in lines of maize. In order to be maintained in populations, it has a drive mechanism consisting of nondisjunction at the pollen mitosis that produces the two sperm cells, and then the sperm with the two B chromosomes has a preference for fertilizing the egg as opposed to the central cell in the process of double fertilization. The sequence of the B chromosome coupled with B chromosomal aberrations has localized features involved with nondisjunction and preferential fertilization, which are present at the centromeric region. The predicted genes from the sequence have paralogues dispersed across all A chromosomes and have widely different divergence times suggesting that they have transposed to the B chromosome over evolutionary time followed by degradation or have been co-opted for the selfish functions of the supernumerary chromosome.
1. Introduction
Thousands of plants and animals have extra dispensable chromosomes called supernumerary or B chromosomes [1]. They typically do not associate with the normal set during meiosis and are of variable number between members of a population. It is assumed that they possess properties to maintain themselves despite being nonvital, although this has only been investigated in a few examples, which do indeed have drive mechanisms. The drive mechanisms of different B chromosomes are varied and can operate pre-meiotically, meiotically or post-meiotically, but all place more copies of themselves into the next generation than present in the previous generation. Thus, while B chromosomes are not needed, they maintain themselves in populations by these non-Mendelian mechanisms.
One of the most thoroughly studied B chromosomes is the one present in maize [2,3]. The supernumerary B chromosome of maize (figure 1) has several properties to perpetuate itself [2,3]. In most lines, the presence of the B is not detrimental to plant growth or development unless at high copy numbers beyond about 15 [4]. The B chromosome has a drive mechanism consisting of nondisjunction at the second pollen mitosis [5–7], which produces the two sperm cells, and then the B containing sperm preferentially fertilizes the egg in the process of double fertilization [8] (figure 2). The B chromosome modulates gene expression across the genome [9,10] and increases the rate of meiotic recombination on all chromosomes, particularly in heterochromatic regions and especially in male meiosis [11–18]. This process would foster the transmission of the B chromosome itself by insuring recombination in this largely heterochromatic chromosome, which would aid its own meiotic transmission [19]. The B chromosome has also evolved a mechanism to prevent its loss as a univalent in meiosis [20–22]. The nondisjunction mechanism requires two components: the centromere of the B [23], which has an additional specific repeat [24–26], and at least two trans-acting factors present at different sites on the chromosome [5,27–29]. The trans-acting factors are thought to delay further the replication of heterochromatin at the second pollen mitosis beyond its usual late duplication [30]. Thus, the B chromosome has manipulated cellular processes to ensure proper segregation in male meiosis by increasing recombination in heterochromatin, by ensuring its transmission if it is in a univalent state, by delaying replication of the centromere at the one mitosis that produces the two sperm and by mediating fertilization of the egg by the B containing sperm.
Figure 1.
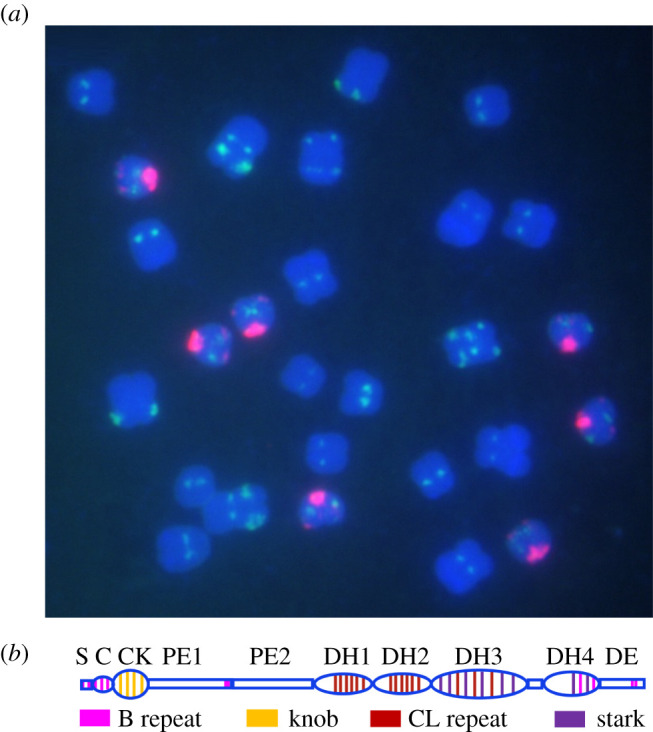
The maize B chromosome. (a) Mitotic metaphase spread of a line with seven B chromosomes (magenta). The magenta signal identifies the ZmBs (B specific repeat) in the centromere and short arm with a minor representative at the distal tip of the B long arm. Green signal identifies two chromosomal features, namely the CentC centromeric satellite and TAG microsatellite clusters. The green signal on the B chromosome represents CentC clusters, which have no centromere activity [26]. DAPI stains the chromosomes (blue). (b) Schematic view of the acrocentric maize B chromosome at pachynema of meiosis. The chromosome is divided into the B short arm (S), B centromere (C), centromeric knob (CK), proximal euchromatin (PE1-2), four blocks of distal heterochromatin (DH1-4) and the distal euchromatin (DE). Four representative repeats on the B chromosome including the B-specific repeat ZmBs, knob-180, CL-repeat and Stark repeat cluster are colour coded along with the length of the chromosome. Knob is present in the centromeric knob region. CL repeat is present at DH1, 2 and 3. The Stark repeat is present in most of DH3 and the distal portion of DH4.
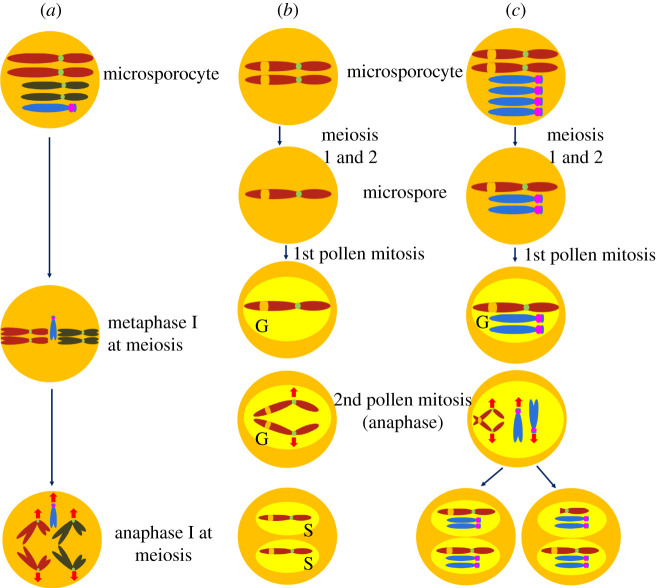
Figure 2.
