Abstract
Background:
Female breast cancer is the most commonly diagnosed cancer in the world, with wide variations in reported survival by country. Women in low-income and middle-income countries (LMICs) in particular face multiple barriers to breast cancer services, including diagnostics and treatment. In this analysis we estimate the potential impact of scaling up the availability of treatment and imaging modalities on global breast cancer survival, together with improvements in quality of care.
Methods:
We used a microsimulation model of global cancer survival which accounts for the availability and stage-specific survival impact of specific treatment modalities (chemotherapy, radiotherapy, surgery, targeted therapy), imaging modalities (ultrasound, X-ray, CT, MRI, PET, SPECT), and quality of cancer care to simulate 5-year net survival for newly diagnosed breast cancer cases in 200 countries. We calibrated the model to empirical data on 5-year net breast cancer survival in 2010-14 from CONCORD-3. We evaluated the potential impact of scaling up specific imaging and treatment modalities and quality of care to the mean level of high-income countries, individually and in combination. We ran 1,000 simulations for each policy intervention and report the means and 95% uncertainty intervals for all model outcomes.
Results:
We estimate that global 5-year net survival for women diagnosed with breast cancer in 2018 is 67·9% (95% UI 62·9-73·4), with a 25-times difference between low-income (3·5% [95% UI 0·4-10·0]) and high-income (87·0% [95% UI 85·6-88·4]) countries. Among individual treatment modalities, scaling up access to surgery would yield the largest survival gains globally (2·7% [95% UI 0·4-8·3]), and CT would have the largest global impact among imaging modalities (0·5% [95% UI 0·0-2·0]). Scaling up a package of traditional modalities (surgery, chemotherapy, radiotherapy, ultrasound, X-ray) could improve global 5-year net survival to 75·6% (95% UI 70·6-79·4), with survival in low-income countries improving to 28·6% (95% UI 4·9-60·1). Adding concurrent improvements in quality of care could further improve global 5-year net survival to 78·2% (95% UI 74·9-80·4), with substantial impact in low-income countries, improving net survival to 55·3% [95% UI 42·2-67·8]). Comprehensive scale-up of access to all modalities and quality of care could improve global 5-year net survival to 82·3% (95% UI 79·3-85·0).
Interpretation:
Comprehensive scale-up of treatment, imaging, and quality of care could improve global 5-year net breast cancer survival by nearly 15 percentage points. Scale-up of traditional modalities and quality of care could achieve 70% of these total potential gains, with substantial impact in LMICs, providing a more feasible pathway to improving breast cancer survival in these settings even without the benefits of future investments in targeted therapy and advanced imaging.
Funding:
Harvard T H Chan School of Public Health, National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center, and Breast Cancer Research Foundation.
INTRODUCTION
Female breast cancer is the most commonly diagnosed cancer in the world, with an estimated 2·3 million new cases and nearly 700,000 deaths each year.1 In low-income and middle-income countries (LMICs) especially, women face barriers to breast cancer care and experience poor survival outcomes. Reported 5-year net survival outcomes for women with breast cancer are 40-60% in LMICs versus over 85% in many high-income countries (HICs),2 which have experienced improvements in breast cancer mortality rates due to improvements in early detection and adjuvant systemic therapy.3 In contrast to higher income settings, a large proportion of patients in LMICs present with advanced stage breast cancer, due to delays in diagnosis associated with both patient-level barriers (e.g. lack of education, traditional medicine care-seeking) and health system barriers (e.g. healthcare provider education, lack of access to diagnostic imaging).4,5
To improve survival outcomes, the World Health Organization (WHO)6 and the Breast Health Global Initiative (BHGI)7 have prioritized the implementation of breast cancer early diagnosis as the first phase of early detection program development in resource-limited settings, as a prerequisite to screening.8 Early diagnosis requires accessible diagnostic breast imaging and biopsy capability, which are used for cancer detection and tissue diagnosis, which is needed for treatment planning.6
Improvements in access to breast cancer education, diagnostic imaging, pathologic tissue diagnosis, and effective cancer treatment will all be needed to improve global breast cancer survival. Resource-stratified clinical practice guidelines for women with breast cancer have been published by the Breast Health Global Initiative9 and the National Comprehensive Cancer Network,10 which provide guidance on recommended care by cancer stage at various levels of healthcare capability. However, a recent review of 49 distinct guidelines for breast cancer treatment standards finds that the majority are not context appropriate, and that further research on the formulation of cancer treatment standards is needed.11 Estimating the survival impact of specific breast cancer treatment components can therefore help provide guidance to policy-makers to prioritize investments in their own context. In this analysis we estimate the potential impact of scaling up the availability of treatment and imaging modalities on global breast cancer survival, together with improvements in quality of care.
METHODS
Overview
We used a previously developed microsimulation (individual-level) model of stage-specific breast cancer survival for newly diagnosed patients in 2018 in 200 countries/territories (see Appendix pg 2-13). The model takes into account the availability and survival impact of specific treatment modalities (chemotherapy, radiotherapy, surgery, targeted therapy), and imaging modalities (ultrasound, X-ray, computed tomography [CT], magnetic resonance imaging [MRI], positron-emission tomography [PET], single-photon emission computed tomography [SPECT]) (see Appendix pg 11-12).12 We modelled targeted therapies as a group, which includes hormonal therapy, but for the purposes of this study individual therapies were not analysed separately. The model also simulates quality of care, capturing health-system and facility-level factors that account for residual differences in survival not explained by cancer stage or treatment and imaging availability (e.g. quality of image acquisition, nursing standards, infection control, etc) (see Appendix pg 13).12 We used hierarchical models to synthesize data for each model input and estimate parameters for countries for which no data were available (see Appendix pg 2). We simulated breast cancer survival in each country, and evaluated the potential impact of scaling up access to treatment and imaging modalities, as well as improving quality of care.
Survival impact of treatment/imaging modalities
In addition to treatment modalities, imaging also plays an important role in the management of breast cancer. Due to insufficient data on underlying cancer incidence and stage distribution, especially regarding the total (i.e. diagnosed and undiagnosed) cases in each country, we focused our analysis on the survival impact of treatment and imaging modalities conditional on diagnosis and stage, and did not consider the potential benefits of imaging on early diagnosis or screening. However, aside from screening, increasing the use of imaging can help to improve the quality of breast cancer treatment in a number of ways, from aiding in initial diagnosis and staging to guiding treatment decisions and assessing response.
Although progression-free survival estimates are available from large cohort studies for different targeted treatments, there is little information available for each modality on overall survival impact compared to no treatment, which is the relevant comparator needed to parameterize the model. We therefore used a two-stage survey to elicit expert opinions on the impact of specific treatment and imaging modalities on stage-specific breast cancer 5-year net survival, previously described.12 A sample of actively practicing physicians was selected from collaborating institutions, based on expertise in their field (cancer imaging and/or therapy), with experience in both high- and low-income countries. Respondents were asked to indicate the impact of each treatment/imaging modality on stage-specific five-year net survival for newly diagnosed breast cancer patients using a four-point scale, ranging from ‘necessary for 5-year survival’ to ‘no impact on 5-year survival’. We received between 18-34 responses for each modality. To provide consensus results, responses with at least 75% agreement were accepted as final responses, while responses with lower levels of agreement were discussed by a panel of experts to forge final consensus. However, for modelling purposes all responses (regardless of level of agreement) were used to estimate prior probability distributions for the probability that each modality was necessary by weighting the responses and estimating Beta distributions with the sum of the weighted estimates (see Appendix pg 10-11 for details).