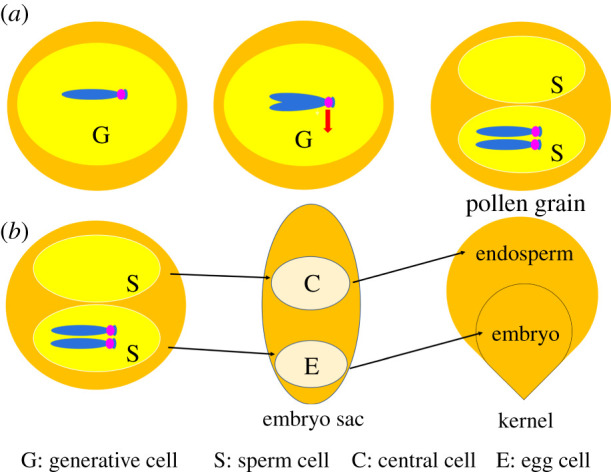
Components of the B drive mechanism. (a) Diagram of nondisjunction of the B chromosome in maize. The B chromosome (blue with a magenta centromere) is shown in the generative nucleus (G) after the first pollen mitosis. After replication, the two chromatids proceed to the same pole (red arrow) at the second pollen mitosis in the vast majority of divisions. Thus, most mature pollen grains contain two sperm cells (S) with only one containing the B chromosomes. (b) Depiction of preferential fertilization. For most lines of maize, the sperm with the two B chromosomes will preferentially fertilize the egg (E), as compared with the central cell (C) in the process of double fertilization. The fertilized egg develops into the next generation embryo and the fertilized central cell develops into the endosperm.
2. B-A translocations: genetic tools for maize
The property of the B chromosome to undergo nondisjunction at the very mitosis that produced the two maize sperm cells has been capitalized upon by maize researchers. Many features of the B chromosome have been deciphered using B-A translocations (figure 3), which add various genetic markers to the chromosome. A large collection of translocations between the B chromosome and A chromosomes has been induced [31,32]. Distal portions of A chromosome arms are joined with the B centromere. Thus, the A portion is also changed in dosage in the sperm and will lead to zygotes with different numbers of the respective chromosome arm. Of particular note and an exceptional advantage to maize is that heterozygous deficiencies can be produced because the nondisjunction occurs at the mitosis that produces the male gametes. Thus, extra or missing chromosome arms can be present in the male gametes at the endpoint of the haploid gametophyte generation that typically would abort if a large deletion were present. Because nondisjunction is not always 100%, some balanced gametes are also produced to generate a dosage series of 1, 2 and 3 copies in the resulting zygotes. B-A translocations have been used for uncovering recessive mutations [31], for numerous types of dosage studies [33–40], for understanding centromere structure [23,25,26,41–44], for examining the nature of the breakage–fusion–bridge (BFB) cycle [45,46], for the discovery of parental imprinting [47] as well as contributing to understanding the B chromosome itself.
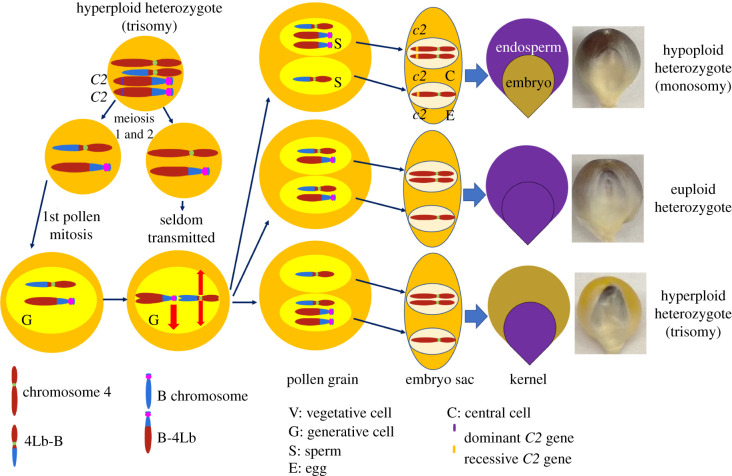
Figure 3.
Diagram of the behaviour of a B-A translocation using TB-4Lb as an example. Hyperploid heterozygote (trisomy; 4 4Lb-B B-4Lb B-4Lb) containing a dominant C2 anthocyanin gene on the B-4Lb was crossed to a female c2 tester, which has no colour (yellow) in the kernel due to the blockage of anthocyanin pathway by the c2 mutation located on 4L. During meiosis, the TB-4Lb trisomy will produce two kinds of microspores, 4 B-4Lb and 4Lb-B B-4Lb. The 4 B-4Lb pollen have two copies of 4L and cannot compete with balanced 4Lb-B B-4Lb during pollen tube growth leading to only the latter being transmitted. During the second pollen mitosis, the nondisjunction of B-4Lb produces one sperm cell with two copies of B-4Lb and the other has zero copies of B-4Lb. Both sperm cells have the 4Lb-B chromosome. After double fertilization, the kernel would show coloured (purple) embryo and colourless endosperm (trisomy), or coloured endosperm and colourless embryo (monosomy). If the B-4Lb disjoins normally (right middle row), the kernel would show full colour (purple embryo and purple endosperm, euploid heterozygote). The copy number of 4L in monosomy, euploid and trisomy should be 1, 2 and 3 copies, respectively. The B portion is shown as blue with a magenta centromere and chromosome 4 is shown as dark red with a green centromere.
3. Structure of the B chromosome
The B chromosome is nearly telocentric consisting mainly of a long arm and a minute short arm. It is highly heterochromatic. Beyond the centromere, it has a proximal euchromatic portion, followed by several large blocks of heterochromatin and terminating the long arm with a short euchromatic region (figure 1). The B chromosome contains a number of B specific repeats. One of these is located in and around the centromere with minor representations near the distal end of the chromosome [24]. The Stark repeat is present in two blocks of heterochromatin [48,49] and the CL repeat is present at several sites along the chromosome, primarily in the heterochromatic blocks [50–54]. The B specific repeat is related on the sequence level to knob heterochromatin present in the normal A chromosomes [24,55]. Knobs are repeated units at high copy number that stain deeply with chromatin stains. There are two major types in maize. The 180 bp knob repeat was the first recognized [56]. As its name implies, it consists of tandem repeats of 180 base pairs. These are typically present at interstitial sites on maize chromosomes [57,58], although a minor representation is present at the ends of all chromosomes [59], which is potentially the progenitor position given this is the place where they reside in relatives of maize such as Zea luxurians and Tripsacum species [60]. The second type of knob repeat is the TR1 repeat that is 350 bp in unit length but is related on the sequence level to the 180 bp repeat [61]. A 180 bp knob is present on the B chromosome in close proximity to the centromere.