We also model the probability that each patient would benefit from ‘modern’ modalities such as CT, based on historical trends in achievable survival before these modalities were available (see Appendix pg 11-12). This helps guard against overestimating the clinical benefit of modern modalities for breast cancer survival.
We assumed that the survival impacts of each modality were independent for each individual patient. However, we assumed that if a modality simulated as ‘needed for survival’ for a patient was unavailable then survival was 0. This model structure allowed for potential interactions between modalities, as multiple modalities may need to be scaled-up before all ‘required’ modalities are available for a given patient.
Quality of care
In addition to the impact of specific treatment and imaging modalities, we also included country-specific parameters for quality of care, defined by the Institute of Medicine as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge”.13 This parameter captures health-system and facility-level factors that account for residual differences in survival not explained by cancer stage or treatment and imaging availability. We set wide priors with increasing probability of quality by income group (25%, 50%, 75%, 95%), and zero-mean priors for the other levels in the hierarchical model. Adequate quality of care is assumed to be a prerequisite for survival in the model, and was thus not included in the expert opinion survey. This parameter can therefore be interpreted as the probability that the quality of care available is adequate to ensure 5-year survival, given the availability of all necessary treatment and imaging modalities.
Stage at diagnosis
Survival estimates from 2010-2016 in the US from the Surveillance, Epidemiology, and End Results (SEER) Program reveal substantial differences in 5-year (net) survival by stage: Stage I = 100%; Stage II = 92%; Stage III = 74%; Stage IV = 27%.14 To estimate the stage distribution of diagnosed breast cancer globally we performed a literature review of reported stage distribution (I-IV) by country, previously described.12 Estimates of breast cancer stage at diagnosis were available from 162 studies in 84 countries (see Appendix pg 3-9). We used a hierarchical modeling approach to regularize the reported estimates and estimate stage distribution for countries for which no data were available.12
Survival estimates
Using our microsimulation model of global cancer survival, we estimated five-year net survival in each country for breast cancer patients diagnosed in 2018 (based on GLOBOCAN 2018 estimates), accounting for the joint distribution of age and stage. Model inputs for the availability of treatment modalities were based on published estimates, and the availability of imaging modalities was estimated using the International Atomic Energy Agency (IAEA) IMAGINE database.15 The model was calibrated to empirical data on five-year net cancer survival in 2010-14 from CONCORD-3 (see Appendix pg 14-19).16 We do not consider mortality from other causes in this analysis as our outcome of net (relative) survival already accounts for other-cause mortality. Full details on the model development are available elsewhere.12 We estimated current stage-specific breast cancer survival in each country, and evaluated the potential impact of individual policy interventions which expand the availability of specific treatment and imaging modalities to the mean level of high-income countries. We also simulated more comprehensive scale-up packages which simultaneously expand the availability of multiple treatment and imaging modalities: treatment only, imaging only, traditional modalities (chemotherapy, radiotherapy, surgery, ultrasound, x-ray), and comprehensive (all treatment and imaging modalities), both with and without concurrent improvements in quality of care. Lastly, to illustrate potential pathways to scale-up, we evaluated the effects of cumulative policy scale-up in which we sequentially added scale-up of specific modalities and improved quality of care. We estimated the cumulative impact of sequentially expanding access to: 1) treatment availability (traditional modalities); 2) imaging availability (traditional modalities); 3) quality of care; 4) targeted therapy; 5) CT; 6) MRI; 7) SPECT; 8) PET.
Although breast cancer mortality may be a better outcome when evaluating the impact of policies which may change when patients are diagnosed (e.g. due to the potential for lead-time bias), as all of our model estimates are conditional on stage and diagnosis (i.e. the modelled policies do not change the point at which patients are diagnosed), we believe that five-year net survival is an appropriate outcome for evaluating the comparative effectiveness of these policies. When calibrating the model we ran 2,000 independent search chains of 1,000 iterations each, and selected the final 100 best-fitting parameter sets to account for uncertainty around the model parameters.12 In each of the final 1,000 policy scenario simulations we sampled a parameter set (from the best-fitting 100 sets) at random, accounting for both first-order (patient-level stochastic) and second-order (parameter) uncertainty. We chose 1,000 simulations as a balance between computational efficiency and the stability of our simulated outcomes. We report the mean and 95% uncertainty intervals (UI), calculated as the 2·5 and 97·5 percentiles of the simulation results. Our estimated 95% UIs, reported for all model outcomes, therefore indicate the sensitivity of our results to different parameter values and account for their joint distribution. To assess the robustness of our predictions, we compared our results to our training set of CONCORD estimates (i.e. used to calibrate the model), as well as to a test set of randomly selected CONCORD estimates withheld from calibration (i.e. not used to fit the model) (see Appendix pg 14-17). The simulation model was developed in Java (version 1.8.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the data, and the corresponding author had the final responsibility to submit for publication.
RESULTS
Expert opinion consensus results for the impact of each treatment and imaging modality on five-year net breast cancer survival are presented in the Appendix (pg 11). The findings suggest that while surgery alone may be used to manage stage I breast cancer (though in some cases systemic therapy is required, and radiation required for patients undergoing partial mastectomy), in addition to surgery the use of chemotherapy, hormonal therapy, and targeted therapies are often required to treat stage II-stage III cancers, while chemotherapy, hormonal therapy, targeted therapy, and radiation are often utilized for patients with stage IV cancers. With respect to imaging, expert opinion indicates that ultrasound is necessary for all stages of breast cancer, MRI can be helpful in selected patients for further breast evaluation, while CT, PET, and SPECT are recommended for more advanced stages.
Assessing the model fit, we found that the model prediction intervals (95% UI) for the training set of breast cancer calibration targets overlapped with the CONCORD 95% CIs 92·6% of the time, contained the reported point estimate (i.e. coverage probability) 83·3% of the time, and that our mean five-year net survival estimates had a mean absolute error of 4·28 (SD 5·87) percentage points compared to the CONCORD point estimates (see Appendix pg 14-16).12 Although the test set of CONCORD estimates (not used for calibration) only comprised five estimates for breast cancer, the model performed well compared to this small test set: the prediction intervals contained these estimates with 80% coverage and a mean absolute error of 2·34 (SD 1·40) percentage points (see Appendix pg 17).12 These predictive accuracy checks on data not used to fit the model help to build confidence in the robustness of the model estimates.
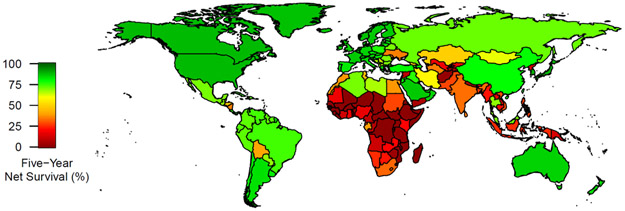
Posterior means and 95% UIs of breast cancer stage distribution and stage-specific survival are reported from the calibrated simulation model by country income group and geographic area in Table 1. We estimate that 72·2% (95% UI 47·6-93·9) (53,830/74,610) of breast cancer cases are diagnosed at advanced stage (III-IV) in low-income countries, compared to 18·8% (95% UI 11·7-26·0) (156,560/834,925) in high-income countries (see Appendix pg 20). We estimate that overall global five-year net survival is 67·9% (95% UI 62·9-73·4) and varies widely by country income group and geographic area, with a nearly 25-times difference in net survival between low-income (3.5% [95% UI 0.4-10.0]) and high-income (87.0% [95% 85.6-88.4]) groups and large variation by country (see Figure 1).