As noted, the B specific centromeric repeat is related to the knob180 and TR1 heterochromatin repeats [24,55], especially to a portion of the knob sequence of highest evolutionary conservation [62]. Also, Cent4 is a repeat cluster found in all maize lines near the centromere of chromosome 4 [60] that is similar to the B repeat, having been isolated based on that homology [63]. Cent4, however, does not undergo nondisjunction in the presence of B chromosomes so there must be some distinction between the B repeat and the Cent4 array.
4. Sequence of the B chromosome
A high-quality sequence of the maize B chromosome has been produced [64]. There are 758 predicted protein-encoding genes. Paralogues in the A chromosome have widely distinct divergence estimates and genomic locations, suggesting that these predicted B genes have transposed to the supernumerary chromosome over evolutionary time [64]. Core genes in the grass family show evidence of synteny but none can be recognized with the B chromosome [64]. Synteny would be predicted whether the origin of the B chromosome occurred within a species or as detritus from a wide cross between species. However, whatever was the ultimate progenitor, the sequences have degenerated beyond recognition. This result suggests that the drive mechanism has propelled the chromosome through millions of years of evolution as a selfish entity. The maize B chromosome is a natural laboratory for transposed duplicate genes that are freed of purifying selection and linkage drag to evolve various selfish functions co-opted from normal. The maize B chromosome has recently been shown to contain active genes [9,10,49,64] and be capable of impacting the expression of genes from the A chromosomes [9,10].
An extensive collection of B centromere misdivision derivatives and other breaks in the surrounding region [44,65,66] made it possible to assemble 543 kb of the B centromere and order the scaffolds in relation to the chromosome, including the knob adjacent to the centromere. This length is close to a fiberFISH estimate of the core region of the B centromere [25]. The B chromosome centromere is very similar in repeat composition and organization to those on the A chromosomes with the exception of the interspersion of the B specific repeat throughout and flanking the core region.
5. Cis factors for nondisjunction
The B chromosome undergoes nondisjunction mainly at the second pollen mitosis, but there is also a low frequency of nondisjunction that occurs at the first pollen mitosis [5,7]. Further, there is a very low level of nondisjunction in the endosperm [41,67] and in the tapetum [68]. With copy numbers starting at about 6, and accelerating with increasing numbers, there is evidence for nondisjunction in sporophytic root tip cells [69]. The high copy number of the B chromosome appears to foster the nondisjunction property in a tissue where it does not otherwise occur at a detectable frequency.
Multiple investigators have examined replication of chromosomes in root tips and found that the 180 bp knobs were late replicating, as was the centromeric region of the B chromosome [30,70–72]. There is a knob near the B centromere, so Pryor et al. [30] postulated that this knob mediated the nondisjunction. This idea creates a conundrum because the knobs on A chromosomes in most backgrounds do not cause nondisjunction or chromosomal breakage. It was not until years later that the B centromere-specific repeat was discovered [24]. It surrounds and is interspersed in the centromere, and also surrounds the centromere-proximal knob [26]. Minichromosomes derived from the full B chromosome that are missing the knob are still capable of undergoing nondisjunction in the presence of the trans-acting factor containing portions of the B [73]. Thus, while the centromere proximal knob might contribute to nondisjunction, it is not solely responsible and the B specific repeat is implicated [64]. Indeed, the B specific repeat is more closely related to the TR1 heterochromatic knob repeat [55]. Wear et al. [72] found that the TR1 repeat was the most enriched in the late replicating sequences, despite its underrepresentation compared to the 180 bp knob [60], suggesting that it is the last or one of the last types of sequences replicated in maize S phase. Together the results imply that the B specific repeat region is very late replicating.
The activity of the B centromere is not needed for the B repeat to mediate nondisjunction. Han et al. [66] recovered an inactive B centromere present on the tip of the short arm of chromosome 9 (9S). It is attached to the normal chromosome 9 centromere so it is inherited through the life cycle and across generations. It is perfectly stable on its own. However, when full B chromosomes, which supply the trans-acting factors, are added to the genotype, this chromosome is induced to undergo nondisjunction at the second pollen mitosis or more frequently to break and set up a BFB cycle [74] in the subsequent endosperm development after fertilization. This is evidenced by a mosaic pattern for the anthocyanin, C1, marker gene on the 9S chromosome when crossed as a male to the recessive tester female stock. These results indicate that the hypothesized B repeat involvement in nondisjunction is independent of centromere function. Numerous lines of evidence point to a delayed or stalled replication of the B repeat cluster, and cytological visualization at the second pollen mitosis indicates that the B repeat array is the site of adherence of the inactive B centromere on separated chromosomes 9 [66].
A series of B chromosome centromere misdivision derivatives were found by Carlson [41]. These different derivatives have different rates of nondisjunction. The amount of the B specific centromere repeat was quantified in this series of chromosomes and the amount was correlated with the rate of nondisjunction [43,64]. These observations provide further evidence that the B specific repeat array is the sight mediating nondisjunction and that the rate of nondisjunction is related to the quantity present around the centromere.
6. Trans-acting factors for nondisjunction
One of the factors that operates in trans and that is required for nondisjunction (figure 3a) is present near the end of the long arm of the chromosome [5,27,29]. A second is present in the proximal euchromatin [28]. The most distal breakpoint of B-A translocations localized on the B chromosome sequence is that of TB-3Sb [64]. The B-3S chromosome contains most of the B chromosome except the very distal tip. This chromosome alone is incapable of nondisjunction at the second pollen mitosis [75]. Thus, the trans-acting factor #1 must reside in the small portion of the B translocated to the short arm of chromosome 3. This region of the sequence has 34 predicted protein-encoding genes [64].