Table 1:
Breast cancer stage at diagnosis and survival by income group and area, means (95% UI)
| Stage I | Stage II | Stage III | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|
| % of cases | 5-year net survival (%) |
% of cases | 5-year net survival (%) |
% of cases | 5-year net survival (%) |
% of cases | 5-year net survival (%) |
|
| GLOBAL | 27·4 (19·2-35·5) | 92·9 (87·0-96·5) | 39·6 (30·7-50·4) | 73·9 (66·3-80·5) | 24·6 (16·8-33·3) | 48·3 (39·6-57·7) | 8·4 (4·2-13·9) | 15·7 (10·6-20·9) |
| Low Income | 11·2 (0·2-38·1) | 8·0 (0·4-37·6) | 16·7 (2·6-41·9) | 4·9 (0·2-17·3) | 48·0 (25·5-70·3) | 2·8 (0·2-9·4) | 24·1 (5·6-47·2) | 2·2 (0·1-7·2) |
| Lower-Middle Income | 7·9 (1·7-16·9) | 47·7 (11·8-83·7) | 41·6 (22·3-58·9) | 36·8 (5·9-65·7) | 36·2 (15·5-59·1) | 26·4 (7·3-48·6) | 14·3 (2·2-30·3) | 10·2 (5·1-15·7) |
| Upper-Middle Income | 22·3 (13·0-35·7) | 95·0 (92·2-97·1) | 43·2 (28·4-57·2) | 82·4 (77·1-87·1) | 28·3 (17·0-41·0) | 61·7 (56·9-67·6) | 6·3 (2·1-11·5) | 19·0 (13·6-23·7) |
| High Income | 43·9 (29·8-51·9) | 98·0 (96·5-99·1) | 37·4 (28·5-49·1) | 89·6 (86·2-92·6) | 13·1 (6·6-21·8) | 69·6 (64·9-73·7) | 5·6 (3·1-9·7) | 24·1 (18·3-28·8) |
| Africa | 11·2 (2·5-29·2) | 44·8 (13·3-73·8) | 24·8 (10·8-45·1) | 35·2 (17·2-55·4) | 44·1 (20·5-65·4) | 17·5 (7·2-31·2) | 19·9 (8·3-41·3) | 6·5 (2·5-11·4) |
| Asia | 19·9 (11·3-30·9) | 89·3 (76·3-96·9) | 44·3 (26·5-57·5) | 66·5 (53·1-77·9) | 27·6 (16·5-41·8) | 46·2 (30·6-59·0) | 8·2 (2·1-16·0) | 14·5 (8·3-20·6) |
| Europe | 36·7 (24·8-47·2) | 96·5 (94·0-98·7) | 38·7 (29·0-53·7) | 84·7 (78·9-90·0) | 18·0 (10·4-28·4) | 61·3 (52·9-67·3) | 6·6 (3·6-10·5) | 20·1 (14·3-25·3) |
| Latin America and the Caribbean | 23·2 (12·2-44·2) | 95·8 (91·0-98·3) | 40·4 (26·9-57·3) | 82·3 (72·3-88·0) | 28·7 (16·7-40·3) | 60·6 (52·1-66·7) | 7·7 (3·2-14·8) | 18·3 (13·5-23·6) |
| North America | 48·0 (33·1-55·7) | 98·8 (97·4-99·7) | 33·9 (25·4-45·4) | 92·2 (87·8-94·8) | 12·1 (5·9-19·2) | 73·4 (67·7-77·6) | 6·0 (3·1-10·0) | 26·8 (21·6-31·4) |
| Oceania | 39·4 (23·0-49·2) | 96·0 (89·6-99·2) | 39·4 (29·8-50·0) | 81·6 (72·1-90·4) | 15·6 (5·7-31·0) | 55·7 (42·3-67·9) | 5·6 (2·9-9·2) | 20·2 (12·7-28·5) |
Figure 1:
Estimated 5-year breast cancer net survival by country
We find that the scale-up of specific treatment modalities that would yield the largest survival gains varies by income group and area (see Table 2). Specifically, we find that scaling up access to surgery would yield the largest survival gains globally (2·7% [95% UI 0·4-8·3]) and in low-income and lower-middle-income countries, and in Africa, Asia, and Oceania in general, while expanding targeted therapy availability would yield the largest gains in Latin America and the Caribbean and upper-middle-income and high-income countries as a whole.
Table 2:
Estimated 5-year net breast cancer survival by income group and area under various single policy interventions, means (95% UI)
| Increased treatment availability | Increased imaging availability | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Chemo- therapy |
Radio- therapy |
Surgery | Targeted therapy |
Quality | Ultrasound | CT (+X-ray) | MRI | PET | SPECT | ||
| Global | Survival (%) | 67·9 (62·9-73·4) | 69·0 (63·8-74·6) | 69·4 (64·2-74·0) | 70·6 (65·1-75·4) | 68·7 (63·4-74·1) | 68·9 (63·5-73·9) | 68·1 (63·0-73·5) | 68·4 (63·2-73·8) | 68·3 (63·0-73·8) | 68·2 (63·2-73·8) | 68·2 (63·2-73·6) |
| Gain (%) | --- | 1·1 (0·1-3·4) | 1·5 (0·2-4·4) | 2·7 (0·4-8·3) | 0·8 (0·2-2·2) | 1·0 (0·1-3·8) | 0·3 (0·0-1·2) | 0·5 (0·0-2·0) | 0·4 (0·0-1·2) | 0·3 (0·0-0·9) | 0·3 (0·0-0·8) | |
| Low income | Survival (%) | 3·5 (0·4-10·0) | 4·6 (0·5-14·7) | 5·8 (0·6-15·8) | 6·4 (0·7-21·7) | 3·5 (0·4-10·1) | 6·2 (0·9-14·3) | 4·7 (0·7-12·9) | 4·0 (0·5-11·6) | 3·5 (0·4-10·1) | 3·5 (0·4-10·1) | 3·5 (0·4-10·0) |
| Gain (%) | --- | 1·1 (0·0-4·4) | 2·4 (0·2-6·1) | 3·0 (0·1-10·6) | 0·0 (0·0-0·2) | 2·8 (0·0-9·9) | 1·2 (0·0-4·4) | 0·6 (0·0-2·3) | 0·1 (0·0-0·2) | 0·0 (0·0-0·1) | 0·0 (0·0-0·1) | |
| Lower middle income | Survival (%) | 30·2 (8·6-51·6) | 33·4 (9·6-60·8) | 36·0 (13·7-56·0) | 40·7 (16·4-60·7) | 30·9 (8·6-52·1) | 33·8 (9·7-53·6) | 31·2 (8·6-53·2) | 31·6 (8·8-51·9) | 31·0 (8·7-52·6) | 30·5 (8·7-52·3) | 30·4 (8·7-52·4) |
| Gain (%) | --- | 3·2 (0·0-12·5) | 5·8 (0·1-17·9) | 10·4 (0·3-35·9) | 0·6 (0·1-1·9) | 3·5 (0·1-14·1) | 0·9 (0·0-5·7) | 1·3 (0·0-7·9) | 0·7 (0·0-1·8) | 0·3 (0·0-0·7) | 0·2 (0·0-0·6) | |
| Upper middle income | Survival (%) | 75·5 (71·4-79·5) | 76·5 (72·0-79·7) | 76·0 (71·4-80·2) | 76·3 (72·2-80·3) | 76·8 (72·2-80·9) | 75·8 (71·4-79·9) | 75·5 (71·4-79·5) | 75·9 (71·5-80·2) | 76·2 (71·4-80·8) | 76·1 (71·9-80·8) | 76·0 (72·2-80·2) |
| Gain (%) | --- | 1·0 (0·0-2·6) | 0·5 (0·0-1·4) | 0·9 (0·0-2·5) | 1·4 (0·1-4·6) | 0·3 (0·0-1·3) | 0·0 (0·0-0·1) | 0·4 (0·0-1·6) | 0·7 (0·0-2·6) | 0·6 (0·0-1·8) | 0·5 (0·0-2·1) | |
| High income | Survival (%) | 87·0 (85·6-88·4) | 87·1 (85·7-88·6) | 87·1 (85·7-88·6) | 87·2 (85·6-88·5) | 87·5 (85·9-88·9) | 87·2 (85·7-88·6) | 87·0 (85·6-88·4) | 87·1 (85·6-88·5) | 87·1 (85·6-88·4) | 87·2 (85·6-88·4) | 87·2 (85·8-88·7) |
| Gain (%) | --- | 0·1 (0·0-0·5) | 0·1 (0·0-0·5) | 0·2 (0·0-0·7) | 0·5 (0·1-1·0) | 0·2 (0·0-0·7) | 0·0 (0·0-0·0) | 0·1 (0·0-0·4) | 0·1 (0·0-0·2) | 0·2 (0·0-0·6) | 0·2 (0·0-0·8) | |
| Africa | Survival (%) | 22·7 (11·5-34·2) | 25·7 (13·9-38·3) | 27·2 (14·7-40·4) | 27·5 (14·5-43·7) | 23·2 (11·6-34·9) | 25·5 (12·5-39·1) | 23·7 (12·0-34·7) | 23·5 (11·5-37·6) | 23·1 (11·6-35·3) | 22·9 (11·6-34·4) | 22·8 (11·5-34·8) |
| Gain (%) | --- | 3·0 (0·1-8·5) | 4·5 (0·3-15·9) | 4·8 (0·3-15·7) | 0·5 (0·0-1·4) | 2·8 (0·0-10·8) | 1·0 (0·0-4·7) | 0·9 (0·0-5·4) | 0·4 (0·0-1·2) | 0·2 (0·0-0·8) | 0·1 (0·0-0·6) | |
| Asia | Survival (%) | 61·1 (51·3-71·4) | 62·6 (52·0-73·0) | 63·4 (53·0-72·5) | 66·0 (54·0-75·6) | 62·0 (51·7-72·1) | 62·5 (51·8-72·0) | 61·5 (51·3-71·5) | 61·8 (51·5-72·1) | 61·8 (51·5-72·6) | 61·5 (51·4-71·6) | 61·5 (51·9-71·9) |
| Gain (%) | --- | 1·5 (0·0-6·6) | 2·3 (0·0-8·7) | 4·9 (0·1-17·3) | 0·9 (0·0-3·2) | 1·3 (0·0-8·0) | 0·4 (0·0-2·9) | 0·6 (0·0-3·8) | 0·7 (0·0-2·3) | 0·3 (0·0-1·2) | 0·4 (0·0-1·5) | |
| Europe | Survival (%) | 80·6 (78·0-83·1) | 81·0 (78·1-83·3) | 81·2 (78·6-83·5) | 81·0 (78·1-83·6) | 81·6 (78·8-83·8) | 81·3 (78·9-83·2) | 80·7 (78·0-83·2) | 80·9 (78·1-84·3) | 80·9 (78·1-83·4) | 81·0 (78·1-83·7) | 80·8 (78·3-83·2) |
| Gain (%) | --- | 0·4 (0·0-2·2) | 0·6 (0·0-2·3) | 0·4 (0·0-1·6) | 0·9 (0·1-2·0) | 0·6 (0·0-3·5) | 0·0 (0·0-0·3) | 0·2 (0·0-1·2) | 0·3 (0·0-1·1) | 0·4 (0·0-1·2) | 0·1 (0·0-0·6) | |
| Latin America and the Caribbean | Survival (%) | 74·3 (68·5-78·6) | 75·4 (69·6-80·8) | 74·6 (68·8-79·1) | 75·0 (69·1-79·0) | 75·6 (69·6-81·3) | 74·6 (68·8-79·3) | 74·3 (68·5-78·6) | 75·2 (69·6-81·4) | 74·6 (68·6-83·1) | 75·0 (68·7-79·8) | 74·6 (68·5-78·9) |
| Gain (%) | --- | 1·1 (0·0-6·2) | 0·3 (0·0-1·2) | 0·7 (0·0-4·8) | 1·3 (0·0-4·8) | 0·3 (0·0-2·1) | 0·0 (0·0-0·5) | 0·9 (0·0-3·6) | 0·3 (0·0-3·9) | 0·7 (0·0-3·0) | 0·3 (0·0-1·8) | |
| Northern America | Survival (%) | 89·2 (86·7-91·1) | 89·3 (86·7-91·1) | 89·3 (86·7-91·1) | 89·4 (86·7-91·5) | 89·3 (86·7-91·3) | 89·3 (86·7-91·3) | 89·2 (86·7-91·1) | 89·3 (86·7-91·1) | 89·2 (86·7-91·1) | 89·3 (86·7-91·2) | 89·5 (87·0-91·7) |
| Gain (%) | --- | 0·0 (0·0-0·1) | 0·0 (0·0-0·2) | 0·1 (0·0-1·6) | 0·0 (0·0-0·7) | 0·1 (0·0-1·2) | 0·0 (0·0-0·0) | 0·0 (0·0-0·1) | 0·0 (0·0-0·0) | 0·0 (0·0-0·0) | 0·3 (0·0-2·3) | |
| Oceania | Survival (%) | 80·0 (74·2-85·8) | 80·3 (74·5-85·9) | 80·9 (74·7-86·2) | 81·0 (75·2-88·9) | 80·8 (75·4-85·9) | 80·8 (74·2-85·9) | 80·1 (74·2-85·8) | 80·3 (74·2-85·9) | 80·0 (74·2-85·9) | 80·2 (74·2-86·1) | 80·4 (74·7-86·5) |
| Gain (%) | --- | 0·3 (0·0-2·3) | 0·9 (0·0-3·7) | 1·0 (0·0-6·4) | 0·8 (0·0-3·5) | 0·9 (0·0-4·2) | 0·1 (0·0-1·3) | 0·3 (0·0-2·0) | 0·0 (0·0-0·3) | 0·2 (0·0-2·3) | 0·5 (0·0-2·6) | |
For imaging, we also find that the scale-up of individual modalities that would yield the largest survival gains varies by context (see Table 2). Expanding the availability of CT would yield the largest survival gains globally (0·5% [95% UI 0·0-2·0]), and in lower-middle income countries and Latin America and the Caribbean in particular. Expanding ultrasound would yield the largest survival gains in low-income countries and Africa, while MRI is estimated to yield the largest survival benefits in upper-middle-income countries and Asia, with PET (Europe) and SPECT (North America, Oceania) yielding the largest benefits in high income countries. However, the gains from expanding any single treatment or imaging modality individually are small.