7. Preferential fertilization
Preferential fertilization is the second aspect of the B chromosome drive mechanism and is determined by the female parent (figure 2b). In most maize lines, the preference for the egg versus the central cell is 2 : 1. However, some maize varieties do not allow preferential fertilization [76–80]. Multiple researchers [21,76–81] have found lines of maize in which there is a lack of preferential fertilization such that fertilization of the egg or the central cell by the B chromosome containing sperm is random rather than being skewed towards the egg, suggesting that there is a variation for this trait in maize populations. In Argentine maize varieties, there is evidence that this difference is controlled by a single gene [21]. Moreover, Carlson [77] has observed lines that, when used as a female parent for B-A translocations, show a reversal of preferential fertilization such that the polar nuclei are now favoured. Carlson [77] also found that adding several normal B chromosomes to the genotype would eliminate the reversal and change it to a random fertilization for the B-A translocation, when there is no difference between the two sperm for B centromere presence. Preferential fertilization of the egg in most lines is also eliminated by adding B chromosomes to the genotype to eliminate a difference between the two sperm [76]. At a low frequency in maize, two pollen tubes are involved with the fertilization of an embryo sac, a process called heterofertilization. Carlson [82] used this phenomenon to test whether preferential fertilization would follow the same frequency if both the egg and the polar nuclei were given a choice of sperm. The result was that preferential fertilization of the egg was at the usual skewed frequency in favour of the inclusion of the B centromere on a B-A translocation.
The most proximally broken B-A translocation when compared to the B sequence is TB-8Lc [64]. Its breakpoint is very near to the centromere [64]. Because this chromosome exhibits preferential fertilization, the difference between the two sperm cells after nondisjunction would essentially be the centromere. The implication is that the centromere or some associated protein, or modification of an associated protein, mediates preferential fertilization. Taken all together, it is probable that the B specific repeat cluster not only mediates nondisjunction at the second pollen mitosis but is also involved with preferential fertilization. At the very least, the two sites on the B chromosome that are involved with the drive mechanism are in very close proximity [64].
8. Effect on recombination
It has long been known that the presence of B chromosomes will increase the frequency of chiasmata visualized in meiosis across the genome [11]. As noted above, numerous subsequent studies showed that crossing over in the A chromosomes was enhanced by B chromosomes, particularly in centromeric heterochromatic regions where it is usually low. Rhoades [12] described a transposed segment of chromosome arm 3L into 9S, which normally has very low rates of crossing over, but showed a dramatic increase in the 3L region in the presence of B chromosomes.
Regions of the B chromosome have been assayed for the responsible segments involved in this modulation of genomic recombination. These studies have implicated the proximal euchromatin but there is also some evidence that the distal heterochromatin might have an effect as well [15,18]. The distal euchromatic tip has been definitively eliminated so this property of the B chromosome is distinct from the distal trans-acting factor required for nondisjunction [27]. Interestingly, the enhancement of recombination is typically greater on the male side than the female [13,14,17]. Thus, the B chromosome has modified the recombination process to favour its own crossing over, which has been shown to occur [83], in male meiosis to insure its faithful segregation and accurate distribution to all spores, which, of course, immediately precedes the other processes of its drive mechanism of nondisjunction and preferential fertilization. The B chromosome itself is highly heterochromatic so it has been postulated that this modulation of recombination is an evolutionary adaptation to help foster the orderly segregation of the supernumerary chromosome and hence its transmission to the next generation [19].
9. Stabilization of univalent transmission
An additional property of the B chromosome that fosters its transmission is its stabilization as a univalent in meiosis (figure 4a) [20,22]. Often, chromosomes as a singleton will lag in meiosis I anaphase and get lost, thus failing to be included in the products of meiosis. B chromosomes, because of their drive mechanism, can find themselves in odd numbers in meiosis. These multimers can take on bizarre associations [24,57,84], but usually the centromeres are in pairs and segregation proceeds. However, when univalent B chromosomes are present, they have been observed to proceed to the poles in meiotic anaphase I ahead of the regular chromosomes [20,22,57]. In some respects, this behaviour is analogous to the behaviour of neocentromere formation of knob heterochromatin in the presence of Abnormal chromosome 10 (Ab10) [85] but is clearly distinct [22]. Indeed, González-Sánchez et al. [22] presented evidence that the small distal array of B repeats were involved in this process despite it having no detectable CENH3 [26]. This observation might suggest that a poleward migration occurs that is independent of a normal kinetochore as occurs with Ab10 [86].
Figure 4.
(a) Depiction of stabilization of univalent transmission of the B chromosome in meiosis. González-Sánchez et al. showed that a B univalent can be out of the plate and with the orientation to the poles at metaphase I of meiosis [22]. At meiotic anaphase I, the B chromosome proceeds to the poles ahead of the regular chromosomes, as shown by Carlson [20] and González-Sánchez [22]. These behaviours of the B chromosome are thought to facilitate the transmission of the B univalent. (b,c) Diagram of the high loss (HL) phenomenon induced by the presence of B chromosomes. From Rhoades & Dempsey's conclusions [88,89], in the HL line without the B chromosome, chromosome 3 with a large knob on 3L divides normally during pollen meiosis and mitosis. However, when a microsporocyte contains multiple B chromosomes such that the resulting microspores would contain two copies of the B chromosome, chromatids in the knob region on the 3L remain attached at the anaphase of the second pollen mitosis, whereas the centromere of chromosome 3 proceeds towards opposite poles. This process fractures 3L. The pollen grain will have one sperm cell with a truncated chromosome 3 and the normal chromosome 3 in the other sperm cell. In some cases, the knob region can divide before the fracture of 3L and both sperm cells have one copy of an intact chromosome 3. Note that the assortment of the nondisjoined B chromosomes is random, which means the B chromosomes could proceed towards the same or opposite poles (shown here).
There are two systems of neocentromere formation associated with Ab10—one for knob180 and one for TR1 knobs [87]. They both involve neomorphic forms of kinesin motor proteins. In these cases, there is no involvement of CENH3, the histone variant typical of active centromeres, as a foundation for a kinetochore but nevertheless an attachment and movement occurs on the meiotic spindle. It is thus conceivable that the B chromosome has evolved a co-opted related mechanism in which univalents can attach to the spindle and proceed ahead of the normal chromosomes to the poles in meiotic anaphase I. The B specific repeat array has only a small portion of it that shows any association with CENH3 on a normal chromosome [26], but the whole array could act as a neocentromere in meiosis.
Carlson & Roseman [20] localized a B distal heterochromatic region required for the precocious progression of the B specific repeat array to the anaphase I poles of meiosis. If the centromere itself is subject to a novel meiotic progression, it would not result in meiotic drive as occurs with Ab10, which relies on recombination between centromeres and the sites of alternative sizes of knob heterochromatin for drive to occur. Instead, a similar process for the B centromere insures that univalents can succeed in passing through meiosis. The sequence of the B chromosome [64] indicates that it is not related at all to chromosome 10.