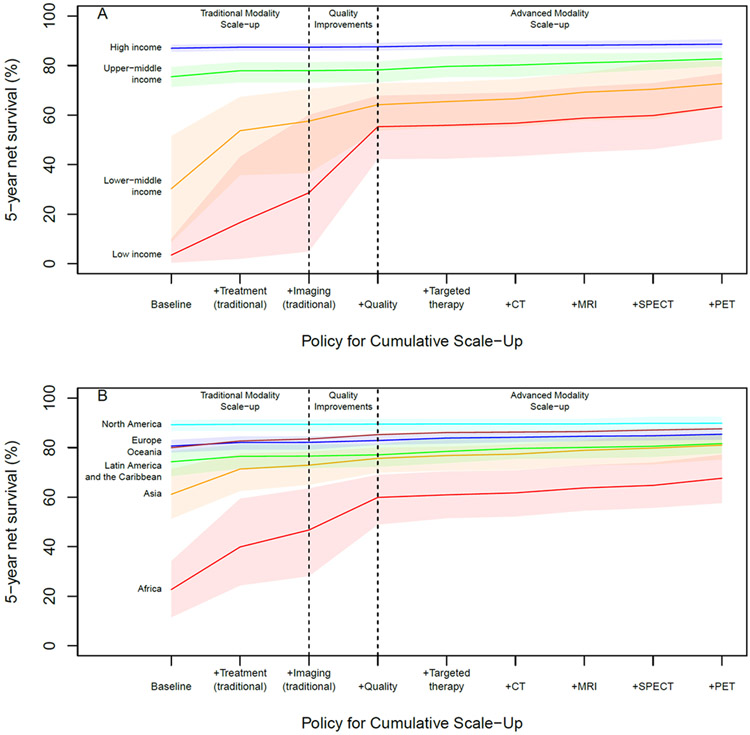
We find that scaling up the availability of all treatment modalities to the mean level of high-income countries could improve global 5-year net breast cancer survival to 75·3% (95% UI 70·4-79·1), while increasing availability of all imaging modalities to the mean level of high-income countries could improve global 5-year net survival to 70·0% (95% UI 64·2-75·2) (see Table 3). Increasing only traditional modalities (chemotherapy, radiotherapy, surgery, ultrasound, x-ray) could improve global 5-year net survival to 75·6% (95% UI 70·6-79·4), and adding quality of care improvements could further improve survival to 78·2% (95% UI 74·9-80·4), while comprehensive scale-up of all modalities and quality of cancer care could raise global 5-year net survival to 82·3% (95% UI 79·3-85·0) (see Table 4), with substantial survival gains in low-income (3·5% [95% UI 0·4-10·0] to 63·4% [95% 50·2-76·8]) and lower-middle income (30·2% [95% UI 8·6-51·6] to 72·7% [95% UI 63.1-81.9]) countries (see Figure 2a) and Africa (22·7% [95% UI 11·5-34·2] to 67·6% [95% UI 57·6-77·2]) and Asia (61·1% [95% UI 51·3-71·4] to 81·0% [95% UI 75·2-85·8]) (see Figure 2b).
Table 3:
Policy packages to scale-up availability of treatment/imaging modalities
| Baseline | No quality improvements | With improvements in quality of care | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment only |
Imaging only |
Traditional only* |
Comprehen sive |
Treatment only |
Imaging only |
Traditional only* |
Comprehen sive |
||
| Global | 67·9 (62·9-73·4) | 75·3 (70·4-79·1) | 70·0 (64·2-75·2) | 75·6 (70·6-79·4) | 79·4 (73·9-83·3) | 77·3 (73·3-80·6) | 71·2 (65·0-76·9) | 78·2 (74·9-80·4) | 82·3 (79·3-85·0) |
| Low income | 3·5 (0·4-10·0) | 16·8 (2·0-43·8) | 6·0 (0·8-14·8) | 28·6 (4·9-60·1) | 32·7 (5·5-65·9) | 30·4 (11·0-53·9) | 11·8 (3·3-31·0) | 55·3 (42·2-67·8) | 63·4 (50·2-76·8) |
| Lower middle income | 30·2 (8·6-51·6) | 54·8 (36·2-68·0) | 34·7 (9·2-59·6) | 57·6 (36·6-70·6) | 65·2 (42·9-78·0) | 61·1 (43·5-73·3) | 38·7 (11·0-62·6) | 64·2 (53·8-72·9) | 72·7 (63·1-81·9) |
| Upper middle income | 75·5 (71·4-79·5) | 79·3 (75·1-83·4) | 78·0 (73·7-82·0) | 77·9 (73·1-81·4) | 82·4 (79·5-85·7) | 79·6 (75·4-83·8) | 78·3 (73·7-82·4) | 78·2 (73·2-81·7) | 82·7 (79·8-85·9) |
| High income | 87·0 (85·6-88·4) | 87·9 (86·4-89·5) | 87·5 (86·0-89·0) | 87·4 (86·0-88·8) | 88·5 (87·1-90·2) | 88·1 (86·5-89·8) | 87·7 (86·3-89·4) | 87·6 (86·1-89·1) | 88·6 (87·1-90·6) |
| Africa | 22·7 (11·5-34·2) | 40·7 (24·8-60·1) | 26·0 (12·6-40·4) | 46·7 (28·1-63·6) | 52·5 (33·6-72·5) | 48·8 (30·2-64·5) | 30·0 (14·8-50·3) | 59·9 (48·9-69·0) | 67·6 (57·6-77·2) |
| Eastern Africa | 6·2 (1·4-16·4) | 19·7 (1·9-55·3) | 8·7 (1·6-23·3) | 31·1 (2·9-68·6) | 35·5 (3·3-75·6) | 31·5 (9·4-68·0) | 14·5 (4·0-38·2) | 55·8 (36·2-73·8) | 63·8 (43·1-82·6) |
| Middle Africa | 5·4 (0·2-23·7) | 24·0 (1·2-63·6) | 7·9 (0·3-29·5) | 34·8 (1·7-65·2) | 39·9 (2·1-76·8) | 35·9 (8·6-66·5) | 11·4 (0·8-53·5) | 56·6 (37·9-70·4) | 65·0 (44·5-79·5) |
| Southern Africa | 32·7 (10·0-70·1) | 63·3 (40·8-76·7) | 35·9 (10·4-76·8) | 63·0 (41·0-77·2) | 70·5 (45·9-84·3) | 65·8 (49·5-79·4) | 37·7 (10·4-76·9) | 65·5 (55·0-79·0) | 73·3 (61·8-85·3) |
| Western Africa | 12·4 (0·5-33·6) | 35·5 (2·8-59·2) | 15·8 (0·8-40·0) | 43·7 (3·6-62·5) | 50·1 (4·1-70·9) | 42·8 (15·5-62·6) | 18·4 (3·5-42·7) | 55·6 (43·2-65·8) | 63·8 (49·4-74·0) |
| Northern Africa | 45·8 (19·2-74·1) | 59·2 (29·5-77·4) | 49·8 (19·8-76·9) | 59·7 (28·5-77·2) | 65·9 (31·1-82·0) | 65·5 (46·3-80·5) | 54·5 (25·7-80·1) | 66·0 (46·4-79·4) | 72·9 (54·5-84·7) |
| Asia | 61·1 (51·3-71·4) | 72·4 (62·7-78·9) | 64·1 (53·1-74·9) | 72·9 (65·0-78·4) | 77·9 (68·1-82·9) | 74·9 (66·5-79·8) | 65·7 (53·6-76·8) | 75·6 (69·8-80·0) | 81·0 (75·2-85·8) |
| Central Asia | 32·6 (4·8-70·6) | 65·2 (38·4-79·3) | 35·4 (5·1-75·3) | 67·3 (37·0-78·4) | 74·3 (39·9-84·9) | 70·3 (46·9-82·1) | 37·4 (5·2-75·9) | 73·1 (65·6-80·5) | 80·9 (72·8-86·5) |
| Eastern Asia | 81·3 (75·1-85·8) | 83·1 (77·8-88·4) | 83·3 (78·2-87·9) | 82·2 (75·8-86·8) | 85·4 (81·5-89·3) | 83·3 (77·8-89·0) | 83·5 (78·5-88·2) | 82·5 (75·8-87·0) | 85·7 (81·7-89·4) |
| South-Eastern Asia | 36·6 (16·4-66·2) | 55·3 (26·7-73·7) | 42·9 (17·7-72·3) | 59·7 (32·6-71·6) | 67·3 (35·8-80·8) | 59·3 (29·8-75·2) | 45·1 (18·4-73·0) | 64·0 (54·1-73·2) | 72·3 (61·8-82·1) |
| Southern Asia | 33·7 (3·2-63·1) | 60·3 (25·3-74·6) | 37·0 (3·3-66·5) | 61·1 (24·7-74·3) | 68·8 (26·9-84·0) | 66·4 (39·5-78·3) | 41·0 (4·6-77·1) | 67·5 (46·0-77·3) | 75·9 (52·2-85·3) |
| Western Asia | 70·5 (61·6-79·4) | 76·6 (66·1-85·7) | 71·7 (62·4-80·9) | 77·1 (67·0-86·6) | 79·6 (69·0-89·0) | 79·0 (68·9-88·1) | 73·0 (63·2-84·8) | 80·5 (73·3-88·6) | 83·5 (76·9-90·7) |