While it might seem counterintuitive to suggest that a centromere sequence would acquire neocentromere activity, there are data in the literature to suggest that is the case. Images of B chromosomes in meiosis have long shown that univalents in meiotic anaphase I progress to a pole before the normal chromosomes (e.g. [57]). Carlson & Roseman [20] studied this feature in detail and found that an intact centromeric region was necessary and that the distal heterochromatin appeared to control this activity. We now understand that the chromosomes that Carlson & Roseman analysed were reduced in the amount of centromeric B-specific repeat [26,43,44]. The B centromere alone does not exhibit this behaviour [85], supporting the claim for a trans-acting factor, which would be analogous to neocentromere formation by Ab10 [86,87].
10. Impact on the A chromosome integrity
Rhoades & Dempsey [88,89] discovered a line of maize (called High Loss (HL)) in which the presence of at least two B chromosomes in a microspore (3 or more per plant) would cause the heterochromatic knobs on the A chromosomes to break at the second pollen mitosis, the same mitosis at which the B chromosome centromere remains adhered during anaphase to produce nondisjunction (figure 4b,c). The line with B chromosomes also produced an unusual frequency of trisomies and triploids when used as a male. They noted that transmission of an extra chromosome through the male is unlikely and that triploid plants are highly sterile so they must arise anew. It seems that the HL characteristic in fact causes nondisjunction of A chromosomes at the second pollen mitosis in order to obtain such individuals in the progeny, although whether this is conditioned by the unreplicated knobs or A centromeric heterochromatin is unresolved. In an attempt to test whether the B chromosomes originally in this line were unique, they introgressed B chromosomes from other lines, which they found were also effective. Their results indicate that the effect occurs in the gametophyte generation but also that there is a single major gene difference between ‘HL’ and other lines harbouring B chromosomes, although there were also numerous modifiers that would affect the severity, as is true of almost any quantitative characteristic. In the HL line, the delay of replication of heterochromatin is so severe as to cause fracture of many chromosomes in the genome and apparent nondisjunction of normal chromosomes [88,89]. The B chromosome relies on this delay for its perpetuation but the normal genome can be severely damaged if it is too late.
11. Conflict between the B and A chromosomes
The B chromosome is widespread in Mexican teosintes, the wild maize relative, as are the major knob positions that have descended into maize [57]. However, multiple investigators have noted that in landraces of maize, there is a negative correlation between the presence of B chromosomes and of many knobs [90,91]. The combination of these studies suggests that teosinte and most maize lines have variants that prevent the HL syndrome from occurring. However, the landraces in which the B chromosomes are prevalent but not knobs might have variants that allow the HL delay of knob replication to occur.
12. Use of the B chromosome as a platform for engineered minichromosomes
Because of its dispensable nature, the B chromosome could serve as a platform to develop engineered minichromosomes. Centromeres in plants are epigenetically based [92] and thus do not rely on DNA sequence for function. Thus, the baker's yeast example of producing artificial chromosomes by assembling centromeres, telomeres and marker genes in vitro with reintroduction to cells will not work in plant species. To overcome this issue, telomere-mediated chromosomal truncation was used to remove the ends of chromosomes and simultaneously place genes at the tip [93]. The B chromosome of maize was an initial target although the procedure can be applied to other chromosomes [94]. The truncation event for the B chromosome removes the terminal trans-acting factor that is needed for nondisjunction, so the engineered minichromosomes disjoin normally at the second pollen mitosis. However, in the presence of full-sized B chromosomes that provide the trans-acting functions, the minichromosomes will undergo nondisjunction [73]. Various applications of B derived engineered minichromosomes have been described [95].
13. More to learn
The B chromosome is a mysterious chromosome. Genes that transpose to the B will degenerate unless they contribute in some way to the perpetuation of the chromosome in the multitude of ways described above. This seems to have occurred to great effect. The B chromosome uses a unique repetitive sequence in and around the centromere to cause nondisjunction at the one mitosis giving rise to the male gametes. There are trans-acting factors that mediate this effect on the repetitive array. How this occurs remains a mystery and is an interesting avenue for further study. Then, the sperm that has the nondisjoined chromosomes somehow preferentially joins with the egg as opposed to the central cell in the process of double fertilization—again by an unknown process. Still further, the B chromosome has manipulated the meiotic recombination process to foster crossing over in its heterochromatic structure and brought an increase in proximal regions across the genome. Increasing recombination between paired B chromosomes would foster faithful distribution into the resulting spores from meiosis and eventual transmission to the next generation. However, if the B chromosome is present as a univalent, it has also developed a mechanism that can foster its transmission in this state, apparently by a distinct mechanism from normal centromere functions. This amazing array of properties illustrates how selfish entities can acquire a multitude of characteristics to propel themselves into future generations when uncoupled from normal chromosomes and having no vital functions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Research supported by NSF grant no. IOS-1545780.
References
- 1.Jones RN, Rees H. 1982. B chromosomes. London, UK: Academic Press. [Google Scholar]
- 2.Longley AE. 1927. Supernumerary chromosomes in Zea mays. J. Agr. Res. 35, 769-784. [Google Scholar]
- 3.Carlson WR. 1978. The B chromosome of corn. Ann. Rev. Genet. 12, 5-5. ( 10.1146/annurev.ge.12.120178.000253) [DOI] [PubMed] [Google Scholar]
- 4.Randolph LF. 1941. Genetic characteristics of the B chromosomes in maize. Genetics 26, 608-631. ( 10.1093/genetics/26.6.608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roman H. 1947. Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics 32, 391-409. ( 10.1093/genetics/32.4.391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi L, Zhu T, Mogensen HL, Keim P. 1996. Sperm identification in maize by fluorescence in situ hybridization. Plant Cell 8, 815-821. ( 10.2307/3870284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusche ML, Mogensen HL, Shi L, Keim P, Rougier M, Chaboud A, Dumas C. 1997. B chromosome behavior in maize pollen as determined by a molecular probe. Genetics 147, 1915-1921. ( 10.1093/genetics/147.4.1915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman H. 1948. Directed fertilization in maize. Proc. Natl Acad. Sci. USA 34, 36-42. ( 10.1073/pnas.34.2.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Du Y, Zhao X, Jin W. 2016. B chromosome contains active genes and impacts the transcription of A chromosomes in maize (Zea mays L.). BMC Plant Biol. 16, 88. ( 10.1186/s12870-016-0775-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong ZJ, Xiao JX, Peng SF, Lin YP, Cheng YM. 2020. Novel B-chromosome-specific transcriptionally active sequences are present throughout the maize B chromosome. Mol. Genet. Genomics 295, 313-325. ( 10.1007/s00438-019-01623-2) [DOI] [PubMed] [Google Scholar]
- 11.Ayonoadu A, Rees H. 1968. The influence of B chromosomes on chiasma frequencies in black Mexican sweet corn. Genetica 39, 75-81. ( 10.1007/BF02324457) [DOI] [Google Scholar]
- 12.Rhoades MM. 1968. Studies on the cytological basis of crossing over. In Replication and recombination of genetic material (eds Peacock WJ, Brock RD), pp. 229-241. Canberra, Australia: Australian Academy of Science. [Google Scholar]
- 13.Hanson G. 1969. B chromosome stimulated crossing over in maize. Am. J. Bot. 43, 18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nel PM. 1973. The modification of crossing over in maize by extraneous chromosomal elements. Theor. Appl. Genet. 43, 196-202. ( 10.1007/BF00306571) [DOI] [PubMed] [Google Scholar]
- 15.Ward EJ. 1973. The heterochromatic B chromosome of maize: the segments affecting recombination. Chromosoma 43, 177-186. ( 10.1007/BF00483377) [DOI] [Google Scholar]
- 16.Chang CC, Kikudome GY. 1974. The interaction of knobs and B chromosomes of maize in determining the level of recombination. Genetics 77, 45-54. ( 10.1093/genetics/77.1.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson DS. 1984. Different frequency in the recovery of crossover products from male and female gametes of plants hypoploid for a B-A translocation. Genetics 107, 117-130. ( 10.1093/genetics/107.1.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson WR, Roseman RR, Zheng YZ. 1993. Localizing a region on the B-chromosome that influences crossing over. Maydica 38, 107-113. [Google Scholar]
- 19.Carlson WR. 1994. Crossover effects of B chromosomes may be ‘selfish’. Heredity 72, 636-638. ( 10.1038/hdy.1994.87) [DOI] [Google Scholar]
- 20.Carlson WR, Roseman RR. 1992. A new property of the maize B chromosome. Genetics 131, 211-223. ( 10.1093/genetics/131.1.211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Sánchez G, González-González E, Molina F, Chiavarino AM, Rosato M, Puertas MJ. 2003. One gene determines maize B chromosome accumulation by preferential fertilization; another gene(s) determines their meiotic loss. Heredity 90, 122-129. ( 10.1038/sj.hdy.6800185) [DOI] [PubMed] [Google Scholar]
- 22.González-Sánchez M, González-García M, Vega JM, Rosato M, Cuacos M, Puertas MJ. 2007. Meiotic loss of the B chromosome of maize in influenced by the B univalent co-orientation and the TR-1 Knob constitution of the A chromosomes. Cytogenet. Genome Res. 119, 282-290. ( 10.1159/000112075) [DOI] [PubMed] [Google Scholar]
- 23.Carlson WR, Chou TS. 1981. B chromosome nondisjunction in corn: control by factors near the centromere. Genetics 97, 379-389. ( 10.1093/genetics/97.2.379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfenito MR, Birchler JA. 1993. Molecular characterization of a maize B chromosome centric sequence. Genetics 135, 589-597. ( 10.1093/genetics/135.2.589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin W, Lamb JC, Vega JM, Dawe RK, Birchler JA. 2005. Molecular and functional dissection of the maize B chromosome centromere. Plant Cell 17, 1412-1423. ( 10.1105/tpc.104.030643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb JC, Kato A, Birchler JA. 2005. Sequences associated with A chromosome centromeres are present throughout the maize B chromosome. Chromosoma 113, 337-349. ( 10.1007/s00412-004-0319-z) [DOI] [PubMed] [Google Scholar]
- 27.Ward EJ. 1973. Nondisjunction: localization of the controlling site in the maize B chromosome. Genetics 73, 387-391. ( 10.1093/genetics/73.3.387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin BY. 1978. Regional control of nondisjunction of the B chromosome in maize. Genetics 90, 613-627. ( 10.1093/genetics/90.3.613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb JC, Han F, Auger DL, Birchler JA. 2006. A trans-acting factor required for non-disjunction of the B chromosome is located distal to the TB-4Lb breakpoint on the B chromosome. Maize Genet. Coop. News Lett. 80, 51-54. [Google Scholar]
- 30.Pryor A, Faulkner K, Rhoades MM, Peacock WJ. 1980. Asynchronous replication of heterochromatin in maize. Proc. Natl Acad. Sci. USA 77, 6705-6709. ( 10.1073/pnas.77.11.6705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckett JB. 1991. Cytogenetic, genetic and plant breeding applications of B–A translocations in maize. In Chromosome engineering in plants: genetics, breeding, evolution (part A) (eds Gupta PK, Tsuchiya T), pp. 493-529. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 32.Birchler JA, Alfenito MR. 1993. Marker systems for B–A translocations in maize. J. Hered. 84, 135-138. ( 10.1093/oxfordjournals.jhered.a111297) [DOI] [Google Scholar]
- 33.Birchler JA. 1979. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics 92, 1211-1229. ( 10.1093/genetics/92.4.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M, Birchler JA. 1994. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science 266, 1999-2002. ( 10.1126/science.266.5193.1999) [DOI] [PubMed] [Google Scholar]