| Europe | 80·6 (78·0-83·1) | 83·1 (80·5-85·4) | 81·8 (78·7-84·7) | 82·1 (79·2-84·6) | 84·6 (81·1-87·2) | 83·8 (81·6-85·8) | 82·4 (79·8-84·9) | 82·9 (81·1-84·9) | 85·4 (83·2-87·4) |
| Eastern Europe | 68·9 (62·1-74·7) | 73·7 (64·5-79·7) | 71·6 (64·7-78·7) | 72·5 (63·3-78·7) | 77·7 (67·1-83·1) | 75·7 (70·1-80·8) | 73·3 (65·8-80·2) | 74·5 (69·3-78·9) | 80·0 (75·6-84·2) |
| Northern Europe | 85·9 (83·7-88·6) | 87·1 (84·9-89·5) | 86·6 (83·7-89·7) | 86·5 (84·1-89·3) | 87·8 (85·2-90·3) | 87·3 (85·3-89·5) | 86·8 (83·7-89·7) | 86·7 (84·2-89·3) | 88·0 (85·8-90·3) |
| Southern Europe | 83·0 (77·9-87·2) | 85·4 (79·9-89·6) | 83·6 (78·6-88·6) | 84·3 (79·6-88·7) | 86·2 (82·1-90·5) | 85·8 (82·4-89·9) | 84·0 (79·7-88·8) | 84·7 (81·0-88·8) | 86·6 (82·7-90·6) |
| Western Europe | 86·7 (83·7-89·9) | 87·5 (83·7-90·4) | 87·0 (84·6-90·0) | 86·9 (83·7-89·9) | 87·8 (84·8-90·5) | 87·7 (84·0-90·5) | 87·2 (84·7-91·2) | 87·1 (83·9-90·4) | 88·0 (85·0-91·2) |
| Latin America and the Caribbean | 74·3 (68·5-78·6) | 77·9 (72·9-82·2) | 76·8 (70·1-84·5) | 76·6 (71·7-81·3) | 81·1 (77·2-85·5) | 78·3 (73·7-84·1) | 77·2 (70·3-84·6) | 77·1 (72·2-81·3) | 81·6 (77·7-85·8) |
| Caribbean | 69·9 (61·6-79·3) | 73·7 (64·2-84·7) | 72·1 (63·1-81·6) | 73·3 (64·0-83·6) | 77·4 (66·9-86·7) | 75·2 (66·8-85·5) | 72·5 (63·4-82·5) | 75·3 (66·9-84·2) | 79·6 (71·2-88·5) |
| Central America | 68·7 (46·8-77·9) | 76·2 (68·2-82·7) | 70·0 (46·9-79·8) | 74·6 (65·8-82·4) | 78·0 (70·9-83·6) | 77·0 (68·2-82·8) | 70·7 (47·9-79·9) | 75·5 (67·8-82·6) | 78·9 (71·0-83·7) |
| South America | 76·0 (70·5-80·3) | 78·6 (73·7-84·3) | 78·9 (72·3-86·9) | 77·3 (72·7-81·2) | 82·1 (77·6-86·9) | 78·9 (73·7-85·3) | 79·2 (72·3-86·9) | 77·6 (72·7-82·0) | 82·5 (78·0-86·9) |
| Northern America | 89·2 (86·7-91·1) | 89·5 (86·7-91·5) | 89·5 (87·0-91·7) | 89·4 (86·7-91·5) | 89·8 (87·0-91·7) | 89·5 (86·7-91·6) | 89·6 (87·1-92·5) | 89·5 (86·7-91·6) | 89·8 (87·1-92·5) |
| Oceania | 80·0 (74·2-85·8) | 83·5 (77·6-89·1) | 81·3 (74·9-87·2) | 83·5 (77·1-89·1) | 85·6 (78·5-90·3) | 85·0 (80·1-89·6) | 82·4 (75·2-89·4) | 85·2 (80·4-89·8) | 87·6 (83·7-91·3) |
| Australia/New Zealand | 86·0 (79·9-91·6) | 87·6 (83·5-91·6) | 86·8 (80·9-92·1) | 86·7 (82·1-91·6) | 88·4 (84·2-92·1) | 88·0 (83·6-91·7) | 87·2 (81·7-92·2) | 87·1 (82·1-91·7) | 88·8 (84·8-92·3) |
| Melanesia | 17·0 (1·6-58·1) | 40·9 (4·6-72·3) | 23·0 (2·5-72·0) | 49·4 (6·3-70·7) | 56·0 (7·1-80·3) | 53·8 (9·1-74·9) | 31·7 (2·9-74·6) | 65·7 (55·6-74·0) | 74·6 (64·5-82·6) |
| Micronesia | 30·2 (4·7-66·1) | 51·6 (11·9-78·0) | 36·2 (6·5-76·1) | 56·6 (13·2-76·9) | 63·5 (15·1-84·4) | 61·6 (21·5-80·4) | 43·2 (10·3-80·2) | 67·5 (54·6-79·4) | 75·9 (63·0-86·9) |
| Polynesia | 35·3 (3·8-77·6) | 58·9 (9·4-83·0) | 43·5 (5·0-85·3) | 65·3 (10·8-82·9) | 72·9 (11·8-90·0) | 65·7 (16·0-84·0) | 48·5 (12·8-86·4) | 72·1 (58·8-83·9) | 80·5 (68·0-90·6) |
Traditional modalities: chemotherapy, radiotherapy, surgery, ultrasound, x-ray
Table 4:
Policy scenarios to scale-up availability of treatment/imaging modalities to the mean level of high-income countries
| Scale-up to mean of high-income countries | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Scale-up Scenarios |
Treatment | Imaging | Estimated global breast cancer 5- year net survival (%), mean (95% UI) |
|||||||||
| Chemo- therapy |
Radio- therapy |
Surgery | Targeted therapy |
Quality of care |
Ultra- sound |
X-ray | CT | MRI | SPECT | PET | ||
| Baseline (status quo) | 67·9 (62·9-73·4) | |||||||||||
| + Treatment (traditional) | X | X | X | 74·4 (69·9-78·2) | ||||||||
| + Imaging (traditional) | X | X | X | X | X | 75·6 (70·6-79·4) | ||||||
| + Quality | X | X | X | X | X | X | 78·2 (74·9-80·4) | |||||
| + Targeted Therapy | X | X | X | X | X | X | X | 79·1 (75·7-81·4) | ||||
| + CT | X | X | X | X | X | X | X | X | 79·6 (76·3-82·1) | |||
| + MRI | X | X | X | X | X | X | X | X | X | 80·6 (76·3-82·1) | ||
| + SPECT | X | X | X | X | X | X | X | X | X | X | 81·2 (78·7-84·2) | |
| + PET | X | X | X | X | X | X | X | X | X | X | X | 82·3 (79·3-85·0) |
Figure 2:
Estimated 5-year breast cancer net survival with cumulative scale-up of treatment and imaging
DISCUSSION
We find that breast cancer outcomes vary substantially by country income group and region, with much worse stage at diagnosis in lower-income settings and a 25-times difference in survival between low-income and high-income countries. Globally, we find that comprehensive scale-up of treatment, imaging, and quality of care to the mean levels in high-income countries could improve five-year net breast cancer survival by 15 percentage points, with traditional modalities and quality of care contributing to 70% of the increase, achieving most of the potential gains in breast cancer survival in LMICs.
Among single treatment modality interventions, our model results suggest that expanding surgery availability would yield the largest breast cancer survival gains in low-income and lower-middle-income countries, while increasing the availability of targeted therapy would yield the largest gains in upper-middle-income and high-income countries. Improving the availability of radiotherapy is estimated to lead to larger survival gains than chemotherapy in low- and lower-middle-income countries, as radiotherapy is generally less likely to be available at present, offering larger potential gains from scale-up. We also find that the relative priority of single imaging modality policies differs by context. Specifically, we find that expanding access to CT would yield the largest survival gains globally, and in lower-middle income and Latin America and the Caribbean in particular, although these gains are smaller than those offered by improving treatment modalities such as surgery or chemotherapy. In low-income countries, scaling up ultrasound would yield the largest gains, especially in Africa. In contrast, in upper middle-income countries and Asia and we find that MRI would yield the largest survival gains, while PET and SPECT would yield the largest gains in high-income countries, although these gains are small as survival is already relatively high in high-income countries.
However, with the exception of expanding surgery in lower-middle-income countries, which is estimated to increase survival by 10 percentage points, we find that the survival impacts of expanding the availability of any single imaging or treatment modality are relatively modest even in lower-income countries. More comprehensive packages of scale-up will therefore be needed to substantially improve breast cancer survival. To achieve such scale-up, investments must be made across disciplines to ensure successful delivery of effective breast cancer care,9 including equipment, infrastructure, and training of engineering and health professionals.
In addition to scaling up treatment availability and quality of care, health system strengthening efforts to improve cancer prevention and early detection will also be needed, as we find that a survival gap still exists by country income group after comprehensive scale-up due to higher stage at diagnosis (see Figure 2A). These findings suggest that about 70% of the survival gap between low- and high-income countries could potentially be addressed by improving treatment availability and quality of care, with earlier detection of cancers needed to further improve survival. Early diagnosis of breast cancer has a major impact on survival, a concern further emphasized by the recent delay in diagnoses of breast cancer even in high-income countries due to the COVID-19 pandemic.17,18 Although mammographic screening has limitations, such as overdiagnosis,19 there may be opportunities to improve the cost-effectiveness and benefit-harm tradeoffs of breast cancer screening through the use of evolving risk prediction models to guide risk-stratified screening strategies.1,20 Especially in low-resource settings, early diagnosis should be prioritized before screening, and programs should be developed to raise public education/awareness, improve clinical knowledge in primary care, and ensure facilities for adequate diagnosis and treatment are in place, including access to diagnostic imaging.21,22 Universal health coverage (UHC) expansion also offers the potential for prevention and early detection efforts, as well as improved access to treatment. Although we did not examine the impact of UHC in this analysis due to data limitations, future research examining the impact of UHC on cancer outcomes could provide valuable information for policymakers.
Although diagnostic mammography is generally the first imaging study performed in high-income countries for women presenting with breast cancer symptoms and/or signs, the general lack of mammography equipment in lower-income settings means ultrasound is often the main imaging modality for initial breast cancer evaluation. Ultrasound is essential for management of breast cancer and can be used to evaluate a breast complaint, identify a mass, suggest benign versus malignant nature, and can also be used to guide diagnostic biopsies.23
Encouragingly, new technologies have yielded mobile ultrasound devices that are safe, simple, and more affordable. However, although LMICs are using such mHealth ultrasound devices for point-of-care imaging, their use for breast cancer capacity building has so far been limited.24-26 Although a shortage of trained ultrasound providers remains a bottleneck in LMICs, recent studies have shown that community health workers and nurses can be taught to effectively use ultrasound to triage women with breast complaints and prioritize access to care for women with suspicious findings.27 Improvements in digital connectivity which allow imaging studies to be transmitted to central sites for interpretation could also improve the accessibility and quality of staging information.
In addition to ensuring treatment availability, establishing basic imaging capacity, such as ultrasound, can have profound effects on treatment efficacy and patient outcomes for women with breast cancer. Imaging can improve survival outcomes through cancer staging and treatment planning, by guiding interventions, and by assessing response to therapy, with modalities having different impacts by stage of breast cancer, as estimated in our model (see Appendix pg 18-19). After scaling-up traditional treatment and imaging modalities and improving quality of care, the introduction of additional modalities, such as CT, etc. offers further incremental benefits, as demonstrated by our model findings, and can be included as resources allow.
Although we synthesized data from multiple sources, data limitations mean that we had to make assumptions when developing the model. For example, data on the distribution of breast cancer stage at diagnosis were only available for selected countries, and are often not available from population-based cancer registries. In addition, we lacked data on the distribution of breast cancer biological subtypes, which are currently implicitly modelled via the treatment impact parameters of targeted therapy, for which estimates of global availability are also scarce. Similarly, although accounting for quality of care is important to control for health-system and facility-level factors not explicitly included in the model, we lacked empirical estimates of specific quality of care indicators which would be useful to inform our model estimates. For example, a recent study found increasing risks of postoperative mortality in LMICs after cancer surgery, although these differences were not statistically significant for breast cancer, perhaps due to the sample size available.28 We also did not model an explicit time horizon for scale-up, instead assuming immediate scale-up to estimate the potential gains in survival that policies could achieve over a longer period of time. Similarly, we did not have global data on longer-term survival outcomes (e.g. 10-year net survival) with which to fit the model. However, given that longer-term breast cancer survival curves (e.g. from SEER)14 are fairly stable after 5 years (i.e. shallower decline), it is unlikely that evaluating longer-term survival instead would substantially change the relative impacts of the policies considered. Lastly, because we modelled survival for newly diagnosed patients (i.e. conditional on stage and diagnosis), we did not consider the impact of mammography for breast cancer diagnosis (or screening), or the impact of other diagnostics (e.g. pathology) on breast cancer outcomes. Although we are not aware of other modelling studies that have estimated the impact of imaging modalities on global breast cancer survival, our results regarding treatment availability are broadly similar to other modelled estimates. For example, we find that expanding treatment availability in Eastern Africa would increase breast cancer survival by about 13 percentage points, which is similar to the 10 percentage point survival increase estimated by Birnbaum et al.29 from expanding the availability of chemotherapy and endocrine therapy for currently detected ER+ cases in the region. Although these estimates are not directly comparable due to differences in modelled outcomes, this type of broad model benchmarking can help to build confidence in the general results.
Overall, we find that comprehensive scale-up of treatment, imaging, and quality of care could improve global 5-year net breast cancer survival by nearly 15 percentage points. Scale-up of traditional modalities and quality of care could achieve 70% of these total potential gains, with substantial impact in LMICs, providing a feasible pathway to improving breast cancer survival in these settings even without the benefits of future investments in targeted therapy and advanced imaging.