- 35.Lee EA, Darrah LL, Coe EH. 1996. Genetic variation in dosage effects in maize aneuploids. Genome 39, 711-721. ( 10.1139/g96-090) [DOI] [PubMed] [Google Scholar]
- 36.Lee EA, Darrah LL, Coe EH. 1996. Dosage effects on morphological and quantitative traits in maize aneuploids. Genome 39, 898-908. ( 10.1139/g96-113) [DOI] [PubMed] [Google Scholar]
- 37.Sheridan WF, Auger DL. 2008. Chromosome segmental dosage analysis of maize morphogenesis using B-A-A translocations. Genetics 180, 755-769. ( 10.1534/genetics.108.091843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunelle DC, Sheridan WF. 2014. The effect of varying chromosome arm dosage on maize plant morphogenesis. Genetics 198, 171-180. ( 10.1534/genetics.114.166330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Shi X, Chen C, Hou J, Ji T, Cheng J, Birchler JA. 2021. Predominantly inverse modulation of gene expression in genomically unbalanced disomic haploid maize. Plant Cell 33, 901-916. ( 10.1093/plcell/koab029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi X, Yang H, Chen C, Hou J, Hanson KM, Albert PS, Ji T, Cheng J, Birchler JA. 2021. Genomic imbalance determines positive and negative modulation of gene expression in diploid maize. Plant Cell 33, 917-939. ( 10.1093/plcell/koab030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson WR. 1970. Nondisjunction and isochromosome formation in the B chromosome of maize. Chromosoma 30, 356-365. ( 10.1007/BF00321067) [DOI] [Google Scholar]
- 42.Lin BY. 1979. Two new B-10 translocations involved in the control of nondisjunction of the B chromosome in maize. Genetics 92, 931-945. ( 10.1093/genetics/92.3.931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaszas E, Birchler JA. 1996. Misdivision analysis of centromere structure in maize. EMBO J. 15, 5246-5255. ( 10.1002/j.1460-2075.1996.tb00910.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaszas E, Birchler JA. 1998. Meiotic transmission rates correlate with physical features of rearranged centromeres in maize. Genetics 150, 1683-1692. ( 10.1093/genetics/150.4.1683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng YZ, Roseman RR, Carlson WR. 1999. Time course study of the chromosome-type breakage-fusion-bridge Cycle in maize. Genetics 153, 1435-1444. ( 10.1093/genetics/153.3.1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han F, Lamb JC, Birchler JA. 2006. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl Acad. Sci. USA 103, 3238-3243. ( 10.1073/pnas.0509650103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kermicle JL. 1970. Dependence of the R-mottled aleurone phenotype in maize on mode of sexual transmission. Genetics 66, 69-85. ( 10.1093/genetics/66.1.69) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark EA, Connerton I, Bennett ST, Barnes SR, Parker JS, Forster JA. 1996. Molecular analysis of the structure of the maize B chromosome. Chromosome Res. 4, 15-23. ( 10.1007/BF02254939) [DOI] [PubMed] [Google Scholar]
- 49.Lamb JC, Riddle NC, Cheng YM, Theuri J, Birchler JA. 2007. Localization and transcription of a retrotransposon-derived element on the maize B chromosome. Chromosome Res. 15, 383-398. [DOI] [PubMed] [Google Scholar]
- 50.Cheng YM, Lin BY. 2003. Cloning and characterization of maize B chromosome sequences derived from microdissection. Genetics 164, 299-310. ( 10.1093/genetics/164.1.299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng YM, Lin BY. 2004. Molecular organization of large fragments in the maize B chromosome: indication of a novel repeat. Genetics 166, 1947-1961. ( 10.1093/genetics/166.4.1947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng YM. 2010. Evolution of the heterochromatic regions on maize B long arm based on the sequence structure of CL-repeat variants. Chromosome Res. 18, 605-619. ( 10.1007/s10577-010-9136-9) [DOI] [PubMed] [Google Scholar]
- 53.Chien YL, Lin CY, Lo KL, Cheng YM. 2014. Development and mapping of CL-repeat display markers on the maize B chromosome. Cytogenet. Genome Res. 144, 227-236. ( 10.1159/000370173) [DOI] [PubMed] [Google Scholar]
- 54.Cheng YM, Feng YR, Lin YP, Peng SF. 2016. Cytomolecular characterization and origin of de novo formed maize B chromosome variants. Chromosome Res. 24, 183-195. ( 10.1007/s10577-015-9516-2) [DOI] [PubMed] [Google Scholar]
- 55.Hsu FC, Wang CJ, Chen CM, Hu HY, Chen CC. 2003. Molecular characterization of a family of tandemly repeated DNA sequences, TR-1, in heterochromatic knobs of maize and its relatives. Genetics 164, 1087-1097. ( 10.1093/genetics/164.3.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peacock WJ, Dennis ES, Rhoades MM, Pryor AJ. 1981. Highly repeated DNA sequence limited to knob heterochromatin in maize. Proc. Natl Acad. Sci. USA 78, 4490-4494. ( 10.1073/pnas.78.7.4490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClintock B, Kato TA, Blumenschein A. 1981. Chromosome constitution of races of maize. Chapingo, Mexico: Colegio de Postgraduados. [Google Scholar]
- 58.Buckler ES, Phelps-Durr TL, Buckler CS, Dawe RK, Doebley JF. 1999. Meiotic drive of chromosomal knobs reshaped the maize genome. Genetics 153, 415-426. ( 10.1093/genetics/153.1.415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamb JC, Meyer JM, Corcoran B, Kato A, Han F, Birchler JA. 2007. Distinct chromosomal distributions of highly repetitive sequences in maize. Chromosome Res. 15, 33-49. ( 10.1007/s10577-006-1102-1) [DOI] [PubMed] [Google Scholar]
- 60.Albert PS, Gao Z, Danilova TV, Birchler JA. 2010. Diversity of chromosomal karyotypes in maize and its relatives. Cytogenet. Genome Res. 129, 6-16. ( 10.1159/000314342) [DOI] [PubMed] [Google Scholar]
- 61.Ananiev EV, Phillips RL, Rines HW. 1998. A knob-associated tandem repeat in maize capable of forming fold-back DNA segments: are chromosome knobs megatransposons? Proc. Natl Acad. Sci. USA 95, 10 785-10 790. ( 10.1073/pnas.95.18.10785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis ES, Peacock WJ. 1984. Knob heterochromatin homology in maize and its relatives. J. Mol. Evol. 20, 341-350. ( 10.1007/BF02104740) [DOI] [PubMed] [Google Scholar]
- 63.Page BT, Wanous MK, Birchler JA. 2001. Characterization of a maize chromosome 4 centromeric sequence: evidence for an evolutionary relationship with the B chromosome centromere. Genetics 159, 291-302. ( 10.1093/genetics/159.1.291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blavet N, et al. 2021. Sequence of the supernumerary B chromosome of maize provides insight into its drive mechanism and evolution. Proc. Natl Acad. Sci. USA 118, e2104254118. ( 10.1073/pnas.2104254118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato A, Zheng ZY, Auger DL, Phelps-Durr T, Bauer MJ, Lamb JC, Birchler JA. 2005. Minichromosomes derived from the B chromosome of maize. Cytogenet. Genome Res. 109, 156-165. ( 10.1159/000082395) [DOI] [PubMed] [Google Scholar]
- 66.Han F, Lamb JC, Yu W, Gao Z, Birchler JA. 2007. Centromere function and nondisjunction are independent components of the maize B chromosome accumulation mechanism. Plant Cell 19, 5243-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianchi A, Bellini G, Contin M, Ottaviano E. 1961. Nondisjunction in presence of interchanges involving B-type chromosomes in maize and some phenotypical consequences of meaning in maize breeding. Z. Vererbungsl. 92, 213-232. [Google Scholar]
- 68.González-Sánchez M, Rosato M, Chiavarino M, Puertas MJ. 2004. Chromosome instabilities and programmed cell death in tapetal cells of maize with B chromosomes and effect on pollen viability. Genetics 166, 999-1009. ( 10.1534/genetics.166.2.999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masonbrink RE, Birchler JA. 2010. Sporophytic nondisjunction of the maize B chromosome at high copy numbers. J. Genet. Genomics 37, 79-84. ( 10.1016/S1673-8527(09)60027-8) [DOI] [PubMed] [Google Scholar]
- 70.Abraham S, Smith HH. 1966. DNA synthesis in the B chromosomes of maize. J. Hered. 57, 78-80. ( 10.1093/oxfordjournals.jhered.a107479) [DOI] [PubMed] [Google Scholar]
- 71.Bass HW, Hoffman GG, Lee TJ, Wear EE, Joseph SR, Allen GC, Hanley-Bowdoin L, Thompson WF. 2015. Defining multiple, distinct, and shared spatiotemporal patterns of DNA replication and endoreduplication from 3D image analysis of developing maize (Zea mays L.) root tip nuclei. Plant Mol. Biol. 89, 339-351. ( 10.1007/s11103-015-0364-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wear EE, et al. 2017. Genomic analysis of the DNA replication timing program during mitotic S phase in maize (Zea mays) root tips. Plant Cell 29, 2126-2149. ( 10.1105/tpc.17.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masonbrink RE, Birchler JA. 2012. Accumulation of multiple copies of maize minichromosomes. Cytogenet. Genome Res. 137, 50-59. ( 10.1159/000339615) [DOI] [PubMed] [Google Scholar]
- 74.McClintock B. 1939. The behavior in successive nuclear division of a chromosome broken at meiosis. Proc. Natl Acad. Sci. USA 25, 405-416. ( 10.1073/pnas.25.8.405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Auger DL, Birchler JA. 2002. Maize tertiary trisomic stocks derived from B-A translocations. J. Hered. 93, 42-47. ( 10.1093/jhered/93.1.42) [DOI] [PubMed] [Google Scholar]
- 76.Carlson WR. 1969. Factors affecting preferential fertilization in maize. Genetics 62, 543-554. ( 10.1093/genetics/62.3.543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlson WR. 1999. Reversal of preferential fertilization. Maize Newslett. 73, 39. [Google Scholar]
- 78.Carlson WR. 2007. Locating a site on the maize B chromosome that controls preferential fertilization. Genome 50, 578-587. ( 10.1139/G07-035) [DOI] [PubMed] [Google Scholar]
- 79.Chiavarino AM, Rosato M, Manzanero S, Jimenez G, González-Sánchez M, Puertas MJ. 2000. Chromosome nondisjunction and instabilities in tapetal cells are affected by B chromosome in maize. Genetics 155, 889-897. ( 10.1093/genetics/155.2.889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosato M, Chiavarino AM, Naranjo CA, Puertas MJ, Poggio L. 1996. Genetic control of B chromosome transmission rate in Zea mays ssp. mays (Poaceae). Am. J. Bot. 83, 1107-1112. ( 10.1002/j.1537-2197.1996.tb13890.x) [DOI] [PubMed] [Google Scholar]
- 81.Chiavarino AM, González-Sánchez M, Poggio L, Puertas MJ, Rosato M, Rosi P. 2001. Is maize B chromosome preferential fertilization controlled by a single gene? Heredity 86, 743-748. ( 10.1046/j.1365-2540.2001.00894.x) [DOI] [PubMed] [Google Scholar]
- 82.Carlson WR. 1970. A test for involvement of the polar nuclei in preferential fertilization. Maize Newslett. 44, 91-92. [Google Scholar]
- 83.Birchler JA, Chalfoun DJ, Levin DM. 1990. Recombination in the B chromosome of maize to produce A-B-A chromosomes. Genetics 126, 723-733. ( 10.1093/genetics/126.3.723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masonbrink RE, Birchler JA. 2012. Multiple maize minichromosomes in meiosis. Chromosome Res. 20, 395-402. ( 10.1007/s10577-012-9283-2) [DOI] [PubMed] [Google Scholar]
- 85.Han F, Gao Z, Yu W, Birchler JA. 2007. Minichromosome analysis of chromosome pairing, disjunction, and sister chromatid cohesion in maize. Plant Cell 19, 3853-3863. ( 10.1105/tpc.107.055905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawe RK, et al. 2018. A kinesin-14 motor activates neocentromeres to promote meiotic drive in maize. Cell 173, 839-850.e18. ( 10.1016/j.cell.2018.03.009) [DOI] [PubMed] [Google Scholar]
- 87.Swentowsky KW, Gent JI, Lowry EG, Schubert V, Ran X, Tseng KF, Harkess AE, Qiu W, Dawe RK. 2020. Distinct kinesin motors drive two types of maize neocentromeres. Genes Dev. 34, 1239-1251. ( 10.1101/gad.340679.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rhoades MM, Dempsey E, Ghidoni A. 1967. Chromosome elimination in maize induced by supernumerary B chromosomes. Proc. Natl Acad. Sci. USA 57, 1626-1632. ( 10.1073/pnas.57.6.1626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhoades MM, Dempsey E. 1972. On the mechanism of chromatin loss induced by the B chromosome of maize. Genetics 71, 73-96. ( 10.1093/genetics/71.1.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bianchi A, Ghatnekar MV, Ghidoni A. 1963. Knobs in Italian maize. Chromosoma 14, 601-617. ( 10.1007/BF00326514) [DOI] [Google Scholar]
- 91.Longley AE. 1938. Chromosomes of maize from North American Indians. J. Agric. Res. 56, 177-195. [Google Scholar]
- 92.Birchler JA, Han F. 2009. Maize centromeres: structure, function, epigenetics. Ann. Rev. Genetics 43, 287-303. ( 10.1146/annurev-genet-102108-134834) [DOI] [PubMed] [Google Scholar]
- 93.Yu W, Han F, Gao Z, Vega JM, Birchler JA. 2007. Construction and behavior of engineered minichromosomes in maize. Proc. Natl Acad. Sci. USA 104, 8924-8929. ( 10.1073/pnas.0700932104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaeta RT, Masonbrink RE, Zhao C, Sanyal A, Krishnaswamy L, Birchler JA. 2013. In vivo modification of a maize engineered minichromosome. Chromosoma 122, 221-232. ( 10.1007/s00412-013-0403-3) [DOI] [PubMed] [Google Scholar]
- 95.Birchler JA. 2015. Promises and pitfalls of synthetic chromosomes in plants. Trends Biotechnol. 33, 189-194. ( 10.1016/j.tibtech.2014.12.010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.