Supplementary Material
Research in context.
Evidence before this study
Recent data on five-year net survival for breast cancer is provided by the CONCORD-3 study. GLOBOCAN 2020, produced by the International Agency for Research on Cancer, also provides modeled mortality estimates for breast cancer. We searched PubMed using the search terms “breast cancer”, “survival”, “global”, and “imaging” on March 25, 2021, without language or publication date restrictions, and found no estimates of the impact of imaging or treatment modalities on global breast cancer survival.
Added value of this study
Using a microsimulation model of global cancer survival for patients diagnosed in 2018, this study provides estimates of breast cancer stage distribution and five-year net survival (stage-specific and overall) for 200 countries and territories. We provide expert opinion consensus on the impact of treatment (chemotherapy, surgery, radiotherapy and targeted therapy) and imaging modalities (ultrasound, X-ray, computerized tomography [CT], magnetic resonance imaging [MRI], positron emission tomography [PET], and single photon emission computed tomography [SPECT]), and estimate the potential breast cancer survival impact of scaling up specific treatment and imaging modalities in different contexts. Among single imaging modalities, we find that expanding CT would yield the largest survival gains globally, while expanding ultrasound would have the largest impact in low-income countries and Africa, with CT in lower-middle, MRI in upper-middle income, and PET and SPECT in high-income countries having the largest impact. Improving surgery availability would yield the largest gains globally among individual treatment modalities, especially in low- and lower-middle income countries, while improving targeted therapy availability would have the largest impact in upper-middle and high-income countries.
Implications of all the available evidence
Breast cancer stage at diagnosis and survival varies substantially by country due to differences in early detection, quality of care, and the availability of treatment and imaging modalities. Comprehensive scale-up of treatment, imaging, and quality of care could improve global 5-year net breast cancer survival by nearly 15 percentage points. Scale-up of traditional modalities (chemotherapy, radiotherapy, surgery, ultrasound, x-ray) and quality of care could achieve 70% of these total potential gains, with substantial impact in LMICs, providing a more feasible pathway to improving breast cancer survival in these settings. Health system strengthening efforts to improve cancer education and early detection will also be needed to reduce global disparities in breast cancer stage at diagnosis and survival.
Acknowledgements
This study was funded by the Harvard T.H. Chan School of Public Health and the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center. This work was supported in part by a grant from the Breast Cancer Research Foundation. AMS is supported by an NHMRC Investigator Fellowship (grant number 1177837).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests. HH receives annual compensation for serving on the Board of Directors of Ion Beam Applications (IBA).
Data Sharing Statement
Country-specific results (means and 95% UIs) are available in a public data repository. [Note: Margin link to Dataverse repository to be provided if accepted for publication]
Contributor Information
Zachary J. Ward, Center for Health Decision Science, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA.
Rifat Atun, Department of Global Health and Population, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA; Department of Global Health and Social Medicine, Harvard Medical School, Harvard University, Boston, MA, USA.
Hedvig Hricak, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Kwanele Asante, African Organisation for Research and Training in Cancer, Cape Town, South Africa.
Geraldine McGinty, Departments of Radiology and Population Science, Weill Cornell Medical College, New York, USA.
Elizabeth J. Sutton, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Larry Norton, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Andrew M. Scott, Olivia Newton-John Cancer Research Institute, Melbourne, Australia; Department of Molecular Imaging and Therapy, Austin Health, Melbourne, Australia; School of Cancer Medicine, La Trobe University, Melbourne, Australia; Department of Medicine, University of Melbourne, Melbourne, Australia.
Lawrence N. Shulman, Department of Medicine, Abramson Cancer Center, University of Pennsylvania, Philadelphia, USA.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; doi: 10.3322/caac.21660. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–92. [DOI] [PubMed] [Google Scholar]
- 4.Azubuike SO, Muirhead C, Hayes L, McNally R. Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World J Surg Oncol 2018; 16(1): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace LE, Mpunga T, Hategekimana V, et al. Delays in Breast Cancer Presentation and Diagnosis at Two Rural Cancer Referral Centers in Rwanda. Oncologist 2015; 20(7): 780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Breast cancer: prevention and control. Available from: http://www.who.int/cancer/detection/breastcancer/en/ (accessed Apr 26, 2021). [Google Scholar]
- 7.Ginsburg O, Yip CH, Brooks A, et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020; 126 Suppl 10(Suppl 10): 2379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Assembly. WHA70.12. Cancer Prevention and Control in the Context of an Integrated Approach. World Health Assembly; 2017. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R12-en.pdf (accessed Feb 4, 2021). [Google Scholar]
- 9.Eniu A, Carlson RW, El Saghir NS, et al. Guideline implementation for breast healthcare in low- and middle-income countries: treatment resource allocation. Cancer 2008; 113: 2269–81. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Framework for Resource Stratification of NCCN Guidelines. Breast Cancer: core resources. Version 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed Feb 12, 2021). [Google Scholar]
- 11.Trapani D, Douillard JY, Winer EP, et al. The global landscape of treatment standards for breast cancer. J Natl Cancer Inst 2021; January 27:djab011. doi: 10.1093/jnci/djab011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Ward ZJ, Scott AM, Hricak H, et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers – global, regional, and country-level simulation results. Lancet Oncol 2020; 21(8): 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Medicare: a strategy for quality assurance. Washington, DC: National Academy Press, 1990. [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 18 Reg Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000-2016) – Linked To County Attributes – Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, release April 2019, based on the November 2018 submission.
- 15.International Atomic Energy Agency. IMAGINE – IAEA Medical imAGIng and Nuclear mEdicine global resources database. https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINE.html [Accessed Feb 13, 2020]. [Google Scholar]
- 16.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020; 21(8): 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gathani T, Clayton G, MacInnes E, Horgan K. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer 2021; 124(4): 710–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puliti D, Duffy SW, Miccinesi G, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 2012; 19 Suppl 1 :42–56. [DOI] [PubMed] [Google Scholar]
- 20.Pal Choudhury P, Wilcox AN, Brook MN, et al. Comparative Validation of Breast Cancer Risk Prediction Models and Projections for Future Risk Stratification. J Natl Cancer Inst 2020; 112(3): 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Heath Global Initiative Global Summit 2007. Cancer 2008; 113(8 Suppl): 2221–43. [DOI] [PubMed] [Google Scholar]
- 22.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer 2008; 113(8 Suppl): 2244–56. [DOI] [PubMed] [Google Scholar]
- 23.Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018; 16(11): 1362–1389. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol 2011; 12(4): 387–98. [DOI] [PubMed] [Google Scholar]
- 25.Ag Ahmed MA, Gagnon MP, Hamelin-Brabant L, Mbemba GIC, Alami H. A mixed methods systematic review of success factors of mhealth and telehealth for maternal health in Sub-Saharan Africa. mHealth 2017; 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker DM, Tafoya CA, Becker SL, Kruger GH, Tafoya MJ, Becker TK. The use of portable ultrasound devices in low- and middle-income countries: a systematic review of the literature. Trop Med Int Health 2016; 21(3): 294–311. [DOI] [PubMed] [Google Scholar]
- 27.Pace LE, Dusengimana JV, Keating NL, et al. Impact of Breast Cancer Early Detection Training on Rwandan Health Workers' Knowledge and Skills. J Glob Oncol 2018; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet 2021; 397(10272): 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birnbaum JK, Duggan C, Anderson BO, Etzioni R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Glob Health 2018; 6(8): e885–e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.